Abstract
Several recent studies reported that Krüppel-like factor 2 (KLF2) controls trafficking, development, and function of B cells. Conditional B cell KLF2 knockout mice have increased numbers of MZ B cells and decreased numbers of B1 phenoytpe cells. However, it was unclear whether KLF2 is required for B1 B cell development, survival or phenotypic maintenance. We show that B1 phenotype B cells are present in neonatal mice with B-cell specific KLF2 deficiency, suggesting B1 differentiation can occur even in the absence of KLF2. Furthermore, by use of an inducible knockout strategy, we show that deletion of KLF2 in mature B1 cells causes loss of phenotypic markers associated with B1 cell identity, but has a minimal effect on short term cell survival. Together, our findings suggest that KLF2 is necessary for the maintenance of B1 cell identity rather than differentiation or survival of the population.
INTRODUCTION
B1 B cells are thought to comprise a distinct subset of the B cell pool, with unique developmental, phenotypic, functional and tissue distribution characteristics(1–5). This population arises during fetal development, and hence precede the B2 B cells (which includes follicular and marginal zone B cells). B1 cells are enriched in certain tissue locations, such as the peritoneal and pleural cavities, but are also present as a minority population in the spleen and blood (5). Studies in mice suggest B1 cells often bear polyspecific B cell receptors and are the major source of “natural” IgM antibodies, which offer initial protective immunity against various infections. The B1 pool mediates rapid responses to viral and bacterial pathogens, especially following infection at mucosal surfaces (2, 5, 6). B1 B cells are also associated with self reactivity and may contribute to certain autoimmune conditions (1, 7)(5). At the same time, the IL-10 producing “Regulatory B cell” subset may comprise a subset of the B1 pool (8). Recent studies suggest that cells with characteristics of B1 cells are present in humans and expanded in patients with Lupus (9, 10), although this issue remains controversial.
Although first identified over 35 years ago (1), considerable controversy surrounds the basis for B1 differentiation (3, 4, 11). While some studies indicate that B cell receptor (BCR) specificity dictates the differentiation of B cell precursors into B1 versus B2 fates, there is also considerable evidence that distinct precursor populations may exist for B1 and B2 lineage cells (4). Current models suggest that the majority of B1 B cells (especially the prototypical CD5high B1a B cell pool) (4) differentiate before or just after birth, and that these cells are maintained throughout adult life by self renewal, rather than the continuous generation characteristic of B2 B cells (3–5). Several studies have focused on the role of signals through the BCR and survival receptors in their generation (12), and have suggested that this pool may be induced by exposure to strong BCR signals with self-ligands (5, 7, 13). This is in keeping with the fact that B1 cells display an activated phenotype (including elevated CD43 and CD5 expression) at steady state. On the other hand, in contrast to B2 B cells, the development and survival of B1 cells is minimally affected by disruption of BAFF receptor signaling (14)(4). Hence the signals and timing of B1 versus B2 development appear quite distinct.
However, the role of transcription factors in mediating the distinct development of B1 and B2 B cells is unclear. Recently, we and others showed that members of the krüppel-like family, KLF2 and KLF3, dramatically affect B cell subset development (15–19). Intriguingly, while KLF2 and KLF3 appear to play opposing roles in generation of Marginal Zone (MZ) B cells, deficiency in either factor leads to profound loss of the peritoneal B1 population (15–17, 19). Whether the B1 pool in other tissues (such as the spleen) are similarly effected was less clear, although the decreased levels of serum IgA, IgG1, and IgE in B-cell specific KLF2 deficient mice (15) is consistent with a global loss of B1 cells, since the B1 population is responsible for a large fraction of these antibodies in unimmunized mice (20–22).
However, existing studies have not addressed the stage or mechanism by which KLF2 influences the B1 subset: KLF2 may be needed for initial B1 differentiation, maintenance of the mature B1 pool or preservation of the unique B1 phenotype. The fact that B1 differentiation peaks at or before birth makes these issues difficult to address in adult animals. Hence, in this study we explore the requirement for KLF2 in development of B1 cells in neonatal mice, and use an inducible Klf2 deletion model to test the function of this factor in mature B1 B cell maintenance. Our studies demonstrate that KLF2 is, unexpectedly, not required for B1 B cell generation but plays a key role in maintenance of mature B1 phenotype, as indicated by loss of typical B1 markers with KLF2 deletion. Hence, our data indicate KLF2 is required for preservation of B1 B cell identity.
