Abstract
Objectives
We assess microvascular integrity as a marker of myocardial viability after coronary stenting, using only a pressure guidewire.
Background
Microvascular integrity generally is not assessed using pressure-only guidewires because the transducer lies upstream of microvasculature. We partially inflate a balloon inside a coronary stent to achieve a specific normalized pressure drop at rest (distal coronary/aortic pressure=0.8) and then infuse a vasodilator, to render the wire sensitive to microvascular function. We hypothesize that the further decline in presssure (ΔFFR0.8) predicts MRI myocardial viability.
Methods
We studied 29 subjects with acute coronary syndrome including myocardial infarction. After successful culprit stenting, the resting coronary/aortic pressure was set to 0.8 using temporary balloon obstruction. ΔFFR0.8 was defined as 0.8-(distal coronary/aortic pressures) during adenosine-induced hyperemia. The average transmural extent of infarction was defined as the average area of MRI late gadolinium enhancement (after 2.8±1.5 days) divided by the corresponding full thickness of the gadolinium enhanced sector in short axis slices, and was compared with ΔFFR0.8.
Results
ΔFFR0.8 corresponded inversely and linearly with the average transmural extent of infarction (r2=0.65, p<0.001). We found that a transmural extent of infarction of 0.50 corresponded to a ΔFFR0.8 threshold of 0.1, and had high sensitivity and specificity (100% and 94.4%, respectively).
Conclusions
Using only an upstream pressure-sensitive guidewire and a partially obstructing balloon during pharmacologic hyperemia, we were able to predict MRI myocardial viability with high accuracy after relief of epicardial stenosis. With further validation, this may prove a useful clinical prognostic tool after percutaneous intervention.
Keywords: Coronary artery physiology, Microvascular resistance, Magnetic resonance imaging, Interventional cardiology, Intravascular diagnostics
Introduction
Pressure-wire based fractional flow reserve better predicts the physiological significance of epicardial coronary artery obstruction than does angiographic severity alone (1,2). However, fractional flow reserve alone does not necessarily predict preservation or recovery of contractile reserve in states of distal myocardial disease, whether infarct, myopathy, or hypertrophy (1). A test of distal microvascular integrity using only a pressure transducer would be a valuable tool.
Conventional pressure-wire measurements do not reflect microvascular function because the transducer is upstream of the subtended myocardium. Previous guidewire-based measurements of microvascular function therefor require an additional transducer element (often integrated into the same guidewire detector) to measure flow. We propose a simple invasive pressure-only test of microvascular function by imposing a calibrated resistance inside a coronary stent (a partially inflated balloon to achieve a specified pressure drop), followed by pressure measurement during vasodilator stress. In this test, resting Pd/PAo is set to 0.8 by the partially obstructing balloon, and downstream pressure drop during hyperemia (designated ΔFFR0.8) reflects microvascular integrity. We hypothesized that ΔFFR0.8 inversely corresponds to extent of infarction in the territory subtended by the lesion. We validate this simple test of risk area microvascular function against magnetic resonance late gadolinium enhancement in subjects undergoing stent treatment of acute coronary syndromes.
Materials and Methods
Human subjects
This trial was conducted according to the principles of the Declaration of Helsinki regarding investigation in humans, and was approved by the Institutional Review Board of Pusan National University Yangsan Hospital.
Subjects were recruited prospectively during the period Feb 2009 through March 2010. Candidates were eligible for inclusion if they were undergoing percutaneous coronary intervention for acute coronary syndromes, non-ST or ST-elevation myocardial infarction, if the culprit lesion was found in the proximal or middle segments of a major epicardial coronary artery with a reference vessel diameter between 2.75mm and 4.0mm, and if the culprit lesion was successfully treated with a coronary stent. Subjects were excluded if they had significant obstructive coronary artery lesion (>50%) in the target vessel distal to the culprit site, if they had a previous infarction other than in the culprit vessel, chronic kidney disease requiring renal replacement therapy, left ventricular hypertrophy with wall thickness > 12mm or hypertrophic cardiomyopathy on subsequent MRI or echocardiography, cardiogenic shock or requirement for catecholamine infusion, collateral flow to the target vessel more than angiographic grade 1, or excessive baseline variability (when the variation of FFR while obtaining data is greater than ± 0.01) of baseline distal coronary artery pressure during investigational balloon obstruction. All subjects consented in writing before participation.
