Abstract
Objective
To evaluate loss of the B cell specific marker CD19 with addition of rituximab (RTX) to healthy donor blood and to determine the role of complement mediated cytotoxicity in these cells.
Methods
Healthy donor whole blood and peripheral blood mononuclear cells (PBMC) were evaluated for loss of CD19 in the presence of RTX using flow cytometry. The effect of complement on CD19 loss was examined using serum free media, C3- and C5- deficient sera, and a C5 blocking antibody. Evidence for B cell death was evaluated by measuring RNA levels as well as by flow cytometry. Demonstration of transfer of CD19 antigen to monocytes and neutrophils was evaluated by flow cytometry and confocal microscopy.
Results
RTX induced a rapid drop in CD19 count (mean 51%, n=37) in PBMC. This reduction occurred in the absence of complement. Despite the drop in CD19 expression, B cell death was absent as evidenced by no change in CD19 or CD20 mRNA, no change in CD19 levels by intracellular staining, and through use of viability dyes. The CD19 antigen was shown to be transferred to monocytes and neutrophils in an Fc-dependent fashion.
Conclusion
RTX added to healthy donor PBMC in vitro results in complement independent loss of CD19 without causing B cell death. CD19 is transferred from B cells to monocytes and neutrophils during shaving of the RTX-CD20 complex in an Fc dependent manner. These data suggest that monitoring the effect of RTX by measuring CD19+ cell counts may be compromised by this activity.
Rituximab is a monoclonal antibody targeting CD20, a B cell specific marker, which has shown great clinical efficacy in B cell malignancies and many autoimmune diseases, including rheumatoid arthritis (RA) and the ANCA associated vasculitides. Rituximab has three purported actions in effecting B cell depletion: antibody-dependent cellular cytotoxicity (ADCC), complement dependent cytotoxicity (CDC), and induction of apoptosis (1). Despite each of these effects being documented in vitro, the relative contribution of each of these effects in vivo remains obscure.
Patients with RA who are given RTX uniformly have near complete to complete depletion of circulating B cells as measured by flow cytometry using another B cell specific surface protein, CD19 (2). Despite this depletion, only 50–70% of patients respond to RTX treatment (2,3,4). There is evidence suggesting that B cell depletion in the synovium predicts RTX response (5). We hypothesized that CDC played a major role in synovial depletion of B cells, and set out to develop a novel whole blood assay to determine variation in RTX CDC in healthy donors and patients.
Interestingly, while we were able to show reductions in CD19+ cells as a function of RTX treatment of whole blood, we were unable to demonstrate that this effect was complement-dependent. To better define this observation, we examined the effect of RTX-dependent complement-dependent killing of normal human B cells in peripheral blood mononuclear cells (PBMC) in vitro. Surprisingly, we observed that human B cells are resistant to complement-dependent killing by RTX. Moreover, the apparent reduction in CD19+ B cells was due to loss of CD19 from the cell surface, not killing, and was also complement independent. Rather, the loss of surface CD19 appeared to accompany the loss of CD20 triggered by RTX. We subsequently showed that RTX addition to PBMC in vitro induced the rapid loss of CD19; this effect of RTX required an intact Fc region and was mediated by both monocytes and neutrophils. These results suggest that reliance of CD19+ expression to evaluate peripheral B cell depletion by RTX may be compromised.
MATERIALS AND METHODS
Cells and Serum
Blood was obtained from healthy volunteer donors following informed consent and PBMC purified by discontinuous gradient isolation using Ficoll-Paque PLUS (GE Healthcare Biosciences), which were resuspended in RPMI plus 10% serum or Aim V serum-free media (Gibco). Neutrophils were isolated by dextran sedimentation from the blood pellet and erythrocytes lysed using BD PharmLyse RBC lysing buffer (BD Biosciences). After washing, neutrophils were resuspended in RPMI.
B cells were isolated by negative selection, using Invitrogen’s Untouched B-Cell Isolation Kit (#113.51d). This protocol involves adding biotinylated monoclonal antibodies targeting non-B cells to the PBMC, followed by addition of streptavidin coated magnetic beads to separate non-B cells from B cells.
Antibodies/Sera
FITC anti-CD45 and APC anti-CD19 were purchased from BD Biosciences. PE anti-CD14 was purchased from eBioscience. AlexaFluor 647 anti-CD19 was purchased from BioLegend. Rituximab (Genentech) and eculizumab (Alexion Pharmaceuticals) were obtained from the hospital pharmacy. Complement C5- and C3-deficient sera were purchased from Sigma Life Science and Calbiochem respectively. Heat inactivation (HI) serum refers to treatment of a donor’s autologous serum (56°C for 45 minutes.).
