Abstract
The ability to selectively process relevant stimuli is a fundamental function of the primate visual system. The best understood correlate of this function is the enhanced response of neurons in visual cortex to attended stimuli1,2. However, recent results show that the superior colliculus (SC), a midbrain structure, also plays a crucial role in visual attention3–5. It has been assumed that the SC acts through the same well-known mechanisms in visual cortex3,5. Here we tested this hypothesis by transiently inactivating the SC during a motion-change detection task and measuring responses in two visual cortical areas. We found that despite large deficits in visual attention, the enhanced responses of neurons in visual cortex to attended stimuli were unchanged. These results show that the SC contributes to visual attention through mechanisms that are independent of the classic effects in visual cortex, demonstrating that other processes must play a major role in visual attention.
Visual attention is a fundamental brain function that makes it possible to base perceptions and actions on the relevant parts of the environment. In the laboratory, visual attention is typically studied by asking subjects to respond to the properties of a cued stimulus while simultaneously ignoring the content of irrelevant, distracting stimuli. Twenty-five years ago, it was shown that in primate visual cortex, the activity of neurons responsive to cued visual stimuli was higher than the activity evoked by uncued distracters6. This finding, later coined `gain modulation', has been subsequently observed in many different areas of the cerebral cortex2,7,8, in many variants of the cueing task8. Visual attention is now understood to involve a network of areas including frontal and parietal cortex, as well as visual cortex9, and gain modulation of sensory responses is commonly considered to be the keystone of the neuronal mechanisms of attention1,2.
Correlates of visual attention are not restricted to cortex, but have also been found in subcortical structures such as the superior colliculus (SC)10,11 and thalamus12–14. Some of these effects could be inherited from cortex. However, manipulation of neuronal activity in the SC alters or disrupts performance in tasks that test visual attention3–5, showing that the SC plays a causal role. In a recent study using pharmacologic inactivation of the SC, monkeys had to report the direction of motion in a stimulus at a cued location, while ignoring equivalent motion in a `foil' stimulus located elsewhere4. Following SC inactivation, the animals exhibited profound deficits in visual attention – they largely failed to report the direction of motion of the cued stimulus when it was placed in the part of the visual field affected by SC inactivation, and instead reported the direction of motion of the foil stimulus. Activity in the SC is therefore not simply updated about visual attention but appears to be necessary for its normal operation.
Previous studies have generally assumed that the SC plays a part in attention by influencing the well-known mechanisms in visual cortex3,5. If so, then disrupting visual attention by inactivating the SC should change attention-related effects in visual cortex. Here we tested this hypothesis by recording the activity of single neurons in MT and MST, two cortical visual areas well-known for their roles in processing motion signals15 and their modulation by visual attention16, while monkeys performed a motion-change detection task. We measured how neuronal activity was modulated by spatial cues before and during temporary pharmacological inactivation of SC. Contrary to the hypothesis, we found that attention-related effects in MT and MST remained intact even though SC inactivation caused major deficits in the visual attention task.
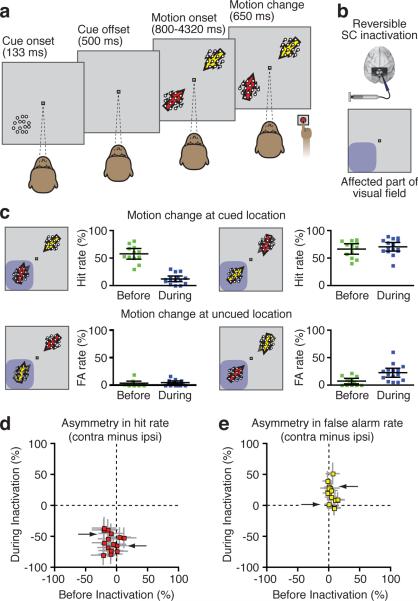
Monkeys performed a motion detection task in which they were rewarded for pressing a button when they correctly detected a change in the direction of motion of the stimulus at the cued location, and ignored changes in the direction of motion of a foil stimulus located diagonally opposite the cued stimulus (Fig. 1a). In trials where the change occurred in the cued stimulus, the animals pressed the button correctly in about 50–60% of the trials (Fig. 1c, pre-injection “hit rates” were 57±21% for M and 53±26% for J). Conversely, they correctly refrained from responding in most of the trials where the change occurred in the distractor stimulus (pre-injection “false alarm” rates were 9±7% for M and 9±15% for J).