MATERIALS AND METHODS
Mice
Klf2Fl/Fl and CD19-Cre/Klf2Fl/Fl mice were described earlier (15). Mice carrying the ROSA26-(floxed STOP)-YFP reporter (23) were kindly provided by Frank Costantini at Columbia University. Other animals were purchased from NCI. All animal studies were conducted under approval from the University of Minnesota IACUC committee.
Flow Cytometry
All fluorochrome and biotin conjugated antibodies were purchased from eBioscience, BD BioScience, or BioLegend. Flow cytometry samples were prepared by staining single cell suspensions with antibodies in FACS buffer (PBS, 1% FCS, 0.02% Sodium Azide) for 30 minutes at 4° and washing cells twice with FACS buffer. Flow cytometry data was collected using an LSR-II cytometer (BD Biosystems), and analyzed using FlowJo software (Tree Star, Inc.). Peripheral B cell subsets were defined with the following markers: B1 (CD19+, B220Int/lo, CD43+, IgM+, CD21−, and CD23−) (24), while “B2” B cells were identified as CD19+, B220high CD93−ve. The typical gating scheme is illustrated in Supplementary Fig. 1.
Tat-Cre transduction
Tat-cre (HTNC) was generated as previously described and either purchased from Excellgen (Rockville, MD) or generously provided by Kevin Otipoby and Klaus Rajewsky (Harvard University) (25). Single cell suspensions were washed 3 times in ADCF-Mab serum-free media and resuspended in Hyclone serum-free media. Cells (5×106 cells/ml, final density) were then incubated with Tat-cre (50µg/ml or 1.25 µM) in the presence of Polymyxin B Sulfate (50 µg/ml final concentration) in Hyclone media. for 45 minutes at 37°C. Cells were then washed in Hyclone media containing 10% serum, prior to in vivo transfer.
Cell isolation, sorting and adoptive transfers
CD19CreTg/+, Klf2Fl/Fl (B cell specific KLF2 deficient) and CD19CreTg/+, Klf2+/+ (“WT” controls), of indicated ages, were sacrificed and cells isolated from spleen, peritoneal cavity and pleural cavity. For studies involving Tat-cre treatment, peritoneal cells were isolated from Klf2+/+ CD45.2/CD45.1 ROSA26-(floxed STOP)-YFP transgenic mice (as controls) and Klf2Fl/Fl CD45.2/CD45.2, ROSA26-(floxed STOP)-YFP transgenic mice (as the experimental group). Peritoneal lavages (from at least 10 mice) were obtained, mixed and the cells cultured with or without Tat-cre. An aliquot was subjected to FACS analysis to assess the input KO to WT ratio. Approximately 106 cells were transferred interperitoneally into B6.SJL (CD45.1/CD45.1) mice. Peritoneal and splenic cells were assessed via flow cytometry 3 and 10 days later. In some experiments, B1 B cell phenotype cells were sorted (on a FACSAria: BD Biosciences) for lack of “Dump” markers (CD4, CD8, Gr-1, and F4/80) and for expression of B1 characteristics (CD19+, B220low, CD43+, CD23−) prior to Tat-cre treatment. Post-sort analysis showed >99% efficiency of sorting. Approximately 5×104 cells were transferred interperitoneally (I.P.) into B6.SJL mice and assessed by flow cytometry 10 days later. From compiled Tat-cre transduction experiments, YFP+ve cells were 32% (+/− 5%) for the Klf2+/+ controls and 41% (+/− 7%) for the Klf2Fl/Fl groups.
Statistical methods
P-values were calculated by Student’s unpaired t-test using Prism software (Graphpad Software Inc.). Where values varied by 10-fold or more, they were converted to Log10 prior to the t-test.
