Abstract
Endothelial cell activation in the process of tumor angiogenesis and in various aspects of vascular biology has been extensively studied. However, endothelial cells also function in other capacities, including in immune regulation. Compared to the more traditional immune regulatory populations (Th1, Th2, Treg, etc.), endothelial cells have received far less credit as being immune regulators. Their regulatory capacity is multifaceted. They are critical in both limiting and facilitating the trafficking of various immune cell populations, including T cells and dendritic cells, out of the vasculature and into tissue. They also can be induced to stimulate immune reactivity or to be immune inhibitory. In each of these parameters (trafficking, immune stimulation and immune inhibition), their role can be physiological, whereby they have an active role in maintaining health. Alternatively, their role can be pathological, whereby they contribute to disease. In theory, endothelial cells are in an ideal location to recruit cells that can mediate immune reactivity to tumor tissue. Furthermore, they can activate the immune cells as they transmigrate across the endothelium into the tumor. However, what is seen is the absence of these protective effects of endothelial cells and, instead, the endothelial cells succumb to the defense mechanisms of the tumor, resulting in their acquisition of a tumor-protective role. To understand the immune regulatory potential of endothelial cells in protecting the host versus the tumor, it is useful to better understand the other circumstances in which endothelial cells modulate immune reactivities. Which of the multitude of immune regulatory roles that endothelial cells can take on seems to rely on the type of stimulus that they are encountering. It also depends on the extent to which they can be manipulated by potential dangers to succumb and contribute toward attack on the host. This review will explore the physiological and pathological roles of endothelial cells as they regulate immune trafficking, immune stimulation and immune inhibition in a variety of conditions and will then apply this information to their role in the tumor environment. Strategies to harness the immune regulatory potential of endothelial cells are starting to emerge in the non-tumor setting. Results from such efforts are expected to be applicable to being able to skew endothelial cells from having a tumor-protective role to a host-protective role.
Keywords: Endothelial cells, Cancer, Immunoregulation, Immune trafficking
Endothelial cell control of immune cell trafficking
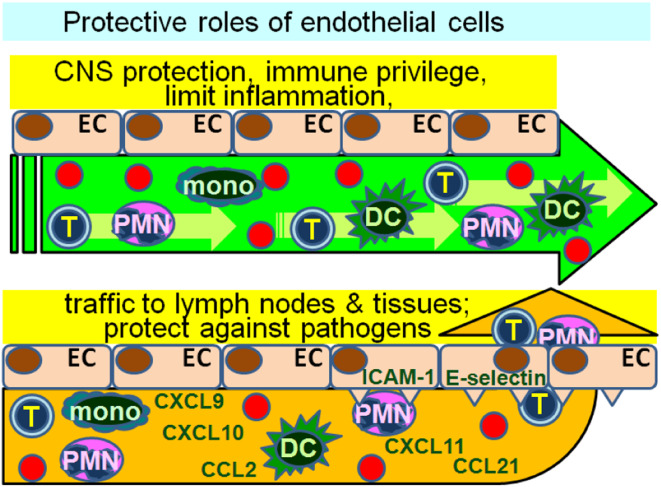
The role of endothelial cells in the recruitment of lymphocytes, neutrophils, monocytes and dendritic cells into lymph nodes and tissues establishes an intimate relationship between them and immune cells. This trafficking of immune cells through the vascular endothelium is not a passive process whereby “leaky” structures allow immune cell passage through the endothelial cell layer. Instead, trafficking involves a finely orchestrated and coordinated partnership by both the endothelial cells and the immune cells. Furthermore, the recruitment and transendothelial cell migration by immune cells are selective and are influenced by both the stimulus that triggers the trafficking process and the mediators produced by the endothelial cells (Fig. 1). For example, bacterial activation of endothelial cell TLR2 results in selective recruitment of neutrophils [1]. Also, through their production and presentation of chemokines, endothelial cells are critical to the movement of T cells and dendritic cells from the periphery and toward lymph nodes in the process of immune surveillance. Endothelial cell production of CCL5 (RANTES), a potent T cell attractant, creates a chemotactic gradient for an influx of T cells [2]. Release of CCL21 following endothelial cell activation stimulates dendritic cell chemotactic migration and thus, contributes to migration into draining lymph nodes [3]. In addition to endothelial cell production of T cell and dendritic cell chemoattractants, their production of heparin sulfate is a critical contributor to mediating the trafficking of T cells and dendritic cells to lymph nodes. A deficiency in heparin sulfate results in reduced adhesion and impaired homing of lymphocytes, reduced T cell and dendritic cell transmigration to lymph nodes, and reduced T cell hypersensitivity responses [4].
Fig. 1.