Figure 1.
Task design and behavioral performance. a. Following a brief static cue, two motion stimuli moving in opposite directions were displayed in diagonally opposite locations. After a variable delay, the motion direction of one of the stimuli changed slightly. The monkey had to press a button when the change occurred at the cued location. b. We recorded single neurons in areas MT/MST while we injected muscimol in the intermediate/deep layers of the SC. The extent of the effect of the inactivation was assessed by mapping saccade velocities across the visual field. The affected part of the visual field is shown here schematically in blue. c. Motion change detection performance in all experiments, before (green) and during SC inactivation (blue). The red arrow indicates the cued patch while the yellow arrow corresponds to the uncued patch. The affected part of the visual field is illustrated by blue shading. Error bars indicate 95% confidence intervals of the mean. d & e. Difference in cued change detection rate (red) and false alarm rate for uncued motion changes (yellow) between the sides contralateral and ipsilateral to the injection before (X-axis) and during (Y-axis) SC inactivation. Each dot corresponds to a different experiment and the grey lines show the 95% confidence interval (whose computation is based on a method described in29). The arrows point to the data corresponding to the two sample experiments shown in Figure 2.
To test the effects of SC inactivation on attention and sensory cortex activity during this task, we injected muscimol, a GABAA agonist, in the intermediate and deep layers of the SC (Fig. 1b). The extent of the neuronal inhibition caused by the injection was assessed at the beginning and the end of each session, by measuring eye peak velocity during visually-guided saccades17. Each session included two data collection phases – one before and one during SC inactivation.
Consistent with previous results4, we found that SC inactivation caused large and spatially specific deficits in the ability of the animal to detect changes in the cued stimulus with post-injection hit rates dropping to about 10–15% in the part of the visual field affected by SC inactivation (Fig. 1 c–e; see also Supplementary Information). We then tested whether SC inactivation induced comparable changes in the cue-related modulation of activity in MT and MST.
Neurons were recorded in either area MT or MST while the monkeys performed the task, during the same behavioral sessions documented above. The location and direction of motion of the stimuli were based on the tuning properties of the neurons and the size of the motion patch was adjusted to the size of the receptive fields (see Methods). Briefly, either the cued or foil stimulus was placed in the receptive field of the neuron under study, and the direction of motion on each trial was set as the preferred or anti-preferred direction of the neuron, and was always opposite in the two stimulus patches. We recorded a total of 69 MST (31/38, monkey J/monkey M) and 44 MT (34/10) neurons before inactivation and 77 MST (26/51, monkey J/monkey M) and 55 MT (47/8) neurons during inactivation. Some of these neurons were isolated continuously throughout the experiment (MST: n=36 cells, MT: n=18 cells). We provide additional analyses for this particular set of neurons in the Supplementary Information.
Before SC inactivation, as expected from previous studies demonstrating attention-related modulation of visual responses in MST and MT16, we found that neurons recorded in MST (Fig. 2c) and MT (Fig. 2g) exhibited higher discharge rates when the motion stimulus in their receptive field was cued (Cue in, darker lines) than when it was not cued (Cue out, lighter lines). As in previous studies, we quantified this modulation by measuring the discharge rate during the delay period of the task (300–800 ms after motion stimuli onset), and computed a Modulation Index, defined as the difference in discharge rates between “cue in” and “cue out” conditions, divided by their sum. For the two sample neurons shown in Fig. 2, the Modulation Indexes were .16 and .07 for the MST and MT neuron, which correspond to increases in discharge rate of 39% and 15%, respectively.
Figure 2.
Sample neuronal activity before and during SC inactivation. a,b,e,f. Receptive field and tuning properties of a sample MST (a–b) and MT (e–f) neuron recorded both before (a,e) and during SC inactivation (b,f). The blue shading illustrates the extent of the effect of the muscimol injection in these experiments, based on saccade velocities.
c,d,g,h. Response of the same sample MST (c,g) and MT (d,h) neurons during the task, before (c,g, in green) and during (d,h, in blue) SC inactivation, for trials in which the cued patch was in the RF (darker line) or out of the RF (lighter line). The vertical tics mark the onset of the motion stimuli. The gray box illustrates the time epoch used to compute the cue-related modulation analyses.