Theory
The objective of this experiment is to assess the microvascular reactivity in the risk area subtended by a specific coronary lesion using a pressure-transducer coronary guidewire and pharmacologic vasodilation, and without Doppler ultrasound or thermodilution flow measurement. For the purpose of this experiment, microvascular integrity is considered directly related to myocardial viability.
A simplified coronary hydrodynamic model assumes two serial resistances in an atherosclerotic coronary artery, one in the obstructed epicardial coronary segment and a second in the distal microvasculature. Non-viable or dysfunctional myocardium has impaired distal microvascular flow reserve (1,3–6). Successful stent therapy of an isolated culprit conductance coronary artery stenosis essentially normalizes pressure-based fractional flow reserve (the ratio of distal coronary to aortic pressures during hyperemia), fixes the local cross sectional area despite pulsatile flow, and abates the pressure contribution of collateral arteries.
The epicardial coronary artery distal to the stent is still upstream the microvascular circulation and contributes minimally to energy (pressure) loss during vasodilation. Partially inflating an angioplasty balloon inside the stent, calibrated to an arbitrary resting pressure, imposes a resistance proximal to the pressure-transducer. This resistance creates additional pressure loss only if pharmacologic vasodilatation augments blood flow, ie in a preserved myocardial territory. The pressure-transducer is thereby rendered sensitive to microvascular flow changes.
Equation 1 defines fractional flow reserve as the arteriovenous pressure drop beyond the stenotic coronary artery (Pd) divided by the arteriovenous pressure drop from the aorta (PAo), during a state of hyperemia. For convenience low myocardial venous (Pv) pressures are ignored.
| Equation (1) |
| Equation (2) |
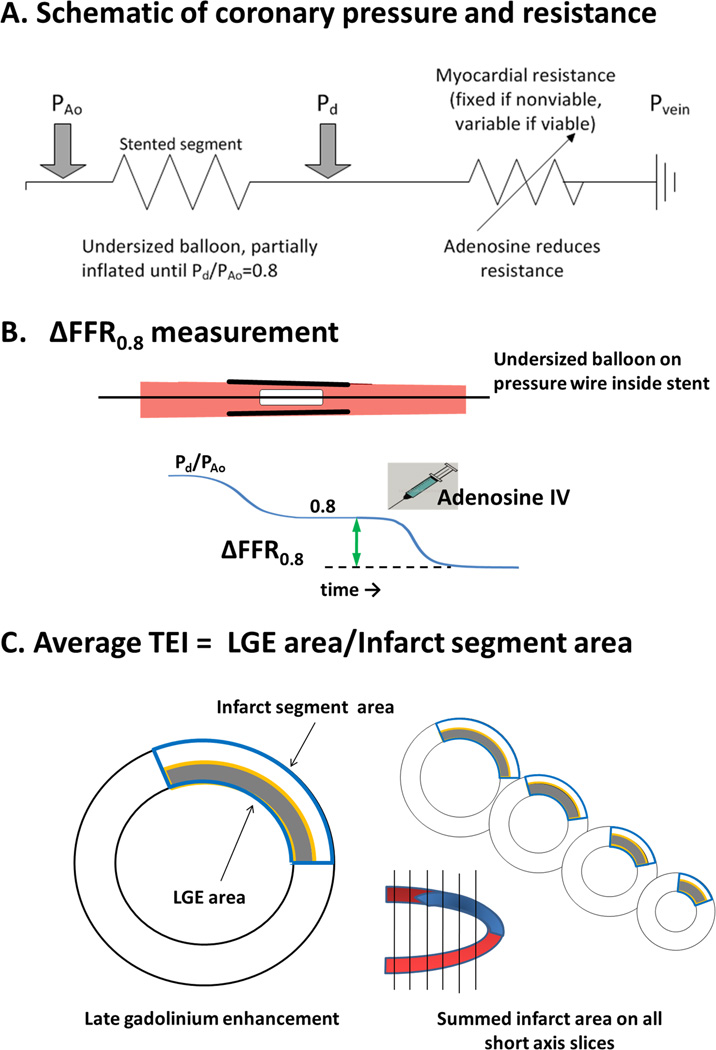
We define our measure of microvascular function (ΔFFR0.8) as the change in Pd/PAo between rest and pharmacological hyperemia when a partially obstructing balloon is inflated at rest to 80% of the inflow pressure (Figure 1A and B, and Equation 2). Pd(B) and Pd(H) indicate distal coronary artery pressure at baseline and during hyperemia, respectively. The first component Pd(B)/PAo(B) is set to 0.8. This threshold was selected for patient tolerability and for convenience. This experiment assumes that venous pressures are negligible, that balloon-in-stent and other coronary segments are noncompressible, and that the non-target coronary artery is normal.