Reagents
Hirudin was obtained from the hospital pharmacy. Propidum iodide (PI) was purchased from Sigma Life Science. Cell Tracker CM DiI and Green LIVE/DEAD viability dye were from Invitrogen. Protein A Sepharose beads were purchased from GE Healthcare. PBS/BSA/azide was prepared at concentrations of BSA 0.1% and azide 0.05%.
Effect of RTX on CD19+ Cell Numbers
Whole blood in 50µg/ml hirudin was left untreated or treated with RTX at room temperature (RT) for 15 minutes followed by RBC lysis using BD PharmLyse (BD Biosciences) for an additional 15 minutes. Following two washes with ice cold PBS/BSA/azide, the cell pellet was stained with fluorochrome labeled antibodies on ice for 15 minutes, washed, and fixed using 1% paraformaldehyde before evaluation by flow cytometry using FACSCalibur (BD Biosciences).
PBMC (2 million cells/ml) in RPMI and 10% serum were treated with specified concentrations of RTX for 30 minutes at RT, washed in ice cold PBS/BSA/azide, pelleted (1500rpm for 5 minutes at 4°C), incubated on ice for 15 minutes with fluorochrome labeled antibodies, washed and fixed using 1% paraformaldehyde. Flow cytometry analysis was performed using FACSCalibur (BD Biosciences). Initial gating of lymphocytes was based on side scatter and forward scatter properties, with subsequent quadrant or histogram analysis based on fluorochrome positivity. In addition to measuring a percent of CD19 positivity, the mean fluorescence intensity (MFI) was determined by measuring the geoMean of CD19. Gating on the monocyte population and the neutrophil population was performed based on side scatter and forward scatter properties. All tests were performed in duplicate.
The effect of eculizumab, a C5 blocking antibody, was examined by pre-incubation for 15 minutes prior to RTX addition at 10 µg/ml, 100 µg/ml, and 1 mg/ml, with equivalent results, so remaining eculizumab experiments were performed using 1 mg/ml. For experiments requiring RTX F(ab’)2 fragments, RTX was treated with immobilized pepsin (Thermo Scientific) per manufacturer’s instructions, followed by removal of Fc fragments using protein A Sepharose beads and purity confirmed by SDS-PAGE.
Measurement of the effect of RTX on B cell viability was performed following RTX treatment through the addition of propidium iodide (1 µg/ml) and evaluated for number of positive dead cells using flow cytometry. As a positive control, PBMC were treated with 20% ethanol for 15 minutes. Separately, the Green LIVE/DEAD viability dye (Invitrogen) was used with 250 µl of a 1:5000 stock solution added to a 0.5 million PBMC pellet and incubated on ice for 30 minutes following RTX treatment, then washed and fixed with 1% paraformaldehyde, prior to flow cytometry.
Intracellular staining for CD19 following RTX incubation utilized a PBS-0.01% paraformaldehyde fixation for 10 min at room temperature before permeabilization in 0.5% Tween-20 in PBS for 15 min at room temperature. Subsequent staining with antibodies was done in PBS with 0.1% Tween-20.
RTX-Complement Dependent Cytotoxicity of Daudi Cells
The human Daudi B cell line was adapted to and maintained in the serum-free media X-Vivo 15 (Lonza) in a 5% CO2 incubator at 37°C. Daudi cells were brought to a concentration of 250,000 cells/ml with the specified serum concentrations ranging from 1% to 10%, along with RTX 10 µg/ml and incubated at RT for 30 minutes. PI 1 µg/ml was added for an additional 30 minutes, followed by flow cytometry to determine cell death. In some experiments, eculizumab 1mg/ml was added for 15 minutes prior to addition of RTX.
Quantitative RT-PCR Analysis
RNA was isolated from whole blood using the PAXgene blood RNA isolation kit per manufacturer’s instructions (PreAnalytiX). RNA was extracted and purified from PBMC as described by Gough (6). RNA was quantified using the NanoDrop spectrophotometer (NanoDrop Technologies) and 0.5 µg reverse-transcribed using the SuperScript III Reverse Transcriptase (Invitrogen). Quantitative real-time PCR was performed using an iCycler iQ Real-Time PCR detection system using iQ SYBR Green Supermix (BioRad). Expression levels of target genes (CD19 and CD20) were quantified relative to the expression of the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin housekeeping genes.
Confocal Microscopy
Isolated neutrophils were stained using CellTracker CM-DiI (Invitrogen) at 1µM concentration, and after incubation were washed generously. The stained neutrophils were combined with purified human B cells, and in the correct setting, RTX 10 µg/ml was added. After 15 minutes, CD19 AlexaFluor 647 was added for an additional 15 minutes, then washed in 15 ml volume PBS/BSA/Azide. The cells were examined on a Zeiss Confocal Microscope using red for the CellTracker and green for CD19.