During SC inactivation, this modulation was intact. Neurons in MST (Fig. 2d) and MT (Fig. 2h) continued to show higher discharge rates for the motion stimulus in their receptive field when it was cued than when it was not cued. The post-injection Modulation Indexes were 0.21 and 0.08 for the MST and MT neuron respectively, which were not significantly different from their pre-injection values, but remained significantly greater than chance (both p<0.0001, Wilcoxon rank-sum test “cue in” versus “cue out”). This cue-related modulation in discharge rate was intact despite the significant decreases in detection performance observed simultaneously during the SC inactivation (Fig. 1d, arrows).
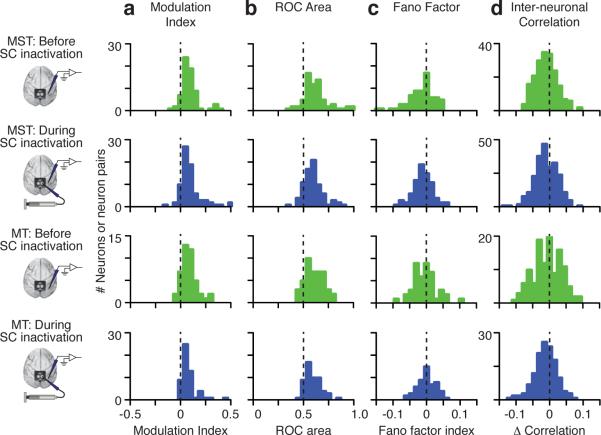
To quantify the effect of SC inactivation across our population, we measured a Modulation Index for each neuron, before and during inactivation. Pre-injection, the average Modulation Index in our sample of neurons was .075±.029 (mean ± 95% ci, median=0.051) in MST and .061±.023 in MT (median=0.048; green bars in Fig. 3a; significantly greater than zero, Wilcoxon signed-rank test, all p < 0.001), corresponding to average increases in discharge rate of 24% and 15%. Post-injection, the average Modulation Index was .071±.025 (median=0.048) in MST and .057±.022 in MT (median=0.041; blue bars in Fig. 3a); these values remained significantly greater than zero (Wilcoxon signed-rank test, all p < 0.001), and were not different from the values before inactivation (Wilcoxon rank-sum test, p > .5; Bayesian p(H0): MST: .99, MT: .985). Thus, SC inactivation produced no appreciable change in the cue-related modulation of average discharge rate across our sample of MST and MT neurons. Similar results were found when the non-preferred stimulus was presented inside the RF (Supplementary Information).
Figure 3.
Population results before and during SC inactivation. Distribution of Modulation Indexes (a), area under ROC curves (b), Fano Factor Indexes (c), and difference in Interneuronal Correlations (d) during the delay epoch before (green) and during (blue) SC inactivation for all MST and MT neurons.
We considered whether SC inactivation might have altered other aspects of cue-related changes in MST and MT neuronal activity. Although modulation of average discharge rate is the standard method for documenting attention-related changes in neuronal activity, it does not measure how noise or variability of discharge rate might change with attention.
To address this point, we computed three additional values for each neuron. First, we computed the area under the ROC curve18, which indicates how well an ideal observer could classify the condition based on the neuron's activity – in our case, whether the cued or uncued stimulus was in the neuron's receptive field. Second, we computed the Fano factor, the ratio of the variance over the mean of the response, which has been found to be lower for cued stimuli than for uncued stimuli19, suggesting that attention decreases the variability of neuronal activity. Third, we computed the noise correlation between pairs of simultaneously recorded neurons, which has recently been found to decrease with attention20,21, improving the signal-to-noise ratio of visual signals across the population of neurons.
These additional measurements were also unchanged by SC inactivation. The ROC areas were significantly higher than chance (Fig. 3b), both before (green) and during (blue) SC inactivation in both MST and MT (Wilcoxon signed-rank test, all p < 0.001), and were not changed by SC inactivation (Wilcoxon rank-sum test, MST: p = 0.30, MT: p = 0.28; Bayesian p(H0): MST: .978, MT: .981); this result indicates that the ability of an ideal observer to discriminate the cued location was unchanged by SC inactivation.
The Fano factor index was significantly less than zero (Fig. 3c) both before (Wilcoxon signed-rank test, MST: p < 0.0001, MT: p =.007) and during inactivation (MST: p < 0.0001, MT: p =.04), and not different from each other (MST p = .66, MT p = .23; Bayesian p(H0): MST: .988, MT: .979); this result shows that the variability in discharge rate was reduced by spatial cueing both before and during SC inactivation.