Figure 1.
- The circuit diagram schematizes the hemodynamic measurement. Upstream resistance is created by a partially inflated balloon in the epicardial coronary artery. The pressure transducer guidewire is distal to this artificial resistance, and measures the pressure drop created between the aorta and the coronary artery. The microvascular bed serves as a downstream variable resistance sensitive to adenosine.
- How ΔFFR0.8 is measured. A balloon dilatation catheter is positioned into a schematic stented coronary artery segment and inflated until the resting Pd/PAo value is 0.8. Thereafter intravenous adenosine is administered to induce maximal coronary hyperemia and ΔFFR0.8 is measured as the further drop in Pd/PAo from 0.8.
- How the average transmural extent of infarction (TEI) is measured. The area of late gadolinium enhancement is measured in a stack of six late gadolinium enhancement MRI images. The risk area is measured as the corresponding full thickness area of myocardium. The average transmural extent of infarction is the ratio of the two.
Measurement of ΔFFR0.8
After the culprit clinical lesion was successfully treated with a coronary stent, a 0.014” coronary guidewire incorporating a pressure transducer at the spring coil junction (PressureWire, Radi, St Jude Medical) was positioned 2–3cm distal to the implanted stent. An undersized balloon shorter (8–12mm length) and narrower (0.5mm less than the nominal stent delivery balloon diameter) was positioned inside the stent lumen. The undersized balloon was then inflated to achieve a mean distal coronary pressure 80% of the mean aortic pressure at rest (Figure 1 A and B). Subjects (n=5) were excluded if Pd(B)/PAo(B) fluctuated more than ±0.01 at baseline. Coronary hyperemia was then induced using intravenous adenosine 140 mcg/kg/min infusion and ΔFFR0.8 calculated after two minutes, according to Equation 2.
Cardiac magnetic resonance imaging
All patients underwent cardiac magnetic resonance imaging after PCI with invasive hemodynamic assessment. Imaging was performed at 1.5T (Avanto, Siemens, Erlangen, Germany) with electrocardiographic gating and a standard torso phased array cardiac receiver coil. A balanced steady-state free precession pulse sequence during repeated breath-holds of approximately 10 seconds was used for cine images with multiple short axis views every 10 mm covering the entire left ventricle. Typical scan parameters were: voxels 1.8 × 1.8 × 6.0 mm3; repetition time/ echo time, 2.8/1.4ms; flip angle 79°; matrix 192 × 156. Late gadolinium enhancement images were obtained approximately 15 minutes after (0.02 mmol/kg) intravenous administration of gadopentate-dimeglumine (Magnevist, Bayer-Schering, Berlin, Germany). The inversion time was set to null the signal of normal myocardium and ranged from 240 to 300 ms. Typical scan parameters were: repetition time/echo time, 8.1/3.1ms; flip angle 25°; triggering to every other heart beat; achieving voxels 1.8 × 1.3 × 8.0 mm2. T2-based assessment of myocardial edema or risk area was not performed. Images were analyzed on a dedicated workstation (Argus VF, Siemens) by consensus of two independent observers, each with 7 years experience in cardiac MRI. Late gadolinium enhancement was defined as signal activity greater than two standard deviations from remote normal myocardium (7), summed from all slices. The risk segment area was defined as the transmural area extent bounded by the lateral margins of the late enhancement area (Figure 1C). Average transmural extent of infarction (TEI) is defined as the ratio of late gadolinium enhancement area to risk segment area, each summed from all slices(8).
Statistics
Continuous parameters were compared using a Student t-test (paired when appropriate) and their correlation measured using a Pearson product-moment correlation coefficient r. Categorical and ordinal parameters were compared using a Pearson chi square test. They are reported as mean ± standard deviation. The association between ΔFFR0.8 and other parameters was determined using univariate and multivariate logistic regression models (Generalized Linear Model, SPSS version 16, IBM). Receiver-operator analysis of the ΔFFR0.8 relationship with transmural extent of infarction was performed using MedCalc for Windows, version 10.0.0 (MedCalc Software, Mariakerke, Belgium).
Results
Human subjects
A total of 333 candidates were screened during the study period. Of 72 eligible candidates, 34 consented to participate. Of these, five were excluded because distal coronary pressure was unstable during partial balloon obstruction (0.8 ±0.03). Ultimately, 29 subjects with acute coronary syndrome or myocardial infarction were enrolled. Of these, more than half of patients (n= 18, 62%) were undergoing primary PCI for ST segment elevation myocardial infarction (STEMI). The left anterior descending artery was the culprit vessel in half (Table 1).
