Abstract
Culturing cells in 3D provides an insight into their characteristics in vivo. We previously reported that human mesenchymal stem/stromal cells (hMSCs) cultured as 3D spheroids acquire enhanced anti-inflammatory properties. Here we explored the effects of hMSC spheroids on macrophages that are critical cells in the regulation of inflammation. Conditioned medium from hMSC spheroids inhibited LPS-stimulated macrophages from secreting pro-inflammatory cytokines TNFα, CXCL2, IL6, IL12p40, and IL23. Conditioned medium also increased the secretion of anti-inflammatory cytokines IL10 and IL1ra by the stimulated macrophages, and augmented expression of CD206, a marker of alternatively activated M2 macrophages. The principal anti-inflammatory activity in conditioned medium had a small molecular weight, and microarray data suggested that it was PGE2. This was confirmed by the observations that PGE2 levels were markedly elevated in hMSC spheroid-conditioned medium, and that the anti-inflammatory activity was abolished by an inhibitor of COX-2, a silencing RNA for COX-2, and an antibody to PGE2. The anti-inflammatory effects of the PGE2 on stimulated macrophages were mediated by the EP4 receptor. Spheroids formed by human adult dermal fibroblasts produced low levels of PGE2 and displayed negligible anti-inflammatory effects on stimulated macrophages, suggesting the features as unique to hMSCs. Moreover, production of PGE2 by hMSC spheroids was dependent on the activity of caspases and NFκB activation in the hMSCs. The results indicated that hMSCs in 3D-spheroid cultures are self-activated, in part by intracellular stress responses, to produce PGE2 that can change stimulated macrophages from a primarily pro-inflammatory M1 phenotype to a more anti-inflammatory M2 phenotype.
Keywords: MSC, COX2, LPS, macrophage, spheroid, caspase
Introduction
Many reports (1–4) have explored the therapeutic potentials of the cells from bone marrow referred to initially as colony forming units-fibroblastic (5), then as marrow stromal cells, subsequently as mesechymal stem cells, and most recently as multipotent mesenchymal stromal cells or MSCs (6). The cells are relatively easy to isolate from human donors or patients, they expand rapidly for 30 or more population doublings in culture, they differentiate into several cellular phenotypes in vitro and in vivo, and they are not tumorigenic (1). Administration of MSCs produced beneficial effects in a series of animal models for human diseases and have prompted tests of human MSCs (hMSCs) or similar cells in a number of clinical trials (see www.clinicaltrials.gov). The initial assumption in exploring the therapeutic benefits of MSCs was that they might engraft and differentiate to replace injured cells (7, 8). Engraftment and differentiation was observed in rapidly grown embryos, with extreme tissue injury, or after local administrations of large concentrations of the cells (1). More frequently however therapeutic benefits were observed without evidence of engraftment. The cells instead enhanced repair or limited tissue destruction by paracrine secretions or cell-to-cell contacts that modulated inflammatory or immune reactions, or enhanced propagation and differentiation of tissue endogenous stem cells. MSCs in culture secrete a large number of cytokines (9) but, in addition, they are activated in vivo to express high levels of a large number of additional factors (10). Some of the secreted factors enhanced conversion of macrophages to an anti-inflammatory phenotype (11, 12). Others enhanced clearance of bacteria (11, 13). However, the cells disappear from tissues with a half-life of about 24 hours as they are being activated (14). Therefore pre-activation of the cells in culture may improve their therapeutic effects.
There are several indications that culturing cells in 3D may more closely mimic their developmental progression and properties in vivo than commonly employed 2D cultures (15). Recent reports demonstrated that aggregation of MSCs into 3D spheroids increased their ability to differentiate and some of their potential therapeutic properties (15–27). We observed (25) that as hMSCs from bone marrow aggregated in hanging drops to form spheroids, the cells up-regulated expression of a number of genes, including genes for the chemokine receptor CXCR4; three anti-cancer proteins (TRAIL, IL-24, and CD82); an anti-apoptotic protein STC-1; and an anti-inflammatory protein TSG-6. Most importantly, hMSC spheroids and spheroid-derived cells were therapeutically more effective than monolayer cultures of the same cells in a murine model of zymosan-induced peritonitis (25). One critical observation was that the anti-inflammatory effects of the spheroid hMSCs were rapid, suggesting that they could reduce the cascade of inflammatory mediators released by macrophages at the onset of the injury (28, 29). In exploring the anti-inflammatory properties of hMSCs cultured as spheroids, we found that one major anti-inflammatory factor secreted by the cells was prostaglandin E2 (PGE2). PGE2 was secreted through a novel self-activation process in hMSCs that was dependent on the activity of caspases and NFκB activation. The secreted PGE2, by interacting with the EP4 receptor of stimulated macrophages, inhibited the secretion of pro-inflammatory cytokines and increased the secretion of anti-inflammatory cytokines by the stimulated macrophages. The results suggested that hMSC spheroid-conditioned medium promoted a transition of macrophages from a primarily pro-inflammatory M1 to a more anti-inflammatory M2 phenotype.
Materials and Methods
hMSC culture
hMSCs, isolated from bone marrow aspirates and cultured as previously described (25), were obtained as frozen vials in passage 1 from the Center for the Preparation and Distribution of Adult Stem Cells (http://medicine.tamhsc.edu/irm/msc-distribution.html). A frozen vial with approximately 1 million hMSCs was thawed and the cells were re-suspended in complete culture medium (CCM) consisting of α-Minimum Essential Medium (MEM, Gibco) supplemented with 17% fetal bovine serum (FBS, Atlanta Biologicals), 100 units/ml penicillin (Gibco), 100 μg/ml streptomycin (Gibco), and 2 mM L-glutamine (Gibco) to promote optimal growth, and plated in a 152 cm2 culture dish (Corning). After 24 h, the adherent viable cells were washed with phosphate buffered saline (PBS) and harvested using 0.25% trypsin and 1 mM ethylenediaminetetraacetic acid (EDTA, Gibco) for 5 min at 37°C, plated at 100 cells/cm2, and expanded for 7 days before freezing as passage 2 cells in 1 ml of α-MEM containing 30% FBS and 5% dimethylsulfoxide (DMSO, Sigma). For the experiments described here, passage 2 hMSCs were recovered by plating in CCM on a 152 cm2 culture dish for a 24 h period, re-seeded at 100–150 cells/cm2 (Adh Low), and incubated for 7–8 days in CCM. Culture medium was changed every 2–4 days and 1 day before harvest.
Human adult dermal fibroblast culture
Human adult dermal fibroblasts (hDFs) were obtained from Dr. Carl Gregory (30) and from 3 commercial sources (American Type Culture Collection (ATCC), Lonza, and Gibco). Frozen vials of the cells were thawed and plated on adherent T-175 flasks (Corning) in CCM for 24 h. After medium change, the cells were expanded until approximately 70–90% confluent. Cells were harvested with trypsin/EDTA for 5 min at 37°C and re-plated at 1,000–3,000 cells/cm2 for expansion. Medium was changed every 2–4 days and cells were harvested at 70–90% confluence for assays.
Spheroid generation and dissociation
To generate multi-cellular spheroids (25), hMSCs or hDFs were suspended in CCM at 714 cells/μl and placing 35 μl drops (25,000 cells) on the inverted lid of a cell culture dish. The lid was then rapidly re-inverted onto the culture dish that contained PBS to prevent evaporation of the drops. The hanging drop cultures were incubated for 3 days at 37°C in a humidified atmosphere with 5% CO2. In some experiments, hMSCs in hanging drops were cultured in the presence of 0.04–5 mM of the nitric oxide synthesis inhibitor L-NAME (Sigma), 0.04–5 μM of the non-selective cyclooxygenase (COX) inhibitor indomethacin (Sigma), 0.04–1 μM of the cyclooxygenase-1 (COX-1) inhibitor SC-560 (Cayman Chemical), 0.04–1 μM of the cyclooxygenase-2 (COX-2) inhibitor NS-398 (Cayman Chemical), 0.4–10 μM of the broad-spectrum caspase inhibitor Q-VD-OPh (EMD Chemicals), or 1 μM of the NFκB transcriptional activation inhibitor QNZ (Cayman Chemical). Spheroids were collected from the tissue culture dish lid using a cell lifter (Corning), transferred to a 15 ml conical tube (Falcon), and centrifuged at 453 ×g for 5 min. To obtain spheroid derived cells, spheroids were incubated with trypsin/EDTA at 37°C for approximately 10 min with pipetting every 2–3 min. When no cell aggregates were visible, spheroid derived cells were collected by centrifugation at 453 ×g for 10 min.
Reverse transfections with siRNA targeting COX-2
Reverse transfections in suspension were performed using Lipofectamine RNAiMAX reagent according to the manufacturer’s instructions (Invitrogen). hMSCs were plated at 150 cells/cm2 and expanded for 7 days. The cells were lifted with trypsin/EDTA and collected by centrifugation at 453 ×g for 10 minutes followed by washing with antiobiotic-free CCM. Total of 4.5 nmol negative control siRNA duplex (Low GC content, Invitrogen), or different COX-2 siRNA duplexes (Invitrogen) alone or as a combination were mixed with 15 ml of Opti-MEM medium (Gibco). For each reaction, 225 μl of Lipofectamine RNAiMAX was added and the combination was gently mixed and incubated for 10 min in RT. Total of 3.1 × 106 hMSCs in 75 ml of antibiotic-free CCM were added for each reaction. The final reactions contained 50 nM of siRNAs, 1:400 of Lipofectamine RNAiMAX, 17% Opti-MEM, and 83% antibiotic-free CCM. Transfection reagent control did not contain any siRNA. The suspensions were mixed gently, and hMSCs were plated at 5,000 cells/cm2 in 152 cm2 dishes and incubated at 37°C and 5% CO2. After 24 h, transfected cells were lifted with trypsin/EDTA and cultured in hanging drops for 3 days to generate spheroids. Knock-down of COX-2 gene expression was validated by real-time PCR for PTGS2 and ELISA for PGE2.
Collection of conditioned medium and cell lysate
hMSCs and hDFs were plated at a high (5,000 cells/cm2, 25.5 cells/μl, Adh High) or very high density (200,000 cells/cm2, 714 cells/μl, Adh VH) on adherent dishes in CCM, or in hanging drops (714 cells/μl) in CCM. After 3 days, images were captured on a Nikon Eclipse Ti-S inverted microscope using a Ds-Fi1 camera (Nikon) and processed with NiS Elemnts AR 3.0 Software (Nikon), and conditioned medium was harvested and centrifuged at 453 ×g for 5–10 min. The supernatant was clarified by centrifugation at 10,000 ×g for 10 min before using for assays. For some experiments, Adh High conditioned medium was concentrated 28x, using an Amicon Ultra-15 (Millipore) centrifugal filter (3 kDa molecular weight cutoff), to match the initial cell concentration used to produce spheroid and Adh VH conditioned medium. Centrifugation was performed at 3,000 ×g at +4°C after washing the filter with cold PBS.
For cell lysis, the cultures were washed 2x with PBS and lysed on the adherent dishes with RLT buffer (RNAeasy Mini Kit, Qiagen) containing β-mercaptoethanol. To obtain spheroid cell lysates, spheroids were centrifuged at 453 ×g for 5 min, washed with PBS, centrifuged at 453 ×g for 5 min, and lysed with RLT buffer containing β-mercaptoethanol.
Fractionation of hMSC spheroid-conditioned medium
hMSC spheroid-conditioned medium was fractionated using Amicon Ultra-4 centrifugal filters (Millipore) with different molecular weight cutoffs. All the centrifugation steps were performed at 3,000 ×g at +4°C. Before use, filters were washed with cold PBS. To start the fractionation, 5 ml of spheroid-conditioned medium was added to a centrifugal filter with a molecular weight cutoff of 100 kDa and centrifuged until approximately 200 μl remained. Cold PBS (5 ml) was added to the concentrated sample twice followed by centrifugation each time until approximately 200 μl remained. The concentrated sample (>100 kDa fraction) was diluted to 15 ml with cold PBS. The filtrate was applied on to a centrifugal filter with a molecular weight cutoff of 50 kDa and centrifuged until approximately 200 μl remained. The concentrated sample (100–50 kDa fraction) was diluted to 15 ml with cold PBS. The filtrate was applied on to a centrifugal filter with a molecular weight cutoff of 3 kDa and centrifuged until approximately 200 μl remained. The concentrated sample (50–3 kDa fraction) was diluted to 15 ml with cold PBS. Filtrate (<3 kDa fraction) contained the low molecular weight molecules. To denature the spheroid-secreted proteins, spheroid-conditioned medium was immersed in boiling water bath for 10 min and cooled on ice for 5 min (Boil sample). The samples were assayed for PGE2 by ELISA and for anti-inflammatory activity in the macrophage assay.
