Abstract
Bone disorders are of significant concern due to increase in the median age of our population. Traditionally, bone grafts have been used to restore damaged bone. Synthetic biomaterials are now being used as bone graft substitutes. These biomaterials were initially selected for structural restoration based on their biomechanical properties. Later scaffolds were engineered to be bioactive or bioresorbable to enhance tissue growth. Now scaffolds are designed to induce bone formation and vascularization. These scaffolds are often porous, biodegradable materials that harbor different growth factors, drugs, genes or stem cells. In this review, we highlight recent advances in bone scaffolds and discuss aspects that still need to be improved.
Keywords: bone scaffolds, biomolecule delivery, osteoinduction, vascularization, biomechanical properties, in vitro and in vivo studies
Bone scaffolds
Bone tissue engineering is a complex and dynamic process that initiates with migration and recruitment of osteoprogenitor cells followed by their proliferation, differentiation, matrix formation along with remodeling of the bone. Major advances in bone tissue engineering with scaffolds are achieved through growth factors, drugs and gene deliveries. Bone scaffolds are typically made of porous degradable materials that provide the mechanical support during repair and regeneration of damaged or diseased bone [22]. Requirements for an ideal scaffold are highlighted in Box 1.
Box 1. Requirements for an ideal scaffold.
The biomechanical system of bone is complex so that the following requirements for an ideal scaffold are diverse.
Biocompatibility One of the primary requirements of any bone scaffolds is biocompatibility a term which has been described in many ways. Biocompatibility of a scaffold is described as its ability to support normal cellular activity including molecular signaling systems without any local and systematic toxic effects to the host tissue [78]. An ideal bone scaffold must be osteoconductive where the scaffold lets the bone cells to adhere, proliferate and form extracellular matrix on its surface and pores. The scaffold should also be able to induce new bone formation through biomolecular signaling and recruiting progenitor cells, a property known as osteoinduction. Furthermore, an ideal scaffold needs to form blood vessels in or around the implant within few weeks of implantation to actively support nutrient, oxygen and waste transport [1].
Mechanical properties The mechanical properties of an ideal bone scaffold should match host bone properties and proper load transfer is important as well. Mechanical properties of bone vary widely from cancellous to cortical bone. Young’s modulus of cortical bone is between 15 and 20 GPa and that of cancellous bone is between 0.1 and 2 GPa. Compressive strength varies between 100 and 200 MPa for cortical bone, and between 2 and 20 MPa for cancellous bone. The large variation in mechanical property and geometry makes it difficult to design an “ideal bone scaffold” [1].
Pore size A must have property for scaffolds is interconnected porosity where pore size should be at least 100 μm in diameter for successful diffusion of essential nutrients and oxygen for cell survivability [25]. However, pore sizes in the range of 200 to 350 μm are found to be optimum for bone tissue in-growth [79]. Furthermore, recent studies have indicated that multi-scale porous scaffolds involving both micro and macro porosities can perform better than only macro porous scaffold [43]. Unfortunately, porosity reduces mechanical properties such as compressive strength, and increases the complexity for reproducible scaffold manufacturing. Researchers have explored porous scaffolds using polymers, ceramics, composites and metals. Strength of dense bioceramic materials matches close to the cortical bone, and different polymers to that of cancellous bone, however ceramic-polymer composite scaffolds are typically weaker than bone. Porous metallic scaffolds meet the mechanical requirements of bone, but fail to provide the necessary implant-tissue integration and add the concern related to metal ion leaching [35].
Bioresorbability Bioresorbability is another crucial factor for scaffolds in bone tissue regeneration [78]. An ideal scaffold should not only have similar mechanical properties that of the host tissue, but also be able to degrade with time in vivo, preferably at a controlled resorption rate and eventually creating space for the new bone tissue to grow. The degradation behavior of the scaffolds should vary based on applications such as 9 months or more for scaffolds in spinal fusion or 3 to 6 months for scaffolds in cranio-maxillofacial applications. Naturally, design and manufacturing of multi-scale porous scaffolds having ideal composition including targeted biomolecules, mechanical properties and related bioresorbability are some of the key challenges today towards their successful implementation in bone tissue engineering [1,50].
Biomolecule delivery
Scaffolds are also used to deliver biomolecules that can facilitate bone tissue engineering. Biomolecules integrated into scaffolds are proteins / growth factors such as TGF-β, BMP, IGF, FGF, and VEGF. These growth factors control osteogenesis, bone tissue regeneration and ECM formation via recruiting and differentiating osteoprogenitor cells to specific lineages [23]. Therefore, incorporating different growth factors and other biomolecules are of special interest for bone tissue engineering. For example, IGF helps in migration of different bone cells required for bone healing while BMPs induces early stage proliferation and differentiation of osteoprogenitor cells [9,10]. In animal models, it has been shown that introducing specific biomolecules can enhance the union of nonunion type (a fracture that does not heal by itself after several months) bone fractures [24]. The effective incorporation of biomolecules and growth factors in scaffolds could reduce wound healing time and thus help in patient recovery.
Angiogenesis in bone scaffolds
Bone is highly vascularized; therefore, the performance of a bone scaffold is dictated by its ability to induce new blood vessel formation [25–27]. In vivo conditions, supply of oxygen and nutrients are essential for the survival of growing cells and tissues within scaffolds [27]. The inflammatory wound healing response induces spontaneous vascularization after scaffold implantation [25], though it takes weeks to form a complex network of blood vessels. Osteoconductive or osteoinductive bone scaffolds do not induce vascularization. Moreover, improper and insufficient vascularization leads to oxygen and nutrient deficiency, which may result in non-uniform cell differentiation and cell death [28]. VEGF can be used to induce a complex network of blood vessels throughout a scaffold [25,29–32].
This review focuses on recent advances in biomolecule-incorporated scaffolds and their osteogenic and angiogenic properties. We first discuss physical requirements of bone scaffolds and their fabrication techniques. We then discuss in vitro responses and in vivo osteoconductive properties of these scaffolds. Finally, we discuss the role of various biomolecules delivery on osseointegration and angiogenesis in the bone tissue engineered scaffolds. We conclude with critical issues and future developments of scaffolds for next generation bone tissue engineering.
