Abstract
Background
Hyperinsulinemia has been associated with hepatic fat deposition and ensuing insulin resistance. It is unknown if treatment with exogenous insulin in patients with type 2 diabetes, who are most prone to hepatic fat accumulation, would promote the occurrence or worsening of nonalcoholic fatty liver disease (NAFLD).
Methods
Patients with treatment-naïve type 2 diabetes (N=16) were treated with insulin and metformin for a 3-month lead-in period, then assigned triple oral therapy (metformin, glyburide and pioglitazone) or continued treatment with insulin and metformin. Hepatic triglyceride content (HTC) – measured by magnetic resonance spectroscopy, serum lipids, glucose, liver function tests, and inflammatory and thrombotic biomarkers were followed for a median of 31 months.
Results
The 45% decline in HTC during the lead-in period persisted through the follow-up period with no difference between treatment groups at the end of the study (5.26±4.21% in the triple oral therapy versus 7.47±7.40% for insulin/metformin), while glycemic control was comparable.
Conclusions
Improvements in HTC with initial insulin/metformin therapy persisted through the median 31-month follow-up period regardless of the treatment. More importantly, insulin-based treatment does not appear to promote or worsen NAFLD.
Keywords: hepatic steatosis, type 2 diabetes, insulin, NAFLD, triglyceride
Introduction
Non-alcoholic fatty liver disease (NAFLD), the most common cause of liver disease in the United States, is characterized by the intra-cytoplasmic accumulation of hepatic triglycerides.1 NAFLD encompasses a histopathological spectrum from simple steatosis to non-alcoholic steatohepatitis (NASH), and represents the fastest growing etiology for cirrhosis and end-stage liver disease. Insulin resistance and obesity both associate with NAFLD and type 2 diabetes, and it was noted that up to 78% of patients with type 2 diabetes also have hepatic steatosis.2 Given the significant consequences of NAFLD, especially in patients with type 2 diabetes, it is important to evaluate the effect of diabetes treatment on occurrence or progression of hepatic steatosis.
Insulin sensitizers approved for diabetes treatment, including thiazolidinediones (TZDs) and biguanides, have demonstrated short-term hepatic triglyceride content (HTC) improvement. Pioglitazone, a second generation TZD, increases adiponectin levels and blocks sterol regulatory element binding protein-1c.3 Patients with type 2 diabetes treated with pioglitazone demonstrate reduced HTC,4 as well as improvements in histology and biochemistry in studies with follow-up of up to 1 year.5,6 Metformin reduces expression of hepatic TNF-α,7–8 but its use in NAFLD has produced conflicting results.9–12 Currently, no approved therapies exist for the treatment of NAFLD.
Insulin stimulates intracellular triglyceride synthesis while inhibiting lipolysis. In one study,13 8 patients with type 2 diabetes underwent a 72-hour hyperinsulinemic-euglycemic clamp and demonstrated increased hepatic steatosis compared to prior baseline measurements. In contrast, a three-month pilot study in 19 newly-diagnosed type 2 diabetes patients14 using insulin/metformin combination treatment demonstrated improved hepatic steatosis. A three-month combination therapy with insulin/metformin has previously resulted in a 45% reduction in HTC (P<0.001) and resolved hepatic steatosis in 75% of patients15. Whether long-term exogenous insulin therapy promotes hepatic fat deposition accelerating the progression of steatosis is uncertain.
As pancreatic β-cell function and insulin sensitivity deteriorate in type 2 diabetes, combinations of oral anti-diabetic (OAD) agents, with or without exogenous insulin, may help patients achieve satisfactory glycemic control.16 Interventional studies for NAFLD historically exclude patients with type 2 diabetes due to the confounding effects of OADs and insulin therapy. These studies do not evaluate the long-term impact of therapy on hepatic steatosis. We performed an open-label, prospective clinical trial of patients with newly-diagnosed type 2 diabetes to investigate the effects of insulin/metformin versus combination OADs, specifically metformin/pioglitazone/glyburide (“triple oral therapy”) to characterize the long-term progression of hepatic steatosis as measured by localized proton magnetic resonance spectroscopy (MRS).