The change in inter-neuronal correlation was significantly less than zero (Fig. 3d) both before (MST: p<0.0001, MT: p=0.0001) and during inactivation (MST: p=0.0001, MT: p=0.0005), and not different from each other (MST: p=0.76, MT: p=0.71; Bayesian p(H0): MST: .996, MT: .995); this result indicates that spatial cues reduced the correlation in activity between neurons, and this reduction was unchanged by SC inactivation. Similar findings were made with a wide range of bin sizes used to compute the correlations (Supplementary Information).
Finally, we examined cue-related modulations in neuronal activity during other epochs of the task, as well as changes in neuronal activity unrelated to the cue – these were also unchanged during SC inactivation (Supplementary Information). We also confirmed that neuronal activity in the parts of MST we recorded were indeed necessary for the performance of the attention task (Supplementary Information).
In summary, we found that during SC inactivation, the enhanced responses of neurons in visual cortex to attended stimuli were preserved despite large behavioral impairments in a covert attention task. This result was found in two visual areas well-known for their roles in processing motion signals15 and their modulation by visual attention16. Moreover, the attention deficit induced by SC inactivation not only preserved the cue-related changes in visual responses, it also left intact the other known correlates of attention in visual cortex: the ability of neurons to discriminate cued from uncued spatial locations, the reliability of neuronal discharge (i.e. Fano factor), and cue-related changes in noise correlations between neurons. These effects cannot be explained by a sensory impairment, because previous studies have shown that attention deficits during SC inactivation are not caused by changes in local motion perception4. The effects also cannot be explained by a motor deficit, because the single-button response in our task was unimpaired for stimuli outside the affected region of the visual field (Fig. 1c).
These findings demonstrate that the known modulations of activity in visual cortex are not the only mechanisms involved in the control of attention and that other processes must play a major role. One possibility is that visual attention involves other aspects of neuronal activity in these same visual areas. For example, although we found no changes in correlations between nearby neurons, there could be changes between more distant sites or across different areas. A second possibility is that the crucial steps take place in other brain areas entirely – for example, in parietal or prefrontal cortex22, the SC or basal ganglia23. In particular, the frontal eye fields (FEF) exert effects on attention qualitatively similar to the SC24,25. However, because of prominent feedback from FEF to visual cortex, SC-induced changes in FEF might have been expected to also change responses in visual cortex. Finally, it is possible that distinct circuits mediate different aspects of attention. For example, changes in visual cortex might be important for feature-based attention26 and for regulating the perceptual appearance of stimuli27, whereas the mechanism targeted by SC inactivation is important for the all-or-none aspects of spatial attention (e.g., change-blindness28).
Methods
Monkey preparation
We performed neuronal recording in MT/MST and reversible inactivation of the intermediate and deep layers of the superior colliculus in two adult rhesus monkeys (subjects F and M) that were 12–16 years of age and weighed 14–16 kg. The monkeys were prepared using standard surgical techniques described in detail previously1. All experimental protocols were approved by the Institutional Animal Care and Use Committee and complied with US Public Health Service policy on the humane care and use of laboratory animals. The laboratory setup for behavioral control and monitoring was identical to that described previously1.
Attentional task
Trials began with the appearance of a central dot that the monkey had to fixate during the whole trial duration. Achievement of fixation triggered the display of a peripheral stimulus, the cue, consisting of a 5 to 7 degree-wide patch of static dots. The actual size of the patch was chosen as not to exceed the size of the receptive fields of the neurons being recorded. On each trial, the cue could be displayed at one of two possible locations, chosen randomly. One of these locations was chosen to be in the center of the receptive fields of the recorded neurons and the other one was the symmetric location across the fixation point. The cue was displayed for 133 ms and was followed by a 500 ms delay during which only the fixation point was displayed. Two patches of moving dots were then displayed at the two previously described locations. The dots were moving in opposite direction in the two patches, one of which being the preferred direction of the neurons being recorded. The characteristics of the stimulus have been described elsewhere2. In the present case, the dots had an 8-frame lifetime (corresponding to 107 ms). The direction of motion of each dot was drawn from a normal distribution centered on the direction of motion of the patch and with a 16° standard deviation. The direction of motion of the patches remained constant for 800 ms plus a geometrically distributed delay of mean 480 ms (range: 0–3520 ms). This distribution allowed the hazard function to remain flat during the delay. Following this delay, the direction of motion of one of the patches changed. The monkey had to press a button whenever the change in direction occurred at the previously cued location. The change varied from 16° to 20° and was adjusted on the basis of the performance of the monkey at the beginning of each session to keep a global performance of about 75%.