Design and fabrication of scaffolds
Bone is a natural composite of collagen and hydroxycarbonate apatite with 10 to 30% porous hard outer layer i.e., cortical bone; and 30 to 90% porous interior i.e., cancellous bone. Mechanical properties of bone vary widely from cancellous to cortical bone which along with complex geometry makes it difficult to design an “ideal bone scaffold” (Box 1). The key factors for an ideal scaffold for bone tissue engineering are - i) macro- (pore size >100 μm) and micro-porosity (pore size < 20 μm); ii) interconnected open porosity for in vivo tissue in-growth; iii) sufficient mechanical strength and controlled degradation kinetics for proper load transfer to the adjacent host tissue, iv) initial strength for safe handling during sterilizing, packaging, transportation to surgery, as well as survival through physical forces in vivo; and v) sterile environment for cell seeding [1,25].
Fabrication techniques
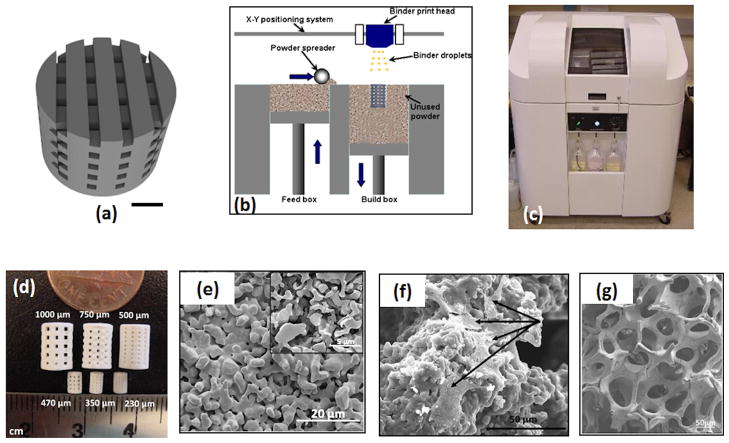
Among various fabrication techniques, SFF based techniques are probably the most widely studied for fabricating three dimensionally (3D) interconnected porous scaffolds [2–4]. SFF is a general approach in which 3D parts are printed layer-by-layer based on a computer aided design (CAD) file. There are many commercial SFF techniques available for different materials. Figure 1(a–b) show schematics of the 3D printing process. First a CAD file is created according to the geometry and porosity of the scaffold (Figure 1a). The 3D printing system has a deposition bed, a feed bed, a powder spreader, a print head and a drying unit (Figure 1b). Figure 1c shows a 3D ceramic scaffold printer (R-1 R&D printer by ProMetal, Ex One Company, Irwin, PA). Initially, the printer head sprays the binder on the loose powder according to the specific CAD file, followed by lowering the deposition bed and raising the feeder bed. A metallic roller then evenly spreads the powder over the binder which then goes to the dryer. The process is repeated, layer-by-layer, until the specific part is built [33]. Ceramic parts can then be densified at high temperature to achieve higher mechanical strength. Figure 1d shows 500, 750 and 1000 μm designed pore sized ceramic scaffolds before and after sintering [34]. Along with macro pores, the scaffolds were characterized for micropores along the struts of the scaffolds (Figure 1e). SFF methods can also be applied to metallic or polymeric materials.
Figure 1.
(a) CAD image of a porous scaffold. Square channels are oriented at 0°/90° for succeeding layers. The scale bar represents 1cm [33]. (b) Schematic drawing of the SFF 3D printing process. In this process, a printer head sprays the binder on the loose powder bed according to a specific CAD file. A layer of powder is then laid over the binder with a metallic rod followed by binder drying. The process is repeated number of times to build the desired part. (c) The ExOne (Ex One Company, Irwin, PA) 3D printer to create interconnected porous 3D ceramic objects. (d) Digital photograph showing 3D printed TCP scaffolds after sintering. The larger samples are for mechanical characterization and small samples for in vivo testing [34]. (e) Surface morphology of 3D printed TCP scaffolds after microwave sintering at 1250 °C showing a porous scaffold strand. Inset scanning electron micrograph images shows the presence of microporosity in the scaffold [34]. (f) Micrographs of hFOB cells showing the cell morphology and adhesion behavior inside the macropores of 3D printed Si/Zn doped TCP scaffolds after 7 days of culture. Osteoblast cells are indicated by arrow [33]. (g) SEM morphologies of the TCP scaffolds coated with 2.5% PCL w/v in dichloromethane prepared by lost mold method shows the interconnected porosity [54].
LENS™ has been used to produce porous metallic scaffold using titanium, tantalum and their alloys [5,6]. In this process, a high power laser locally melts metal powder particles that are injected at the focal point of the laser on the substrate, and the liquid metal is used to build parts layer-by-layer [5]. The process is repeated until a 3D porous scaffold is formed. Other SFF techniques to prepare porous scaffolds have been discussed elsewhere [4].
Common practices to fabricate 3D composite scaffold are thermally induced phase separation (TIPS), solvent casting / particle leaching, microsphere sintering and scaffold coating [35][36]. Another approach, electrospinning the polymeric scaffold, shows great promise. Here, polymer solution is injected through a needle under an electric field where a spinning surface gives shape to the scaffold [36,37]. Although it was primarily designed for polymeric scaffolds, ceramic-polymer composites were also successfully fabricated using this approach [38–40].
In vitro and in vivo evaluation of bone scaffolds
The following summary highlights scaffolds made with different materials that have been tested under in vitro and in vivo conditions.