Materials and Methods
Study participants
Treatment-naïve patients aged 21–70 years diagnosed with type 2 diabetes within the preceding two months were recruited in to a parent study from Parkland Memorial Hospital diabetes services and self-referral at the Clinical Diabetes Research Clinic at the University of Texas Southwestern Medical Center (UTSW) in Dallas, Texas. Exclusion criteria included: type 1 diabetes–related antibodies, HbA1c < 7%, serum creatinine > 1.5 mg/dL, women pregnant or not utilizing contraception, and history of heart failure or lactic acidosis. All patients met additional inclusion criteria of weight < 160 kg, absence of metallic implants, claustrophobia, and known chronic liver diseases of any etiology. Additionally, patents met exclusion criteria prohibiting illicit drug use within the past six months, or consumption of two or more alcoholic drinks daily. The study protocol was approved by the local Institutional Review Board, and participants provided written informed consent prior to enrollment.
Treatment
Patients were treated for a three-month lead-in period with a combination of NovoLog Mix 70/30 and metformin (1000 mg twice daily).15
Following the three-month lead-in period, patients either continued insulin/metformin or discontinued insulin and began triple oral therapy at visit #2 (month “0”). Treatment was assigned in the parent study using a block randomization scheme as described previously.17 Patients assigned to triple oral therapy were treated with metformin (1000 mg twice daily), pioglitazone (45 mg once daily), and glyburide (1.25 mg twice daily). Dose titration of insulin and glyburide (up to 10 mg daily) was performed by the study physician throughout the study to maintain target glycemic control (HbA1c ≤ 6.5%). Initiation and dose adjustment of antihypertensive and lipid-lowering agents were permitted after the treatment allocation visit if medically necessary.
Measurements
Biochemical evaluations, performed at baseline and within 3 months of each MRS, included blood glucose, insulin, HbA1c, liver function tests, and lipid profile. All blood samples were obtained while fasting (10–14 hrs), in the morning, processed immediately and analyzed within 24 hrs. Liver function tests, glucose levels, and lipid profiles were measured by a commercial laboratory (Quest Diagnostics, Irving, TX). Insulin levels were measured by radioimmunoassay (Coat-A-Count Insulin TKIN1, Siemens Healthcare Diagnostics, Tarrytown, NY) and HbA1c by high performance liquid chromotography in the UTSW Clinical Diabetes Laboratory.
Hepatic triglyceride content (HTC) was measured by MRS at baseline (month “−3”), at the treatment assignment visit (month “0”) and compared with follow-up studies performed after short-term treatment (months 10–23) or long-term treatment (months 24–42). MRS is the preferred method for non-invasive measurement of hepatic steatosis in vivo as it permits precise and reproducible quantification of intracellular HTC.18–28 MRS evaluation of HTC is now broadly accepted in clinical studies as fast, safe, and reliable. We evaluated HTC using a1.5 Tesla Gyroscan Achieva whole body clinical system (Philips Medical Systems, Cleveland, USA) equipped with software for localized spectroscopy as described previously.20,27 High-resolution morphological images were collected to define a testing volume of 27 cm3 within the upper right hepatic lobe, avoiding major blood vessels, intrahepatic