Table 1.
Baseline clinical and angiographic characteristics
| Clinical Characteristics (n=29) | |
|---|---|
| Age | 61±12 |
| Sex, (M/F) | 24/5 |
| Coronary risk factors, n (%) | |
| Diabetes | 6 (20) |
| Hypertension | 9 (31) |
| Smoking | 14 (48) |
| Hypercholesterolemia | 5 (17) |
| STEMI/NSTEMI/UAP, n (%) | 18 (62) / 8 (28) / 3 (10) |
| LV ejection fraction (%) | 55±9 |
| Time interval between PCI and MRI (days) | 2.8±1.5 |
| Angiographic Data (n=29) | |
| Culprit Vessel, n (%) | |
| LAD | 15 (51) |
| LCx | 5 (17) |
| RCA | 9 (32) |
| Location of lesion, n(%) | |
| Proximal | 15 (51) |
| Mid | 14 (49) |
| Infarct related artery diameter stenosis (%) | 95±6 |
| Reference vessel size (mm) | 3.27±0.30 |
| Stent length (mm) | 25.6±5.8 |
| Thrombolysis in Myocardial Infarction flow after PCI, n (%) | |
| Grade 2 | 1 (3) |
| Grade 3 | 28 (97) |
| Thrombolysis in Myocardial Infarction angiographic myocardial blush grade after PCI, n (%) | |
| Grade 0 or 1 | 4 (14) |
| Grade 2 | 3 (10) |
| Grade 3 | 22 (76) |
Predictors of ΔFFR0.8
Figure 2 shows a roughly linear inverse relationship between transmural extent of myocardial infarction, assessed by MRI late gadolinium enhancement, and ΔFFR0.8 (r2=0.65, p<0.001). This remained largely unchanged after excluding the four subjects without evident late gadolinium enhancement on MRI (n=25, r2=0.60, p<0.001).
Figure 2.
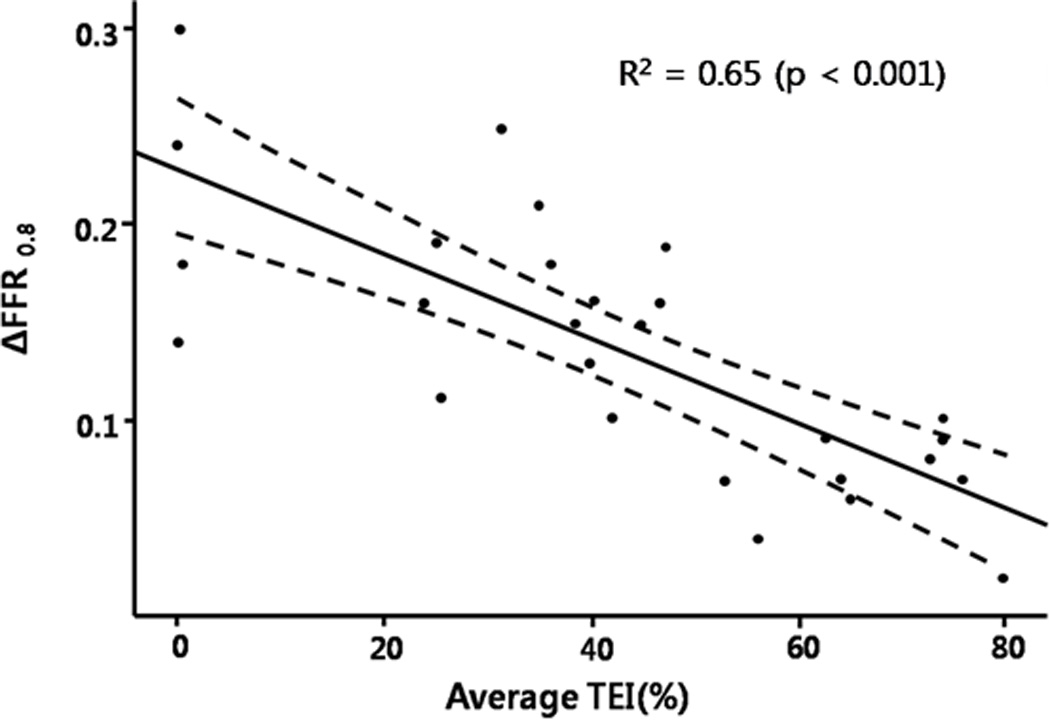
The relationship between ΔFFR0.8 and average transmural extent of infarction (TEI).