Following the beginning of the change in direction, stimuli remained on the screen for 650 ms or until the animal's response. Monkeys received a liquid reward only for correct responses in completed trials (button press after change occurred at cued location or absence of response when no change occurred or change occurred at uncued location). If the monkey broke fixation midtrial, the trial was aborted and repeated later in the session. This paradigm has been referred to as a “filtering” task, because it requires the monkey to actively ignore stimulus changes at the uncued location. The advantage of this task design is that correct performance requires the filtering out of signals from irrelevant distracter stimuli. This paradigm is similar to that used originally to demonstrate attentional modulation in areas MT and MST19 and more recently to show a causal role of the SC in the control of spatial attention2; it is also similar to that described by Palmer and Moore, 20093.
All stimuli were displayed on a cathode ray tube (CRT) display with a refresh rate of 75 Hz. The background luminance of the monitor was 14 cd m–2. Luminance of the fixation dot and of each dot in the patches was 50 cd m–2. Subjects pushed buttons mounted on a button box at waist level within easy reach of the left hand. Each subject used only its left hand to push buttons.
Procedure
At the beginning of each inactivation session, we lowered a recording tetrode in a track selected on the basis of previous recording sessions. After identification of a good recording spot for MT/MST neurons, we mapped the receptive fields (see examples in Fig. 2a, b, e and f; 50–80 trials) and motion direction tuning properties (Fig. 2a, b, e and f; 30–60 trials) of the isolated neurons and recorded them during performance of the attentional task (232–366 trials). After completion of the pre-injection data collection, an injectrode, whose tip was previously sitting above the quadrigeminal cistern, was lowered into the intermediate and deep layers of the Superior Colliculus and muscimol was injected, following a procedure described earlier2. Following the injection, the extent of the effect of the inactivation was evaluated by measuring eye peak velocity during visually-guided saccades (60–120 trials).
By carefully choosing the injection site on the basis of exploratory recordings, by adjusting the volume of muscimol injected (between 0.4 and 0.6 μl) and the orientation of the bevel of the injection cannula, we were able to localize the affected region of visual space such as to encompass in all experiments the contralateral visual stimulus location used during the attentional task. The affected part of the visual field was defined as the portion of space where velocities were inferior to the lower bound of the confidence interval (alpha = 0.05) of the baseline velocities. Then the MT/MST neurons were recorded again during the receptive field and tuning mapping procedures and during the attentional task. The pre-injection part, including isolation and mapping of the MT/MST neurons lasted 1.5–2.5 hours, the lowering of the injectrode and the injection lasted together about 45 minutes and the post-injection part lasted between 1 and 2 hours. The duration of a whole session lasted between 3.5 and 4.5 hours. There was a total of 12 successful sessions with SC inactivation combined with recordings before and during SC inactivation. For the MST injection experiment (Supplementary Fig. 3), we first lowered the injectrode to a depth previously recognized as being 500 μm above the lower limit of MST. We then injected a first muscimol dose of .5 μl, moved the injectrode up 500 μm, injected again, and so forth up to the upper limit of the area. This led to a total of 4 injections.
Behavioral analysis
Performance in the task was evaluated by the number of correct and incorrect trials (binomial variable) in each condition (ipsilateral versus contralateral and before versus during inactivation). The statistical tests used to assess the significance of the behavioral change induced by the inactivations were logistic regressions, with each condition and their interaction used as categorical predictor variables. Inactivations were considered as having a significant effect when the p-value for the interaction between the conditions ipsilateral versus contralateral and before versus during was inferior to 0.05.
When conducting these analyses on all sessions together, subject identity was added as a random categorical predictor to take into account repeated measurements.
Neuronal recordings
Recordings were conducted with a tetrode (Thomas Recording GmBH). Neuronal signals were amplified, band-pass filtered and digitized (Plexon ® recording system). Neurons were isolated during the experiment to allow for online mapping of their receptive fields and motion direction tuning properties. In parallel, all waveforms passing a manually set threshold were stored for offline sorting. Offline sorting was conducted first automatically (Klustakwik sorting algorithm4) and was then refined manually. On average, we recorded 7.5 neurons per experimental session.