RESULTS AND DISCUSSION
B1 B cells develop in the absence of KLF2
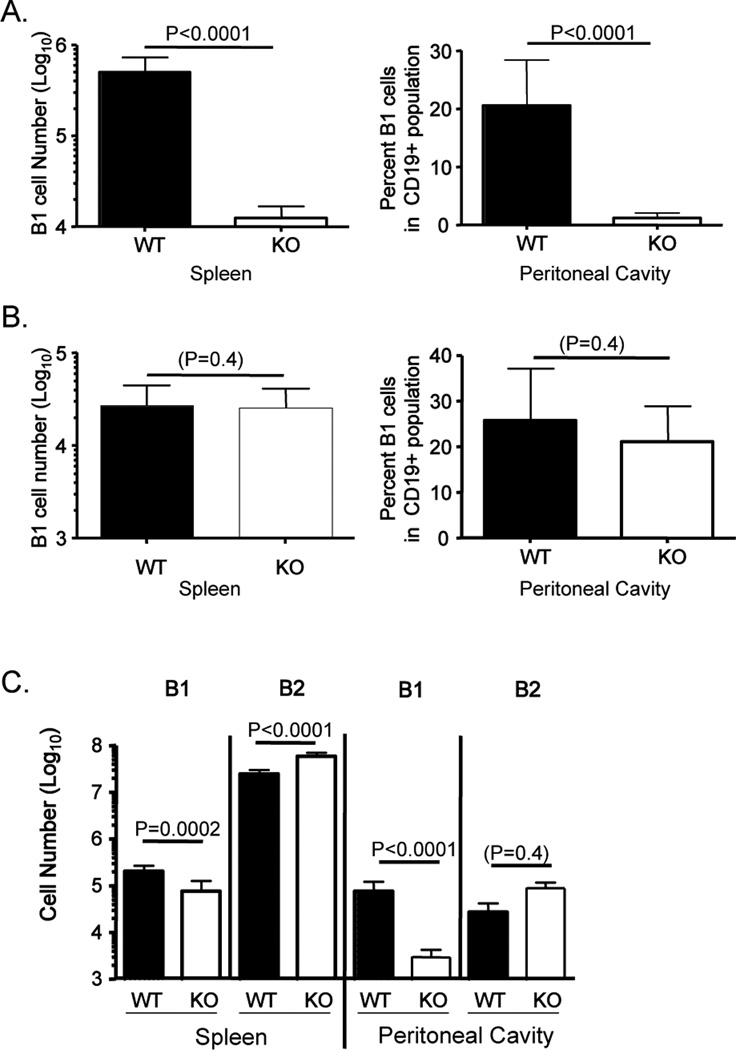
We and others have reported that conditional KLF2 deficiency in B cells leads to dramatic reduction of peritoneal cavity B1 B cells (15–17). It was less clear whether a residual population of B1 cells exist in the spleen of adult CD19CreKlf2Fl/Fl mice, with reports differing on whether this pool was reduced (15, 17) or elevated (16). To address this issue we used a rigorous gating strategy to identify mature B cell subsets (Supplementary Figure 1A), and observed a dramatic reduction in the number of cells expressing typical B1 B cell markers (CD19+, B220Int, CD43+, IgM+, CD21−, CD23−) in the spleen of adult CD19CreKlf2Fl/Fl mice (Fig 1A). As reported previously (15–17), CD19CreKlf2Fl/Fl animals also show a substantially lower percentage of peritoneal B1 B cells (Fig 1A) and corresponding low numbers of B1 cells in both peritoneal and pleural cavities (Supplementary Figure 1B,C). Accordingly, these data confirm and extend our previous observations that KLF2 deficiency in B cells leads to a severe deficiency of B1 phenotype cells in adult mice.
Figure 1. B1 B cells are present in young but not adult KLF2-deficient mice.
B1 B cells were analyzed in the spleen and peritoneal cavity of CD19Cre Tg/+, Klf2+/+ mice (WT) or CD19Cre Tg/+, Klf2Fl/Fl (KO) mice that were (A) >10 weeks old (B) 8–11 days old or (C) 30 days old. The number or percentage of B1 B cells is indicated for each tissue. In (C), the number of B2 B cells is also indicated. These data are compiled from at least 3 experiments (the number of animals represented are 9 WT and 8 KO in A, 8 WT and 12 KO in B, 9 WT and 7KO in C).
Such findings might suggest KLF2 is important for B1 B cell differentiation, maintenance, or expression of characteristic B1 phenotypic markers (or a combination of these roles). This issue is compounded by the fact that the B1 B cell differentiation is largely limited to fetal and neonatal life (4, 5, 13, 26) – hence analysis of adult CD19CreKlf2Fl/Fl mice cannot distinguish between defects in generation versus maintenance of B1 B cells.