Calcium phosphate (CaPs) based bioactive ceramic scaffolds
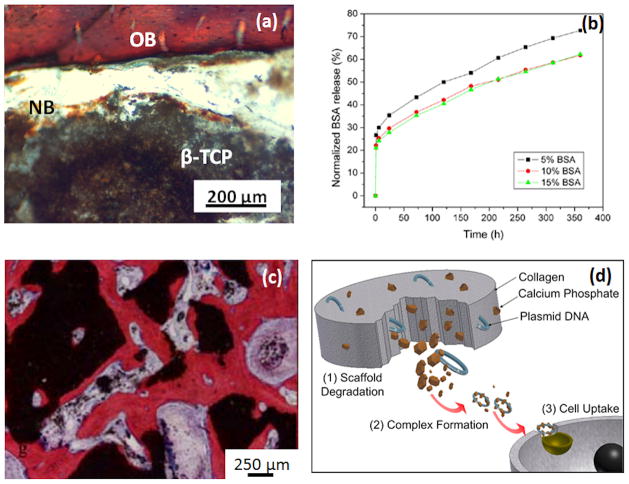
Being a major constituent of bone, CaPs have been extensively studied as scaffold material for bone tissue engineering. Among different CaPs, the majority of research has been focused on hydroxyapatite (HA), beta-tricalcium phosphate (β-TCP) or mixture of HA and β-TCP, known as biphasic calcium phosphate (BCP). These materials have long been studied to fabricate porous scaffolds for bone tissue engineering [41][35]. The polymer replica method can be used to prepare BCP scaffolds (80 ± 3% HA and 20 ± 3% β-TCP) with 70% interconnected porosity (68% pores are 400 μm, and ~ 3% are 0.7μm in size), which successfully support new bone formation in immune-deficient male mice [42]. A combination of macro-porosity (250–350 μm) and micro-porosity (2–8 μm) results in lamellar and woven bone formation in HA scaffolds, a property absent in scaffolds without micro-porosity [43]. Microwave sintered 3D printed β-TCP scaffolds with >60% porosity not only facilitated osteoblast activity but also aided in new bone formation in the pores [34]. High compressive strength of 10.95 ± 1.28 MPa was reported considering ~ 50% porosity by volume in CaP scaffold. The scaffolds also supported new bone formation when implanted in rat femur as shown in Figure 2(a) [34].
Figure 2.
(a) Photomicrograph of the 3D printed TCP scaffolds of 350 μm pore size showing development of new bone formation after 2 weeks implantation in the rat femur. Modified Masson’s Goldner trichrome staining of a transverse section: OB: old bone; NB: new bone; MC: mesenchymal cell; NB: osteoid-like new bone [34]. (b) BSA release pro le from a PCL-coated TCP scaffolds. It is evident that the presence of PCL helped in achieving a sustained release of BSA [54]. (c) Micrographs showing bone formation and scaffold degradation of a TCP scaffold loaded with BMSCs [62]. (d) Schematic representation showing degradation behavior and delivery of VEGF from a CaP/collagen scaffold without a non-viral vector. The concept is that the degradation of scaffold will release the plasmid DNA along with CaP. Both CaP and DNA will form a complex that can be up taken by targeted cell and express VEGF and lead to angiogenesis [72].
Recent results on CaPs indicate dopant addition in scaffolds can control dissolution rates, densification behavior, mechanical strength, and biocompatibility [33,44,45]. 0.5 % SiO2 and 0.25 % ZnO doping in β-TCP scaffold can result in a 2.5 fold increase in compressive strength and up to 92% increase in cell viability by day 11 [33]. Figure 1(f) shows the human fetal osteoblast (hFOB) cell attachment inside the macropores of a 3D printed Si/Zn doped β-TCP scaffold [33]. The cells anchor to the micropores within the macroporous struts. Both Zn and Si doping can increase type 1 collagen (COL1) gene expression and extracellular signal regulated kinases (ERK) secretion that positively regulates angiogenesis, osteoblast proliferation, differentiation and morphogenesis [46].
Bioglass based bioresorbable scaffolds
Since the development of resorbable 45S5 Bioglass®, many different compositions have been explored over the years [47]. When tested in vitro, a 70% porous 3D bioglass scaffold with 300 to 400 μm pore size showed hydroxy carbonate apatite (HCA) layer formation on its surface that significantly enhanced osteoblast activity [48]. The HCA layer also adsorbs protein and growth factors that facilitated new bone formations in vivo. [48]. In a recent work, cobalt (Co) was introduced in meso-porous bioglass scaffold to induce hypoxia (low oxygen pressure) that increased bone marrow-derived stem cells (BMSCs) proliferation, differentiation, VEGF secretion, HIF-1α expression and bone related gene expression [49].
Polymeric scaffolds
Polymers can be both bioactive and biodegradable [50]. Commonly used natural polymers for bone tissue engineering are collagen, fibrin, alginate, silk, hyaluronic acid, and chitosan [51]. Flexibility in processing and ability to tailor the chemistry of polymers are added advantages. Degradation of synthetic polymers such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and polycaprolactone (PCL) produces monomers which are readily removed by the natural physiological pathway. Some polymers such as poly(propylene fumarate) (PPF) show high compressive strength that is comparable to cortical bone and their degradation time can be controlled over a wide range [52]. However, the polymeric scaffolds show rapid strength degradation in vivo even with high initial strength [53]. Degradation of certain polymers (PLA, PGA) creates a local acidic environment that can also have adverse tissue responses.
Composite scaffolds
Composites are those that are made of two or more distinctly different materials such as ceramics and polymers. Development of an interconnected CaP-polymer scaffold takes advantages of both CaPs and polymers to meet mechanical and physiological requirements of the host tissue. Polymer in CaP scaffolds increases toughness and compressive strength similar to bone. Similarly, mechanical integrity and bioactivity of polymers can be improved adding CaP. Figure 1(g) shows interconnected TCP scaffold coated with PCL that has been fabricated using a sacrificial polymer foam [54]. A PGA/β-TCP (1:3 weight ratios) 3D porous composite scaffold, prepared by solvent casting and particulate leaching method, with 88.4 ± 0.7 % open porosity having pore size 483.3 ± 113.6 μm can degrade up to 96.2 ± 3.3 % after 90 days of implantation in Sprague-Dawley male rats [23]. The HA/ poly(ester-urethane) (PU) composite scaffolds are also known to adsorb higher amounts of bovine serum albumin (BSA), bovine fibrinogen and fetal calf serum (FCS) in vitro compared to PU scaffold [55]. The micro-computed tomography (μCT) reconstruction study showed that a 200μm sized porous HA/PU scaffolds with 90 ± 2 % volume fraction porosity could be fabricated using traditional salt leaching /phase inversion process. Although there was no significant difference in angiogenesis, HA/PU and PU scaffolds showed 49 and 55 cm cm−2 blood vessel growth in the border zones and between 0.4 and 3 cm cm−2 in the center zones at day 14 after implantation in mice dorsal skinfold chamber model. Even a surface modification of BCP porous scaffold with HA/PCL composite has shown to increase the compressive strength by a factor of two [56]. Surface modification also encouraged differentiation of primary human bone derived cells, with substantial upregulation of osteogenic gene expression (Runx2, collagen type I, osteocalcin (OC) and bone sialoprotein) and alkaline phosphatase (ALP) activity. TCP/PCL composite scaffolds are also studied for possible protein delivery. Figure 2 (b) shows the release profile of BSA from a porous TCP/PCL scaffold. A sustained release of BSA was noticed even after 2 weeks due to PCL coating [54]. Polymer/Bioglass composite scaffolds also have shown promise in bone tissue engineering. A nano composite of collagen and Bioglass have shown early mineralization within 3 days of immersion in SBF along with increased ALP expression after 21 days of culture [44].