A receiver-operator analysis was performed to determine a threshold ΔFFR0.8 value corresponding to an average transmural extent of infarction of 50%. A threshold ΔFFR0.8 value of 0.1 predicted a 50% transmural extent of myocardial infarction (Figure 3), with sensitivity 100%, specificity 94.4%, area under curve 0.997, positive predictive value 91.7%, and negative predictive value 100%. Excluding the four subjects without late gadolinium enhancement on MRI, the receiver-operator relationship was largely unchanged (sensitivity 100%, specificity 92.9%).
Figure 3.
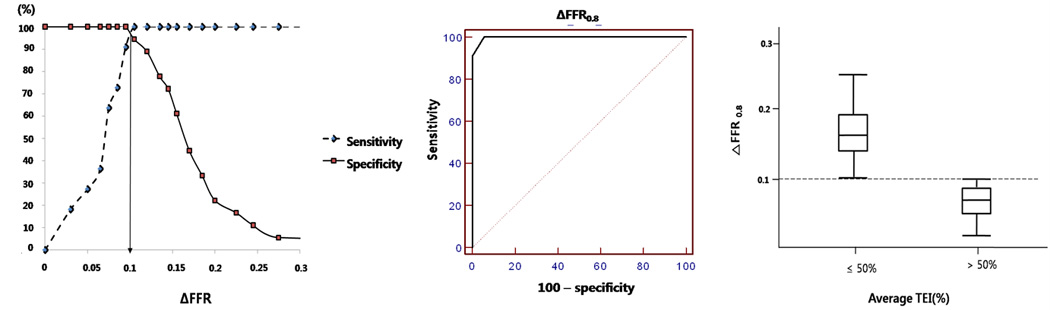
Receiver-operator characteristics of ΔFFR0.8. The threshold ΔFFR0.8 =0.1 corresponds to 50% average transmural extent of infarction (TEI).
Clinical, laboratory, and MRI parameters were considered using this threshold ΔFFR0.8 greater and less than 0.1 (table 2). Six patients had evidence of microvascular obstruction on MRI, five of whom had ΔFFR0.8 < 0.1. By univariate analysis, only overall myocardial ejection fraction, peak serum troponin value, MRI evidence of microvascular obstruction, TIMI angiographic myocardial blush score, and average transmural MRI extent of infarction were different between the high and low ΔFFR0.8 strata. By multivariate analysis (table 3-A), only average transmural extent of infarction predicted ΔFFR0.8.
Table 2.
Clinical and laboratory parameters according to ΔFFR 0.8 threshold of 0.1.
| ΔFFR 0.8 > 0.1 (n=17) |
ΔFFR 0.8≤0.1 (n=12) |
p | |
|---|---|---|---|
| Age | 59±13 | 65±12 | 0.746 |
| Sex, Male | 14 (82.4) | 10 (83.3) | 0.671 |
| Coronary risk factors, n | |||
| Diabetes | 4 (23.5) | 2 (16.7) | 0.513 |
| Hypertension | 9 (52.9) | 2 (16.7) | 0.053 |
| Dyslipidemia | 2 (11.8) | 2 (16.7) | 0.556 |
| Smoking | 8 (47.1) | 6 (50) | 0.587 |
| Clinical diagnosis | 0.437 | ||
| STEMI / NSTEMI / UAP | 9/5/3 | 9/3/0 | |
| LV EF (%) | 59.5±8.4 | 49.8±7.6 | 0.004* |
| Peak Troponin I | 36.2±58.6 | 121.1±106 | 0.024* |
| Time to reperfusion time in STEMI | 4.8±2.3 | 6.6±5.4 | 0.011* |
| Time interval of MRI and PCI (days after PCI ) | 2.7±1.3 | 3.0±1.8 | 0.651 |
| Culprit vessel, n | 0.108 | ||
| LAD / LCX / RCA | 9/1/7 | 6/4/2 | |
| Location of lesion | 0.254 | ||
| Proximal / Mid | 8/9 | 8/4 | |
| Infarct related artery diameter stenosis | 0.94±0.07 | 0.97±0.03 | 0.275 |
| Reference Vessel Size (mm) | 3.32±0.30 | 3.20±0.31 | 0.560 |
| Stent length (mm) | 26.8±5.3 | 23.9±6.3 | 0.532 |
| Thrombolysis in Myocardial Infarction flow grade before PCI, n | 0.812 | ||
| Grade 0/1/2/3 | 8/0/3/6 | 7/0/2/3 | |
| Average transmural extent of infarction (TEI) by MRI (%) | 27.8±17.3 | 66.6±11.7 | <0.001* |
| Microvascular obstruction by MRI (n,%) | 1 (8.3%) | 5 (29.4%) | 0.054 |
| Thrombolysis in Myocardial Infarction angiographic myocardial blush grade after PCI | 0.037* | ||
| Grade 0 or 1 (n, %) | 0 (0) | 4 (33.3) | |
| Grade 2 (n,%) | 2 (11.8) | 1 (8.3) | |
| Grade 3 (n,%) | 15 (88.2) | 7 (58.3) |
Parameters marked with an asterisk have p<0.05 on univariate analysis.