Statistical Analysis
Analysis was performed using STATA software version 12.0 (StataCorp). Student’s t-tests were performed to calculate statistical differences between the means of the different variables. Two-tailed P values less than 0.05 were considered significant.
RESULTS
Rituximab results in rapid and robust loss of measured CD19
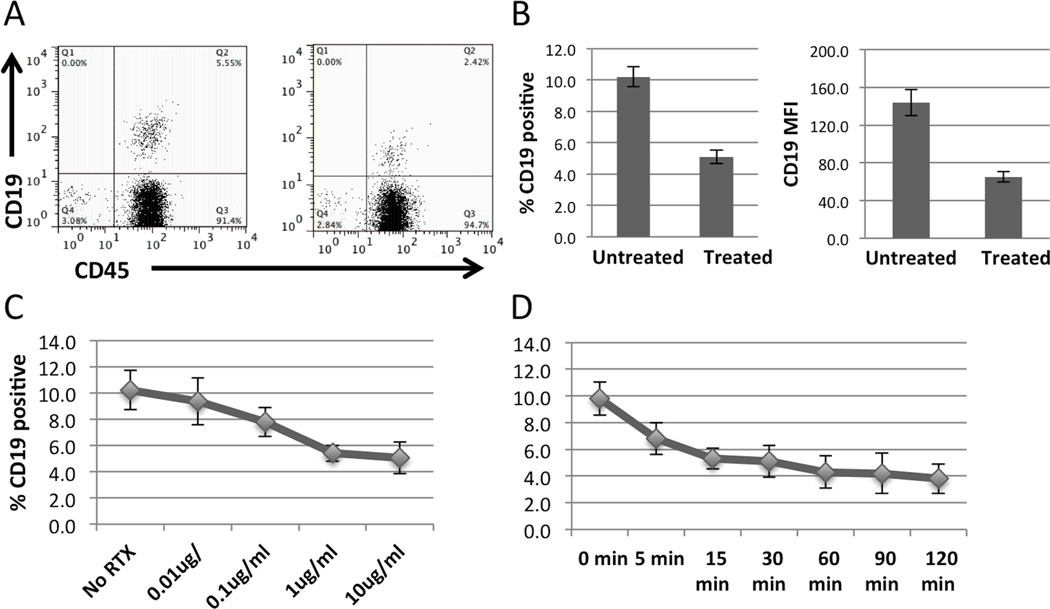
Following hirudin (50 µg/ml) anticoagulation, the effect of RTX addition to whole blood on CD19+ cell number and MFI was examined (Data not shown). RTX treatment rapidly reduced CD19+ cell number (mean 37%, n=32). Surprisingly, this effect of RTX on CD19+ cell expression was not accompanied by a reduction in CD19 or CD20 mRNA as measured by quantitative RT-PCR (Data not shown). To better characterize this unexpected result, we examined the relative CD19 depleting effect of RTX in PBMC cultured in 10% autologous serum (Figure 1). A consistently greater (mean 51%, n=37, range 21–84%) degree of CD19 depletion was observed. This RTX-dependent reduction in CD19+ B cells was accompanied by a comparable decline in CD19 surface expression as measured by changes in mean fluorescent intensity (MFI). This effect of RTX was dose-dependent, becoming maximal at 1 µg/ml (Figure 1C). The changes in CD19 expression occurred very rapidly (5’) at 25°C (Figure 1D).
Figure 1.
RTX added to PBMC in 10% serum results in rapid loss of CD19 as measured by flow cytometry. A, Representative dot plot of untreated and RTX treated lymphocytes as determined by forward and side scatter properties. B, Bar graph representing mean percent positive CD19 cells without and with RTX treatment (p<0.0001) and change in mean fluorescence intensity (MFI) (p<0.0001, n=37). C, RTX promotes depletion of CD19 in a dose-dependent manner with an incubation of 30 minutes (n=4). D, CD19 depletion occurs as rapidly as 5 minutes at RTX dose of 10 µg/ml (n=5). Error bars represent standard error of the mean.