Endothelial cell regulation of immune cell trafficking. Endothelial cells (EC) can form a barrier that prevents transmigration of immune cells, thereby protecting tissue from immune injury. This is particularly important in protecting the blood–brain barrier or in maintaining immune privilege. However, depending on their state of activity, endothelial cells can also recruit immune cells through their expression of adhesion molecules and chemokine. This often occurs in response to exposure to pathogens, such as bacteria or viruses where recruitment of innate immune response is the first line of defense against the invading organism. Whether immune cell transmigration is prevented or facilitated depends on the state of endothelial cell activity and the endothelial cell stimuli
It has become clear that trafficking is a bidirectional interplay between immune and endothelial cells. For example, the vascular growth that is critical to lymph nodes as they enlarge during infection is not only regulated by the endothelial cells in response to the invader, but is also controlled immunologically through several distinct steps [5]. The initial step of endothelial cell proliferation is dependent on CD11c+ cells but not on T or B cells, while the later step of expansion is dependent on both T and B cells. How these intimate interactions of endothelial cells with T cells or dendritic cells impact on immune reactivity is not straightforward since endothelial cells can be either immune stimulatory or immune inhibitory. This contrasts with the better defined immune regulatory roles such as of Treg, Th1, Th2 cells or myeloid-derived suppressor cells (MDSC). The direction of immune regulation is largely driven by the level of endothelial cell activation, the nature of the stimulus and the endothelial cell microenvironment. This, in turn, impacts on whether endothelial cells contribute to an environment that facilitates or limits immune cell trafficking.
Endothelial cells as immune stimulatory cells
The physical intimacy of endothelial cells with T cells at the blood–tissue interface creates an environment that is important for the process of immune surveillance. Whether or not this interaction leads to a primary adaptive immune response is not certain, although there is indication that endothelial cells can effectively present antigen to re-stimulate memory/effector T cells. In this process, T cells form invadosome-like protrusions, which are microcontacts with the antigen-presenting endothelial cells [6]. It has been proposed that these microcontacts serve as sensory organelles to facilitate antigen recognition by the T cells.
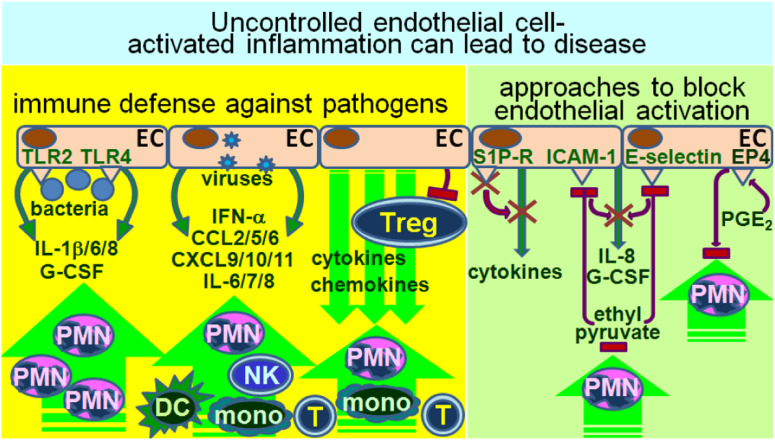
The role of endothelial cells in stimulating adaptive immune responses has not been as extensively studied as their role in stimulating inflammatory or innate responses (Fig. 2). In response to pathogens, there is a tendency for endothelial cells to become activated and to produce inflammatory mediators. For example, exposure of endothelial cells to peptidoglycan of gram-positive Staphylococcus epidermidis causes a transient increase in both IL-6 and the pathogen-binding, Toll-like receptor TLR2 [7]. In response to endothelial cell TLR2 activation by bacterial lipoproteins, endothelial cells upregulate levels of cytokines such as IL-6, IL-8 and G-CSF, which are associated with inflammation and with neutrophil recruitment [1]. In addition to activation via TLR2, endotoxin activation of TLR4 also triggers vein endothelial cells to produce inflammatory cytokines that are associated with sepsis, including IL-1β, IL-6 and IL-8 [8]. The consequences of endothelial cell production of inflammatory cytokines and their enhancement of neutrophil trafficking to sites of infection is that they can not only protect against pathogens but, in over-responding, can contribute to organ injury that is sepsis-associated and inflammation-mediated.
Fig. 2.
Endothelial cells as immune stimulatory cells. Activation of endothelial cells by various pathogens can stimulate release of cytokines and chemokines, leading to recruitment of immune cells. If uncontrolled, this first line of immunological defense against invading pathogens can result in injury. For example, excessive inflammation induced by endothelial cells can result in a cytokine storm, which can contribute to tissue damage. In recognition of the injury that can result from uncontrolled endothelial cells stimulation of immune reactivity, various pharmacological approaches are being tested to limit this excessive inflammation
Endothelial cell contribution to activation of innate immune responses is not limited to bacterial infections. Influenza infection brings on an early cytokine/chemokine storm and recruitment of macrophages, neutrophils and NK cells in the lungs [9]. These inflammatory mediators include, but are not limited to IFN-α, CCL2, IL-6 and CXCL10, and they play a direct role in influenza-mediated lung disease. The production of the inflammatory cytokines and chemokines, as well as recruitment of innate immune cells in response to influenza infection, is driven by pulmonary endothelial cells. Another example of viral-initiated endothelial cell–mediated immune stimulation occurs when endothelial cells are infected by dengue virus. Infection of endothelial cells with dengue virus results in an early explosion of a multitude of inflammatory cytokines and chemokines (such as BAFF, CXCL9/10/11, CCL5 and IL-6/7/8), as well as complement factors (properdin factor B), which function as a double-edged sword [10]. Early secretion of IFN-α by infected endothelial cells limits viral spread to adjacent endothelial cells. However, endothelial cell activation leading to the release of immune enhancing mediators and complement factors is felt to contribute to dengue disease, including dengue hemorrhagic fever and dengue shock syndrome.