Table 3.
| A. Regression analysis of ΔFFR 0.8 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Average transmural extent of infarction (TEI) |
LV ejection fraction |
Late gadolinium enhancement area |
Infarct segment area |
Troponin I |
MRI Microvascular obstruction |
Time-to perfusion |
LAD vs non LAD |
TIMI myocardial blush score |
||
| r2 | 0.65 | 0.29 | 0.23 | 0.00 | 0.35 | 0.10 | ||||
|
Univariate analysis |
p | <0.001 | 0.002 | 0.010 | 0.985 | 0.001 | 0.016 | 0.208 | 0.701 | 0.016 |
|
Multivariate analysis |
p | 0.005 | 0.380 | 0.099 | 0.344 | 0.964 | 0.922 | |||
| B. Regression analysis of average transmural extent of infarction (TEI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Δ FFR0.8 | LV ejection fraction |
Troponin I |
MRI Microvascular obstruction |
Time-to perfusion |
LAD vs non LAD |
TIMI myocardial blush score |
||
| r2 | 0.65 | 0.44 | 0.46 | 0.17 | ||||
|
Univariate analysis |
p | <0.001 | <0.001 | <0.001 | 0.006 | 0.100 | 0.750 | 0.007 |
|
Multivariate analysis |
p | 0.008 | 0.024 | 0.092 | 0.774 | 0.932 | ||
When stratified according to average transmural extent of infarction as a marker of viability, the following parameters were found significant on univariate analysis (Table 3A): ΔFFR0.8, left ventricular ejection fraction, troponin I, MRI evidence of microvascular obstruction, and TIMI angiographic myocardial blush score. By multivariate analysis (Table 3B), ΔFFR0.8 and LV ejection fraction remained significant predictors of average transmural extent of infarction.
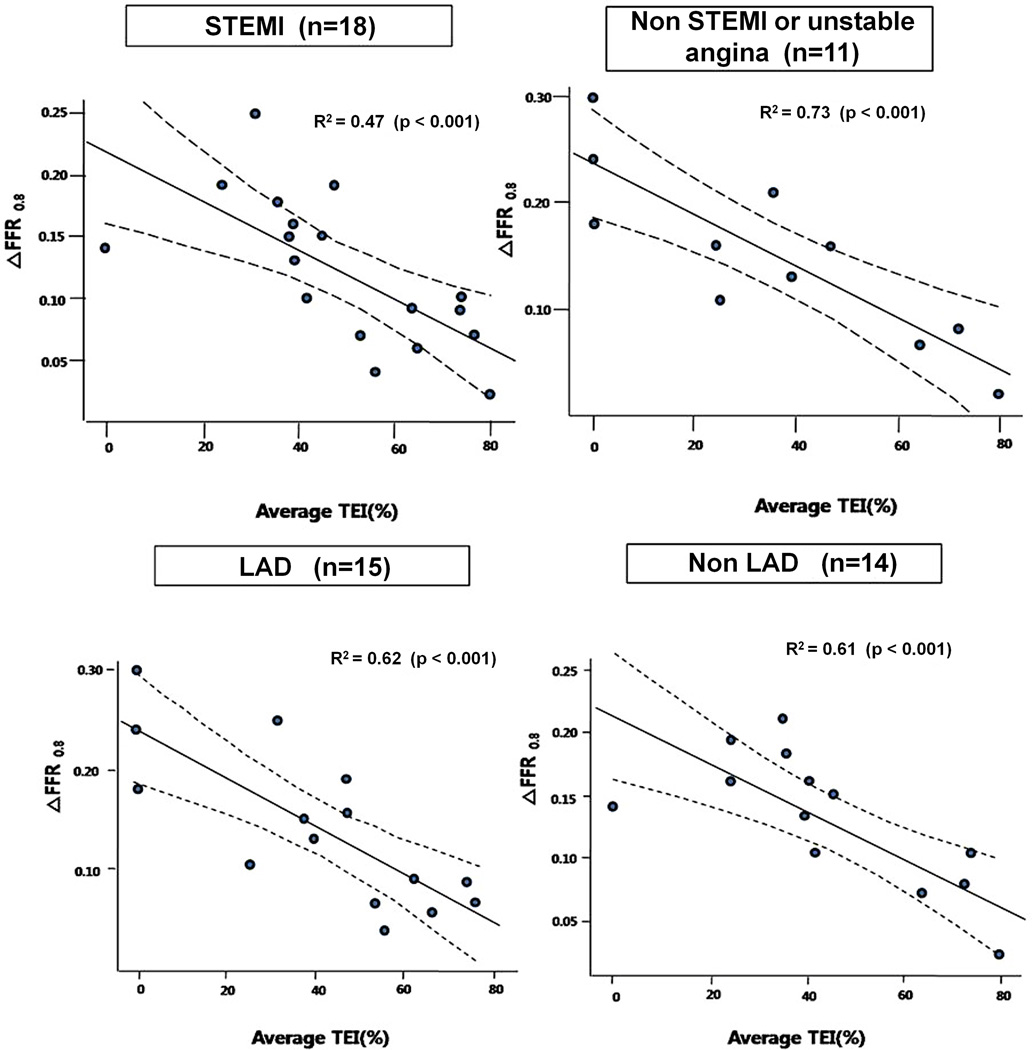
The subgroups ST-elevation versus non-ST-elevation myocardial infarction, or LAD versus non-LAD showed the same consistent relationship between ΔFFR0.8 and average transmural extent of infarction (Figure 4).
Figure 4.
The relationship between ΔFFR0.8 and average transmural extent of infarction (TEI) according to subgroups. In the lower right panel (non-LAD lesions), the rightmost circle represents two overlapping data points.
Discussion
We found that a pressure-wire-only measure of microvascular function, applied immediately after coronary artery stenting, predicts myocardial viability by MRI after acute coronary syndromes or myocardial infarction. We observed a linear relation between our index, ΔFFR0.8, and extent of viable myocardium subtended by the index coronary artery lesion. Traditionally, pressure-wire-only assessment of microvascular integrity is not possible in the absence of epicardial stenosis. Our calibrated in-stent balloon obstruction creates a temporary upstream resistance that allows downstream coronary artery pressure to fall during pharmacologic hyperemia. This renders the pressure-wire sensitive to alterations in microvascular function. Moreover, we identified a convenient threshold value of ΔFFR0.8=0.1 that corresponds to a transmural extent of infarction of 50%, which predicts recovery of myocardial contractile function after revascularization (9). These findings appeared consistent irrespective of coronary artery territory or clinical syndrome.