bile ducts, and neighboring adipose tissue. All spectra were collected using PRESS sequence (PointRESolvedSpectroscopy) for spatial localization and signal acquisition with the following data acquisition parameters: Te = 27 ms, Tr= 3s. All data were collected without water suppression. Sixteen acquisitions were averaged. Areas of resonances from protons in water molecules and in methylenes of fatty acid chains were evaluated with line-fit procedure using a commercial software (NUTS-ACORNNMR, Freemont, CA). The unit of measurement is the ratio of signal from fat (f) to the total signal from fat (f) and water (w) [f/(f+w)] expressed as percent. The upper limit of normal for HTC is 5.56%.27 The coefficient of variation (CV) for MRS is 8.5%27 which is superior to the reproducibility of liver biopsy in the determination of HTC. 29–31 Although liver biopsy is considered the gold standard to measure hepatic steatosis, the procedure carries significant morbidity and mortality, as well as risk for sampling error. Previous studies demonstrate high validity and reproducibility for hepatic steatosis measurements obtained by MRS and biopsy both in animals 21 and humans.6
Statistical Analysis
Summary statistics are means and standard deviations (SD) for continuous variables or counts and percentages for categorical variables. Correlations between variables at the treatment allocation visit were calculated using the nonparametric Spearman method. We tested for the intervention effect using a mixed linear regression model of the HTC outcome with fixed treatment group, visit, and treatment group × visit effects, assuming a first-order autoregressive process for the residual errors. A significant treatment group × visit interaction was considered evidence for an intervention effect. We fit an exploratory longitudinal model to the HTC visit series by using backward variable selection in a mixed linear regression model including cross-sectional and longitudinal weight, BMI, HbA1c, glucose, cholesterol, fasting triglycerides, fibrinogen and PAI-1. The selected model included within- and between-subjects versions of any covariates that were significant within subjects and any other significant between-subjects covariates. Insulin resistance was measured using the HOMA-IR calculator provided at the Diabetes Treatment Unit, University of Oxford (http://www.dtu.ox.ac.uk/). Significance was denoted by p < 0.05. We used SAS/STATR, version 9.2, (SAS Institute, Inc., Cary, NC) for all analyses.
Results
Twenty-four patients were enrolled initially; one patient was excluded due to motion artifact on the baseline MRS and 7 patients did not return for subsequent MRIs. Of the 16 patients with at least one baseline and one follow-up post-treatment MRS, 10 patients were in the insulin/metformin arm and 6 patients in triple oral therapy arm. The baseline (at the time of randomization) characteristics of the treatment groups are shown in Table 1A.
Table 1.
Baseline Characteristics and Post-treatment Follow-up
| 1A. Baseline | 1B. Post-treatment Follow-up | |||
|---|---|---|---|---|
| (n=16) | Insulin/Metformin (n=10) | Triple Oral Therapy (n=6) | Insulin/Metformin (n=10) | Triple Oral Therapy (n=6) |
| Age (years) | 45.4 ± 8.32 | 42 ± 7.27 | ||
| Male | 8 | 5 | ||
| Ethnicity: Hispanic | 4 | 5 | ||
| Caucasian | 2 | 1 | ||
| African-American | 4 | 0 | ||
| Statin Use: None | 5 | 3 | 7 | 3 |
| Current | 5 | 3 | 3 | 3 |