Rather than endothelial cell–triggering of immunopathogenesis via their production of inflammatory cytokines and chemokines, an alternative means by which immune regulatory activities of endothelial cells can lead to disease is through their downregulation of Treg activity. For example, through their production of leptin, endothelial cells can downregulate Treg activity [11]. Correlative studies suggest that this contributes to the inflammation associated with idiopathic pulmonary arterial hypertension. The inter-relationship between endothelial cells and Treg is bidirectional, as inducible Treg have also been shown to reduce endothelial cell activity and their recruitment of T cells [12]. This may reflect a delicate balance between triggering inflammation in response to an alert and the need to prevent uncontrolled inflammation-mediated tissue damage. The conditions under which this balance is tilted to result in unrestricted or insufficient inflammation are still being defined.
Strategies to control endothelial cell activity so as to prevent inflammation-mediated tissue damage
The recognition of the contribution of endothelial cells in activating innate inflammatory responses that lead to tissue injury and disease has led to efforts to develop therapeutic approaches to block this activation. For example, exploitation of endothelial cell expression of the sphingosine-1-phosphate (S1P) receptor has resulted in demonstration that S1P antagonism significantly diminishes the cytokine storm and innate immune cell infiltration into the lungs that otherwise occurs in response to influenza infection [9]. An alternate approach to dampen endothelial cell–driven inflammation is based on the anti-inflammatory effects of ethanol. In order to avoid symptoms of intoxication that result from ethanol, ethyl pyruvate has been experimentally compared to ethanol for the capacity to block endothelial cell activation [13]. Such studies have shown ethyl pyruvate to be highly effective at reducing endothelial cell production of IL-8 and G-CSF, reducing their expression of the adhesion molecules E-selectin and intercellular adhesion molecule 1 (ICAM-1; CD54), and reducing the binding of neutrophils to endothelial cells. Endothelial cell–driven inflammation has also been shown to be tempered through agonists to the E-type prostanoid 4 (EP4) receptor. The anti-inflammatory effects of endothelial cell EP4 receptor stimulation is associated with impairment in neutrophil adhesion to endothelial cells and reduced transendothelial migration [14]. While increases in levels of prostaglandin E2 (PGE2) are classical hallmarks of inflammatory responses, the dampening of endothelial cell pro-inflammatory activity upon EP4 stimulations provides another example of a delicate balance in the regulatory controls of endothelial cell–triggered inflammation.
Endothelial cells as immune inhibitory cells
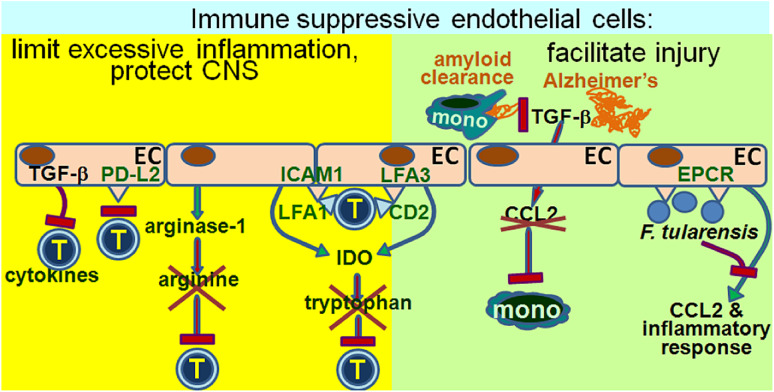
Just like endothelial cells are in a unique physical position to mediate immune stimulation through their expression of adhesion molecules and chemoattractants to orchestrate leukocyte activation and trafficking, their physical contact with immune cells also empowers them to mediate immune suppression. This immune suppression occurs through secretion of soluble inhibitory mediators as well as by direct cell contact (Fig. 3). A physiologically beneficial effect of immune inhibitory endothelial cells is to maintain the blood–brain barrier by limiting T cell transmigration into the central nervous system (CNS). Studies with endothelial cells from normal brain tissue showed their expression of a ligand for programmed cell death-1, PD-L2 [15]. The PD-L2 expressed on endothelial cells is functionally immune inhibitory as blocking PD-L2 results in increased transmigration by both CD4+ and CD8+ T cells. In contrast to the uniform expression of PD-L2 by endothelial cells from normal brain tissue, PD-L2 expression by endothelial cells from multiple sclerosis lesions is significantly reduced. This suggests the critical role of the inhibitory function of endothelial cells in protecting the CNS from inflammatory disease.
Fig. 3.