Invasive guidewire probes combining pressure transducers with Doppler ultrasound or thermistors have been used for combined assessment of resting and provoked pressure gradients as well as of velocities or cold-saline transit times (1). These allow combined invasive assessment of epicardial lesion severity and of microvascular function, applicable to the locally subtended myocardium. The Index of Microvascular Resistance (10–14) is a validated thermodilution measure that is independent of epicardial stenosis severity when corrected for collateral artery pressure (11). Indeed Aarnoudse and colleagues (11) used partially obstructive balloons inflated to different nominal cross-sectional areas inside coronary stents to simulate varying epicardial stenosis severity. The additional Doppler and thermodilution transducer elements are subjectively more cumbersome to use but provide epicardial and microvascular indices attainable both before and after revascularization. Our alternative pressure-based measurement permits microvascular assessment and prognostication only after relief of the epicardial obstruction.
Why would ΔFFR0.8 correspond to the proportion of viable myocardium? We hypothesized that recruitable microvascular flow would relate linearly with the mass of subtended viable muscle (15). Absent epicardial stenosis, changes in our calibrated Pd/PAo during pharmacologic provocation reflect flow augmentation related only to recruited microvascular flow. Indeed we found the unitless measures ΔFFR0.8 to be inversely related to the transmural extent of infarction.
Kocaman et al(16) have reported clinical prognostic utility of an index they describe as “delta FFR,” which is the magnitude in normalized pressure drop between resting and hyperemic conditions. This index is attractive because it is obtained before revascularization, and uses data otherwise routinely obtained during ordinary fractional flow reserve measurement. However, the epicardial component reflects a variable intrinsic baseline resistance (from the native lesion), compared with our calibrated epicardial coronary resistance, and therefore does not reflect purely on downstream microvascular integrity. Further comparison of the Kocaman “delta FFR” and our ΔFFR0.8 would be interesting. We did not obtain “delta FFR” in our study because of the large proportion of patients undergoing primary PCI for ST-elevation myocardial infarction.