| Hepatic Triglyceride Content (%) | 6.59 ± 7.43 | 5.14 ± 5.38 | 7.47 ± 7.40 | 5.26 ± 4.21 |
| Weight (kilograms) | 108.27 ± 19.31 | 95.62 ± 10.35 | 110.94 ± 24.44 | 103.45 ± 4.08 |
| Body mass index | 35.77 ± 4.30 | 34.18 ± 5.42 | 37.13 ± 9.01 | 37.91 ± 4.07 |
| Insulin dose (units) | 64.2 ± 22.0 | 50.3 ± 15.9 | 85.2 ± 60.52 | NA |
| Hemoglobin A1c (%) | 5.99 ± 0.45 | 6.40 ± 0.19 | 6.47 ± 1.20 | 6.70 ± 2.01 |
| Fasting Glucose (mg/dl) | 107.50 ± 20.78 | 121.67 ± 19.79 | 118.00 ± 18.40 | 166.50 ± 122.21 |
| Fasting Insulin (uU/ml) | 35.27 ± 55.55 | 16.97 ± 10.76 | 45.08 ± 36.40 | 5.21 ± 2.06 |
| HOMA-IR | 3.53 ± 4.71 | 1.98 ± 1.21 | 4.95 ± 3.52 | 1.09 ± 1.27 |
| Cholesterol (mg/dl) | 166.7 ± 36.4 | 172.2 ± 23.6 | 160.1 ± 37.6 | 190.8 ± 29.6 |
| Fasting Triglycerides (mg/dl) | 152.9 ± 96.1 | 140.8 ± 75.4 | 180.2 ± 134.5 | 167.0 ± 101.3 |
| Cardiac CRP (mg/l) | 6.00 ± 6.50 | 8.70 ± 11.42 | 8.14 ± 9.94 | 4.89 ± 6.04 |
| Fibrinogen (mg/dl) | 389.1 ± 61.4 | 402.2 ± 95.9 | 429.0 ± 79.7 | 394.3 ± 126.9 |
| Plasminogen activator inhibitor-1 (IU/ml) | 22.56 ± 18.65 | 17.98 ± 8.19 | 18.90 ± 15.08 | 8.06 ± 8.58 |
| Aspartate aminotransferase (U/l) | 16.9 ± 4.84 | 17.2 ± 5.34 | 18.1 ± 4.04 | 13.3 ± 4.50 |
Lead-In Period
Treatment-naïve patients with newly-diagnosed type 2 diabetes completed a three-month lead-in period with insulin/metformin treatment. HTC declined on average 45.6% (from 11.83+/−7.6% to 6.1+/−6.6%, P<0.001) during the lead-in period compared to the baseline measurements at enrollment. More details on the lead-in period results are provided in a previous publication.15
Intervention Period
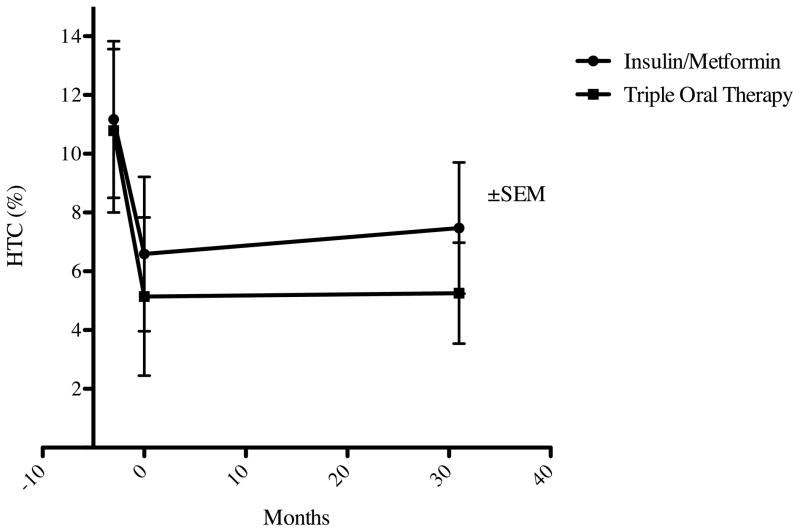
Following the lead-in period, patients either continued insulin/metformin therapy or discontinued insulin and began triple oral therapy. No difference in the between-visit changes in HTC was observed in the two treatment groups (Table 1B). There was also no difference in HbA1c, HOMA-IR, total cholesterol, fasting serum triglycerides, cardiac CRP, fibrinogen, PAI-1, and AST. Following allocation to triple oral therapy versus continued insulin/metformin treatment, no difference in HTC between groups was observed (5.26±4.21% versus 7.47±7.40% at a median of 31 months from treatment allocation). These results are summarized in Figure 1.
Figure 1. Hepatic Triglyceride Content in Newly-Recognized Type 2 Diabetes Treated with Insulin/Metformin or Triple Oral Therapy.
Treatment-naïve patients with type 2 diabetes underwent lead-in treatment with insulin and metformin (months −3 through 0) and were subsequently assigned to Triple Oral Therapy (n=6) versus continued treatment with insulin/metformin (n=10). Hepatic triglyceride content (HTC) did not differ between treatment groups in long term follow-up (median: 31 months).
An exploratory longitudinal model of HTC suggests that within-subjects changes in glucose (coefficient = 0.21 % per mg/dL, p < 0.001) and fibrinogen (coefficient = 0.04 % per mg/dL, p = 0.002) are positively associated with changes in HTC, controlling for corresponding between-subjects effects and between-subjects effect of weight. Neither the dose of insulin nor statin use, whether concurrent or initiated during the study, correlated with HTC improvement.
Spearman rank correlation showed that baseline HTC correlated with BMI (r=0.577, p=0.019) while no significant association was found between HTC and cholesterol, triglyceride, fibrinogen, PAI-1, AST or ALT levels.