Metallic scaffolds
Metals have high compressive strengths and excellent fatigue resistance. Porous metallic scaffolds, predominantly made of titanium (Ti) and tantalum (Ta), have been studied as bone replacement materials [5,57]. LENS™ processed 17 to 58 vol% porous Ti with an average pore size of 800 μm allowed strong osteoblast cell attachment and proliferation [6]. However, unlike CaP or polymeric scaffolds, biomolecules cannot be integrated into these scaffolds and they are not biodegradable. Moreover, there are concerns related to metal ion release. Surface modification techniques are often employed to improve bioactivity of Ti scaffolds [58]. Recent developments in bioactive metals report an orthopaedic biodegradable material substitute which can be used especially for load bearing applications [59]. The early stages of in vivo biocompatibility of magnesium (Mg) scaffolds have recently been established [60].
Third generation scaffolds
Although CaP scaffolds allow new bone formation and biomineralization, next generation scaffolds are predicted to be osteoinductive. Different approaches have been investigated to make CaP scaffold osteoinductive, which includes but is not limited to modifying scaffold chemistry, seeding bone marrow stem cells and incorporation of different growth factors such as TGF-β, BMP, and VEGF in the scaffold. Osteoinduction is associated with both materials compositions and porosity. Si-TCP/HA with 60% porosity showed better bone - scaffold integration than 80% porous pure HA scaffold [61]. Both scaffolds were similarly seeded with BMSC and ectopically implanted in immune-deficient mice.
Cell seeded CaP scaffolds can be as good as autograft, if not better, which is considered the gold standard for bone substitute materials. BMSC seeded 70 ± 5% porous BCP (80±5wt% HA and 20±5wt% β-TCP) and 100% β-TCP scaffold results in higher bone formation compared to natural bone grafts [62]. Figure 2 (c) shows abundant bone formation when the BCP scaffolds are seeded with BMSC [62]. Mesenchymal stem cells (MSCs) are used along with collagen hydrogels to form bone-like tissue [63]. Interestingly, the tissue stiffness and ultimate burst strength increased in a time dependent manner due to differentiation of MSCs into osteoblast. Collagen hydrogel also increased OC secretion, Ca deposition, and Runx2/osterix mRNA levels under in vitro culture conditions [63]. Collagen-CaP cements were used to carry human umbilical cord mesenchymal stem cells (hUCMSCs) for rapid bone tissue engineering [64]. MSCs are also used in combination with endothelial cells to support the complexity of bone dynamics in bone replacement scaffolds. A complex 3D vascular network was noticed in decalcified processed bovine cancellous bone (allograft) when implanted in mice seeded with both MSC and human umbilical vein endothelial cells (HUVECs) [65].
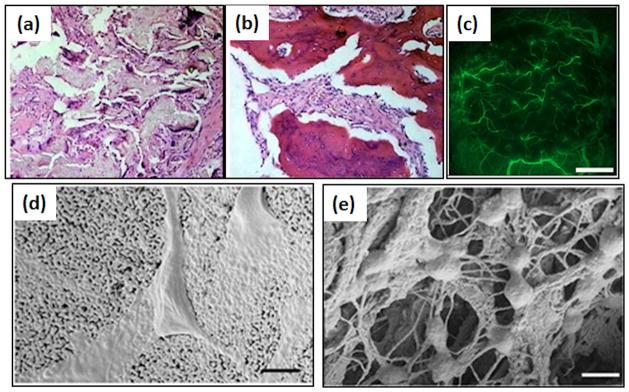
Major advancements in bone tissue engineering are also achieved through growth factors, drugs and gene delivery. Figure 3(a) and (b) shows that when nano-hydroxyapatite / collagen/poly (L-lactic acid) scaffolds are loaded with BMP-2, new bone deposits faster than pure scaffolds [24]. A 5μg/ml VGEF resulted in an increase in blood vessel density (counts/mm3) of 83.8 ± 16.5 compared to 53.8 ± 10.9 after 28 days in Balb/c mice, but also resulted in a 3-fold increase in bone formed inside the macropores [30]. Enhanced vascularization provided abundant osteoprogenitor cells to the defect site along with direct stimulating effects on osteoblast migration and differentiation, leading to higher bone deposition. Figure 3 (c) shows prominent vessel formation after 28 days of implantation due to VEGF incorporation in BCP scaffolds [30]. Recent studies with BMP-2 and VEGF co-loaded scaffold resulted in both enhanced vascularization and new bone formation [66–68]. Another growth factor, IGF, gained significant interest in fracture healing due to its ability to stimulate proliferation and chemotactic migration of different bone regeneration cells [10]. ESCs, which can potentially differentiate into all types of somatic cells, can be led to osteogenic differentiation with the addition of IGF2 [11]. Bone replacement scaffolds are often loaded with drugs and growth factors to treat bone defects along with introducing osteoinductivity. Commonly used drugs include Gentamicin, Vancomycin, Alendronate, Methothrexate, and Ibuprofen [69,70].
Figure 3.
(a) and (b) represents photomicrograph of 12 weeks post-operative histological samples of nano-hydroxyapatite/collagen/poly (L-lactic acid) and nano-hydroxyapatite/collagen/poly (L-lactic acid)/BMP-2, respectively. BMP-2 loading results in larger area of new bone formation (dark red regions) (magnification: ×200) [24]. (c) Visualization of blood vessel formation in effect of VEGF added BCP ceramics implanted into the cranial window for 2 days, using a vertical illumination fluorescence microscope. Plasma marker fluorescein-isothiocyanate-labeled (FITC) dextran was used to study the microcirculation. Scale bars represent 1mm [30]. Cellular interactions with (d) HA and (e) HA-collagen scaffolds indicate the differences between cellular adhesion behaviors. Bars: 500 nm. The cells anchored on the collagen nanofibers in HA-collagen scaffold [77].