To begin addressing this, we assessed whether B1 B cells were generated in young CD19CreKlf2Fl/Fl mice. Surprisingly, a population of B1 B cells was readily detected in the spleen of neonatal CD19CreKlf2Fl/Fl mice, similar in numbers to those found in control mice of the same age (Fig. 1B). Furthermore, B1 B cell phenotype B cells were found at normal frequencies in the peritoneal cavity of young CD19CreKlf2Fl/Fl mice (Fig 1B). More than 50% of the peritoneal B1 B cells from both CD19CreKlf2Fl/Fl and control (CD19CreKlf2+/+) neonatal mice were CD5+ (data not shown) indicating similar generation of B1a and B1b populations. By 30 days after birth, the number of B1 cells was significantly reduced in both the spleen and peritoneal cavity of CD19CreKlf2Fl/Fl mice, yet this population was still clearly detectable, especially in the spleen (where the average number of CD19CreKlf2Fl/Fl B1 B cells was < 3-fold fewer than the CD19CreKlf2+/+ controls) (Fig 1C). At this time point, the increase in splenic B2 B cells reported previously (15–17) was already observed (Fig 1C). Hence, our data suggested that KLF2 is not essential for B1 development, but rather may be important for maintenance of the B1 pool.
Inducible KLF2 deficiency leads to loss of the mature B1 B cell pool
Although these findings suggested KLF2 was dispensable for B1 B cell differentiation, it was possible that KLF2 was important for a step in final maturation of B1 cells (e.g. acquiring the capacity for self renewal) rather than playing a role in maintenance of fully mature B1 cells. To test this hypothesis further, we sought a model by which KLF2 could be deleted after maturation of B1 B cells. To achieve this, we used the documented ability of the fusion protein Tat-cre to induce deletion of “floxed” genes, following protein transduction (25). In this system, the HIV Tat peptide induces entry of the Cre cargo into the cell cytosol, and the presence of a nuclear localization signal directs the active Cre enzyme to the nucleus, prompting floxed gene excision (25, 27). In order to track Cre activity, we also employed the ROSA26 floxed-STOP-YFP allele, which offers the ability to use flow cytometry to identify cells that have been successfully transduced by Tat-cre protein (see methods).
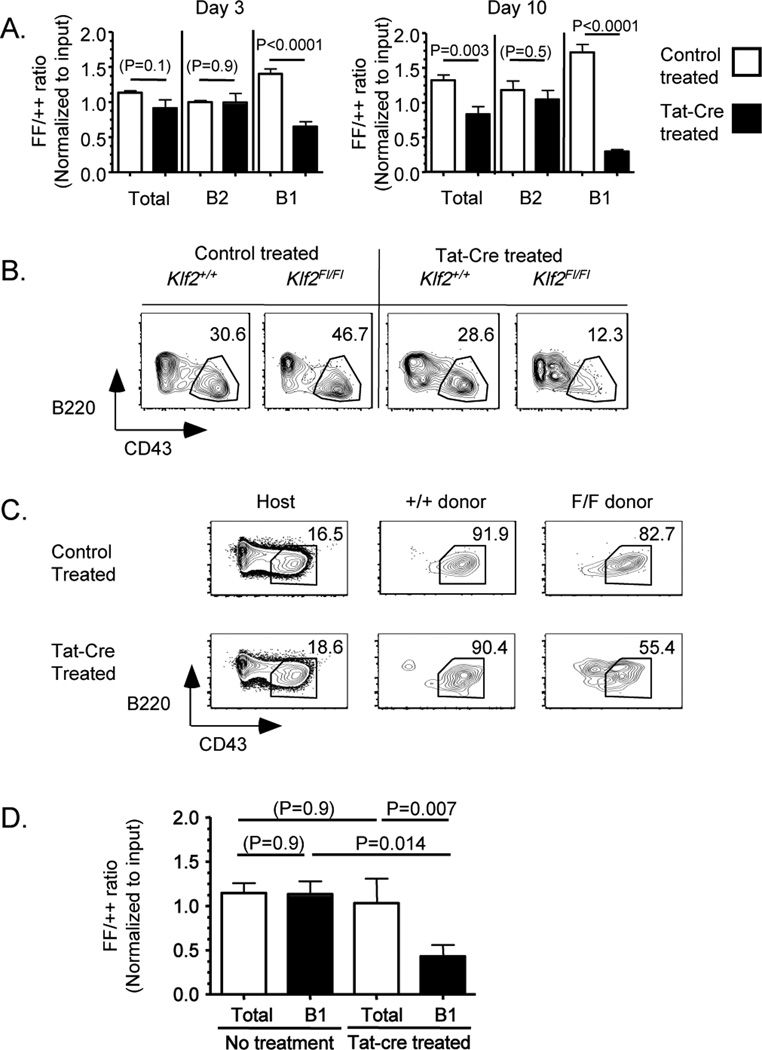
We applied this system to induce KLF2 deletion in mature B1 B cells. Peritoneal cavity cells were isolated from congenically distinct Klf2+/+ and Klf2Fl/Fl mice, the cells were mixed, transduced with Tat-Cre (or incubated with PBS as control), and injected interperitoneally into congenic recipient mice. Using this approach, we could assess the impact of inducible KLF2 deletion, by measuring the ratio of Klf2Fl/Fl to Klf2+/+ donor cells within distinct B cell populations. Since the populations are mixed prior to Tat-Cre transduction and transfer, this approach minimizes non-specific effects (e.g. potential toxicity of Tat-Cre treatment). The data were further normalized to the input ratio of Klf2Fl/Fl to Klf2+/+ cells to allow compilation of distinct experiments.