Discussion
Type 2 diabetes is a cluster of metabolic abnormalities that coexist and compound cardiovascular risk. It is most important to study the impact of treatments not only on the condition for which they are being primarily prescribed, but also on the associated co-morbidities, ensuring their safety profile and ultimately a positive effect on global cardiovascular risk reduction. Our study aimed to evaluate the long-term effect of the two most commonly used treatment regimens for type 2 diabetes on a co-morbidity known to exist in over 70% of these patients--hepatic steatosis.
We found that newly-diagnosed patients with type 2 diabetes initiated on insulin/metformin therapy showed a 45% improvement in HTC content within three months, which was sustained over the subsequent 31 months regardless of whether patients continued insulin/metformin or resumed triple oral therapy. Neither treatment with insulin nor the insulin dose required for glycemic control accelerated HTC progression. Our findings suggest that therapeutically-induced hyperinsulinemia (i.e. exogenous treatment) does not have a deleterious effect on hepatic steatosis occurrence or progression, in contrast to the well-established positive association between hepatic steatosis and endogenous hyperinsulinemia.
Although conclusions from our study are limited by the small sample size and randomization in the parent study, to our knowledge, this is the longest study to chart the progression of hepatic steatosis in newly-diagnosed patients with type 2 diabetes treated with insulin or OADs. Previously, the longest study evaluated pioglitazone monotherapy versus placebo in type 2 diabetes and hepatic steatosis for one year.5 The longest study to define the contribution of insulin treatment to hepatic steatosis progression lasted only seven months,33 and no studies sufficiently model real-life diabetes treatment, in which patients receive multiple OADs or a combination of insulin and metformin. Our study confirms, complements, and extends the findings of these previous studies by having longer follow-up (median follow-up of 31 months) and by comparing the effect of TZD-based therapy with insulin regimens most commonly employed to treat type 2 diabetes. Furthermore, both groups had comparable and sustained glycemic control throughout the study period, allowing for the treatment effect to be observed independent of changes in glycemic control.
The selection of insulin injections over OADs has bearing on the clinical management of patients with newly-diagnosed type 2 diabetes. Our study specifically addresses the effects of treatment, suggesting that both insulin and OADs provide equivalent and sustained improvements on HTC, and that concern for worsening HTC with exogenous insulin is unfounded. Further larger studies are warranted to evaluate the effect of diabetes treatment on other metabolic co-morbidities, including hepatic steatosis.
Acknowledgments
Sources of Support: none
The parent study was supported by an investigator-initiated trial grant from NovoNordisk, Inc. IL was supported by NIH K23RR024470 and 3UL1 RR024982-05S1. LS was supported by National Heart, Lung, and Blood Institute Grants K08 HL083101. We thank Rogers MRI for their skillful help in conducting the study.
Footnotes
The Authors have no potential conflicts of interest to disclose.
References
- 1.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004 Dec;40(6):1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 2.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003 Oct;285(4):E906–916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj M, Suraamornkul S, Piper P, et al. Decreased plasma adiponectin concentrations are closely related to hepatic fat content and hepatic insulin resistance in pioglitazone-treated type 2 diabetic patients. J Clin Endocrinol Metab. 2004 Jan;89(1):200–206. doi: 10.1210/jc.2003-031315. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj M, Suraamornkul S, Pratipanawatr T, et al. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003 Jun;52(6):1364–1370. doi: 10.2337/diabetes.52.6.1364. [DOI] [PubMed] [Google Scholar]