Assay for PGE2 production
PGE2 was assayed with an ELISA kit (R&D Systems). The samples were diluted 1:5–1:100. For all assays, optical density was determined on a plate reader (FLUOstar Omega; BMG Labtech) at an absorbance of 450 nm with wavelength correction at 540 nm to correct for the optical imperfections in the plate.
Cell concentration measurement
The end cell numbers after conditioning the medium were determined with CyQUANT Cell Proliferation Assay Kit (Invitrogen) based on DNA amount alone. The end cell concentrations were calculated from the CyQUANT cell counts and the amount of conditioned medium recovered from the 3 day cultures. End PGE2 concentration was determined with the PGE2 ELISA. The results are summarized in Supplemental Table 1.
Macrophage inflammatory assay
Frozen vials of J774A.1 mouse macrophages (ATCC) were thawed and expanded as adherent cultures on 15 cm diameter petri dishes (Falcon) in high glucose Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen) supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Subcultures were prepared every 2–3 days by washing the cells from the dishes and re-plating at a ratio of 1:6–1:12. For the inflammatory assay, macrophages were harvested, centrifuged at 200–250 ×g for 5 min, and stimulated with LPS (Sigma). After 5–10 min, un-stimulated or stimulated macrophages were transferred at 25,000 cells/cm2 onto 12 or 24-well culture plates containing test reagents. The test reagents were 1:10–1:10,000 dilutions of hMSC or hDF conditioned medium, 0.01–100 ng/ml PGE2 (Sigma), 0.1–10 μg/ml of a PGE2 neutralizing antibody (Cayman Chemical), 10–1,000 ng/ml of prostaglandin D2 (PGD2, Cayman Chemical), 0.01–10 μM of EP1 (SC-19220, Cayman Chemical), EP2 (AH 6809, Cayman Chemical), EP3 (L-798,106, Tocris Bioscience), and EP4 (L-161,982 and GW627368X, Cayman Chemical) receptor antagonists, 0.0001–10 ng/ml of EP4 receptor agonists (CAY10589 and CAY10580, Cayman Chemical), or 0.4–50 μM cAMP analog (8-Bromo cAMP, Tocris Bioscience). The final concentration of LPS was 100 ng/ml. After 18–24 h, images were captured on a Nikon Eclipse Ti-S inverted microscope using a Ds-Fi1 camera and processed with NiS Elemnts AR 3.0 Software. Medium conditioned by the macrophages was collected and clarified by centrifugation at 500 ×g for 5 min. Levels of pro-inflammatory (TNFα, CXCL2/MIP-2, IL6, IL23, IL12p40) and anti-inflammatory (IL-10, IL-1ra) mouse cytokines were assayed with ELISA kits (R&D systems). At time points specified, mouse macrophages were washed with PBS and harvested for RNA. For some assays, the J774A.1 macrophages were stimulated with 20 μg/ml of zymosan (Sigma).
Raw264.7 mouse monocytes/macrophages (ATCC) were cultured in high glucose DMEM containing 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. For the inflammatory assay, Raw264.7 cells were suspended in culture medium and stimulated with 100 ng/ml LPS or 20 μg/ml zymosan. After 5–10 min, un-stimulated or stimulated macrophages were transferred at 25,000 cells/cm2 onto 12-well culture plates. Spheroid-conditioned medium was added at 1:100 dilution. After overnight culture, medium was harvested for mouse TNFα ELISA.
The human monocytic cell line U-937 (ATCC) was cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were suspended in 15 cm diameter culture plates at 500,000 cells/ml in growth medium supplemented with 100 ng/ml phorbol-12 myristate 13-acetate (PMA, Sigma) for induction of monocyte/macrophage differentiation. After 2 days, the PMA was cleared from the cultures by medium change and the adherent cells incubated for an additional 24 h. For inflammatory assays, the adherent PMA-pretreated U-937 cells were scraped from the culture dish, re-suspended at 200,000 cells/ml, and stimulated with 100 ng/ml LPS. After 5–10 min, un-stimulated or stimulated macrophages were transferred at 50,000 cells/cm2 onto 24-well culture plates. Spheroid-conditioned medium was added at 1:10 dilution. After 18 h, medium was collected and used for human TNFα ELISA (R&D Systems).
Analysis of macrophage CD206 surface expression
Cell surface expression of the M2 macrophage marker CD206 (mannose receptor) was determined by flow cytometry. J774A macrophages were stimulated with 100 ng/ml LPS for 5–10 min then plated at 25,000 cells/cm2 on 10 cm culture plates (Corning). The stimulated cells were incubated for 24 h with spheroid conditioned medium or control CCM (1:100 dilution). After 24 h, the cells were harvested, collected by centrifugation, and re-suspended at 500,000 cells/ml in HBSS containing 2% FBS. For flow cytometric analysis, the macrophages were pre-incubated for 15 min with 1.0 μg of anti-mouse CD16/CD32 Fc receptor blocking antibody (ebioscience) and then labeled with 0.5 μg of phycoerythrin (PE)-conjugated anti-mouse CD206 antibody (R&D Systems) or isotype control (R&D Systems). After 30 min, the cells were washed twice with PBS and the surface expression was analyzed on a FC500 flow cytometer (Beckman-Coulter). The percentage of cells staining positive for CD206 was determined by comparing the test samples to macrophages labeled with isotype control antibody.
Microarrays
For the macrophage microarray assays, J774A.1 mouse macrophages (MΦ sample) were stimulated with LPS (sMΦ sample) and treated with either CCM (sMΦ + CCM sample) or spheroid-conditioned medium (sMΦ + Sph CM sample) for 6h. Cells were lysed, RNA isolated with RNeasy Mini Kit, and the isolated RNA was quantified with Nanodrop spectrophotometer (Thermo Scientific). Total of 150 ng of each RNA sample was used to prepare labeled amplified RNA (aRNA) according to manufacturer’s instructions for GeneChip 3′ IVT Express Kit (Affymetrix). Total of 15 μg of labeled aRNA was fragmented and hybridized (GeneChip Hybridization Oven 640, Affymetrix) onto mouse arrays (MG-430 2.0, Affymterix) followed by washing and staining (GeneChip Fluidics Station 450, Affymetrix) with GeneChip Wash and Stain Kit (Affymetrix). Arrays were scanned with GeneChip Scanner (Affymetrix) and raw data files (CEL-files) were transferred into Partek Genomics Suite 6.4 (Partek). Data were normalized using robust multi-array (RMA) algorithm and gene level analysis and comparisons were done using the Partek software. For hierarchical clustering, genes were filtered based on significant changes in the expression between the sMΦ + Sph CM and sMΦ samples. Data were searched for inflammation-related genes and relative quantities were calculated comparing all the samples to MΦ sample. GeneOntology analysis was performed on the 172 differentially expressed genes with the Partek software and significance was calculated using exact hypergeometric distribution and recorded as enrichment score [−log10 (p-value)].
For the hMSC microarray, the previously published data on 2 hMSC donors grown in adherent dishes at low density for 7 days (Adh Low sample), or in adherent dishes at high density (Adh High sample) and in hanging drops (Sph sample) for 3 days (25), were searched for PGE2 synthesis related genes. Relative quantities were calculated comparing all the samples to Adh Low (D1) sample.
For the hDF microarray, hDFs (Lonza) were grown at high density on adherent dishes (hDF Adh High sample) or in hanging drops (hDF Sph sample) for 3 days. Total of 2 μg of RNA from each sample was applied for microarrays using Whole Transcript Sense Target Labeling Assay Kit (Affymterix) according to manufacturer’s directions. Total of 5.5 μg of cDNA was used for fragmentation followed by labeling. The labeled and fragmented cDNA was hybridized on Human Exon 1.0 ST arrays (Affymetrix) followed by washing, staining, and scanning. Raw data files (CEL-files) were transferred into Partek software and analyzed together with CEL-files from the previously published hMSC samples for donor 1 grown in adherent dishes at a low density for 7 days (hMSC Adh Low sample), or for 3 days on adherent dishes at high density (hMSC Adh High sample) and in hanging drops (hMSC Sph sample) (25). For hierarchical clustering, the normalized data were filtered based on significant changes in the expression between the hMSC Sph and hMSC Adh Low samples. The data showed that there were 1192 up-regulated and 1428 down-regulated genes.
Real-time PCR assays
Total RNA was isolated from cells using RNeasy Mini Kit (Qiagen) with DNase (RNase-Free DNase Set; Qiagen) digestion step and the isolated RNA was quantified with Nanodrop spectrophotometer. RNA was converted to cDNA with High-Capacity cDNA RT Kit (Applied Biosystems). Real-time PCR was performed in triplicate for expression of human PTGS2, PTGES, PLA2G4A, and PLA2G4C, or mouse Tnf, Cxcl2, Csf2, Il10, Il1rn, and Tgm2, using Taqman® Gene Expression Assays (Applied Biosystems) and Taqman® Fast Master Mix (Applied Biosystems). Total of 10–20 ng of cDNA was used for each 20 μl reaction. Thermal cycling was performed with 7900HT System (Applied Biosystems) by incubating the reactions at 95°C for 20 s followed by 40 cycles of 95°C for 1 s and 60°C for 20 s. Data were analyzed with Sequence Detection Software V2.3 (Applied Biosystems) and relative quantities (RQs) were calculated with comparative critical threshold (CT) method using RQ Manager V1.2 (Applied Biosystems). The protein and gene symbols used in the publication are shown in Supplemental Table 2.
Data analysis
Data are summarized as mean ± SD. One-way analysis of variance with Bonferroni’s Multiple Comparison Test was used to calculate the levels of significance (ns, P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001) for data with at least 3 samples. Unpaired two-tailed t-test was used when the data consisted of only 2 samples. Statistical analysis was performed with GraphPad Prism 5 (GraphPad Software).
Results
hMSC spheroid-conditioned medium drives stimulated macrophages into M2 phenotype
To study the anti-inflammatory effects of hMSC spheroids, we used a test system of LPS-stimulated J774A mouse macrophage cultures, as interactions of hMSCs with macrophages might explain their beneficial effects in vivo. hMSCs for the assays were first expanded as adherent monolayers at a low density (100–150 cells/cm2) for 7–8 days in CCM and then cultured in hanging drops (25,000 cells/drop) in CCM for 3 days to permit spheroid formation (25). As controls, the hMSCs were plated on adherent dishes in CCM at a high density (5,000 cells/cm2), or on adherent dishes at a very high density (200,000 cells/cm2), corresponding to the very high concentration of the cells in spheroids, and incubated for 3 days. The hMSCs cultured under each of these conditions were then transferred into multi-well plates and the stimulated macrophages, on a transwell insert, were added to the cultures. The hMSC spheroids and spheroid derived cells were more effective than standard cultures of adherent hMSCs in decreasing secretion of the pro-inflammatory cytokine TNFα by LPS-stimulated macrophages (Fig. 1A). Of special importance was that spheroid-conditioned medium showed a profound effect in reducing TNFα secretion by the stimulated macrophages in a dose dependent manner (Fig. 1B). Moreover, with spheroid-conditioned medium treatment we observed increases in the total number of LPS-stimulated macrophages and in macrophages with refractile morphology (Fig. S1A and B). The effects on TNFα secretion were not dependent of the ligand used to stimulate the macrophages, the species of the macrophages, or the line of macrophages (Fig. S1C–E).