Donor cells were analyzed in the peritoneum at both 3 and 10 days following adoptive transfer (very few donor cells appeared in the spleen at either time point – data not shown). Treatment with Tat-Cre induced substantial changes in the Klf2Fl/Fl to Klf2+/+ ratio: At day 3, Cre treatment led to a significant reduction in the proportion of Klf2Fl/Fl cells in the B1 phenotype population and this effect was even more notable by day10 (Fig 2A). This effect was further magnified because (for unknown reasons) untreated Klf2Fl/Fl donor B1 cells displayed a slight advantage over Klf2+/+ control cells at both time points after adoptive transfer (white bars for B1 cells in Fig 2A). In contrast, the proportion of Klf2Fl/Fl donor cells in the “B2” phenotype pool (CD19+, B220high, CD93−, CD23+, CD43−) cells was not changed by Tat-cre treatment (Fig 2A). Although these data would appear consistent with a requirement for KLF2 in maintenance of B1 B cells, closer inspection revealed that Tat-Cre treatment of Klf2Fl/Fl cells led to the appearance of a novel CD43int pool, as the canonical CD43high B1 phenotype population declined (Fig 2B). Such findings raised the possibility that the B1 pool is not eliminated but rather alters its phenotype after KLF2 loss.
Figure 2. Induced KLF2 deletion in mature peritoneal B1 B cells leads to loss of B1 B cell phenotype.
In (A,B), peritoneal cells were isolated from congenic mice of either Klf2+/+ (++) or Klf2Fl/Fl (FF) genotype, and treated with Tat-cre prior to adoptive transfer. The data in (A) represent the ratio of Klf2Fl/Fl to Klf2+/+ donor cells, for the B cell subsets indicated, among the peritoneal lavage population at the time point indicated. These data are compiled from 3 experiments. (B) Representative B220/CD43 staining of the indicated donor populations from day 10 post-transfer. In (C,D) peritoneal B1 B cells were sorted from Klf2+/+ (++) or Klf2Fl/Fl (FF) mice and treated with Tat-cre or control prior to adoptive transfer. At day 10, cells were isolated from the peritoneum of the host. (C) Representative data for B220/CD43 expression on the Klf2+/+ and Klf2Fl/Fl donor populations (as well as host cells, for reference). In (D) the ratio of Klf2Fl/Fl to Klf2+/+ donor cells among the total B cell and B1 phenotype population is shown, for control and Tat-cre treated populations. Data were compiled from 3 experiments. (The number of animals represented are 9 mice per group in A; 3 untreated and 8 Tat-cre treated in D).