Loss of CD19 in healthy donor PBMC in vitro is not complement dependent
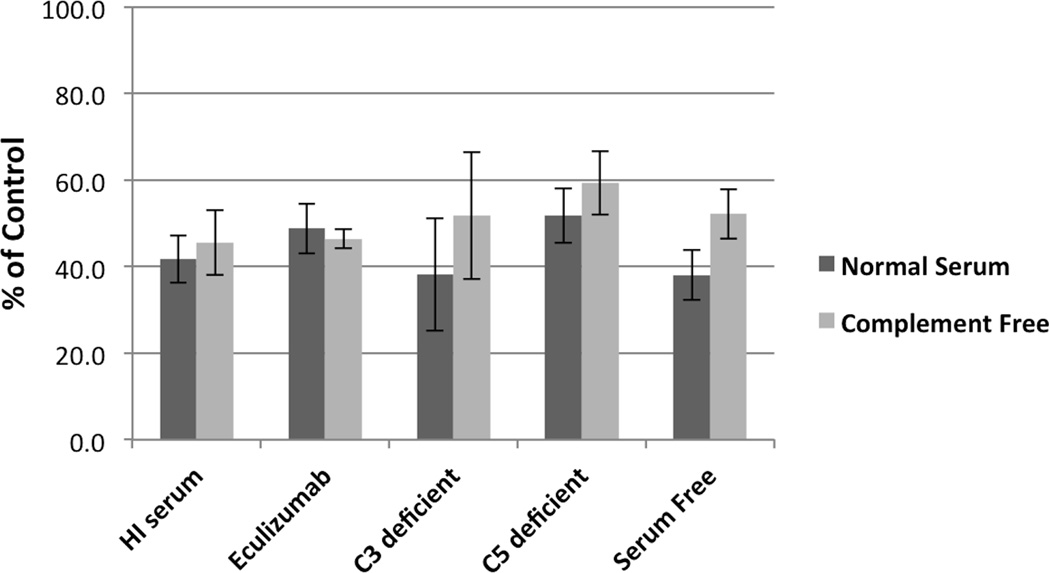
The rapidity of the decline in CD19+ cells prompted further consideration that this represented RTX mediated CDC. Surprisingly, the RTX dependent reduction in CD19+ B cells was conserved despite the use of serum-free media (Figure 2). Moreover, this effect of RTX did not require an intact or functional complement cascade as we saw equivalent effects using heat-inactivated (56°C, 45’) human serum as well as C3- or C5-deficient sera. Blockade of the C5 activity with the anti-C5 monoclonal antibody eculizumab also did not alter this effect.
Figure 2.
RTX incubation for 30 minutes consistently results in CD19 depletion in multiple complement independent conditions (displayed as the % of the CD19 value obtained from untreated PBMC). Error bars represent standard error of the mean.
The activity of RTX in mediating CDC with human cells was confirmed using the Daudi B cell line. With concentrations of 5% serum or less, RTX addition resulted in the rapid killing of >80% of Daudi cells (Data not shown). No RTX-dependent killing was seen in the absence of serum as a source of complement. Moreover, no Daudi cell death was seen using 5% C3- or C5-deficient sera, or when eculizumab, a C5 blocking antibody, was added (data not shown). Thus, in contrast to that seen with human B cell lines, normal human B cells exhibit little, if any, sensitivity to RTX-CDC.
Loss of measured CD19 does not represent cell death, but represents loss of the surface protein
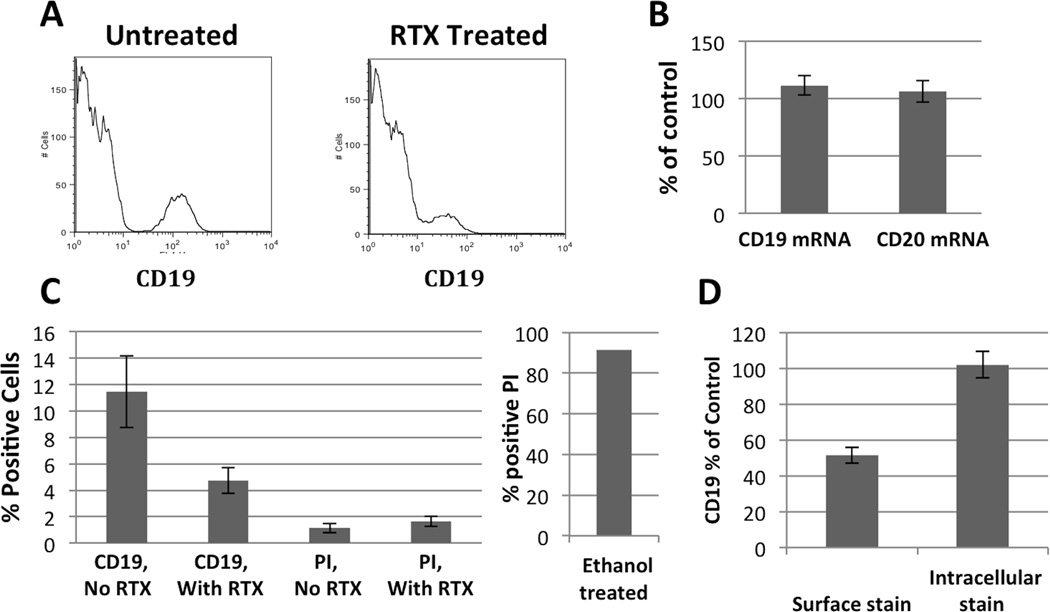
A consistent effect of RTX was the decline (~50% by MFI) in CD19 surface expression (Figure 3A). This suggested that CD19 expression was being lost from the cell surface rather than by cell death. Consistent with this interpretation, RT-PCR analysis of PBMC demonstrated no decline in CD19 or CD20 mRNA with RTX treatment, as seen in whole blood (Figure 3B). Furthermore, propidium iodide uptake was not increased as a function of RTX treatment (Figure 3C). Identical results were obtained with LIVE/DEAD green viability dye (Data not shown). Subsequent studies determined that intracellular CD19 expression was unaffected by RTX treatment (Figure 3D). Thus, the rapid decline in CD19 positive cells occurring with RTX treatment was due to its rapid loss from the cell surface.