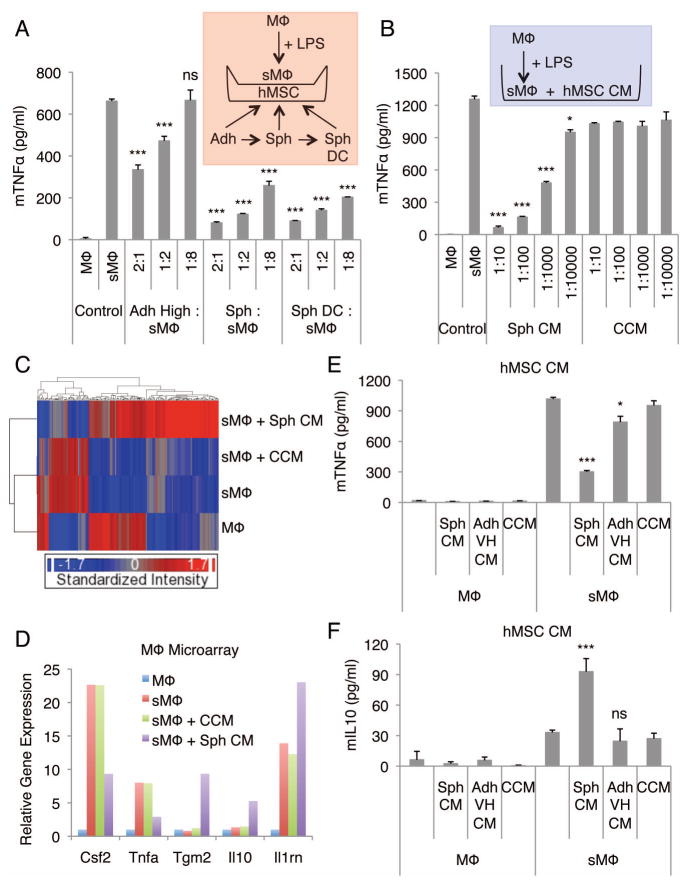
Figure 1. hMSC spheroids and spheroid-conditioned medium promote anti-inflammatory macrophage phenotype.
(A) hMSC spheroids and spheroid-derived cells reduce the secretion of mTNFα by LPS-stimulated macrophages. Transwell co-cultures of macrophages and hMSCs at different cell ratios. (B) hMSC spheroid-conditioned medium reduces the secretion of mTNFα by LPS-stimulated macrophages at a dose-dependent manner. (C) Hierarchical clustering of mouse macrophage microarray data. (D) Relative gene expression levels of selected inflammatory related genes from the mouse macrophage microarray data. Un-stimulated mouse macrophages were used as a baseline. (E,F) Comparison of the spheroid and monolayer hMSC conditioned medium effect in production of mTNFα (E) and mIL10 (F) by LPS-stimulated macrophages. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to control (sMΦ) in A, compared to corresponding vehicle controls (CCM) in B, and compared to vehicle control (CCM) in E and F. Abbreviations: Adh High, adherent monolayer hMSCs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d; CM, conditioned medium; CCM, complete culture medium; hMSC, human mesenchymal stem/stromal cell; LPS, lipopolysaccharide; mTNFα, mouse TNFα; MΦ, macrophage; sMΦ, stimulated macrophage; Sph, spheroid hMSC from 3 d hanging drop cultures (25,000 cells/drop); Sph DC, spheroid-derived cell.
To further explore the effects of spheroid-conditioned medium on stimulated macrophages, microarray assays were used. Exposure to spheroid-conditioned medium extensively altered the transcriptome of the stimulated macrophages in that 172 genes were differentially expressed (Fig. 1C). Many of the genes down-regulated by the spheroid-conditioned medium were the same as those up-regulated by LPS stimulation (Fig. 1C). In contrast, incubation of the stimulated macrophages with vehicle control CCM had very little effect (Fig. 1C). In addition, the down-regulated group of genes contained several genes related to cell adhesion and apoptosis whereas the up-regulated group of genes contained many genes associated with signal transduction and wounding (Table S3 and S4). Both up- and down-regulated groups of genes included genes for secreted cytokines and other factors involved in immune responses (Table S3 and S4). Of special note was that culture with spheroid-conditioned medium down-regulated expression of the pro-inflammatory genes Tnf and Csf2, up-regulated the expression of the anti-inflammatory molecules Il10 and Il1ra, and up-regulated the expression of Tgm2 that has been implicated in apoptotic cell clearance (31) (Fig. 1D). The microarray data were validated by real-time PCR assays of the time-dependent changes in the stimulated macrophages (Fig. S2). The results demonstrated that the peak levels of expression of the genes in the stimulated macrophages varied between 1–8 h but that the spheroid-conditioned medium consistently down-regulated Tnf and Csf2 and up-regulated Il10 and Il1rn (Fig. S2).
We next compared the anti-inflammatory effects of spheroid-conditioned medium with conditioned medium from monolayer cultures on stimulated macrophages. To obtain the different conditioned medium preparations, hMSCs were cultured for 3 days in hanging drops (25,000 cells/drop, 714 cells/μl) in CCM, or for 3 days on adherent dishes at a very high density (200,000 cells/cm2, 714 cells/μl), corresponding to the very high initial concentration of the cells in spheroids. Spheroid-conditioned medium was more effective than monolayer conditioned medium (both used at 1:100 dilution) in inhibiting secretion of pro-inflammatory cytokines TNFα (Fig. 1E), CXCL2 (Fig. S3A), IL6 (Fig. S3B), IL12p40 (Fig. S3C), and IL23 (Fig. S3D). Spheroid-conditioned medium was also more effective in increasing the secretion of anti-inflammatory cytokines IL10 (Fig. 1F and S3F) and IL1ra (Fig. S3E). In addition, spheroid-conditioned medium increased the number of CD206+ macrophages recovered from stimulated macrophage cultures (Fig. S4). Moreover, conditioned medium from hMSCs expanded on adherent dishes in CCM at a standard high density (5,000 cells/cm2, 25.5 cells/μl) for 3 days and concentrated 28x to match the initial cell concentration (714 cells/μl) used to produce spheroid and adherent very high conditioned medium, did not reduce the TNFα (Fig. S5A) or increase the IL10 (Fig. S5B) secretion by the LPS-stimulated macrophages when used at 1:100 dilution. These results suggested that spheroid-conditioned medium changed the stimulated macrophage cultures from a primarily pro-inflammatory M1 phenotype to a more anti-inflammatory M2 phenotype.
Identification of PGE2 as a potential anti-inflammatory factor secreted by spheroid hMSCs
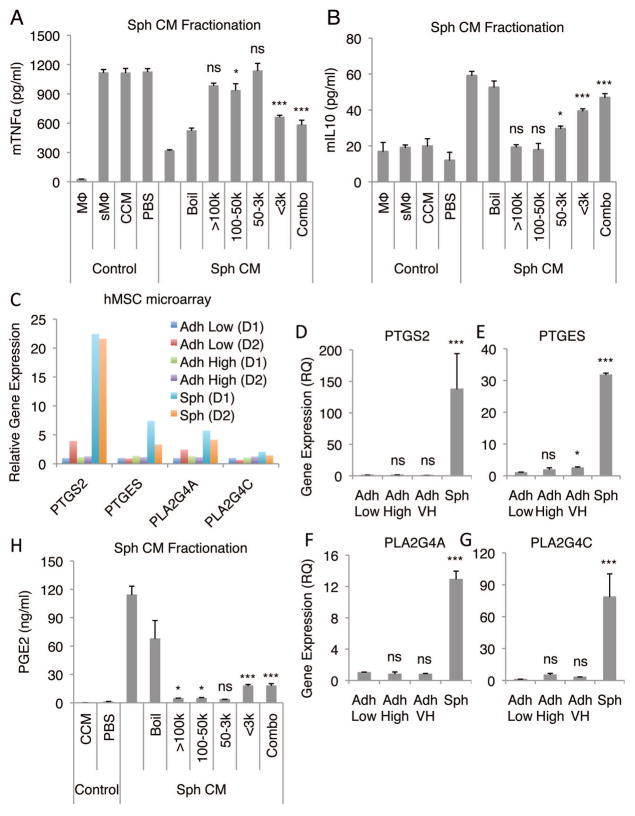
To identify anti-inflammatory factors secreted by spheroid hMSCs, spheroid-conditioned medium was crudely fractionated based on molecule size. The major anti-inflammatory activity that decreased TNFα secretion (Fig. 2A) and increased IL10 secretion (Fig. 2B) by stimulated macrophages, was recovered in the fraction of less than 3 kDa. The activity was only partially inactivated by boiling under conditions that would completely denature most proteins (Fig. 2A and B). Both IDO and NO has been implicated as important anti-inflammatory molecules secreted by MSCs, although NO appears to be secreted only by mouse MSCs (12, 32), However, since the major anti-inflammatory activity was in the fraction of less than 3 kDa we tested the effects of inhibiting NO synthesis in hMSC spheroids with L-NAME rather than inhibiting the effects of the much larger molecule IDO. Inhibition of NO synthesis did not have any effect on the anti-inflammatory activity of spheroid-conditioned medium (Fig. S5B). We then examined our previously published microarray data from spheroid hMSCs (25) for up-regulation of genes involved in the synthesis of other candidate small molecules. The data revealed up-regulation of several genes in the pathway for the synthesis of PGE2 (Fig. 2C). The microarray data were confirmed by real-time PCR of PTGS2, PTGES, PLA2G4A, and PLA2G4C (Fig. 2D–G). Also, ELISA demonstrated that PGE2 was present in the unfractionated spheroid-conditioned medium and in the low molecular weight fraction (Fig. 2H). In addition, hMSC spheroids and spheroid-derived cells secreted large amounts of PGE2 even after transfer into adherent dishes (Fig. S6). In contrast, adherent monolayer hMSCs expressed considerably lower levels of PGE2 synthesis related molecules (Fig. 2D–G) and did not secrete high levels of PGE2 (Fig. S6).
Figure 2. Fractionation of hMSC spheroid-conditioned medium identifies PGE2 as a candidate anti-inflammatory molecule.
(A,B) Effects of fractions of hMSC spheroid-conditioned medium on production of mTNFα (A) and mIL10 (B) by LPS-stimulated macrophages. (C) Relative gene expression levels of molecules involved in PGE2 synthesis from microarray data from 3 different preparations of hMSCs from 2 different donors. hMSCs plated at a low density (100–150 cells/cm2) and grown for 7 d were used as a baseline. (D–G) Real-time PCR expression data for PTGS2 (D), PTGES (E), PLA2G4A (F), and PLA2G4C (G) in hMSC monolayers and spheroids. hMSCs plated at a low density and grown for 7 d were used as a baseline. (H) PGE2 content of hMSC spheroid-conditioned medium fractions. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to vehicle control (CCM) in A, B, and H and compared to Adh Low in D, E, F, and G. Abbreviations: Boil, denatured hMSC Sph CM; Combo, equal combination of all the fractions; D1, donor 1; k, kDa; PBS, phosphate buffered saline. Other abbreviations as in Fig. 1.
COX-2-dependent production of PGE2 in spheroid hMSCs is a major mechanism for their anti-inflammatory effect
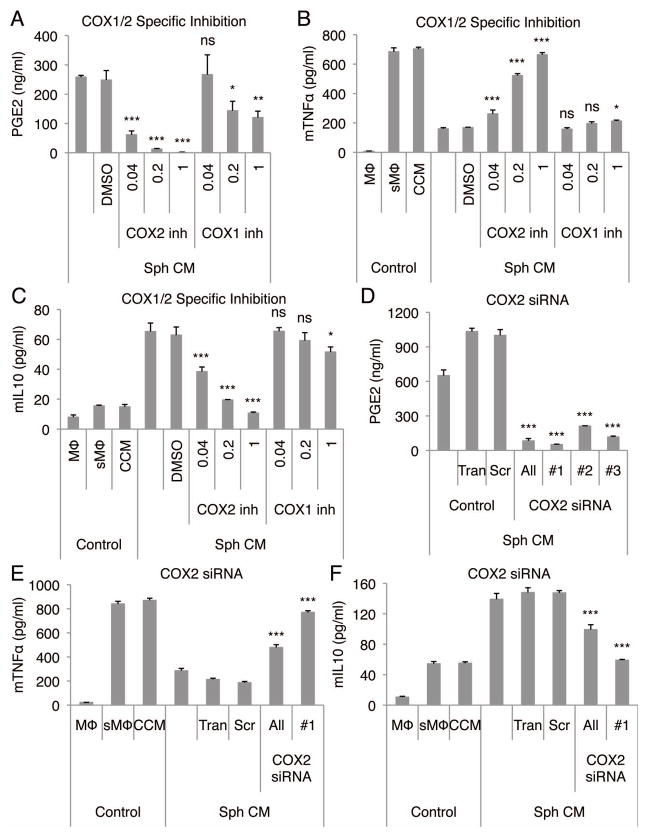
To confirm the role of PGE2, we tested the effects of inhibitors of COX-2 an essential enzyme in PGE2 synthesis. When added to hanging drop cultures of hMSCs, the non-selective COX inhibitor, indomethacin, abolished the production of PGE2 by the spheroid hMSCs (Fig. S7A). Also, conditioned medium from indomethacin treated spheroids failed to reduce TNFα (Fig. S7B) and increase IL10 secretion (Fig. S7C) by the stimulated macrophages. When COX-2 specific inhibitor (NS398) was applied to the hanging drop cultures, the production of PGE2 was decreased (Fig. 3A) and the spheroid-conditioned medium was unable to reduce TNFα (Fig. 3B) and increase IL10 secretion (Fig. 3C) by the stimulated macrophages. COX-1 inhibitor (SC560) had a minor effect only at very high doses (Fig. 3A–C) in which the inhibitor has also been reported to inhibit some COX-2 activity (33, 34). To confirm the role of COX-2, the gene was knocked down in the hMSC spheroids with 3 different COX-2 specific siRNA duplexes (Fig. S8A). As expected, the COX-2 siRNAs decreased the amount of PGE2 produced by the hMSC spheroids (Fig. 3D). Also, conditioned medium from COX-2 knockdown spheroids failed to reduce the secretion TNFα (Fig. 3E) and CXCL2 (Fig. S8B), and failed to increase the secretion of IL10 (Fig. 3F) and IL1ra (Fig. S8C) by stimulated macrophages.