Sustained KLF2 expression is needed for maintenance of the B1 B cell phenotype
The origin of the CD43int population induced by KLF2 deletion was impossible to determine from studies using transfer of bulk peritoneal B cells, since these cells could arise from either B1 or B2 cells in the inoculum. Hence, we performed similar Tat-cre treatment experiments, but used sorted peritoneal B1 B cells. Post-sort analysis indicated highly efficient sorting of CD19+, B220low, CD43high, CD23− B1 cells (see Methods). In the absence of Tat-Cre transduction, adoptively transferred B1 (whether Klf2Fl/Fl or Klf2+/+) maintained their phenotype for at least 10 days (Fig 2C). However, treatment with Tat-Cre lead to a dramatic change in the Klf2Fl/Fl donor cells: A large fraction of this population showed reduced expression of CD43 and elevated B220 staining, suggesting loss of two standard phenotypic traits defined for murine B1 B cells (Fig 2C). This effect was observed for the bulk donor B cell population (Fig. 2C), but gating on YFP-reporter expressing cells confirmed that this change in phenotype was a feature of Klf2Fl/Fl (but not Klf2+/+) donor B cells in which cre had been active (Supplementary Figure 2A). Predictably, these effects led to a reduction in the frequency of B1 phenotype-gated cells in the Tat-Cre treated Klf2Fl/Fl population, compared to their WT counterparts (Fig 2D, black bars). Interestingly, however, the ratio of Klf2Fl/Fl to Klf2+/+ cells in the total donor B cell pool was not affected by Tat-Cre treatment, and matched the input ratio (Fig 2D, white bars), suggesting similar maintenance of the Tat-Cre transduced Klf2Fl/Fl donor B1 cells (at least for the 10 day period studied). It is very unlikely that these findings represent contamination with B2 B cells in the B1 sorted population, since very few of the Tat-cre treated Klf2Fl/Fl cells gained expression of CD23 (Supplementary Figure 2B). Furthermore, while induced Klf2 deletion led to some loss of CD5, this marker was retained on the majority of cells (Supplementary Figure 2C). Hence, these data support a model in which KLF2 is important primarily for maintenance of some (but not all) phenotypic characteristics of B1 B cells.
Previous studies indicated that B cell specific KLF2 deficiency leads to a substantial loss of B1 B cells, yet there was considerable discordance about whether this effect was uniform in diverse tissues (15–17). Our data help resolve this issue by demonstrating that the B1 B cell pool is significantly reduced in adult but not very young KLF2-deficient mice. Furthermore, in mice of intermediate ages (e.g. 1 month of age [Fig 1C]) the loss of the B1 pool is much more profound in the peritoneal cavity than the spleen. Hence, depending on the timing of analysis (and possibly other aspects of the conditional KLF2 knockout system), the extent of apparent B1 B cell deficiency would vary widely in different tissues. The fact that B1 generation in the neonate (and presumably the fetus) is independent of KLF2 may also address another unexpected feature of the described KLF2-deficient models: Previous studies have argued that >80% of serum IgM derives from B1 B cells (5), yet we and others noted minimal impact of KLF2 deficiency on IgM levels (15–17). While this result could be explained in other ways (e.g. as a consequence of the increased MZ B cell pool in KLF2 deficient mice), our current findings show that KLF2 deficient B1 cells are present (at least through the neonatal phase) and so may contribute to the levels of “natural” circulating IgM antibodies found in adults.
Rather than a role in initial B1 B cell development, our data suggest that KLF2 is essential for preservation of the B1 phenotype. Using an inducible gene ablation system we could show that KLF2 deletion induces loss of typical B1 markers without affecting short term survival. This raises the possibility that cells selected into the B1 lineage are actually present in adult CD19CreKlf2Fl/Fl but that these cells lack typical B1 phenotypic markers. We were unable to identify a distinct pool of CD43int B220high CD23low cells in such mice (data not shown), suggesting that the “ex-B1” population is not stable. It is possible that KLF2 deficient B1-lineage cells accumulate further phenotypic changes that make them difficult to distinguish from the B2 cell subsets (for example, upregulation of CD23 and further decline of CD43 expression would cause this population to be included in the follicular B cell phenotype pool). On the other hand, it is possible that KLF2 ablation causes a loss of B1 B cell survival not detected within our 10 day timecourse. In either case, it is apparent that KLF2 is not simply required for acute B1 B cell survival but plays a role in sustaining characteristic phenotypic features of B1 cells.