Figure 3.
Loss of CD19 with RTX treatment is not due to cell death. A, Flow cytometry histogram of PBMCs without and with RTX (gating on lymphocyte population based on forward and side scatter properties) showing representative leftward shift of CD19 count into the negative population. B, CD19 and CD20 mRNA message from PBMC does not change with addition of RTX. C, Viability dye, propidium iodide, indicates that there is no increase in cell death with addition of RTX despite large decline in CD19 count. Ethanol treated cells were >90% positive for propidium iodide. D, While surface staining supports decrease in CD19 count, intracellular staining indicates that B cells are not lost with addition of RTX. All graphs represent a 30 minute RTX incubation. Bar graphs represent the mean of at least three separate experiments; error bars represent standard error of the mean.
Rituximab induces loss of CD19 through the shaving process
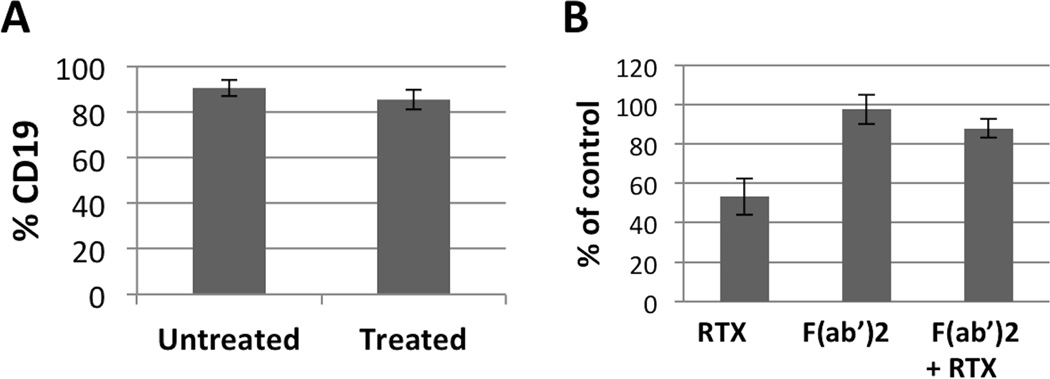
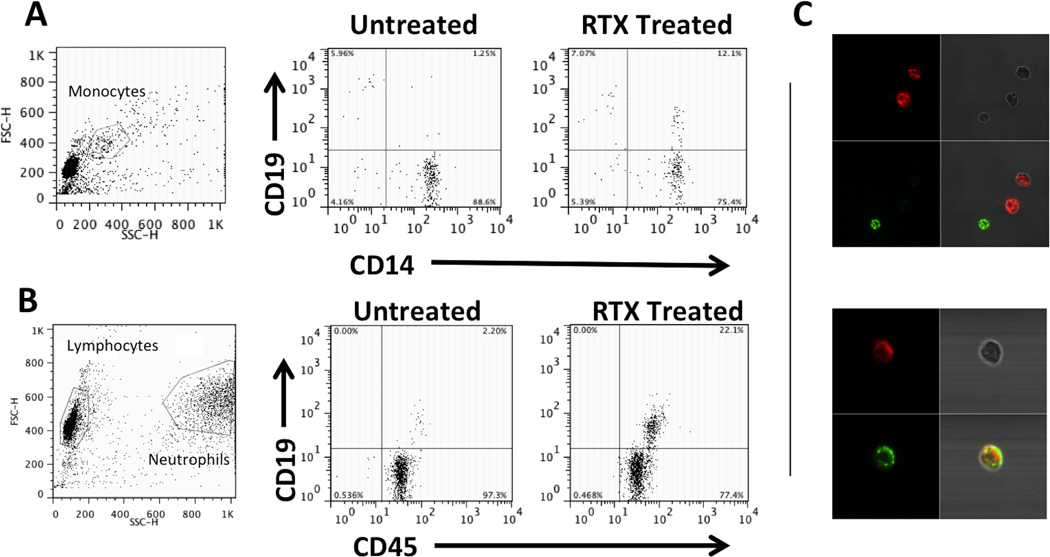
Previous studies have demonstrated that RTX binding to CD20 enables monocytes to mediate trogocytosis or “shaving” and internalization of the RTX-CD20 complex and accompanying cell membrane through an Fcγ receptor dependent mechanism (7, 8). We tested the potential role of shaving by first demonstrating that the CD19-depleting effect of RTX was lost using partially (~90%+) purified B cells (Figure 4A) indicating a requirement for a non-B cell. Second, RTX mediated loss of CD19 was completely inhibited by 0.05% azide, which would inhibit the cellular respiration for shaving to occur (data not shown). Third, in PBMC, using an F(ab’)2 fragment of RTX we observed a requirement for an intact human Fc portion (Figure 4B). In addition, prior incubation with the F(ab’)2 of RTX blocked the RTX-induced decline in CD19 (Figure 4B), establishing a requirement for both the F(ab) and Fc portions of RTX for a reduction in CD19 expression. Finally, flow cytometry demonstrated that, following incubation of PBMC with RTX, CD19 expression was acquired by CD14+ monocytes, thereby directly indicating shaving (Figure 5A). Similar results were obtained when purified human peripheral blood neutrophils were added to B cells in the presence of RTX (Figure 5B). Using confocal microscopy, purified neutrophils (color red) were combined with purified B cells, then stained with anti-CD19 (color green). Untreated cells showed clear separation of neutrophils and CD19 positivity, however, following treatment with RTX, the neutrophils had acquired CD19 from B cells (Figure 5C).
Figure 4.
RTX induced loss of CD19 is dependent on Fc-FcγR interaction. A, Highly purified (~90%) B cells do not lose CD19 in the presence of RTX. B, In PBMC, RTX F(ab’)2 does not result in loss of CD19, but does prevent RTX mediated loss of CD19. Error bars represent standard error of the mean.
Figure 5.