Figure 3. Anti-inflammatory effect of hMSC spheroid-conditioned medium is dependent on COX-2.
(A-C) COX-2 inhibition in hMSC spheroids reduces the production of PGE2 by the hMSCs (A), and the conditioned medium fails to reduce mTNFα (B) and increase mIL10 (C) secretion by the stimulated macrophages. COX-1 and 2 inhibitor concentrations are shown in μM. (D–F) COX-2 knockdown in hMSC spheroids reduces the production of PGE2 by the hMSCs (D), and the conditioned medium fails to reduce mTNFα (E) and increase mIL10 (F) secretion by the stimulated macrophages. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001 compared to vehicle control (DMSO) in A, B, and C and compared to control (Scr) in D, E, and F. Abbreviations: All, combination of COX-2 siRNA #1, 2, and 3; DMSO, dimethyl sulfoxide; inh, inhibitor; Scr, negative control siRNA; Tran, Transfection reagent control; #1, 2, and 3, three different siRNAs for COX-2. Other abbreviations as in Fig. 1.
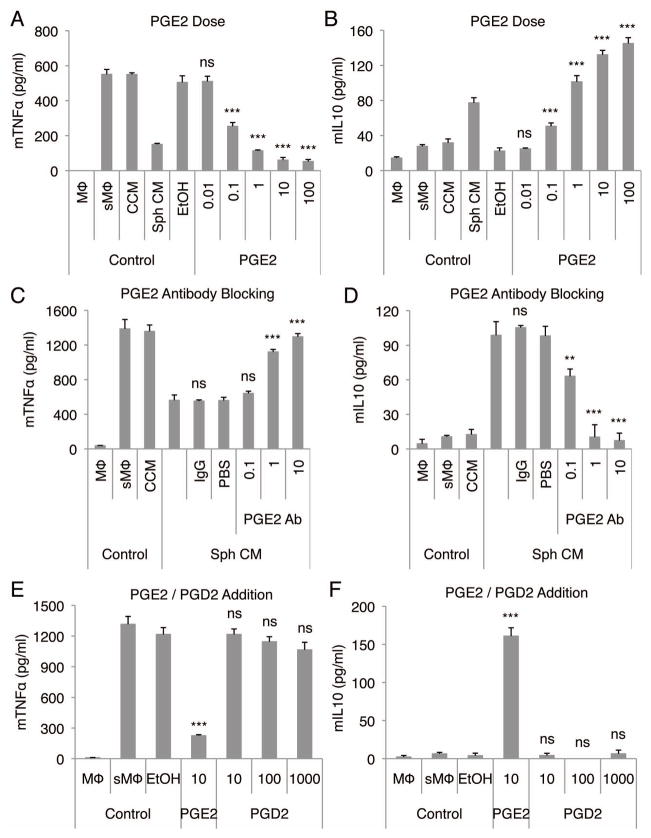
The role of PGE2 was further confirmed by the observations that synthetic PGE2 reproduced the effects of spheroid conditioned-medium on stimulated macrophages (Fig. 4A, 4B, S9A, and S9B) in dose-dependent manner. Synthetic PGE2 at 1 ng/ml was equivalent in the assay with stimulated macrophages as a 1:300 dilution of spheroid-conditioned medium (Fig. 4A) and therefore at a concentration comparable to the average concentration of PGE2 detected in 1:300 diluted spheroid-conditioned medium by ELISA (Fig. 3A). Most importantly, when a blocking antibody to PGE2 was added to the cultures, spheroid-conditioned medium failed to decrease the secretion of TNFα (Fig. 4C), and failed to increase the secretion of IL10 (Fig. 4D) and IL1ra (Fig. S10A) by stimulated macrophages. The effect of synthetic PGE2 on stimulated macrophages was also lost with the addition of PGE2 antibody (Fig. S10B). Moreover, the inhibition observed with indomethacin was reversed in a dose-dependent manner by addition of synthetic PGE2 (Fig. S11).
Figure 4. Spheroid hMSC produced PGE2 has a strong anti-inflammatory effect on stimulated macrophages.
(A,B) PGE2 reduces the secretion of mTNFα (A) and increases the secretion of mIL10 (B) by LPS-stimulated macrophages in a dose-dependent manner. PGE2 doses are shown in ng/ml. (C,D) PGE2 neutralization in hMSC spheroid-conditioned medium reduces the anti-inflammatory effect of the conditioned medium on LPS-stimulated macrophages measured as mTNFα (C) and mIL10 (D) secretion. PGE2 antibody doses are shown in μg/ml. (E,F) PGD2 does not reduce the secretion of mTNFα (E) or increase the secretion of mIL10 (F) by LPS-stimulated macrophages. PGE2 and PGD2 doses are shown in ng/ml. Values are mean ± SD (n = 3). ns P ≥ 0.05, **P < 0.01, ***P < 0.001 compared to vehicle control (EtOH) in A, B, E, and F and compared to control (IgG) in C and D. Abbreviations: Ab, antibody; EtOH, ethanol; PBS, phosphate buffered saline. Other abbreviations as in Fig. 1.
An increase in expression of prostaglandin D synthase was detected in hMSC spheroids by microarrays (Fig. S12). However, addition of synthetic PGD2 did not decrease the secretion of TNFα (Fig. 4E) or increase the secretion of IL10 (Fig. 4F) by stimulated macrophages even at very high doses, suggesting that PGD2 had no role in the anti-inflammatory effect of spheroid-conditioned medium.
The roles of PGE2 and COX-2 were confirmed with other macrophages and ligands. Spheroid-conditioned medium inhibited TNFα secretion in macrophages (J774A) stimulated with zymosan (Fig. S13A) and in another line of mouse macrophages (Raw) stimulated with LPS (Fig. S13B). As expected, inhibition of COX activity in spheroid hMSC with indomethacin negated the anti-inflammatory activity of spheroid-conditioned medium, but addition of PGE2 restored the activity in both cell lines (Fig. S13). More specifically, conditioned medium from hMSC spheroids cultured with COX-2 inhibitor, failed to reduce the secretion of TNFα by zymosan-stimulated J774 macrophages (Fig. S13A) and LPS-stimulated Raw macrophages (Fig. S13B).
hMSC spheroid-conditioned medium signals through the PGE2 receptor EP4 on stimulated macrophages
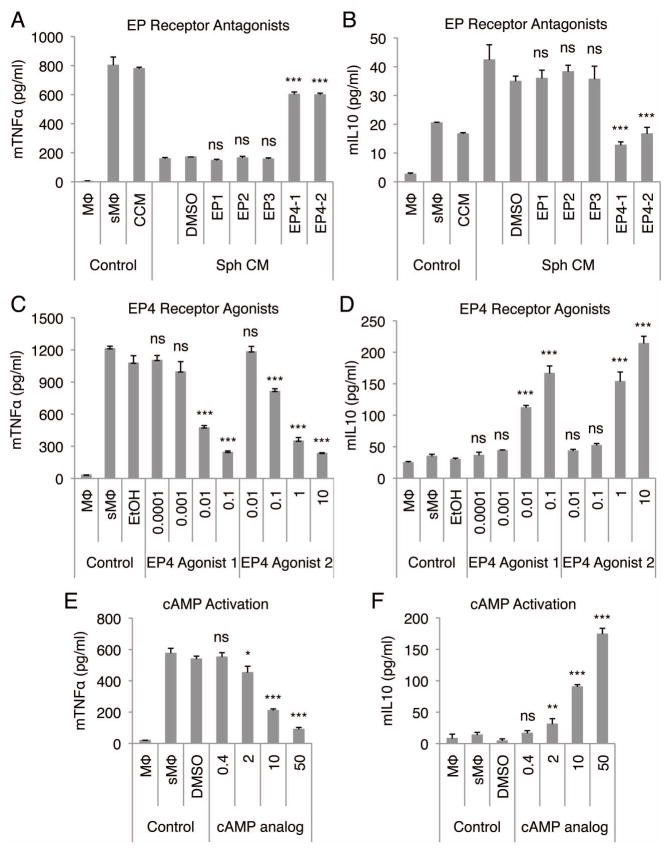
Since PGE2 can elicit effects through 4 different EP receptors (35, 36), we used receptor antagonists for EP1, 2, 3, and 4 to determine the receptor that mediates the observed anti-inflammatory effects of PGE2 in hMSC spheroid-conditioned medium on stimulated macrophages. Only EP4 receptor antagonists were able to inhibit the reduction in TNFα (Fig. 5A) and inhibit the increase in IL10 (Fig. 5B) and IL1ra (Fig. S14A) secretion by stimulated macrophages in cultures with spheroid-conditioned medium. Moreover, the effect of EP4 receptor antagonists on TNFα (Fig. S14B) and IL10 (Fig. S14C) secretion was dose dependent. Also, the anti-inflammatory effects of synthetic PGE2 were inhibited by EP4 receptor antagonist (Fig. S14D). To verify the EP4 receptor mediated signaling we showed that 2 EP4 receptor agonists demonstrated a dose dependent anti-inflammatory effect on stimulated macrophages by reducing the secretion of TNFα (Fig. 5C) and CXCL2 (Fig. S15A), and increasing the secretion of IL10 (Fig. 5F) and IL1ra (Fig. S15B). Since PGE2 binding to EP4 receptor leads to the activation of adenylate cyclase and subsequent increase in cAMP (35, 36), we used a cAMP analog (8-Bromo cAMP) on stimulated macrophages to determine if the anti-inflammatory effects of spheroid-conditioned medium could be reproduced. The cAMP analog reduced the secretion of TNFα (Fig. 5E) and increased the secretion of IL10 (Fig. 5F) by the stimulated macrophages in a dose dependent manner similar to the hMSC spheroid-conditioned medium.
Figure 5. Anti-inflammatory effect of hMSC spheroid-conditioned medium is mediated through EP4 receptor on stimulated macrophages.