Endothelial cells as immune suppressive cells. Through a variety of approaches, both cell contact–dependent and cell contact–independent, endothelial cells can inhibit immune reactivity. The physiological benefit of this immune suppressive capability of endothelial cells is that it limits excessive inflammation that could otherwise lead to tissue injury. However, the immune inhibitory activity of endothelia cells can also be induced by pathogens to as a protective strategy from immune destruction. Furthermore, it can prevent the normal immunological processes that maintain health, such as in the tightly regulated recruitment of monocytes to limit amyloid accumulation that otherwise occurs in Alzheimer’s disease
In contrast to the beneficial effect of immune inhibitory endothelial cells in the protection of the CNS, as mentioned above, immune inhibitory endothelial cells can also prevent some of the immunological functions of cells such as perivascular macrophages that maintain CNS health. For example, in Alzheimer’s disease, brain levels of the immune regulatory cytokine TGF-β are increased. In response to TGF-β, endothelial cells produce reduced levels of chemokines, including the monocyte chemoattractant CCL-2, and they inhibit the capacity of monocytes to prevent cerebrovascular amyloid accumulation and to clear insoluble amyloid [16]. Also, TGF-β-exposed endothelial cells inhibit IFN-γ production, which is the hallmark of a Th1 response, and would otherwise contribute to reducing amyloid accumulation. Thus, while dogma would indicate the critical role of endothelial cells in protecting the CNS by maintaining the blood–brain barrier so as to prevent immune-mediated injury, a carefully controlled level of immune infiltration is in fact needed to prevent toxic accumulation of cerebrovascular amyloid.
Another important role for the inhibitory function of endothelial cells is in maintaining the cornea as an immune privileged site, although several mechanisms have been identified through which endothelial cells maintain an immune suppressed environment. One mechanism, which is cell contact–independent, is mediated through corneal endothelial cell expression of arginase I, which hydrolyzes arginine to consequently result in arginine depletion [17]. Depletion of arginine impairs T cell function. That this mechanism of T cell inhibition is truly cell contact–independent is supported by the capacity of soluble extracts from corneas to inhibit T cell proliferation through an arginase-dependent manner. The in vivo biological role of endothelial cell–derived arginase I in maintaining the immune privilege of the cornea is demonstrated by the acceptance of corneal allografts, but the rejection of allografts when arginase I is inhibited. However, maintaining the immune privileged status of the cornea by endothelial cell inhibition of T cells can also occur through a cell contact–dependent mechanism, when co-cultured, corneal endothelial cells inhibit proliferation of CD4+ T cells [18]. In addition to proliferation, corneal endothelial cells inhibit T cell activation to produce cytokines. This inhibition is most prominent for IL-17 production, suggesting an increased targeting of Th17 responses. These inhibitory effects were lost when endothelial cells were separated via an insert from T cells. In addition, the cell contact–dependent inhibition of T cells requires TGF-β, presumably membrane-bound TGF-β, since the inhibitory effect was lost upon transfection of the endothelial cells with TGF-β siRNA. Thus, endothelial cells play a significant role in protecting the cornea from inflammatory activity and in maintaining an immune privileged status in the eye.
In stark, contrast to the pro-inflammatory effects of endothelial cells following their exposure to multiple types of pathogens (as summarized in an earlier section), endothelial cell exposure to Francisella tularensis, the agent that causes tularemia, leads to suppression of the pro-inflammatory effects of endothelial cells [19]. Using production of the chemokines CCL2 and CXCL8 as indicators of endothelial cell pro-inflammatory activity, the suppression of endothelial cell–mediated inflammation requires live F. tularensis, but is not associated with its replication as endothelial cells do not support the replication of F. tularensis. Live F. tularensis also blocks the pro-inflammatory effects that would otherwise be induced by the killed pathogen. This suppression of endothelial cells’ inflammatory activity does not occur through soluble mediators, but instead requires contact between the pathogen and the endothelial cells, and can be prevented by the blockage of the endothelial protein C receptor (EPCR). This suggests that use of EPCR by F. tularensis to prevent endothelial cell chemokine release induces endothelial cells that contribute to the lethality of the infection through their failure to recruit monocytes, T cells and dendritic cells. Similar to these findings with F. tularensis, the inflammatory response to an infection with the protozoan parasite Leishmania major results in endothelial cells tempering the inflammatory response in response to the infection [20]. However, this tempering of inflammation is mediated through endothelial cell nitric oxide synthase (eNOS). Paradoxically, and in contrast with the response to F. tularensis, is the observation that the parasite load, level of granulocyte infiltration and severity of lesions is enhanced in eNOS-deficient mice. This suggests that endothelial cells counteract the inflammatory response that otherwise exacerbates the infection and disease.
In contrast to expectations, activation of lymphatic endothelial cells with IFN-γ induces them to inhibit T cell proliferation in response to alloantigens [21]. This is despite the expression of co-stimulatory molecules ICAM-1 (CD54) and lymphocyte function-associated antigen (LFA)-3 (CD58). T cells adhere to the co-stimulatory molecules of endothelial cells through the respective ligands, LFA-1 and CD2. Despite this adhesion, IFN-γ-stimulated endothelial cells mediate their inhibition of T cell proliferation through soluble mediators, although suppression is not through cytokines, such as TGF-β or IL-10, that are traditionally associated with immunosuppression. Instead, IFN-γ stimulates the upregulation of indoleamine 2,3 dioxygenase (IDO) in endothelial cells. While other immune inhibitory mediators are possible, the increased expression of IDO can contribute to T cell inhibition through depletion of tryptophan.