For inactivation experiments, the four-channel waveforms and interspike interval distributions (ISI) of each neuron isolated before muscimol injection was correlated with the waveforms and ISI distribution of each neuron isolated after injection5. We then used these correlation values to identify the neurons that were putatively the same before and during inactivation (see also Supplementary Material).
Motion direction tuning and receptive field mapping
Following isolation of the neurons, the motion direction tuning of the cells was first evaluated, following a procedure similar to Schoppmann et al.6. In brief, the monkey had to fixate a central fixation dot while a whole-screen patch of dots was moving coherently in a direction changing on every frame, leading to a circular motion. The direction of rotation (clockwise or counterclockwise) was selected randomly on every trial. The response of the isolated neurons as a function of the direction of motion of the patch was used to determine their preferred direction of motion.
After the direction tuning procedure, the receptive fields of the neurons were assessed. The monkey had to fixate a central dot while patches of dots moving coherently in the preferred direction of motion of the cells were displayed in quick succession at locations selected randomly from a grid encompassing the whole screen. Typically, 48 different locations were probed.
Bayesian analysis
In order to estimate the probability of an absence of difference between the pre- and post-injection data, we computed the Bayes Factor for the comparison between a model assuming a change in mean value during inactivation and a model assuming no change (null hypothesis, or H0). When necessary, data was transformed to achieve a normal distribution. We computed the Bayes Factor by means of different methods: fractional Bayes factor7, Bayesian Information Criterion8 and Bayesian t-test based on the Savage-Dickey ratio test9. These different methods provided comparable results. We mention in the main text only the posterior probability of the null hypothesis (p(H0)) computed with the fractional Bayes factor method.
Interneuronal correlations
Interneuronal correlations were computed following the same procedure as described in Mitchell et al., 200910. Briefly, the delay period (between 300 and 800 ms following stimuli onset) was divided into non-overlapping bins (4, 6, 7, 11, 16, 22, 31, 45, 63, 83, 125, 250 or 500 ms long) in which spike counts were computed. The average spike count in each bin was subtracted out from the spike count values to remove any stimulus-locked response variation. Similarly, the slow variation in discharge rate over consecutive trials was also removed by subtracting the Gauss weighted smoothing of spike count changes (sigma=5 trials). Pearson correlations were computed for all pairs of units having a minimum discharge rate of 5 spikes per second (MT before inactivation: 122 pairs, MT during: 134 pairs, MST before: 194 pairs, MST during: 235 pairs).
We then estimated the effect of attention on interneuronal correlations by computing the CUE IN – CUE OUT difference in correlations for MST and MT. These differences are shown in Supplementary material for all bin sizes. To illustrate these results in the main article, we chose a bin size of 31ms (shown in Fig. 3), based on the timescale of interneuronal correlations estimated in Bair et al., 200111. Because the spike counts obtained with this bin size were not always normally distributed, we also performed the same analysis using non-parametric Spearman correlations and obtained similar results.
Supplementary Material
Acknowledgements
We thank E. Boehle and N. Dill for technical assistance, and R. Wurtz for critical discussions and reading of the manuscript. This work was supported by the F.M. Kirby Foundation, Inc., and the National Eye Institute Intramural Research Program at the National Institutes of Health.
Footnotes
Author Contributions A.Z. and R.J.K. designed and conducted the experiments and wrote the manuscript. A.Z. analyzed the data.
The authors declare no competing financial interests.