(A,B) EP4 receptor antagonists inhibit the anti-inflammatory effect of hMSC spheroid-conditioned medium on stimulated macrophages. mTNFα (A) and mIL10 (B) ELISA. EP receptor antagonists were used at 10 μM. (C,D) Two different EP4 receptor agonists decrease the production of mTNFα (C) and increase the production of mIL10 (D) by stimulated macrophages in a dose-dependent manner. Doses are shown in ng/ml. (E,F) cAMP analog decreases the production of mTNFα (E) and increases the production of mIL10 (F) by stimulated macrophages in a dose-dependent manner. Doses are shown in μM. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001 compared to vehicle control (DMSO) in A, B, E, and F and compared to vehicle control (EtOH) in C and D. Abbreviations: DMSO, dimethyl sulfoxide; EtOH, ethanol. Other abbreviations as in Fig. 1.
hDFs do not produce large quantities of PGE2 when aggregated into spheroids
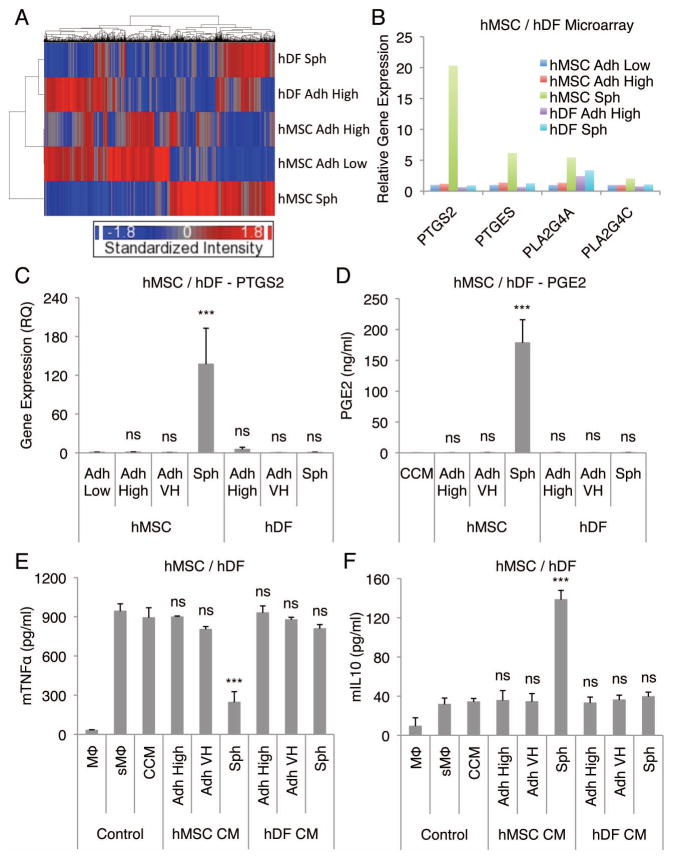
In control experiments, hDFs were cultured in CCM for 3 days in hanging drops (25,000 cells/drop) and in adherent cultures at high (5,000 cells/cm2) and very high (200,000 cells/cm2) density. Similar to hMSCs, hDFs aggregated into a single spheroid in a hanging drop (Fig. S16). Transcriptome analysis demonstrated that although hDF spheroids acquired some of the characteristics of hMSC spheroid, they still remained very different (Fig. 6A). Specifically, many of the enzymes involved in PGE2 synthesis were expressed in much lower levels in hDF spheroids (Fig. 6B). The differences were confirmed by real-time PCR assays of PTGS2, PTGES, PLA2G4A, and PLA2G4C (Fig. 6C, S17A, S17B, and S17C). Therefore, not surprisingly, the amount of PGE2 produced in 3 days by hDFs cultured in hanging drops was much lower than the amount produced by hMSC cultured in hanging drops (Fig. 6D). Moreover, hDF conditioned medium obtained from hanging drop (714 cells/μl), adherent high (25.5 cells/μl), and adherent very high (714 cells/μl) cultures (at 1:100 dilution) failed to reduce the secretion of TNFα (Fig. 6E) and CXCL2 (Fig. S18A), and failed to increase the secretion of IL10 (Fig. 6F) and IL1ra (Fig. S18B) by the stimulated macrophages. The secretion of PGE2 was extremely low with spheroids formed from 4 different hDF donors (Fig. S19). In contrast, spheroids from 5 preparations of hMSCs from 5 different donors consistently secreted high levels of PGE2 (Fig. S19).
Figure 6. hDFs do not produce high levels of PGE2 and do not have an anti-inflammatory effect on stimulated macrophages.
(A) Hierarchical clustering of hMSC and hDF microarray data. (B) Relative gene expression levels of molecules involved in PGE2 synthesis from the hMSC and hDF microarray data. hMSCs plated at a low density and grown for 7 d were used as a baseline. (C,D) hDF spheroids do not express high levels of PTGS2 (C) or secrete PGE2 (D). (E,F) Conditioned medium from hDF spheroids does not lower the secretion of mTNFα (E) or increase the secretion of mIL10 (F) by LPS-stimulated macrophages. Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to Adh Low in C and compared to vehicle control (CCM) in D, E, and F. Abbreviation: hDF, human adult dermal fibroblast. Other abbreviations as in Fig. 1.
The self-activation of hMSCs in spheroids to produce PGE2 is dependent on caspases and NFκB
We previously demonstrated that hMSC aggregation in hanging drops resulted in a time-dependent increase in the number of apoptotic cells (25). We also showed that the aggregation of hMSCs into spheroids up-regulated the expression of many genes related to cellular stress including the apoptosis inducer TRAIL. Thus we hypothesized that hMSCs in spheroids are responding to signals released by stressed and apoptotic cells by secreting anti-inflammatory molecules and that these signals are mediated through caspases and NFκB, important molecules in the cellular stress response. To study in more detail the effect of cellular stress in self-activation process of spheroid hMSCs to produce PGE2, caspase and NFκB inhibitors were used in the hanging drop cultures. Broad-spectrum caspase inhibitor (Q-VD-OPh) reduced the production of PGE2 by the hMSCs in spheroids (Fig. S20A), and the spheroid-conditioned medium was unable to reduce TNFα (Fig. S20B) and increase IL10 secretion (Fig. S20C) by the stimulated macrophages. Another inhibitor of stress signaling, NFκB transcriptional activation inhibitor (QNZ), also reduced the production of PGE2 by the hMSC spheroids (Fig. S21A) and the conditioned medium failed to reduce TNFα (Fig. S21B) and increase IL10 secretion (Fig. S21C) by the stimulated macrophages. These results suggested that the 3D cultures activated hMSCs to produce PGE2 through caspase and NFκB dependent mechanism.
Discussion
Administration of MSCs has produced functional improvements in a series of animal models for human diseases but the effects have been difficult to explain on the basis of either engraftment and differentiation of the cells or the paracrine factors they produce in culture (1, 37). Instead, many of the beneficial effects appear to arise as a result of cross-talk in vivo between MSCs and the cells and tissues in which they either engraft or lodge (1). For example, hMSCs infused intravenously into mice were efficiently trapped in the lungs (38, 39) and after a delay, the cells were activated to express a large number of genes they do not express in culture (14). Our initial interpretation of the observations was that the cells trapped in the lung produced micro-emboli and that the resulting tissue injury released pro-inflammatory cytokines that activated the hMSCs (40). The conclusion that the infused hMSCs formed microemboli was consistent with observations that the cells were detected in afferent vessels of the lung and that intravenous infusions of partially aggregated hMSCs produced lethal emboli (40). However, we recently reexamined the histological sections and were impressed that the hMSCs in the afferent vessels were largely present in clusters of multiple cells (40). Therefore the activation of the hMSCs trapped in lung may have in part been explained by the cells forming aggregates that caused self-activation of the cells similar to the self-activation seen when the cells aggregated into spheroids in hanging drops.
The results presented here demonstrated that as hMSCs aggregated into spheroids, they began to secrete an anti-inflammatory activity that dramatically altered LPS or zymosan stimulated macrophages. The activity was found in a small molecular weight fraction of spheroid-conditioned medium. Examination of microarray for possible candidates suggested the hypothesis that the anti-inflammatory factor might be PGE2. The hypothesis was confirmed by the observations that large quantities of PGE2 were present in the spheroid-conditioned medium, and that the anti-inflammatory activity of spheroid-conditioned medium was abolished by an inhibitor of COX-2, by siRNAs for COX-2, and by an antibody to PGE2. Also, the anti-inflammatory activity was reproduced by PGE2 and the effects on stimulated macrophages were inhibited by an antagonist for the EP4 receptor for PGE2. Furthermore, the production of PGE2 by hMSC spheroids was dependent on activity of caspases and NFκB activation.
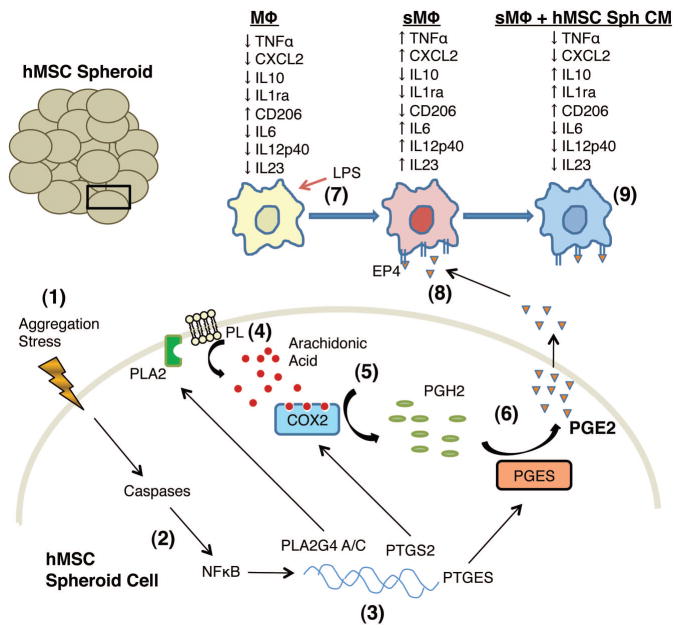
The results were consistent with the proposed sequence of events summarized in Fig. 7 in which aggregation of human MSCs into spheroids generated a “stress” on the cells (Step 1). The stress resulted in the activation of caspases and NFκB (Step 2) leading into up-regulation of the genes for phospholipase A2, COX-2, and prostaglandin E synthase (Step 3). The increase in phospholipase A2 increased release of arachidonic acid from the plasma membrane phospholipids (Step 4), the arachidonic acid was processed by COX-2 to prostaglandin H2 (Step 5) and then by prostaglandin E synthase (Step 6) to yield more PGE2. In cultures with LPS-stimulated macrophages (Step 7), the PGE2 interacted with the EP4 receptor on the stimulated macrophages (Step 8) to convert them to a more anti-inflammatory M2 phenotype (Step 9). The transition to M2-type macrophages was characterized by a decrease in secretion of pro-inflammatory cytokines TNFα, CXCL2, IL6, IL12p40, and IL23 and increase in secretion of anti-inflammatory cytokines IL10 and IL1ra by the stimulated macrophages, and increase in the number of macrophages that expressed CD206 in line with previous reports (41, 42).
Figure 7. Schematic of the proposed signaling in spheroid hMSCs promoting anti-inflammatory phenotype in stimulated macrophages.
(1) hMSCs aggregate in a hanging drop to form a spheroid. (2) Aggregation and the resulting stress result in activation of caspases and NFκB. (3) The expression of PLA2G4A/C, PTGS2, and PTGES genes is up-regulated. (4) Phospholipases (PLA2) release arachidonic acid from plasma membrane phospholipids (PL). (5) Arachidonic acid is converted into PGH2 by COX-2. (6) PGH2 is converted into PGE2 by PGE synthase (PGES) followed by secretion of PGE2 (7) LPS stimulation of macrophages increases the secretion of pro-inflammatory cytokines TNFα, CXCL2, IL6, IL12p40, and IL23. (8) hMSC spheroid produced PGE2 binds to the EP4 receptor on LPS-stimulated macrophages. (9) PGE2 binding results in decreased secretion of TNFα, CXCL2, IL6, IL12p40, and IL23 and increased secretion of anti-inflammatory cytokines IL10 and IL1ra, and increased surface expression of M2 macrophage marker CD206 by the stimulated macrophages. Abbreviations as in Fig. 1.
Nemeth et al. (12), originally demonstrated that MSCs could produce anti-inflammatory effects by being activated to synthesize and secrete PGE2. They demonstrated that intravenous administration of murine MSCs to mice before or shortly after inducing sepsis by cecal ligation and puncture reduced mortality and improved organ function. Their results indicated that MSCs activated by either LPS or TNF-α reprogrammed macrophages by releasing PGE2 that interacted with EP2 and EP4 on the macrophages. The reprogramming of the macrophages was NO-dependent and reflected in part by increased levels in serum of the anti-inflammatory cytokine IL10. Others have since shown that murine MSCs inhibit local inflammation in experimental arthritis through IL-6-dependent production of PGE2 (43), and could turn activated macrophages into a regulatory-like profile through production of PGE2 in co-cultures (44). hMSCs have been shown to produce PGE2 upon stimulation with pro-inflammatory cytokines (45), and have an ability to inhibit monocyte-derived dendritic cell maturation and function through PGE2 (46). In related experiments, we recently found that hMSCs suppressed LPS-induced glial activation in organotypic hippocampal slice cultures by being activated to secrete PGE2 (47). The results here are consistent with these previous observations (12, 47), except that we described a novel self-activation process of hMSCs to secrete PGE2 simply by aggregation into spheroids in NO-independent mechanism and without cross-talk with the macrophages.