Strategies to activate endothelial cells so as to lessen their immune inhibitory capacity
Various approaches have been tested to dampen endothelial activation that contributes to the pathogenesis of inflammatory disorders. Some of these were summarized above. However, far less emphasis has been placed on developing means to activate endothelial cells so as to stimulate innate immune responses against pathogens and cancer. Recent improvements in synthesis of small molecule libraries and high-throughput screening have led to the identification of endothelial cell–activating compounds, as defined by the capacity of endothelial cells to stimulate primary human monocytes to produce increased levels of macrophage inflammatory protein 1α (MIP1α) and MIP1β [22]. A group of small molecule octahydro-1, 6-naphthyridin-4-ones has been shown to be capable of stimulating endothelial cells to trigger a cascade in which they enable macrophages to release chemokines that play a role in attracting lymphocytes and monocytes to presumably function in a protective capacity against pathogens.
Immune modulation by endothelial cells in the tumor microenvironment
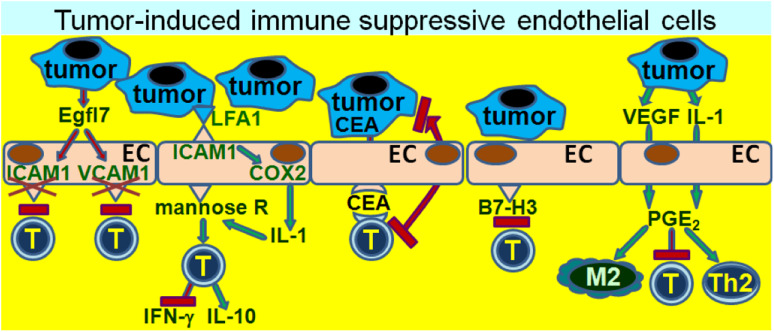
Given the above sections summarizing the broad immune regulatory potential of endothelial cells that can either protect or injure the host, it is possible to envision them as being either enablers of host defenses against cancer, or succumbing to protecting the tumor from immunological surveillance. Within the tumor environment, endothelial cell activation in the process of tumor-induced angiogenesis has been extensively studied. However, the immunological role that endothelial cells play in defending either the tumor or the host has received far less emphasis. As a means to evade immunological recognition and destruction, cancers have been shown to induce endothelial cells to assist in cancer evasion from immune defense mechanisms (Fig. 4). Once again, their location puts endothelial cells in a unique position to inhibit T cell reactivity due to their exposure to T cells during extravasation from the circulation into the tumor tissue. As described in the sections above, endothelial cells can upregulate their expression of leukocyte adhesion molecules that are involved in a complex set of interactions that facilitate transendothelial cell migration and extravasation into tissue. Among the endothelial cell adhesion molecules are ICAM-1 and vascular cell adhesion molecule 1 (VCAM-1). However, through their secretion of Egfl7, lung and breast carcinoma cells downregulate endothelial cell expression of these adhesion molecules [23]. The consequence of this downregulation is the inability of endothelial cells to participate in the recruitment of a T cell infiltrate into the tumor tissue. Tumors having endothelial cells with downregulated expression of adhesion molecules have a reduced T cell content and are clinically more aggressive than those in which adhesion molecule expression is not downregulated via Egfl7.
Fig. 4.
Immune inhibitory endothelial cells in the tumor environment. The intimate proximity of endothelial cells to immune cells attempting to transmigrate into tumor tissue or regional lymph nodes places them in a precarious position of being able to either promote or prevent immune reactivity to tumor. Through multiple means, tumors have developed schemes by which to manipulate the endothelial cells to take on a role that prevents immune reactivity to tumor and, thus, facilitates tumor development. These means include downregulation of endothelial cell adhesion molecules that otherwise facilitate immune cell trafficking into tissue, or inducing endothelial cells that either tolerize T cells or skew various immune cell populations into inhibitory cells
The interactions between endothelial cell adhesion molecules such as ICAM-1 and tumor are bidirectional. The interaction of LFA-1 of colorectal tumor cells with ICAM-1 of tumor-activated liver sinusoidal endothelial cells leads to tumor upregulation of COX-2 and, in turn, induction of endothelial cell production of IL-1 [24]. IL-1 then upregulates endothelial cell mannose receptor, which is important in antigen presentation. The result of this tumor-endothelial cell interaction is an inhibition of liver sinusoidal lymphocyte production of IFN-γ, an increase in their production of the inhibitory mediator IL-10, and reduced lymphocyte cytolytic activity toward tumor cells. Each of these effects is dependent on the increased mannose receptor expression and endocytosis by tumor-exposed endothelial cells. What has not yet been resolved is the mechanism by which increased mannose receptor levels leads to these immune inhibitory effects.