Loss of CD19 with RTX treatment is not due to cell death. A, Flow cytometry histogram of PBMCs without and with RTX (gating on lymphocyte population based on forward and side scatter properties) showing representative leftward shift of CD19 count into the negative population. B, CD19 and CD20 mRNA message from PBMC does not change with addition of RTX. C, Viability dye, propidium iodide, indicates that there is no increase in cell death with addition of RTX despite large decline in CD19 count. Ethanol treated cells were >90% positive for propidium iodide. D, While surface staining supports decrease in CD19 count, intracellular staining indicates that B cells are not lost with addition of RTX. All graphs represent a 30 minute RTX incubation. Bar graphs represent the mean of at least three separate experiments; error bars represent standard error of the mean.
DISCUSSION
We report that both the analysis and effect of RTX on human peripheral blood B cells are more complex and nuanced than previously thought. Notably, RTX addition to PBMC results in a rapid loss of CD19 from the B cell surface in a complement-independent manner. This effect is not associated with a decline in either CD19 or CD20 mRNA and is observed in whole blood as well as in PBMC. This process is IgG Fc dependent and blocked by azide. Moreover, it requires the presence of Fcγ receptor-expressing cells such as monocytes and/or PMNs, which acquire the CD19 molecule on their cell surface as a consequence of RTX treatment. This effect of RTX occurs without affecting B cell viability. The importance of this finding to monitoring B cell recovery is underscored by the demonstration that CD20 shaving occurs in vivo, where a CD20-negative population of B cells arises in minutes following rituximab treatment (9). Our data would suggest that CD19 might be similarly affected.
Other potential mechanisms contributing to the loss of surface CD19 have been considered. For example, CD19 internalization could be occurring following capping of CD20 by RTX. This model is not consistent with several findings. First, we do not see a significant increase in intracellular CD19 with intracellular staining of PBMC. Second, surface CD19 expression is resistant to internalization, even with direct antibody ligation (10, 11). Third, both internalization and shedding models for CD19 loss would be expected to be replicated by RTX F(ab’)2 treatment. In contrast, our data (Figure 4) demonstrate a clear requirement of CD19 loss for an intact rituximab antibody and Fc portion. The Fc-dependence of CD19 loss with RTX argues against the model of CD19 shedding or internalization. These conclusions are supported by other studies, where trogocytosis plays the dominant role in loss of CD20 from the B cell (7, 8, 17). Despite these reservations, determining the contribution, if any, of internalization and membrane shedding of CD19 is an area of future study, especially in vivo. Whatever the result of these investigations, our primary conclusion remains: resting human B cells lose CD19 from their cell surface following RTX treatment and this is not associated with cell death.