Aggregation of hMSC into spheroids may improve the therapeutic benefits of the cells. The cells compact in size to approximately one-quarter the volume of hMSCs lifted from monolayer cultures (25). Therefore, single cells obtained from hMSC spheroids by trypsination more readily escape being trapped in the lung after intravenous infusion (14, 25, 38–40). Also, hMSC spheroids are self-activated to express a number of potentially beneficial factors that include not only PGE2 but also the anti-inflammatory protein TSG-6, the anti-reactive oxygen species protein STC-1 and several putative anti-cancer genes (TRAIL, IL24, and CD82) (25). Therefore, hMSC spheroids and spheroid-derived cells may generate larger amounts of these factors in vivo than hMSCs from monolayer cultures that require several hours to be activated and disappear with a half life of as short as 24 h after intravenous infusion (14). However, current protocols for the preparation of spheroid hMSCs have the disadvantage of requiring further manipulation after the cells are expanded in monolayer cultures. The further manipulations might become manageable with different conditions for 3D aggregation of hMSCs (4, 15, 48). Alternatively, administration of hMSC spheroids locally may be useful for repair of tissues such as bone and cartilage or intraperitoneal administration for systemic modulation of inflammatory or immune responses. For example, we recently observed that intraperitoneal administration of hMSCs reduced chemical injury to the cornea without evidence of the cells engrafting in the cornea (49). At the same time, it is apparent that spheroid hMSCs are self-activated to up-regulate expression of large number of genes (25), more than one of which may be therapeutically beneficial.
Supplementary Material
Supplemental Figure 1. hMSC spheroid-conditioned medium reduces the secretion of TNFα by stimulated macrophages. (A) Images of non-stimulated and LPS-stimulated J774 mouse macrophages after 18 h incubation with hMSC spheroid-conditioned medium. (B) hMSC spheroid-conditioned medium increases the growth of LPS-stimulated J774 mouse macrophages. (C) hMSC spheroid-conditioned medium decreases the secretion of mTNFα by zymosan-stimulated mouse macrophages (J774). (D) hMSC spheroid-conditioned medium decreases the secretion of mTNFα by LPS-stimulated mouse macrophages (Raw). (E) hMSC spheroid-conditioned medium decreases the secretion of hTNFα by LPS-stimulated human macrophages (U937). Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to control sMΦ in B and vehicle control (CCM) in C, D, and E. Scale bar: 50 μm. Abbreviations: CCM, complete culture medium; CM, conditioned medium; LPS, lipopolysaccharide; MΦ, macrophage; sMΦ, stimulated macrophage; Sph, spheroid hMSC from 3 d hanging drop cultures (25,000 cells/drop).
Supplemental Figure 2. hMSC spheroid-conditioned medium down-regulates the expression of pro-inflammatory genes and up-regulates the expression of anti-inflammatory genes in stimulated macrophages. (A–C) hMSC spheroid-conditioned medium down-regulates the expression of Tnf (A), Cxcl2 (B), and Csf2 (C) in LPS-stimulated macrophages. (D–F) hMSC conditioned medium up-regulates the expression of Il10 (D), Il1rn (E), and Tgm2 (F) in LPS-stimulated macrophages. Real-time PCR measurements shown as mean (assay triplicates) relative quantities (RQ) compared to time 0 value of each gene. Abbreviations as in Fig. S1.
Supplemental Figure 3. hMSC spheroid-conditioned medium has an anti-inflammatory effect on stimulated macrophages. (A, B, C, D, E) Comparison of the spheroid and monolayer hMSC conditioned medium effect in production of mCXCL2 (A), mIL6 (B), mIL12p40 (C), mIL23 (D), and mIL1ra (E) by LPS-stimulated macrophages. (F) hMSC spheroid-conditioned medium increases the secretion of mIL10 by LPS-stimulated macrophages in a dose dependent manner. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001 compared to sMΦ with vehicle control (CCM) in A, B, C, D, and E and compared to corresponding vehicle control (CCM) in F. Abbreviations: hMSC, human mesenchymal stem/stromal cell; Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d. Other abbreviations as in Fig. S1.
Supplemental Figure 4. hMSC spheroid-conditioned medium increases the number of CD206 positive macrophages. hMSC spheroid-conditioned medium increases the number of CD206 positive LPS-stimulated macrophages measured with flow cytometry. Histogram overlays of representative samples (A) and combined data (B) are shown. Values are mean ± SD (n = 4). ***P < 0.001 compared to vehicle control (CCM) in B. Abbreviations as in Fig. S1.
Supplemental Figure 5. Conditioned medium from adherent hMSC does not have an anti-inflammatory effect and the anti-inflammatory effect of hMSC spheroid-conditioned medium is not mediated by nitric oxide. (A,B) Concentrated conditioned medium from adherent hMSC does not lower the mTNFα (A) or increase the mIL10 (B) secretion by LPS-stimulated macrophages. (C) Inhibition of nitric oxide synthesis in hMSC spheroids does not prevent the effect of spheroid-conditioned medium on production of mTNFα by LPS-stimulated macrophages. Nitric oxide synthesis inhibitor concentrations are shown in μM. Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to corresponding vehicle control (CCM, or CCM conc) in A and compared to corresponding vehicle control (Wtr) in B. Abbreviations: Adh High, adherent monolayer hMSCs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d; conc, 28x concentrated conditioned medium; inh, inhibitor; Wtr, water. Other abbreviations as in Fig. S1.
Supplemental Figure 6. hMSC spheroids and spheroid-derived cells produce large amounts of PGE2. The production of PGE2 by hMSC monolayers, spheroids, or spheroid-derived cells was determined at time points indicated. Values are mean ± SD (n = 3). ns P ≥ 0.05, **P < 0.01, ***P < 0.001 compared to compared to control (Adh VH) in each time point. Abbreviations: Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d; hMSC, human mesenchymal stem/stromal cell; Sph DC, spheroid-derived cell. Other abbreviations as in Fig. S1.
Supplemental Figure 7. Anti-inflammatory effect of hMSC spheroid-conditioned medium on stimulated macrophages is dependent on hMSC COX activity. (A–C) COX inhibition in hMSC spheroids reduces the production of PGE2 (A) and the anti-inflammatory effect of conditioned medium on LPS-stimulated macrophages measured as mTNFα (B) and mIL10 (C) secretion. Indomethacin doses are in μM and dose of 1 μM was used in C. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001 compared to vehicle control (MetOH) in A, B, and C. Abbreviations: Indo, Indomethacin; MetOH, methanol. Other abbreviations as in Fig. S1.
Supplemental Figure 8. COX2 knockdown in spheroid hMSCs reduces the anti-inflammatory effect on stimulated macrophages. (A) Real-time PCR to asses the knockdown of COX2 gene, PTGS2, in hMSC spheroids with siRNA. hMSCs plated at a low density and grown for 7 d were used as a baseline. Biological triplicates were pooled and technical triplicates with 95% upper confidence intervals are shown. (B,C) COX2 knockdown in hMSC spheroids reduces the anti-inflammatory effect of conditioned medium on LPS-stimulated macrophages measured as mCXCL2 (B) and mIL1ra (C) secretion. Values are mean ± SD (n = 3). ***P < 0.001 compared to control (Scr) in B and C. Abbreviations: All, combination of COX2 siRNA #1, 2, and 3; Scr, negative control siRNA; Tran, Transfection control. Other abbreviations as in Fig. S1.
Supplemental Figure 9. PGE2 can elicit an anti-inflammatory effect on stimulated macrophages. PGE2 reduces the secretion of mCXCL2 (A) and increases the secretion of mIL1ra (B) by LPS-stimulated macrophages in a dose-dependent manner. PGE2 doses are in ng/ml. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to vehicle control (EtOH). Abbreviation: EtOH, ethanol. Other abbreviations as in Fig. S1.
Supplemental Figure 10. PGE2 antibody reduces the anti-inflammatory effect of hMSC spheroid-conditioned medium on stimulated macrophages. (A) PGE2 neutralization in hMSC spheroid-conditioned medium reduces the anti-inflammatory effect of the conditioned medium on LPS-stimulated macrophages measured as mIL1ra secretion. PGE2 antibody doses are shown in μg/ml. (B) PGE2 neutralizing antibody inhibits the anti-inflammatory effect of added synthetic PGE2 on LPS-stimulated macrophages. PGE2 was used at 10 ng/ml and PGE2 antibody at 10 μg/ml. Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to vehicle control (PBS) in A and compared to vehicle control (EtOH) and PGE2 with PGE2 Ab in B. Abbreviations: Ab, antibody; EtOH, ethanol; PBS, phosphate buffered saline. Other abbreviations as in Fig. S1.
Supplemental Figure 11. PGE2 addition restores the anti-inflammatory effect of conditioned medium from Indomethacin treated spheroids. Indomethacin doses are in μM and PGE2 doses in ng/ml. Indomethacin was used at 1 μM with PGE2. Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to corresponding vehicle control (MetOH or EtOH). Abbreviations: EtOH, ethanol; Indo, Indomethacin; MetOH, methanol. Other abbreviations as in Fig. S1.
Supplemental Figure 12. PGD2 synthase is up-regulated in hMSC spheroids. Relative gene expression levels for PGD2 synthase from microarray data for 2 hMSC donors. Abbreviations: Adh High, adherent monolayer hMSCs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh Low, adherent monolayer hMSCs plated at low density (100–150 cells/cm2) and cultured for 7 d; D1, donor 1; hMSC, human mesenchymal stem/stromal cell. Other abbreviations as in Fig. S1.
Supplemental Figure 13. hMSC spheroid-conditioned medium has a COX2-dependent anti-inflammatory effect on both LPS and zymosan stimulated macrophages. (A) hMSC spheroid-conditioned medium reduces the production of mTNFα by zymosan-stimulated mouse macrophages (J774) in a COX2-dependent manner. (B) hMSC spheroid-conditioned medium reduces the production of mTNFα by LPS-stimulated mouse macrophages (Raw) in a COX2-dependent manner. Indomethacin, COX2 inhibitor, and COX1 inhibitor were used at 1 μM. PGE2 was used at 10 ng/ml. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to corresponding vehicle control (MetOH, Indo EtOH, or DMSO). Abbreviations: DMSO, dimethyl sulfoxide; EtOH, ethanol; Indo, Indomethacin; inh, inhibitor; MetOH, methanol. Other abbreviations as in Fig. S1.
Supplemental Figure 14. Anti-inflammatory effect of hMSC spheroid-conditioned medium on stimulated macrophages is mediated through EP4 receptor. (A) EP4 receptor antagonists inhibit the anti-inflammatory effect of hMSC spheroid-conditioned medium on stimulated macrophages measured as mIL1ra secretion. All EP receptor antagonists were used at 10 μM. (B,C) EP4 receptor antagonists inhibit the anti-inflammatory effect of hMSC spheroid-conditioned medium in a dose dependent manner measured as mTNFα (B) and mIL10 (C) secretion. Doses for the two different EP4 receptor antagonists are shown as μM. (D) EP4 receptor antagonist inhibits the anti-inflammatory effect of PGE2 on stimulated macrophages measured as mTNFα secretion. EP4 antagonist was used at 10 μM. Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to vehicle control (DMSO) in A, B, and C and compared to corresponding vehicle control (DMSO, or PGE2 DMSO) in D. Abbreviation: DMSO, dimethyl sulfoxide. Other abbreviations as in Fig. S1.
Supplemental Figure 15. EP4 receptor agonist has an anti-inflammatory effect on stimulated macrophages. (A,B) EP4 receptor agonist decreases the production of mCXCL2 (A) and increases the production of mIL1ra (B) by stimulated macrophages in a dose-dependent manner. Doses are shown in μM. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to vehicle control (EtOH). Abbreviation: EtOH, ethanol. Other abbreviations as in Fig. S1.
Supplemental Figure 16. hMSC and hDF spheroids have similar morphology. Microscopy images of hMSC and hDF adherent and hanging drop cultures at 3 days and after 1 day transfer of spheroids. Scale bar 100 μm. Abbreviations: Adh High, adherent monolayer hMSCs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d.hDF, human adult dermal fibroblast; hMSC, human mesenchymal stem/stromal cell. Other abbreviations as in Fig. S1.