Although not very popular and efficient, gene therapy has also been explored to modulate osteoinductive properties of growth and transcription factors [50][71][72]. Here, genes encoding growth factor delivery to specific cells are used to express exogenous genes and proteins in the surrounding tissues [73] [30]. Figure 2 (d) shows a schematic presentation of CaP/polymer degradation in vivo, the release of loaded VEGF and cellular uptake of VEGF without a non-viral vector and a possible future angiogenesis [72]. Gene therapy showed promising bone gap bridging results when a collagen sponge was seeded with BMP-9 gene transfected MSCs and placed in mice [71].
Critical issues in bone tissue engineering scaffolds
Several in vitro and in vivo studies have demonstrated excellent biocompatibility and new bone formation for a variety of bone scaffolds, however, some key challenges still remain: i) biocompatibility and biomechanical strength in polymer scaffolds, ii) metal ion release, limited bioactivity and biodegradation for metallic scaffolds and iii) toughness as well as reliable and reproducible manufacturing techniques for ceramic scaffolds. Moreover, controlling the degradation rate of any scaffold material to match that of the regeneration rate of the replacing bone tissues requires better understanding and future development.
Several growth factors have been identified that have positive effects on osteogenesis and angiogenesis. Appropriate dosage and release profiles are very important for optimized biomolecule delivery. For example, BMP-2 induces osteoinductivity in a dose dependent manner: μg quantities act as differentiation stimuli for direct endochondral ossification and chondrogenesis of MSCs, while ng levels recruit stem cells through chemotaxis [51][74][75]. Similarly, slow and sustained release of VEGF can produce well-functioning blood vessels whereas uncontrolled release of VEGF lead to malformed and non-functional blood vessels [66,76]. Controlling the release of VEGF or other growth factors is one of the primary concerns in bone tissue engineering.
Scaffolds are designed with interconnected porosity in which osteogenic and angiogenic agents are added. However, organization of porosity in the scaffolds can play a significant role in the quality of bone formation (Figure 3(d) and (e)) [77]. Ordered geometry of HA foams deposited and led to self-assembly of collagen in the pores, which resulted in a compact lamellar bone [77]. In comparison, an HA/collagen composite with a disordered pore structure, initiates collagen deposition in a nematic phase and eventually yields a woven isopotic bone [77]. Therefore, understanding related to effects of pore orientation on quality and quantity of bone formation is needed to design optimally performing bone scaffolds.
Concluding remarks and future direction
Research on bone tissue engineering over the past decade has inspired innovation in new materials, processing techniques, performance evaluation and applications. Significant progress has been made towards scaffold materials for structural support with desired osteogenesis and angiogenesis abilities. Bioresorbable scaffolds with controlled porosity and tailored properties are possible today due to innovation in scaffold fabrication using advanced technologies, e.g. SFF. One of the drawbacks of porous scaffolds is that, independent of composition, it is mechanically weak. Porosity in most of these scaffolds is uniformly distributed throughout the scaffold dimension. However, the scaffold may not need to be uniformly porous. Natural bone does not have a uniform distribution of porosity: it has higher porosity in the core with a strong and dense outer shell. A gradient distribution of porosity from the center to the periphery of the scaffold can be achieved through complex design and manufacturing that will ensure mechanical integrity and scaffold interconnectivity.
We also need to develop new material combinations that are strong but can have, timed bioresorption. Normally polymer-ceramic composite scaffolds are designed and fabricated to address these issues. However, polymeric materials degrade faster than most of the ceramic materials thereby making the scaffold degradation uneven which can also create issues such as osteolysis. To achieve a uniform resorption of the scaffolds, degradation of polymer and ceramic materials should match. One approach can be using amorphous calcium phosphate (ACP) which degrades faster than its crystalline counterpart. Degradation of ACP creates a calcium rich environment for faster apatite deposition. Alternatively slower degrading polymeric scaffold materials can also be developed.
New frontiers of research should be directed towards better mimicking the natural process of bone tissue regeneration such as coupling between angiogenesis and osteogenesis which may require progenitor cell recruitment and differentiation. An orchestrated performance of each of these biomolecules is required for successful development of bone tissue within the scaffold. It is not only the combinational therapy of biomolecules that is required for optimized bone tissue engineering, sequential and sustained delivery of the biomolecules are also important. Scaffold pore size may play an important role in controlling the delivery efficiency and rate of delivery. It may be possible that micropores in scaffolds are optimized such that the capillary action will prevent the entrapped biomolecules from burst release. Degradation of the scaffold will therefore release the biomolecules in a time dependent manner, a much anticipated and essential requirement of the third generation scaffolds. A sequential delivery of biomolecules can also play a significant role in modulating the natural bone remodeling process. An initial release of angiogenic growth factor can induce new blood vessels at an early stage of bone healing whereas later stage release of BMP, IGF can induce the osteogenic properties. A combination of these factors can reduce the amount of growth factors needed, and simultaneously enhance tissue integration in vivo. However, the mechanism of action to which these parameters may influence bone and the healing process is still a mystery. Some knowledge towards specific signaling pathways and regulatory factors can shed light towards our understanding mechanisms at the cellular and molecular level for osteoblastic differentiation and ECM mineralization.
While it is difficult to mimic nature, recent scientific and technological findings show potential to achieve bone scaffolds that would encourage local and systemic biological functions. Proper selection of scaffold materials, their geometry, pore size and size distribution and ability to release biomolecules at a desired rate will play critical roles in future development of bone scaffolds. Effective optimization of those properties towards scaffold development in the future can only be possible using interdisciplinary approaches at multiple length-scales.