- 5.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006 Nov 30;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 6.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004 Jan;39(1):188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 7.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000 Sep;6(9):998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001 Sep 15;358(9285):893–894. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 9.Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005 May;100(5):1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 10.Uygun A, Kadayifci A, Isik AT, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004 Mar 1;19(5):537–544. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 11.Tiikkainen M, Hakkinen AM, Korsheninnikova E, Nyman T, Makimattila S, Yki-Jarvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004 Aug;53(8):2169–2176. doi: 10.2337/diabetes.53.8.2169. [DOI] [PubMed] [Google Scholar]
- 12.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004 Jul 1;20(1):23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderwald C, Bernroider E, Krssak M, et al. Effects of insulin treatment in type 2 diabetic patients on intracellular lipid content in liver and skeletal muscle. Diabetes. 2002 Oct;51(10):3025–3032. doi: 10.2337/diabetes.51.10.3025. [DOI] [PubMed] [Google Scholar]
- 14.Zib I, Jacob AN, Lingvay I, et al. Effect of pioglitazone therapy on myocardial and hepatic steatosis in insulin-treated patients with type 2 diabetes. J Investig Med. 2007 Jul;55(5):230–236. doi: 10.2310/6650.2007.00003. [DOI] [PubMed] [Google Scholar]
- 15.Lingvay I, Raskin P, Szczepaniak LS. Effect of insulin-metformin combination on hepatic steatosis in patients with type 2 diabetes. J Diabetes Complications. 2007 May-Jun;21(3):137–142. doi: 10.1016/j.jdiacomp.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garber AJ. Benefits of combination therapy of insulin and oral hypoglycemic agents. Arch Intern Med. 2003 Aug 11–25;163(15):1781–1782. doi: 10.1001/archinte.163.15.1781. [DOI] [PubMed] [Google Scholar]
- 17.Lingvay I, Legendre JL, Kaloyanova PF, Zhang S, Adams-Huet B, Raskin P. Insulin-based versus triple oral therapy for newly diagnosed type 2 diabetes: which is better? Diabetes Care. 2009 Oct;32(10):1789–1795. doi: 10.2337/dc09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boesch C, Slotboom J, Hoppeler H, Kreis R. In vivo determination of intra-myocellular lipids in human muscle by means of localized 1H-MR-spectroscopy. Magn Reson Med. 1997 Apr;37(4):484–493. doi: 10.1002/mrm.1910370403. [DOI] [PubMed] [Google Scholar]
- 19.Schick F, Eismann B, Jung WI, Bongers H, Bunse M, Lutz O. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: two lipid compartments in muscle tissue. Magn Reson Med. 1993 Feb;29(2):158–167. doi: 10.1002/mrm.1910290203. [DOI] [PubMed] [Google Scholar]
- 20.Szczepaniak LS, Dobbins RL, Metzger GJ, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003 Mar;49(3):417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 21.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999 May;276(5 Pt 1):E977–989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 22.McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006 Apr 4;144(7):517–524. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- 23.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007 Sep 4;116(10):1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 24.Reingold JS, McGavock JM, Kaka S, Tillery T, Victor RG, Szczepaniak LS. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab. 2005 Nov;289(5):E935–939. doi: 10.1152/ajpendo.00095.2005. [DOI] [PubMed] [Google Scholar]
- 25.Lingvay I, Raskin P, Szczepaniak LS. The fatty hearts of patients with diabetes. Nat Rev Cardiol. 2009 Apr;6(4):268–269. doi: 10.1038/nrcardio.2009.30. [DOI] [PubMed] [Google Scholar]
- 26.Lingvay I, Esser V, Legendre JL, et al. Noninvasive quantification of pancreatic fat in humans. J Clin Endocrinol Metab. 2009 Oct;94(10):4070–4076. doi: 10.1210/jc.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005 Feb;288(2):E462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 28.Szczepaniak LS, Dobbins RL, Stein DT, McGarry JD. Bulk magnetic susceptibility effects on the assessment of intra- and extramyocellular lipids in vivo. Magn Reson Med. 2002 Mar;47(3):607–610. doi: 10.1002/mrm.10086. [DOI] [PubMed] [Google Scholar]
- 29.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005 Jun;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 30.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005 Jun;128(7):1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 31.Younossi ZM, Gramlich T, Liu YC, et al. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol. 1998 Jun;11(6):560–565. [PubMed] [Google Scholar]
- 32.Juurinen L, Tiikkainen M, Hakkinen AM, Hakkarainen A, Yki-Jarvinen H. Effects of insulin therapy on liver fat content and hepatic insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab. 2007 Mar;292(3):E829–835. doi: 10.1152/ajpendo.00133.2006. [DOI] [PubMed] [Google Scholar]