Previous work has identified ADCC, CDC, and apoptosis as possible mechanisms of B cell depletion induced by RTX that account for its efficacy in autoimmune disease (1). We show that normal human peripheral blood B cells are resistant to RTX-CDC, in contrast to that seen with human B cell lines (12, 13, 14). Indeed, RTX treatment had no apparent effect on B cell viability in vitro, although it dramatically altered B cell phenotype, potentially confounding measures of B cell numbers by CD19 surface expression. These data suggest for the first time that RTX-CDC may not play a major role in the clinical efficacy of B cell targeting in autoimmune disease. It remains uncertain as to whether malignant B cells differ in their sensitivity to RTX-CDC, although reports have suggested that both shaving and CDC occur (9, 15).
The observation of CD20 shaving induced by RTX has been extensively studied by the Taylor laboratory, who have documented the role of the monocyte and Fc-FcγR interactions (7, 15, 16, 17). Evidence supporting the presence of shaving of the RTX-CD20 complex has been described in vivo in mice and in CLL patients (9, 18, 19). Our studies extend this observation in several important ways. First, we demonstrate that shaving of the RTX-CD20 complex from normal human B cells is also associated with a marked loss of CD19. Second, the magnitude of RTX-induced CD19 loss from normal human B cells appears to be greater than that seen with B cell lines (7). This result potentially indicates a greater proximity of CD19 with CD20 in peripheral blood B cells. Third, we observed that shaving can be mediated by both monocytes and neutrophils, the predominant circulating phagocyte. Finally, the CD19-depleting effect of RTX occurs rapidly in whole blood without affecting CD19 mRNA levels, indicating that shaving of CD19 is also likely to occur in vivo.
The ability of RTX to create CD19-deficient B cells through shaving has important potential consequences. The first of these arises from the common use of CD19 as a pan-B cell marker. We have demonstrated that RTX concentrations of 1 µg/ml lead to marked loss of CD19+ cells, yet in RA patients, RTX administration results in serum concentrations of 10 µg/ml or higher for up to 16 weeks, and concentrations of >1µg/ml for at least 24 weeks (20). Thus, previous analyses of the effectiveness of RTX activity by monitoring CD19+ B cells may have overestimated B cell depletion. Finally, persistent shaving of CD19 and CD20 from the B cell surface might alone influence B cell function. CD20 deficiency in humans results in altered humoral immunity through decreased T cell independent antibody production (21). Potentially even more important, CD19 deficiency in humans resulted in severely impaired B cell function as manifested by the syndrome of common variable immunodeficiency (22, 23). Thus, RTX-dependent shaving of CD20 and CD19 from B cells might have profound effects on B cell function and immune responses independent of B cell depletion.
In summary, we have identified loss of the CD19 surface antigen from healthy donor B cells exposed to rituximab in vitro. This effect is complement-independent and occurs without causing B cell death. We have shown that healthy donor B cells are resistant to CDC, raising the possibility that RTX-CDC in vitro or in vivo are restricted to malignant B cells. We have shown that this loss of CD19 with the addition of RTX is mediated by trogocytosis, or shaving, in which the CD19 surface marker accompanies the transfer of the RTX-CD20 complex to monocytes and neutrophils. These findings suggest that the assumption that the measurement of CD19+ cells as a marker of RTX-induced B cell loss may be flawed. Finally, this finding has potential implication for a change in B cell function as a result of RTX induced shaving.
Acknowledgements
The authors would like to thank Xiao-Wei Wang and Whitney Hilton for technical assistance.
Supported by: This work was supported by grants from the National Institutes of Health R01AR49834 (W.F.C.R.) and the American College of Rheumatology Research and Education 'Within Our Reach' program (W.F.C.R.). Dr. Jones was supported by NIH Training Grants (T32 AI07363) and the Hitchcock Foundation. This paper is subject to the NIH Public Access Policy.
Footnotes
Disclosures: The authors report no financial support or other benefits from commercial sources for the work reported on in the manuscript, or any other financial interests that any of the authors may have, which could create a potential conflict of interest or the appearance of a conflict of interest with regard to the work.