References
- 1.Desimone R, Duncan J. Neural Mechanisms of Selective Visual Attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu. Rev. Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 3.Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc. Natl. Acad. Sci. U.S.A. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nature Neuroscience. 2010;13:261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavanaugh J. Subcortical Modulation of Attention Counters Change Blindness. Journal of Neuroscience. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 7.Treue S. Neural correlates of attention in primate visual cortex. Trends in Neurosciences. 2001 doi: 10.1016/s0166-2236(00)01814-2. [DOI] [PubMed] [Google Scholar]
- 8.Roelfsema PR, Lamme VA, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- 9.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 10.Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- 11.Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nature Neuroscience. 2003;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nature Neuroscience. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- 13.Bender DB, Youakim M. Effect of attentive fixation in macaque thalamus and cortex. Journal of Neurophysiology. 2001;85:219–234. doi: 10.1152/jn.2001.85.1.219. [DOI] [PubMed] [Google Scholar]
- 14.Robinson DL, Petersen SE. The pulvinar and visual salience. Trends in Neurosciences. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- 15.Rudolph K. Transient and Permanent Deficits in Motion Perception after Lesions of Cortical Areas MT and MST in the Macaque Monkey. Cerebral Cortex. 1999;9:90–100. doi: 10.1093/cercor/9.1.90. [DOI] [PubMed] [Google Scholar]
- 16.Treue S, Maunsell JHR. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 17.Hafed ZM, Goffart L, Krauzlis RJ. Superior colliculus inactivation causes stable offsets in eye position during tracking. Journal of Neuroscience. 2008;28:8124–8137. doi: 10.1523/JNEUROSCI.1317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JF, Sundberg KA, Reynolds JH. Differential attention-dependent response modulation across cell classes in macaque visual area V4. Neuron. 2007;55:131–141. doi: 10.1016/j.neuron.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MR, Maunsell JHR. Attention improves performance primarily by reducing interneuronal correlations. Nature Neuroscience. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore T. The neurobiology of visual attention: finding sources. Current Opinion in Neurobiology. 2006;16:159–165. doi: 10.1016/j.conb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? NSC. 1999;89:1009–1023. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- 24.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 25.Wardak C, Ibos G, Duhamel J-R, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. Journal of Neuroscience. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treue S, Martínez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- 27.Carrasco M, Ling S, Read S. Attention alters appearance. Nature Neuroscience. 2004;7:308–313. doi: 10.1038/nn1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rensink RA, O'Regan JK, Clark JJ. To See or not to See: The Need for Attention to Perceive Changes in Scenes. Psychological Science. 1997;8:368–373. [Google Scholar]
- 29.Ross TD. Accurate confidence intervals for binomial proportion and Poisson rate estimation. Comput. Biol. Med. 2003;33:509–531. doi: 10.1016/s0010-4825(03)00019-2. [DOI] [PubMed] [Google Scholar]
References
- 1.Hafed ZM, Goffart L, Krauzlis RJ. Superior colliculus inactivation causes stable offsets in eye position during tracking. Journal of Neuroscience. 2008;28:8124–8137. doi: 10.1523/JNEUROSCI.1317-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nature Neuroscience. 2010;13: 261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer J, Moore CM. Using a filtering task to measure the spatial extent of selective attention. Vision Res. 2009;49:1045–1064. doi: 10.1016/j.visres.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsáki G. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. Journal of Neurophysiology. 2000;84:401–414. doi: 10.1152/jn.2000.84.1.401. [DOI] [PubMed] [Google Scholar]
- 5.Dickey AS, Suminski A, Amit Y, Hatsopoulos NG. Single-unit stability using chronically implanted multielectrode arrays. Journal of Neurophysiology. 2009;102:1331–1339. doi: 10.1152/jn.90920.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoppmann A, Hoffmann K-P. Continuous mapping of direction selectivity in the cat's visual cortex. Neuroscience Letters. 1976;2:177–181. doi: 10.1016/0304-3940(76)90011-2. [DOI] [PubMed] [Google Scholar]
- 7.Berger JO, et al. Objective Bayesian methods for model selection: introduction and comparison. Lecture Notes-Monograph Series. 2001:135–207. [Google Scholar]
- 8.Wagenmakers E-J. A practical solution to the pervasive problems of p values. Psychon Bull Rev. 2007;14:779–804. doi: 10.3758/bf03194105. [DOI] [PubMed] [Google Scholar]
- 9.Wetzels R, Raaijmakers JGW, Jakab E, Wagenmakers E-J. How to quantify support for and against the null hypothesis: a flexible WinBUGS implementation of a default Bayesian t test. Psychon Bull Rev. 2009;16:752–760. doi: 10.3758/PBR.16.4.752. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell JF, Sundberg KA, Reynolds JH. Spatial attention decorrelates intrinsic activity fluctuations in macaque area V4. Neuron. 2009;63:879–888. doi: 10.1016/j.neuron.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bair W, Zohary E, Newsome WT. Correlated firing in macaque visual area MT: time scales and relationship to behavior. Journal of Neuroscience. 2001;21:1676–1697. doi: 10.1523/JNEUROSCI.21-05-01676.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.