Table 1.
physical and mechanical properties of bone scaffolds
| Scaffold composition | Porosity (%) | Pore size (μm) | Compressive strength (MPa) | Ref |
|---|---|---|---|---|
| 80 ± 3% HA + 20 ± 3% β-TCP | 70 | 400 | Not available | [42] |
| HA | 41 | 250–350 and 2–8 | 34.4 ± 2.2 | [43] |
| β-TCP | 50 | 400 | 10.95 ± 1.28 | [34] |
| β-TCP + 0.5% SiO2 + 0.25% ZnO | 46.44 | 698.35 ± 3.48 | 10.21 ± 0.11 | [33] |
| 33% HA + 67% Si- β-TCP + BMSC | 60 | - | - | [61] |
| 80 ± 5% HA + 20 ± 5% β-TCP + BMSC | 70 ± 5 | 1–1000 | - | [62] |
| Na2O-K2O-MgO-CaO-B2O3 - P2O5–SiO2 | 70 | 300–400 | - | [48] |
| Bioactive glass (Ca/p/Si=15/5/80 molar raio) | 0.30 cm3/gm | 300–500 and 4–5 nm | - | [49] |
| 6Na2O, 8K2O, 8MgO, 22CaO, 54B2O3, and 2P2O5 | 20 | 5–10 | - | [80] |
| PGA: β-TCP = 1:3 | 88.4 ± 0.7 | 483.3 ± 113.6 | - | [23] |
| HA: PU = 1:5 | 90 ± 2 | 200 ± 16 | - | [55] |
| (40% HA + 60% β-TCP) coated with HA/PCL | 90.8 | 550 | 2.1 | [56] |
| TCP scaffold coated with 5% PCL | 70 | 300–800 | 2.41 | [54] |
| Porous Ti | 17–58 | 800 | 24–463 | [6] |
| Porous Ta | 27–55 | 200–2000 | 100–746 | [5] |
Acknowledgments
The authors like to acknowledge the support from the National Institute of Health, specifically NIBIB (Grant no. R01A1EB 007351).
Glossary
- Cortical bone
the outer part of a bone that is dense with high strength (100 and 200 MPa) and high modulus (15 – 25 GPa). The primary role of cortical bone is to provide structural support to body and protect vital organs [1]
- Cancellous bone
the inner part of a bone that hosts the bone marrow and responsible for blood cell generation; often called “spongy bone” due to its resemblance with a sponge or foam. Cancellous bone is weak in mechanical properties: Young’s modulus is between 0.1 and 2 GPa and the compressive strength is between 2 and 20 MPa [1]
- Osteoconductivity
A materials property that lets the bone cells to adhere, proliferate and form extracellular matrix on its surface and pores [1]
- Osteoinductivity
A materials property to induce new bone formation through biomolecular signaling and recruiting progenitor cells [1]
- Solid freeform fabrication (SFF)
a generic term used to describe three dimensional layer-by-layer printing of any object without any part specific tooling from its computer aided design (CAD) file. The process has been successfully used to fabricate polymer, ceramic, metal and composite scaffolds for bone tissue engineering [2–4]
- Laser engineered net shaping (LENS™)
a layer by layer SFF process that uses a high power laser (between 500 W and 2 KW) to melt metal powders to form three dimensional structures based on CAD data. Laser is focused onto a metal substrate to create a molten metal pool where metal powder is externally fed into the metal pool in controlled environment. Moving the substrate in the X-Y direction creates a pattern and fill material in the desired area forming a layer. The next layer is built on top of the previous layer. This procedure is then repeated until the entire body is produced. Apart from the macrostructure, the pore structure can also be controlled in LENS processed parts [5,6]
- 45S5 Bioglass®
High silica containing glassy bioactive / bioresorbable material that was first proposed in 1969 as an alternative to conventional bioinert materials for bone tissue repair [7]
- Transforming growth factor-β (TGF-β)
protein superfamily related to bone that stimulates recruitment and proliferation of mesenchymal cells, their differentiation into osteoblasts and/or chondrocytes, and ECM production [8]
- Bone morphogenetic protein (BMP)
Critical in embryonic skeletal development, bone formation, maturation and repair. Also known as “growth and differentiation factors (GDFs)”. Activated BMPs induce the transcription of specific genes intracellularly through Smad proteins [9]
- Insulin-like growth factor (IGF)
Regulates several key cellular processes, including proliferation, movement and inhibition of apoptosis. Expression of IGF regulates anchorage independent growth and eventual activation of P13K (phosphatidylinositol 3-kinase) [10,11]
- Fibroblast growth factor (FGF)
Secreted glycoproteins that are sequenced in ECM and cell surface by heparan sulphate proteoglycans. FGFs are released from the ECM by heparinases and regulate cellular proliferation, survival, migration and differentiation [12]
- Vascular endothelial growth factor (VEGF)
An angiogenic signal that increases the permeability of endothelial cell to extravasate and lay down a provisional ECM. VEGF is responsible to generating new blood vessels in the tissue [13,14]
- Extracellular matrix (ECM)
Self assembled macromolecules generally consisting of collagens, non-collagenous glycoproteins, hyaluronan and proteoglycans. It works as a reservoir for different cytokines and growth factors [15,16]
- Alkaline phosphatase (ALP)
An osteoblast differentiation marker [17]
- Bone marrow-derived stem cells (BMSC)
Bone marrow stromal cells. These are adult stem cells isolated from samples of bone marrow [18]
- Mesenchymal stem cell (MSC)
A multipotent cell line capable of differentiating in to osteoblasts, chondrocytes, and adipocytic cells [19]
- Human umbilical cord mesenchymal stem cells (hUCMSC)
multipotent cell line capable of differentiating in to osteoblasts, chondrocytes, and adipocytic cells [20]
- Human umbilical vein endothelial cells (HUVEC)
Derived from umbilical vein and used to study angiogenesis [20]
- Embryonic stem cells (ESC)
These cells are derived from embryos and are pluripotent and can differentiate into all somatic cell types [20]
- Parthenogenetic ESCs (PESC)
ESCs derived from human oocytes that is an alternative stem cell source for tissue repair and regeneration [21]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olszta MJ, et al. Bone structure and formation: A new perspective. Materials Science and Engineering: R: Reports. 2007;58:77–116. [Google Scholar]
- 2.Bose S, et al. Processing of controlled porosity ceramic structures via fused deposition. Scripta Materialia. 1999;41:1009–1014. [Google Scholar]
- 3.Darsell J, et al. From CT Scan to Ceramic Bone Graft. Journal of the American Ceramic Society. 2003;86:1076–1080. [Google Scholar]
- 4.Hutmacher DW, et al. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends in Biotechnology. 2004;22:354–362. doi: 10.1016/j.tibtech.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Balla VK, et al. Porous tantalum structures for bone implants: Fabrication, mechanical and in vitro biological properties. Acta Biomaterialia. 2010;6:3349–3359. doi: 10.1016/j.actbio.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue W, et al. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomaterialia. 2007;3:1007–1018. doi: 10.1016/j.actbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Hench LL. Bioceramics: From Concept to Clinic. Journal of the American Ceramic Society. 1991;74:1487–1510. [Google Scholar]