Supplemental Figure 17. Expression of genes involved in PGE2 synthesis are lower in hDF spheroids than in hMSC spheroids. (A–C) Real-time PCR expression data for PTGES (A), PLA2G4A (B), and PLA2G4C (C) in hMSC and hDF monolayers and spheroids. hMSCs plated at a low density and grown for 7 d were used as a baseline. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to hMSC Adh Low. Abbreviations: Adh High, adherent monolayer hMSCs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh Low, adherent monolayer hMSCs plated at low density (100–150 cells/cm2) and cultured for 7 d; Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d; hDF, human adult dermal fibroblast; hMSC, human mesenchymal stem/stromal cell; RQ, relative quantity. Other abbreviations as in Fig. S1.
Supplemental Figure 18. hDF spheroid-conditioned medium does not have an anti-inflammatory effect on stimulated macrophages. (A,B) hDF spheroid-conditioned medium does not reduce the secretion of mCXCL2 (A) or increase the secretion of mIL1ra (B) by LPS-stimulated mouse macrophages. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to vehicle control (CCM). Abbreviations: Adh High, adherent monolayer hMSCs/hDFs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh VH, adherent monolayer hMSCs/hDFs plated at very high density (200,000 cells/cm2) and cultured for 3 d; hDF, human adult dermal fibroblast; hMSC, human mesenchymal stem/stromal cell. Other abbreviations as in Fig. S1.
Supplemental Figure 19. hMSC but not hDF spheroids produce large quantities of PGE2. Spheroids from 5 hMSC donors (D1–5) produced similar high levels of PGE2 whereas spheroids from 4 hDF donors (D6–9) produced only very small amounts of PGE2. Values are mean ± SD (n = 4). Abbreviations: Adh VH, adherent monolayer hMSCs/hDFs plated at very high density (200,000 cells/cm2) and cultured for 3 d; D1, donor 1; hDF, human adult dermal fibroblast; hMSC, human mesenchymal stem/stromal cell. Other abbreviations as in Fig. S1.
Supplemental Figure 20. Caspase inhibition in hMSC spheroids reduces the anti-inflammatory effect of the conditioned medium on stimulated macrophages. (A–C) Broad-spectrum caspase inhibition reduces the production of PGE2 (A) and the anti-inflammatory effect of conditioned medium on LPS-stimulated macrophages measured as mTNFα (B) and mIL10 (C) secretion. Caspase inhibitor doses are in μM. Values are mean ± SD (n = 3). ns P ≥ 0.05, **P < 0.01, ***P < 0.001 compared to vehicle control (DMSO). Abbreviations: DMSO, dimethyl sulfoxide, inh, inhibitor. Other abbreviations as in Fig. S1.
Supplemental Figure 21. NFκB inhibition in hMSC spheroids reduces the anti-inflammatory effect of the conditioned medium on stimulated macrophages. (A–C) Inhibitor of NFκB transcriptional activation in hMSC spheroids reduces the production of PGE2 (A) and the anti-inflammatory effect of conditioned medium on LPS-stimulated macrophages measured as mTNFα (B) and mIL10 (C) secretion. NFκB inhibitor was used at 1 μM. Values are mean ± SD (n = 3). ***P < 0.001 compared to vehicle control (DMSO). Abbreviations: DMSO, dimethyl sulfoxide; inh, inhibitor. Other abbreviations as in Fig. S1.
Acknowledgments
Supported in part by NIH grant P40 RR 17447.
Footnotes
Author contributions: JHY: conception and design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing; TJB: conception and design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing; KC: provision of study material or patients; DJP: financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
References
- 1.Prockop DJ, Kota DJ, Bazhanov N, et al. Evolving paradigms for repair of tissues by adult stem/progenitor cells (MSCs) J Cell Mol Med. 2010;14:2190–2199. doi: 10.1111/j.1582-4934.2010.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart DJ, Mei SH. Cell-based therapies for lung vascular diseases: lessons for the future. Proc Am Thorac Soc. 2011;8:535–540. doi: 10.1513/pats.201105-035MW. [DOI] [PubMed] [Google Scholar]
- 3.Weiss DJ, Bertoncello I, Borok Z, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8:223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranganath SH, Levy O, Inamdar MS, et al. Harnessing the Mesenchymal Stem Cell Secretome for the Treatment of Cardiovascular Disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 6.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 7.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 8.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 12.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012 Jan 16; doi: 10.1136/thoraxjnl-2011-201176. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saleh FA, Genever PG. Turning round: multipotent stromal cells, a three-dimensional revolution? Cytotherapy. 2011;13:903–912. doi: 10.3109/14653249.2011.586998. [DOI] [PubMed] [Google Scholar]
- 16.Arufe MC, De la Fuente A, Fuentes-Boquete I, et al. Differentiation of synovial CD-105(+) human mesenchymal stem cells into chondrocyte-like cells through spheroid formation. J Cell Biochem. 2009;108:145–155. doi: 10.1002/jcb.22238. [DOI] [PubMed] [Google Scholar]
- 17.Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735–749. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- 18.Potapova IA, Brink PR, Cohen IS, et al. Culturing of human mesenchymal stem cells as three-dimensional aggregates induces functional expression of CXCR4 that regulates adhesion to endothelial cells. J Biol Chem. 2008;283:13100–13107. doi: 10.1074/jbc.M800184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potapova IA, Doronin SV, Kelly DJ, et al. Enhanced recovery of mechanical function in the canine heart by seeding an extracellular matrix patch with mesenchymal stem cells committed to a cardiac lineage. Am J Physiol Heart Circ Physiol. 2008;295:H2257–2263. doi: 10.1152/ajpheart.00219.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potapova IA, Gaudette GR, Brink PR, et al. Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells. 2007;25:1761–1768. doi: 10.1634/stemcells.2007-0022. [DOI] [PubMed] [Google Scholar]
- 21.Qihao Z, Xigu C, Guanghui C, et al. Spheroid formation and differentiation into hepatocyte-like cells of rat mesenchymal stem cell induced by co-culture with liver cells. DNA Cell Biol. 2007;26:497–503. doi: 10.1089/dna.2006.0562. [DOI] [PubMed] [Google Scholar]
- 22.Xie QP, Huang H, Xu B, et al. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differentiation. 2009;77:483–491. doi: 10.1016/j.diff.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang CC, Chen CH, Hwang SM, et al. Spherically symmetric mesenchymal stromal cell bodies inherent with endogenous extracellular matrices for cellular cardiomyoplasty. Stem Cells. 2009;27:724–732. doi: 10.1634/stemcells.2008-0944. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Itaka K, Ohba S, et al. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials. 2009;30:2705–2715. doi: 10.1016/j.biomaterials.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 25.Bartosh TJ, Ylostalo JH, Mohammadipoor A, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jing Y, Jian-Xiong Y. 3-D spheroid culture of bone marrow mesenchymal stem cell of rhesus monkey with improved multi-differentiation potential to epithelial progenitors and neuron in vitro. Clin Experiment Ophthalmol. 2011;39:808–819. doi: 10.1111/j.1442-9071.2011.02560.x. [DOI] [PubMed] [Google Scholar]
- 27.Saleh FA, Whyte M, Genever PG. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur Cell Mater. 2011;22:242–257. doi: 10.22203/ecm.v022a19. discussion 257. [DOI] [PubMed] [Google Scholar]
- 28.Choi H, Lee RH, Bazhanov N, et al. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118:330–338. doi: 10.1182/blood-2010-12-327353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 30.Whitney MJ, Lee A, Ylostalo J, et al. Leukemia inhibitory factor secretion is a predictor and indicator of early progenitor status in adult bone marrow stromal cells. Tissue Eng Part A. 2009;15:33–44. doi: 10.1089/ten.tea.2007.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebe C, Raveneau M, Chevriaux A, et al. Induction of transglutaminase 2 by a liver X receptor/retinoic acid receptor alpha pathway increases the clearance of apoptotic cells by human macrophages. Circ Res. 2009;105:393–401. doi: 10.1161/CIRCRESAHA.109.201855. [DOI] [PubMed] [Google Scholar]
- 32.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 33.Brenneis C, Maier TJ, Schmidt R, et al. Inhibition of prostaglandin E2 synthesis by SC-560 is independent of cyclooxygenase 1 inhibition. FASEB J. 2006;20:1352–1360. doi: 10.1096/fj.05-5346com. [DOI] [PubMed] [Google Scholar]
- 34.Smith CJ, Zhang Y, Koboldt CM, et al. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci U S A. 1998;95:13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furuyashiki T, Narumiya S. Stress responses: the contribution of prostaglandin E(2) and its receptors. Nat Rev Endocrinol. 2011;7:163–175. doi: 10.1038/nrendo.2010.194. [DOI] [PubMed] [Google Scholar]
- 36.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 37.Prockop DJ, Oh JY. Medical therapies with adult stem/progenitor cells (MSCs): A backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem. 2011 Dec 29; doi: 10.1002/jcb.24046. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao J, Dennis JE, Muzic RF, et al. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 39.Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Lee RH, Seo MJ, Pulin AA, et al. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosser DM, Zhang X. Activation of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14(Unit 14):12. doi: 10.1002/0471142735.im1402s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouffi C, Bony C, Courties G, et al. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasanna SJ, Gopalakrishnan D, Shankar SR, et al. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 2010;5:e9016. doi: 10.1371/journal.pone.0009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spaggiari GM, Abdelrazik H, Becchetti F, et al. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576–6583. doi: 10.1182/blood-2009-02-203943. [DOI] [PubMed] [Google Scholar]
- 47.Foraker JE, Oh JY, Ylostalo JH, et al. Cross-talk between human mesenchymal stem/progenitor cells (MSCs) and rat hippocampal slices in LPS-stimulated cocultures: the MSCs are activated to secrete prostaglandin E2. J Neurochem. 2011;119:1052–1063. doi: 10.1111/j.1471-4159.2011.07511.x. [DOI] [PubMed] [Google Scholar]
- 48.Baraniak PR, McDevitt TC. Scaffold-free culture of mesenchymal stem cell spheroids in suspension preserves multilineage potential. Cell Tissue Res. 2012;347:701–711. doi: 10.1007/s00441-011-1215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roddy GW, Oh JY, Lee RH, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. hMSC spheroid-conditioned medium reduces the secretion of TNFα by stimulated macrophages. (A) Images of non-stimulated and LPS-stimulated J774 mouse macrophages after 18 h incubation with hMSC spheroid-conditioned medium. (B) hMSC spheroid-conditioned medium increases the growth of LPS-stimulated J774 mouse macrophages. (C) hMSC spheroid-conditioned medium decreases the secretion of mTNFα by zymosan-stimulated mouse macrophages (J774). (D) hMSC spheroid-conditioned medium decreases the secretion of mTNFα by LPS-stimulated mouse macrophages (Raw). (E) hMSC spheroid-conditioned medium decreases the secretion of hTNFα by LPS-stimulated human macrophages (U937). Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to control sMΦ in B and vehicle control (CCM) in C, D, and E. Scale bar: 50 μm. Abbreviations: CCM, complete culture medium; CM, conditioned medium; LPS, lipopolysaccharide; MΦ, macrophage; sMΦ, stimulated macrophage; Sph, spheroid hMSC from 3 d hanging drop cultures (25,000 cells/drop).
Supplemental Figure 2. hMSC spheroid-conditioned medium down-regulates the expression of pro-inflammatory genes and up-regulates the expression of anti-inflammatory genes in stimulated macrophages. (A–C) hMSC spheroid-conditioned medium down-regulates the expression of Tnf (A), Cxcl2 (B), and Csf2 (C) in LPS-stimulated macrophages. (D–F) hMSC conditioned medium up-regulates the expression of Il10 (D), Il1rn (E), and Tgm2 (F) in LPS-stimulated macrophages. Real-time PCR measurements shown as mean (assay triplicates) relative quantities (RQ) compared to time 0 value of each gene. Abbreviations as in Fig. S1.