Consistent with the above-mentioned shift of endothelial cells to producing immune inhibitory cytokines following exposure to tumor and, thus, a failure to stimulate immune reactivity toward tumor, is their induction of tolerance to tumor antigens. Carcinoembryonic antigen (CEA) is among a group of tumor antigens that could be immunological targets. However, endothelial cells can block reactivity to such tumor antigens by taking up the CEA released from cancer, such as colorectal carcinoma, and presenting the antigen to CD8+ T cells [25]. Rather than stimulating the T cells, endothelial cells facilitate tumor escape from immune defenses as their presentation of CEA results in tolerization of the CD8+ cells.
Another mechanism by which cancer-associated endothelial cells can subvert immune defenses is through their expression of B7 family members of immune regulatory molecules. The vasculature associated with ovarian cancer expresses the co-inhibitory B7 family member, B7-H3 [26]. The clinical significance of expression of this co-inhibitory B7 family member on cancer-associated endothelial cells is that it directly correlates with the level of cancer grade, with the vasculature of most high-stage cancers expressing B7-H3 and the vasculature of fewer low-stage cancers expressing B7-H3. In addition, increased expression of B7-H3 on cancer endothelium is associated with increased recurrence and worse survival. Similar types of results are also found for endometrial cancer, where B7-H3 is overexpressed on endothelial cells of high grade tumors. This overexpression of B7-H3 has been suggested to be associated with downregulation of T cell anti-tumor reactivity by the endothelial cells [27].
In addition to mediating immune inhibition through cell contact routes, several studies have shown that endothelial cells can be stimulated through soluble tumor-derived mediators to become immune inhibitory and to subvert immune competence through soluble endothelial cell–derived mediators. While different mechanisms have been shown to induce endothelial cells to become immune inhibitory, some of these pathways have triggered similar means by which tumor-exposed endothelial cells inhibit immune reactivity. Through their production of IL-1, squamous cell carcinoma cells of the head and neck (HNSCC) induce microvascular endothelial cells to upregulate their expression of microsomal PGE-synthase-1, enabling them to produce the immune inhibitory mediator, PGE2 [28]. In a separate series of studies, endothelial cells isolated from lung carcinoma tissue have been shown to also be inhibitory toward T cell reactivity through their production of PGE2 [29]. This latter series of studies showed vascular endothelial cell growth factor (VEGF) to be the mediator that induced normal microvascular endothelial cells to become immune inhibitory. However, like the induction of suppressive endothelial cells by tumor-derived IL-1, tumor-derived VEGF induces upregulation of PGE2 production by endothelial cells and, in turn, their inhibition of T cell activity [30–32]. Of interest is the possibility of endothelial cell production of PGE2 being a catalyst that can trigger a cascade of inhibitory mechanisms since PGE2 can mediate Th2 and M2 skewing, and induce Tregs [33–35]. This raises the question of the extent to which PGE2-producing endothelial cells contribute to the immune suppression in the cancer setting or are responsible for triggering immune suppressive cascades compared to other more traditional immune inhibitory cells (Th2, MDSC, Treg, etc.) that are more typically studied in the cancer environment. The contribution of endothelial cells triggering this type of cascade in the tumor setting has yet to be explored. Also not known is when, in the course of progression from premalignant lesions to cancer, endothelial cells switch to acquiring an immune inhibitory phenotype.
A multitude of studies have suggested the protective effects of prostaglandin synthesis inhibitors in preventing cancer development or reducing cancer progression. However, the mechanisms for possible protective effects are varied. For example, COX-2 inhibition can block epithelial-mesenchymal transition and cancer stem cell proliferation, and increase sensitivity to chemotherapy [36–38]. Added to the list is the capacity to block endothelial cell immune suppressive activity [32] and their possible induction of other suppressive populations. Yet to be sorted out is the role of endothelial cell COX-2 in the cascade that results in the possible induction of immune suppressor cells and, in turn, immune dysfunction in the developing cancer.
Summary
Historically, studies have focused on the role of tumor-associated endothelial cells in tumor vascularization. However, their unique location that places them in intimate contact with immune cells as they pass through the endothelium into lymph nodes or tissue makes them readily able to also be immune regulators. In that capacity, they can regulate immune cell transmigration into lymph nodes or tissue, and either stimulate or inhibit immune reactivity. To some extent this dichotomy is dictated by their level of activity and the types of stimuli that induce their activity. In general, their response to pathogens tends to favor their stimulation of innate immune responses, although pathogens can also manipulate endothelial cells to function in a way that protects the pathogens from immune defenses. In other instances, endothelial cells have a critical role in dampening immune parameters so as to prevent excessive reactivities and maintain health. This is particularly evident in their limiting immune trafficking into the central nervous system or in maintaining immune privileged sites. Tumors seem to have mastered the skill of manipulating endothelial cells to protect the tumor from immune surveillance. Inappropriate immunosuppression mediated by endothelial cells occurs in the tumor environment where immune trafficking through the vessel walls is critical for passage into tumor tissue or regional lymph nodes. The induction by tumors of endothelial cell expression of a variety of immune subversive tactics that are mediated through cell contact–dependent and cell contact–independent means leads to protection of tumors from immune defenses. Lessons learned from understanding how endothelial cells are skewed to become immune stimulatory versus immune inhibitory in a variety of settings, both physiological and pathological, are critical in harnessing these cells to defend against cancer, while limiting excessive immune reactivity that can lead to additional tissue injury.