- 8.Buijs JT, et al. The role of TGF-[beta] in bone metastasis: novel therapeutic perspectives. BoneKEy Reports. 2012;1 doi: 10.1038/bonekey.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X, Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357:1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nature Reviews Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 11.Kang H, et al. Insulin-Like Growth Factor 2 Promotes Osteogenic Cell Differentiation in the Parthenogenetic Murine Embryonic Stem Cells. Tissue Engineering Part A. 2012;18:331–341. doi: 10.1089/ten.TEA.2011.0074. [DOI] [PubMed] [Google Scholar]
- 12.Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nature Reviews Drug Discovery. 2009;8:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grellier M, et al. Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. Journal of Cellular Biochemistry. 2009;106:390–398. doi: 10.1002/jcb.22018. [DOI] [PubMed] [Google Scholar]
- 14.Nomi M, et al. Principals of neovascularization for tissue engineering. Molecular Aspects of Medicine. 2002;23:463–483. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 15.Badylak SF, et al. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Hynes RO. The Extracellular Matrix: Not Just Pretty Fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman JE. Structure and Mechanism of Alkaline Phosphatase. Annual Review of Biophysics and Biomolecular Structure. 1992;21:441–483. doi: 10.1146/annurev.bb.21.060192.002301. [DOI] [PubMed] [Google Scholar]
- 18.Chanda D, et al. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. Journal of Cellular Biochemistry. 2010;111:249–257. doi: 10.1002/jcb.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kachgal S, Putnam A. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 2011;14:47–59. doi: 10.1007/s10456-010-9194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phinney DG, Prockop DJ. Concise Review: Mesenchymal Stem/Multipotent Stromal Cells: The State of Transdifferentiation and Modes of Tissue Repair Current Views. STEM CELLS. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 21.Lin G, et al. A highly homozygous and parthenogenetic human embryonic stem cell line derived from a one-pronuclear oocyte following in vitro fertilization procedure. Cell Research. 2007;17:999–1007. doi: 10.1038/cr.2007.97. [DOI] [PubMed] [Google Scholar]
- 22.Khan Y, et al. Tissue Engineering of Bone: Material and Matrix Considerations. JBJS. 2008;90:36–42. doi: 10.2106/JBJS.G.01260. [DOI] [PubMed] [Google Scholar]
- 23.Cao H, Kuboyama N. A biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone. 2010;46:386–395. doi: 10.1016/j.bone.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Li J, et al. Repair of rat cranial bone defects with nHAC/PLLA and BMP-2-related peptide or rhBMP-2. Journal of Orthopaedic Research. 2011;29:1745–1752. doi: 10.1002/jor.21439. [DOI] [PubMed] [Google Scholar]
- 25.Rouwkema J, et al. Vascularization in tissue engineering. Trends in Biotechnology. 2008;26:434–441. doi: 10.1016/j.tibtech.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Bramfeldt H, et al. Scaffold vascularization: a challenge for three-dimensional tissue engineering. Curr Med Chem. 2010;17:3944–3967. doi: 10.2174/092986710793205327. [DOI] [PubMed] [Google Scholar]
- 27.Jain RK, et al. Engineering vascularized tissue. Nature Biotechnology. 2005;23:821–823. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 28.Malda J, et al. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng. 2004;86:9–18. doi: 10.1002/bit.20038. [DOI] [PubMed] [Google Scholar]
- 29.Garcia P, et al. Temporal and Spatial Vascularization Patterns of Unions and Nonunions: Role of Vascular Endothelial Growth Factor and Bone Morphogenetic Proteins. J Bone Joint Surg Am. 2012;94:49–58. doi: 10.2106/JBJS.J.00795. [DOI] [PubMed] [Google Scholar]
- 30.Wernike E, et al. VEGF incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur Cell Mater. 2010;19:30–40. doi: 10.22203/ecm.v019a04. [DOI] [PubMed] [Google Scholar]
- 31.Clarkin CE, et al. Evaluation of VEGF-mediated signaling in primary human cells reveals a paracrine action for VEGF in osteoblast-mediated crosstalk to endothelial cells. J Cell Physiol. 2008;214:537–544. doi: 10.1002/jcp.21234. [DOI] [PubMed] [Google Scholar]
- 32.Li R, et al. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. Journal of Orthopaedic Research. 2009;27:8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 33.Fielding GA, et al. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dental Materials. 2012;28:113–122. doi: 10.1016/j.dental.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarafder S, et al. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. Journal of Tissue Engineering and Regenerative Medicine. doi: 10.1002/term.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rezwan K, et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 36.Holzwarth JM, Ma PX. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials. 2011;32:9622–9629. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo CJ, et al. A novel method of selecting solvents for polymer electrospinning. Polymer. 2010;51:1654–1662. [Google Scholar]
- 38.Seyednejad H, et al. An Electrospun Degradable Scaffold Based on a Novel Hydrophilic Polyester for Tissue-Engineering Applications. Macromolecular Bioscience. 2011;11:1684–1692. doi: 10.1002/mabi.201100229. [DOI] [PubMed] [Google Scholar]
- 39.Phipps MC, et al. Increasing the pore sizes of bone-mimetic electrospun scaffolds comprised of polycaprolactone, collagen I and hydroxyapatite to enhance cell infiltration. Biomaterials. 2012;33:524–534. doi: 10.1016/j.biomaterials.2011.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cicotte KN, et al. Synthesis and Electrospun Fiber Mats of Low T(g) Poly(propylene fumerate-co-propylene maleate) J Appl Polym Sci. 2010;117:1984–1991. [Google Scholar]
- 41.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Teixeira S, et al. In vivo evaluation of highly macroporous ceramic scaffolds for bone tissue engineering. J Biomed Mater Res. 2010;93A:567–575. doi: 10.1002/jbm.a.32532. [DOI] [PubMed] [Google Scholar]