Supplemental Figure 3. hMSC spheroid-conditioned medium has an anti-inflammatory effect on stimulated macrophages. (A, B, C, D, E) Comparison of the spheroid and monolayer hMSC conditioned medium effect in production of mCXCL2 (A), mIL6 (B), mIL12p40 (C), mIL23 (D), and mIL1ra (E) by LPS-stimulated macrophages. (F) hMSC spheroid-conditioned medium increases the secretion of mIL10 by LPS-stimulated macrophages in a dose dependent manner. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001 compared to sMΦ with vehicle control (CCM) in A, B, C, D, and E and compared to corresponding vehicle control (CCM) in F. Abbreviations: hMSC, human mesenchymal stem/stromal cell; Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d. Other abbreviations as in Fig. S1.
Supplemental Figure 4. hMSC spheroid-conditioned medium increases the number of CD206 positive macrophages. hMSC spheroid-conditioned medium increases the number of CD206 positive LPS-stimulated macrophages measured with flow cytometry. Histogram overlays of representative samples (A) and combined data (B) are shown. Values are mean ± SD (n = 4). ***P < 0.001 compared to vehicle control (CCM) in B. Abbreviations as in Fig. S1.
Supplemental Figure 5. Conditioned medium from adherent hMSC does not have an anti-inflammatory effect and the anti-inflammatory effect of hMSC spheroid-conditioned medium is not mediated by nitric oxide. (A,B) Concentrated conditioned medium from adherent hMSC does not lower the mTNFα (A) or increase the mIL10 (B) secretion by LPS-stimulated macrophages. (C) Inhibition of nitric oxide synthesis in hMSC spheroids does not prevent the effect of spheroid-conditioned medium on production of mTNFα by LPS-stimulated macrophages. Nitric oxide synthesis inhibitor concentrations are shown in μM. Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to corresponding vehicle control (CCM, or CCM conc) in A and compared to corresponding vehicle control (Wtr) in B. Abbreviations: Adh High, adherent monolayer hMSCs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d; conc, 28x concentrated conditioned medium; inh, inhibitor; Wtr, water. Other abbreviations as in Fig. S1.
Supplemental Figure 6. hMSC spheroids and spheroid-derived cells produce large amounts of PGE2. The production of PGE2 by hMSC monolayers, spheroids, or spheroid-derived cells was determined at time points indicated. Values are mean ± SD (n = 3). ns P ≥ 0.05, **P < 0.01, ***P < 0.001 compared to compared to control (Adh VH) in each time point. Abbreviations: Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d; hMSC, human mesenchymal stem/stromal cell; Sph DC, spheroid-derived cell. Other abbreviations as in Fig. S1.
Supplemental Figure 7. Anti-inflammatory effect of hMSC spheroid-conditioned medium on stimulated macrophages is dependent on hMSC COX activity. (A–C) COX inhibition in hMSC spheroids reduces the production of PGE2 (A) and the anti-inflammatory effect of conditioned medium on LPS-stimulated macrophages measured as mTNFα (B) and mIL10 (C) secretion. Indomethacin doses are in μM and dose of 1 μM was used in C. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, **P < 0.01, ***P < 0.001 compared to vehicle control (MetOH) in A, B, and C. Abbreviations: Indo, Indomethacin; MetOH, methanol. Other abbreviations as in Fig. S1.
Supplemental Figure 8. COX2 knockdown in spheroid hMSCs reduces the anti-inflammatory effect on stimulated macrophages. (A) Real-time PCR to asses the knockdown of COX2 gene, PTGS2, in hMSC spheroids with siRNA. hMSCs plated at a low density and grown for 7 d were used as a baseline. Biological triplicates were pooled and technical triplicates with 95% upper confidence intervals are shown. (B,C) COX2 knockdown in hMSC spheroids reduces the anti-inflammatory effect of conditioned medium on LPS-stimulated macrophages measured as mCXCL2 (B) and mIL1ra (C) secretion. Values are mean ± SD (n = 3). ***P < 0.001 compared to control (Scr) in B and C. Abbreviations: All, combination of COX2 siRNA #1, 2, and 3; Scr, negative control siRNA; Tran, Transfection control. Other abbreviations as in Fig. S1.
Supplemental Figure 9. PGE2 can elicit an anti-inflammatory effect on stimulated macrophages. PGE2 reduces the secretion of mCXCL2 (A) and increases the secretion of mIL1ra (B) by LPS-stimulated macrophages in a dose-dependent manner. PGE2 doses are in ng/ml. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to vehicle control (EtOH). Abbreviation: EtOH, ethanol. Other abbreviations as in Fig. S1.
Supplemental Figure 10. PGE2 antibody reduces the anti-inflammatory effect of hMSC spheroid-conditioned medium on stimulated macrophages. (A) PGE2 neutralization in hMSC spheroid-conditioned medium reduces the anti-inflammatory effect of the conditioned medium on LPS-stimulated macrophages measured as mIL1ra secretion. PGE2 antibody doses are shown in μg/ml. (B) PGE2 neutralizing antibody inhibits the anti-inflammatory effect of added synthetic PGE2 on LPS-stimulated macrophages. PGE2 was used at 10 ng/ml and PGE2 antibody at 10 μg/ml. Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to vehicle control (PBS) in A and compared to vehicle control (EtOH) and PGE2 with PGE2 Ab in B. Abbreviations: Ab, antibody; EtOH, ethanol; PBS, phosphate buffered saline. Other abbreviations as in Fig. S1.
Supplemental Figure 11. PGE2 addition restores the anti-inflammatory effect of conditioned medium from Indomethacin treated spheroids. Indomethacin doses are in μM and PGE2 doses in ng/ml. Indomethacin was used at 1 μM with PGE2. Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to corresponding vehicle control (MetOH or EtOH). Abbreviations: EtOH, ethanol; Indo, Indomethacin; MetOH, methanol. Other abbreviations as in Fig. S1.
Supplemental Figure 12. PGD2 synthase is up-regulated in hMSC spheroids. Relative gene expression levels for PGD2 synthase from microarray data for 2 hMSC donors. Abbreviations: Adh High, adherent monolayer hMSCs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh Low, adherent monolayer hMSCs plated at low density (100–150 cells/cm2) and cultured for 7 d; D1, donor 1; hMSC, human mesenchymal stem/stromal cell. Other abbreviations as in Fig. S1.
Supplemental Figure 13. hMSC spheroid-conditioned medium has a COX2-dependent anti-inflammatory effect on both LPS and zymosan stimulated macrophages. (A) hMSC spheroid-conditioned medium reduces the production of mTNFα by zymosan-stimulated mouse macrophages (J774) in a COX2-dependent manner. (B) hMSC spheroid-conditioned medium reduces the production of mTNFα by LPS-stimulated mouse macrophages (Raw) in a COX2-dependent manner. Indomethacin, COX2 inhibitor, and COX1 inhibitor were used at 1 μM. PGE2 was used at 10 ng/ml. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to corresponding vehicle control (MetOH, Indo EtOH, or DMSO). Abbreviations: DMSO, dimethyl sulfoxide; EtOH, ethanol; Indo, Indomethacin; inh, inhibitor; MetOH, methanol. Other abbreviations as in Fig. S1.
Supplemental Figure 14. Anti-inflammatory effect of hMSC spheroid-conditioned medium on stimulated macrophages is mediated through EP4 receptor. (A) EP4 receptor antagonists inhibit the anti-inflammatory effect of hMSC spheroid-conditioned medium on stimulated macrophages measured as mIL1ra secretion. All EP receptor antagonists were used at 10 μM. (B,C) EP4 receptor antagonists inhibit the anti-inflammatory effect of hMSC spheroid-conditioned medium in a dose dependent manner measured as mTNFα (B) and mIL10 (C) secretion. Doses for the two different EP4 receptor antagonists are shown as μM. (D) EP4 receptor antagonist inhibits the anti-inflammatory effect of PGE2 on stimulated macrophages measured as mTNFα secretion. EP4 antagonist was used at 10 μM. Values are mean ± SD (n = 3). ns P ≥ 0.05, ***P < 0.001 compared to vehicle control (DMSO) in A, B, and C and compared to corresponding vehicle control (DMSO, or PGE2 DMSO) in D. Abbreviation: DMSO, dimethyl sulfoxide. Other abbreviations as in Fig. S1.
Supplemental Figure 15. EP4 receptor agonist has an anti-inflammatory effect on stimulated macrophages. (A,B) EP4 receptor agonist decreases the production of mCXCL2 (A) and increases the production of mIL1ra (B) by stimulated macrophages in a dose-dependent manner. Doses are shown in μM. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to vehicle control (EtOH). Abbreviation: EtOH, ethanol. Other abbreviations as in Fig. S1.
Supplemental Figure 16. hMSC and hDF spheroids have similar morphology. Microscopy images of hMSC and hDF adherent and hanging drop cultures at 3 days and after 1 day transfer of spheroids. Scale bar 100 μm. Abbreviations: Adh High, adherent monolayer hMSCs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d.hDF, human adult dermal fibroblast; hMSC, human mesenchymal stem/stromal cell. Other abbreviations as in Fig. S1.
Supplemental Figure 17. Expression of genes involved in PGE2 synthesis are lower in hDF spheroids than in hMSC spheroids. (A–C) Real-time PCR expression data for PTGES (A), PLA2G4A (B), and PLA2G4C (C) in hMSC and hDF monolayers and spheroids. hMSCs plated at a low density and grown for 7 d were used as a baseline. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to hMSC Adh Low. Abbreviations: Adh High, adherent monolayer hMSCs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh Low, adherent monolayer hMSCs plated at low density (100–150 cells/cm2) and cultured for 7 d; Adh VH, adherent monolayer hMSCs plated at very high density (200,000 cells/cm2) and cultured for 3 d; hDF, human adult dermal fibroblast; hMSC, human mesenchymal stem/stromal cell; RQ, relative quantity. Other abbreviations as in Fig. S1.
Supplemental Figure 18. hDF spheroid-conditioned medium does not have an anti-inflammatory effect on stimulated macrophages. (A,B) hDF spheroid-conditioned medium does not reduce the secretion of mCXCL2 (A) or increase the secretion of mIL1ra (B) by LPS-stimulated mouse macrophages. Values are mean ± SD (n = 3). ns P ≥ 0.05, *P < 0.05, ***P < 0.001 compared to vehicle control (CCM). Abbreviations: Adh High, adherent monolayer hMSCs/hDFs plated at high density (5,000 cells/cm2) and cultured for 3 d; Adh VH, adherent monolayer hMSCs/hDFs plated at very high density (200,000 cells/cm2) and cultured for 3 d; hDF, human adult dermal fibroblast; hMSC, human mesenchymal stem/stromal cell. Other abbreviations as in Fig. S1.
Supplemental Figure 19. hMSC but not hDF spheroids produce large quantities of PGE2. Spheroids from 5 hMSC donors (D1–5) produced similar high levels of PGE2 whereas spheroids from 4 hDF donors (D6–9) produced only very small amounts of PGE2. Values are mean ± SD (n = 4). Abbreviations: Adh VH, adherent monolayer hMSCs/hDFs plated at very high density (200,000 cells/cm2) and cultured for 3 d; D1, donor 1; hDF, human adult dermal fibroblast; hMSC, human mesenchymal stem/stromal cell. Other abbreviations as in Fig. S1.
Supplemental Figure 20. Caspase inhibition in hMSC spheroids reduces the anti-inflammatory effect of the conditioned medium on stimulated macrophages. (A–C) Broad-spectrum caspase inhibition reduces the production of PGE2 (A) and the anti-inflammatory effect of conditioned medium on LPS-stimulated macrophages measured as mTNFα (B) and mIL10 (C) secretion. Caspase inhibitor doses are in μM. Values are mean ± SD (n = 3). ns P ≥ 0.05, **P < 0.01, ***P < 0.001 compared to vehicle control (DMSO). Abbreviations: DMSO, dimethyl sulfoxide, inh, inhibitor. Other abbreviations as in Fig. S1.
Supplemental Figure 21. NFκB inhibition in hMSC spheroids reduces the anti-inflammatory effect of the conditioned medium on stimulated macrophages. (A–C) Inhibitor of NFκB transcriptional activation in hMSC spheroids reduces the production of PGE2 (A) and the anti-inflammatory effect of conditioned medium on LPS-stimulated macrophages measured as mTNFα (B) and mIL10 (C) secretion. NFκB inhibitor was used at 1 μM. Values are mean ± SD (n = 3). ***P < 0.001 compared to vehicle control (DMSO). Abbreviations: DMSO, dimethyl sulfoxide; inh, inhibitor. Other abbreviations as in Fig. S1.