References
- 1.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–3837. doi: 10.1016/j.molimm.2007.06.151. [review] [DOI] [PubMed] [Google Scholar]
- 2.Edwards JCW, Szczepanski L, Szechinski J, Filiposicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Eng J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. for the REFLEX Trial Group. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy. Arth Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 4.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: Results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arth Rheum. 2006;54:1390–1400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 5.Kavanaugh A, Rosengren S, Lee SJ, Hammaker D, Firestein GS, Kalunian K, et al. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann Rheum Dis. 2008;67:402–408. doi: 10.1136/ard.2007.074229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gough NM. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988;173:93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- 7.Beum PV, Kennedy AD, Williams ME, Lindofer MA, Taylor RP. The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. J Immunol. 2006;176:2600–2609. doi: 10.4049/jimmunol.176.4.2600. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen AE, Jungersen MB, Pedersen CD. Monocytes mediate shaving of B-cell-bound anti-CD20 antibodies. Immunology. 2011;133:239–245. doi: 10.1111/j.1365-2567.2011.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kennedy AD, Beum PV, Solga MD, DiLillo DJ, Lindorfer MA, Hess CE, et al. Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J Immunol. 2004;172:3280–3288. doi: 10.4049/jimmunol.172.5.3280. [DOI] [PubMed] [Google Scholar]
- 10.Du X, Beers R, Fitzgerald DJ, Pastan I. Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingle GS, Chan P, Elliott JM, Chang WS, Koeppen H, Stephan JP, et al. High CD21 expression inhibits internalization of anti-CD19 antibodies and cytotoxicity of an anti-CD19-drug conjugate. Br J Hematolog. 2008;140:46–58. doi: 10.1111/j.1365-2141.2007.06883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, van de Winkel JGJ, Parren PWHI, et al. Binding of submaximal C1q promotes complement-dependent cytotoxicity (CDC) of B cells opsonized with anti-CD20 mAbs ofatumumab (OFA) or rituximab (RTX): considerably higher levels of CDC are induced by OFA than RTX. J Immunol. 2009;183:749–758. doi: 10.4049/jimmunol.0900632. [DOI] [PubMed] [Google Scholar]
- 13.Guo B, Ma Z, Li H, Xu G, Zheng P, Zhu B, et al. Mapping of binding epitopes of a human decay-accelerating factor monoclonal antibody capable of enhancing rituximab-mediated complement-dependent cytotoxicity. Clin Immunol. 2008;128:155–163. doi: 10.1016/j.clim.2008.03.507. [DOI] [PubMed] [Google Scholar]
- 14.van Meerten T, van Rijn RS, Hol S, Hagenbeek A, Ebeling SB. Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Canc Res. 2006;12:4027–4035. doi: 10.1158/1078-0432.CCR-06-0066. [DOI] [PubMed] [Google Scholar]
- 15.Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Curr Op Immunol. 2008;20:444–449. doi: 10.1016/j.coi.2008.05.011. [review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beum PV, Lindorfer MA, Taylor RP. Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. J Immunol. 2008;181:2916–2924. doi: 10.4049/jimmunol.181.4.2916. [DOI] [PubMed] [Google Scholar]
- 17.Beum PV, Peek EM, Lindorfer MA, Beurskens FJ, Engelberts PJ, Parren PWHI, et al. Loss of CD20 and bound CD20 antibody from opsonized B cells occurs more rapidly because of trogocytosis mediated Fc receptor-expressing effector cells than direct internalization by the B cells. J Immunol. 2011;187:3438–3447. doi: 10.4049/jimmunol.1101189. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Williams ME, Cousar JB, Pawluczkowycz AW, Lindorfer MA, Taylor RP. Rituximab-CD20 complexes are shaved from Z138 mantle cell lymphoma cells in intravenous and subcutaneous SCID mouse models. J Immunol. 2007;179:4263–4271. doi: 10.4049/jimmunol.179.6.4263. [DOI] [PubMed] [Google Scholar]
- 19.Williams MD, Densmore JJ, Pawluczkowycz AW, Beum PV, Kennedy AD, Lindorfer MA, et al. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol. 2006;177:7435–7443. doi: 10.4049/jimmunol.177.10.7435. [DOI] [PubMed] [Google Scholar]
- 20.Breedveld F, Agarwal S, Yin M, Ren S, Li NF, Shaw TM, et al. Rituximab pharmacokinetics in patients with rheumatoid arthritis: B-cell levels do not correlate with clinical response. J Clin Pharmacol. 2007;47:1119–1128. doi: 10.1177/0091270007305297. [DOI] [PubMed] [Google Scholar]
- 21.Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IAM, Dolman KM, et al. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120:214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJM, van Tol MJD, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354:1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 23.Kanegane H, Agematsu K, Futatani T, Sira MM, Suga K, Sekiguchi T, et al. Novel mutations in a Japanese patient with CD19 deficiency. Gen Immun. 2007;8:663–670. doi: 10.1038/sj.gene.6364431. [DOI] [PubMed] [Google Scholar]