Acknowledgments
This work was supported by the Department of Veterans Affairs through a Clinical Sciences Research & Development award (CX000100) and a Senior Research Career Scientist award, and by grants RO1 CA128837 and RO1 DE018268 from the National Institutes of Health.
Conflict of interest
The author declares that she has no conflict of interest.
References
- 1.Wilhelmsen K, Mesa KR, Prakash A, Xu F, Hellman J. Activation of endothelial TLR2 by bacterial lipoprotein upregulates proteins specific for the neutrophil response. Innate Immun. 2012;18:602–616. doi: 10.1177/1753425911429336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salsman VS, Chow KK, Shaffer DR, et al. Crosstalk between medulloblastoma cells and endothelium triggers a strong chemotactic signal recruiting T lymphocytes to the tumor microenvironment. PLoS ONE. 2011;6:e20267. doi: 10.1371/journal.pone.0020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson LA, Jackson DG. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int Immunol. 2010;22:839–849. doi: 10.1093/intimm/dxq435. [DOI] [PubMed] [Google Scholar]
- 4.Bao X, Moseman EA, Saito H, et al. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity. 2010;33:817–829. doi: 10.1016/j.immuni.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chyou S, Benahmed F, Chen J, Kumar V, Tian S, Lipp M, Lu TT. Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J Immunol. 2011;187:5558–5567. doi: 10.4049/jimmunol.1101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sage PT, Varghese LM, Martinelli R, Sciuto TE, Kamei M, Dvorak AM, Springer TA, Sharpe AH, Carman CV. Antigen recognition is facilitated by invadosome-like protrusions formed by memory/effector T cells. J Immunol. 2012;188:3686–3699. doi: 10.4049/jimmunol.1102594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson J, Lang S, Lambert PA, Martin PE. Peptidoglycan derived from Staphylococcus epidermidis induces Connexin43 hemichannel activity with consequences on the innate immune response in endothelial cells. Biochem J. 2010;432:133–143. doi: 10.1042/BJ20091753. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Deng M, Liu X, Ai W, Tang Q, Hu J. TLR4 activation induces nontolerant inflammatory response in endothelial cells. Inflammation. 2011;34:509–518. doi: 10.1007/s10753-010-9258-4. [DOI] [PubMed] [Google Scholar]
- 9.Teijaro JR, Walsh KB, Cahalan S, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalrymple NA, Mackow ER. Endothelial cells elicit immune-enhancing responses to dengue virus infection. J Virol. 2012;86:6408–6415. doi: 10.1128/JVI.00213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huertas A, Tu L, Gambaryan N et al. (2012) Leptin and regulatory T lymphocytes in idiopathic pulmonary arterial hypertension. Eur Respir J [Epub ahead of print] [DOI] [PubMed]
- 12.Maganto-Garcia E, Bu DX, Tarrio ML, et al. Foxp3+-inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J Immunol. 2011;187:3521–3529. doi: 10.4049/jimmunol.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johansson AS, Johansson-Haque K, Okret S, Palmblad J. Ethyl pyruvate modulates acute inflammatory reactions in human endothelial cells in relation to the NF-κB pathway. Br J Pharmacol. 2008;154:1318–1326. doi: 10.1038/bjp.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konya V, Ullen A, Kampitsch N et al. (2012) Endothelial E-type prostanoid 4 receptors promote barrier function and inhibit neutrophil trafficking. J Allergy Clin Immunol [Epub ahead of print] [DOI] [PubMed]
- 15.Pittet CL, Newcombe J, Prat A, Arbour N. Human brain endothelial cells endeavor to immunoregulate CD8 T cells via PD-1 ligand expression in multiple sclerosis. J Neuroinflammation. 2011;8:155. doi: 10.1186/1742-2094-8-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss R, Lifshitz V, Frenkel D. TGF-β1 affects endothelial cell interaction with macrophages and T cells leading to the development of cerebrovascular amyloidosis. Brain Behav Immun. 2011;25:1017–1024. doi: 10.1016/j.bbi.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Fu H, Khan A, Coe D, Zaher S, Chai JG, Kropf P, Muller I, Larkin DF, George AJ. Arginine depletion as a mechanism for the immune privilege of corneal allografts. Eur J Immunol. 2011;41:2997–3005. doi: 10.1002/eji.201141683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugita S, Kawazoe Y, Yamada Y, Imai A, Horie S, Yamagami S, Mochizuki M. Inhibitory effect of corneal endothelial cells on IL-17-producing Th17 cells. Br J Ophthalmol. 2012;96:293–299. doi: 10.1136/bjophthalmol-2011-300769. [DOI] [PubMed] [Google Scholar]
- 19.Bublitz DC, Noah CE, Benach JL, Furie MB. Francisella tularensis suppresses the proinflammatory response of endothelial cells via the endothelial protein C receptor. J Immunol. 2010;185:1124–1131. doi: 10.4049/jimmunol.0902429. [DOI] [PubMed] [Google Scholar]