- 43.Woodard JR, et al. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials. 2007;28:45–54. doi: 10.1016/j.biomaterials.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee SS, et al. Understanding the influence of MgO and SrO binary doping on the mechanical and biological properties of β-TCP ceramics. Acta Biomaterialia. 2010;6:4167–4174. doi: 10.1016/j.actbio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Bose S, et al. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO2 doped β-TCP. Bone. 2011;48:1282–1290. doi: 10.1016/j.bone.2011.03.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shie MY, et al. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomaterialia. 2011;7:2604–2614. doi: 10.1016/j.actbio.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Jones JR, et al. Optimising bioactive glass scaffolds for bone tissue engineering. Biomaterials. 2006;27:964–973. doi: 10.1016/j.biomaterials.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Miguel BS, et al. Enhanced osteoblastic activity and bone regeneration using surface-modified porous bioactive glass scaffolds. Journal of Biomedical Materials Research Part A. 2010;94A:1023–1033. doi: 10.1002/jbm.a.32773. [DOI] [PubMed] [Google Scholar]
- 49.Wu C, et al. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33:2076–2085. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 50.Lichte P, et al. Scaffolds for bone healing: Concepts, materials and evidence. Injury. 2011;42:569–573. doi: 10.1016/j.injury.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 51.Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Advanced Drug Delivery Reviews. 2007;59:339–359. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 52.Yan J, et al. Cross-linking Characteristics and Mechanical Properties of an Injectable Biomaterial Composed of Polypropylene Fumarate and Polycaprolactone Co-polymer. J Biomater Sci-Polym Ed. 2011;22:489–504. doi: 10.1163/092050610X487765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheung HY, et al. A critical review on polymer-based bio-engineered materials for scaffold development. Composites Part B: Engineering. 2007;38:291–300. [Google Scholar]
- 54.Xue W, et al. Polycaprolactone coated porous tricalcium phosphate scaffolds for controlled release of protein for tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;91B:831–838. doi: 10.1002/jbm.b.31464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laschke MW, et al. In vitro and in vivo evaluation of a novel nanosize hydroxyapatite particles/poly(ester-urethane) composite scaffold for bone tissue engineering. Acta Biomaterialia. 2010;6:2020–2027. doi: 10.1016/j.actbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 56.Roohani-Esfahani SI, et al. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite PCL composites. Biomaterials. 2010;31:5498–5509. doi: 10.1016/j.biomaterials.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 57.Dabrowski B, et al. Highly porous titanium scaffolds for orthopaedic applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2010;95B:53–61. doi: 10.1002/jbm.b.31682. [DOI] [PubMed] [Google Scholar]
- 58.Das K, et al. Surface modification of laser-processed porous titanium for load-bearing implants. Scripta Materialia. 2008;59:822–825. [Google Scholar]
- 59.Yun Y, et al. Revolutionizing biodegradable metals. Materials Today. 2009;12:22–32. [Google Scholar]
- 60.Witte F, et al. Biodegradable magnesium scaffolds: Part II: Peri-implant bone remodeling. Journal of Biomedical Materials Research Part A. 2007;81A:757–765. doi: 10.1002/jbm.a.31293. [DOI] [PubMed] [Google Scholar]
- 61.Papadimitropoulos A, et al. Kinetics of in vivo bone deposition by bone marrow stromal cells within a resorbable porous calcium phosphate scaffold: An X-ray computed microtomography study. Biotechnology and Bioengineering. 2007;98:271–281. doi: 10.1002/bit.21418. [DOI] [PubMed] [Google Scholar]
- 62.Eniwumide JO, et al. Ectopic bone formation in bone marrow stem cell seeded calcium phosphate scaffolds as compared to autograft and (cell seeded) allograft. Eur Cell Mater. 2007;14:30–38. doi: 10.22203/ecm.v014a03. discussion 39. [DOI] [PubMed] [Google Scholar]
- 63.Naito H, et al. The Effect of Mesenchymal Stem Cell Osteoblastic Differentiation on the Mechanical Properties of Engineered Bone-Like Tissue. Tissue Engineering, Part A. 2011;17:2321–2329. doi: 10.1089/ten.TEA.2011.0099. [DOI] [PubMed] [Google Scholar]
- 64.Thein-Han W, Xu HHK. Collagen-Calcium Phosphate Cement Scaffolds Seeded with Umbilical Cord Stem Cells for Bone Tissue Engineering. Tissue Engineering Part A. 2011;17:2943–2954. doi: 10.1089/ten.tea.2010.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koob S, et al. Bone Formation and Neovascularization Mediated by Mesenchymal Stem Cells and Endothelial Cells in Critical-Sized Calvarial Defects. Tissue Engineering Part A. 2011;17:311–321. doi: 10.1089/ten.TEA.2010.0338. [DOI] [PubMed] [Google Scholar]
- 66.Kempen DHR, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 67.Patel ZS, et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young S, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012;8:1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verron E, et al. Calcium phosphate biomaterials as bone drug delivery systems: a review. Drug Discovery Today. 2010;15:547–552. doi: 10.1016/j.drudis.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 71.Kimelman-Bleich N, et al. Targeted Gene-and-host Progenitor Cell Therapy for Nonunion Bone Fracture Repair. Molecular Therapy. 2010;19:53–59. doi: 10.1038/mt.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keeney M, et al. The ability of a collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF165. Biomaterials. 2010;31:2893–2902. doi: 10.1016/j.biomaterials.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 73.Fischer J, et al. Future of local bone regeneration Protein versus gene therapy. Journal of Cranio-Maxillofacial Surgery. 2011;39:54–64. doi: 10.1016/j.jcms.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 74.Lan Levengood SK, et al. The effect of BMP-2 on micro- and macroscale osteointegration of biphasic calcium phosphate scaffolds with multiscale porosity. Acta Biomaterialia. 2010;6:3283–3291. doi: 10.1016/j.actbio.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 75.Bessa PC, et al. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) Journal of Tissue Engineering and Regenerative Medicine. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 76.Patel ZS, et al. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res. 2008;25:2370–2378. doi: 10.1007/s11095-008-9685-1. [DOI] [PubMed] [Google Scholar]
- 77.Scaglione S, et al. Order versus Disorder: in vivo bone formation within osteoconductive scaffolds. Sci Rep. 2012;2 doi: 10.1038/srep00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–2953. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 79.Murphy CM, et al. The effect of mean pore size on cell attachment, proliferation and migration in collagen glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–466. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]
- 80.Fu Q, et al. Three-Dimensional Visualization of Bioactive Glass-Bone Integration in a Rabbit Tibia Model Using Synchrotron X-Ray Microcomputed Tomography. Tissue Engineering Part A. 2011;17:3077–3084. doi: 10.1089/ten.tea.2011.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]