The concept that KLF2 is important for preservation of normal B1 B cell “identity” resonates with previous reports in which we and others concluded that KLF2 deficiency caused follicular B cells to exhibit some gene expression characteristics of MZ B cells (15, 16). Hence, KLF2 may play analogous roles in maintaining the “identity” of B1 B cells and FO B cells. It is interesting to note that KLF2 protein expression in mature B cell subsets follows the hierarchy B1>FO>MZ (15, 16), which would conform with the idea that loss of the KLF2 gene might provoke cells to acquire characteristics of cells that naturally express lower levels of KLF2. Thus, understanding how the levels of KLF2 are dictated by signals (via the BCR or other receptors) during B cell differentiation will be important for determining how stable B cell subsets are generated.
Supplementary Material
Acknowledgments
We thank Klaus Rajewsky and Kevin Otipoby for generous provision of Tat-cre, Frank Costantini for mice and the Jamequist lab for helpful discussions throughout this project.
Footnotes
This work was supported by grants from the NIH (R37 AI38903 to SCJ and Predoctoral Training grant T32 AI07313 to GTH) and a Leukemia and Lymphoma Society Career Development Award (to SEH).
References
- 1.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. The Journal of experimental medicine. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Current opinion in immunology. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 3.Herzenberg LA, Tung JW. B cell lineages: documented at last! Nature immunology. 2006;7:225–226. doi: 10.1038/ni0306-225. [DOI] [PubMed] [Google Scholar]
- 4.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature reviews. Immunology. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 6.Herzenberg LA. B-1 cells: the lineage question revisited. Immunological reviews. 2000;175:9–22. [PubMed] [Google Scholar]
- 7.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science (New York, N.Y. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 8.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunological reviews. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 9.Griffin DO, Rothstein TL. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. The Journal of experimental medicine. 2011;208:2591–2598. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. The Journal of experimental medicine. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annual review of immunology. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 12.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nature reviews. 2002;2:945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 13.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 14.Mackay F, Schneider P. Cracking the BAFF code. Nature reviews. Immunology. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 15.Hart GT, Wang X, Hogquist KA, Jameson SC. Kruppel-like factor 2 (KLF2) regulates B-cell reactivity, subset differentiation, and trafficking molecule expression. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:716–721. doi: 10.1073/pnas.1013168108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkelmann R, Sandrock L, Porstner M, Roth E, Mathews M, Hobeika E, Reth M, Kahn ML, Schuh W, Jack HM. B cell homeostasis and plasma cell homing controlled by Kruppel-like factor 2. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:710–715. doi: 10.1073/pnas.1012858108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoek KL, Gordy LE, Collins PL, Parekh VV, Aune TM, Joyce S, Thomas JW, Van Kaer L, Sebzda E. Follicular B cell trafficking within the spleen actively restricts humoral immune responses. Immunity. 2010;33:254–265. doi: 10.1016/j.immuni.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turchinovich G, Vu TT, Frommer F, Kranich J, Schmid S, Alles M, Loubert JB, Goulet JP, Zimber-Strobl U, Schneider P, Bachl J, Pearson R, Crossley M, Agenes F, Kirberg J. Programming of marginal zone B-cell fate by basic Kruppel-like factor (BKLF/KLF3) Blood. 2011;117:3780–3792. doi: 10.1182/blood-2010-09-308742. [DOI] [PubMed] [Google Scholar]
- 19.Vu TT, Gatto D, Turner V, Funnell AP, Mak KS, Norton LJ, Kaplan W, Cowley MJ, Agenes F, Kirberg J, Brink R, Pearson RC, Crossley M. Impaired B cell development in the absence of Kruppel-like factor 3. Journal of immunology. 2011;187:5032–5042. doi: 10.4049/jimmunol.1101450. [DOI] [PubMed] [Google Scholar]
- 20.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarlinton DM, McLean M, Nossal GJ. B1 and B2 cells differ in their potential to switch immunoglobulin isotype. European journal of immunology. 1995;25:3388–3393. doi: 10.1002/eji.1830251228. [DOI] [PubMed] [Google Scholar]
- 22.Vink A, Warnier G, Brombacher F, Renauld JC. Interleukin 9-induced in vivo expansion of the B-1 lymphocyte population. The Journal of experimental medicine. 1999;189:1413–1423. doi: 10.1084/jem.189.9.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allman D, Pillai S. Peripheral B cell subsets. Current opinion in immunology. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peitz M, Pfannkuche K, Rajewsky K, Edenhofer F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4489–4494. doi: 10.1073/pnas.032068699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. The Journal of experimental medicine. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic acids research. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.