- 20.Fritzsche C, Schleicher U, Bogdan C. Endothelial nitric oxide synthase limits the inflammatory response in mouse cutaneous leishmaniasis. Immunobiology. 2010;215:826–832. doi: 10.1016/j.imbio.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Norder M, Gutierrez MG, Zicari S, Cervi E, Caruso A, Guzman CA. Lymph node-derived lymphatic endothelial cells express functional costimulatory molecules and impair dendritic cell-induced allogenic T-cell proliferation. FASEB J. 2012;26:2835–2846. doi: 10.1096/fj.12-205278. [DOI] [PubMed] [Google Scholar]
- 22.Cruz D, Wang Z, Kibbie J, Modlin R, Kwon O. Diversity through phosphine catalysis identifies octahydro-1,6-naphthyridin-4-ones as activators of endothelium-driven immunity. Proc Natl Acad Sci USA. 2011;108:6769–6774. doi: 10.1073/pnas.1015254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delfortrie S, Pinte S, Mattot V, et al. Egfl7 promotes tumor escape from immunity by repressing endothelial cell activation. Cancer Res. 2011;71:7176–7186. doi: 10.1158/0008-5472.CAN-11-1301. [DOI] [PubMed] [Google Scholar]
- 24.Arteta B, Lasuen N, Lopategi A, Sveinbjornsson B, Smedsrod B, Vidal-Vanaclocha F. Colon carcinoma cell interaction with liver sinusoidal endothelium inhibits organ-specific antitumor immunity through interleukin-1-induced mannose receptor in mice. Hepatology. 2010;51:2172–2182. doi: 10.1002/hep.23590. [DOI] [PubMed] [Google Scholar]
- 25.Hochst B, Schildberg FA, Bottcher J et al. (2012) Liver sinusoidal endothelial cells contribute to CD8+ T cell tolerance towards circulating carcinoembryonic antigen in mice. Hepatology [Epub ahead of print] [DOI] [PubMed]
- 26.Zang X, Sullivan PS, Soslow RA, et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol. 2010;23:1104–1112. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brunner A, Hinterholzer S, Riss P, Heinze G, Brustmann H. Immunoexpression of B7-H3 in endometrial cancer: relation to tumor T-cell infiltration and prognosis. Gynecol Oncol. 2012;124:105–111. doi: 10.1016/j.ygyno.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Casos K, Siguero L, Fernandez-Figueras MT, Leon X, Sarda MP, Vila L, Camacho M. Tumor cells induce COX-2 and mPGES-1 expression in microvascular endothelial cells mainly by means of IL-1 receptor activation. Microvasc Res. 2011;81:261–268. doi: 10.1016/j.mvr.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Mulligan JK, Young MR. Tumors induce the formation of suppressor endothelial cells in vivo. Cancer Immunol Immunother. 2010;59:267–277. doi: 10.1007/s00262-009-0747-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulligan JK, Day TA, Gillespie MB, Rosenzweig SA, Young MR. Secretion of vascular endothelial growth factor by oral squamous cell carcinoma cells skews endothelial cells to suppress T-cell functions. Hum Immunol. 2009;70:375–382. doi: 10.1016/j.humimm.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mulligan JK, Lathers DM, Young MR. Tumors skew endothelial cells to disrupt NK cell, T cell and macrophage functions. Cancer Immunol Immunother. 2008;57:951–961. doi: 10.1007/s00262-007-0425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulligan JK, Rosenzweig SA, Young MR. Tumor secretion of VEGF induces endothelial cells to suppress T cell functions through the production of PGE2 . J Immunother. 2010;33:126–135. doi: 10.1097/CJI.0b013e3181b91c9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baratelli F, Lin Y, Zhu L, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 34.Bao YS, Zhang P, Xie RJ, Wang M, Wang ZY, Zhou Z, Zhai WJ, Feng SZ, Han MZ. The regulation of CD4+ T cell immune responses toward Th2 cell development by prostaglandin E2 . Int Immunopharmacol. 2011;11:1599–1605. doi: 10.1016/j.intimp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Hinz B, Brune K, Pahl A. Prostaglandin E2 upregulates cyclooxygenase-2 expression in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem Biophys Res Commun. 2000;272:744–748. doi: 10.1006/bbrc.2000.2859. [DOI] [PubMed] [Google Scholar]
- 36.Vivaldi A, Ciampi R, Tacito A, Molinaro E, Agate L, Bottici V, Pinchera A, Collecchi P, Elisei R. Celecoxib, a cyclooxygenase-2 inhibitor, potentiates the chemotherapic effect of vinorelbine in the medullary thyroid cancer TT cell line. Mol Cell Endocrinol. 2012;355:41–48. doi: 10.1016/j.mce.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 37.Bocca C, Bozzo F, Cannito S, Parola M, Miglietta A (2011) Celecoxib inactivates epithelial-mesenchymal transition stimulated by hypoxia and/or epidermal growth factor in colon cancer cells. Mol Carcinog 10. doi:10.1002/mc.20846 [DOI] [PubMed]
- 38.Sharma V, Dixit D, Ghosh S, Sen E. COX-2 regulates the proliferation of glioma stem like cells. Neurochem Int. 2011;59:567–571. doi: 10.1016/j.neuint.2011.06.018. [DOI] [PubMed] [Google Scholar]