Pressure-derived coronary flow reserve measurements are similar attempts to assess combined epicardial and microvascular function in the presence of coronary stenoses. Akasaka and colleagues (17) found pressure-derived coronary flow reserve corresponded to Doppler and flow-meter measurements in animals. MacCarthy and colleagues (18) measured pressure-derived coronary flow reserve after stenting using partially-obstructive balloons similar to our approach. They found their measurement underestimated thermodilution-based coronary flow reserve and was insufficient to estimate coronary flow reserve in purely microvascular dysfunction.
We assessed infarcted area using a patient- and vessel-specific index different from standard American Society of Echocardiography segmentation (19). Instead we measured infarct area by MRI late gadolinium enhancement. At the time of our study we were technically unable to use more advanced T2-weighted measures of myocardial edema (20) to determine risk area. Instead we determined the “average transmural extent of infarction” using surrounding healthy myocardial segments, based on O’Regan and colleagues’ (8) evidence that lateral infarct margins approximate the ischemic bed in both partial thickness and transmural infarcts.
Other limitations of this study include our failure to obtain baseline and hyperemic pressure gradients and matching thermodilution transit time measurements, for comparative “delta FFR” and Index of Microvascular Resistance measurements. Our local ethics board did not favor obtaining these comparative measurements in the subject population undergoing primary angioplasty for acute myocardial infarction, because of concerns about prolonged research procedures. Our incidence of slow-flow or no-reflow was low but MRI microvascular obstruction appreciable, reflecting our community reperfusion intervals; conceivably the relationship between ΔFFR0.8 and transmural extent of infarction would differ after late reperfusion (21–23). Only 29 of 333 candidate subjects are reported, although 45% of eligible subjects were enrolled. Our approach is unsuitable for patients not undergoing PCI. We used an undersized balloon for the purpose of this research procedure, however the clinical post-dilatation or stent delivery balloons also may be employed.
We conclude that ΔFFR0.8 is a simple candidate predictor of downstream myocardial viability immediately after percutaneous stenting of acute coronary syndrome and myocardial infarction. A ΔFFR0.8 value of 0.1 corresponds to a transmural extent of infarction of 50%, which predicts recovery of contractile function after revascularization. The test is attractive in its simplicity but applicable only after revascularization. Further consideration of this test might establish its value in a wider range of conditions, especially after later reperfusion of myocardial infarction, and in comparison with the related “delta FFR” before revascularization and the thermodilution-based “index of microvascular resistance (IMR).”
Figure 5.
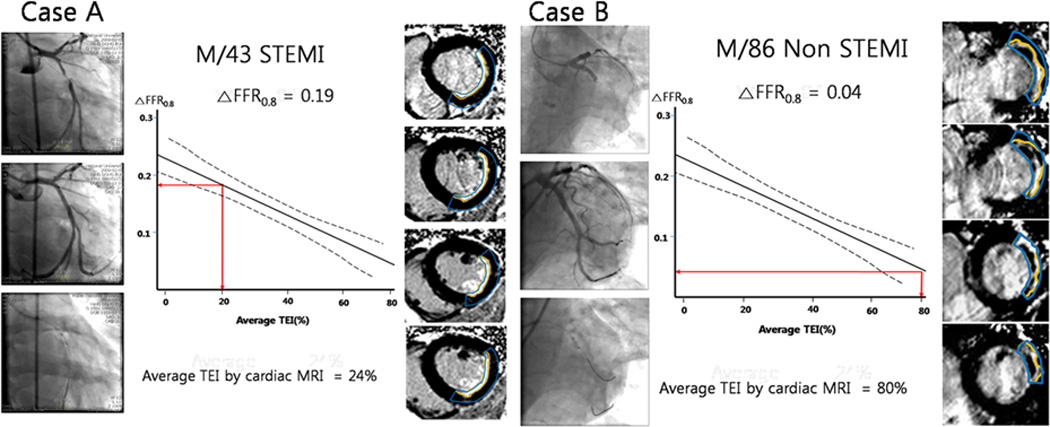
Two case examples showing the relationship between ΔFFR0.8 and average transmural extent of infarction (TEI). In cases A and B, the left panels show representative pre-PCI coronary angiograms. The middle panels show the aggregate relationship of transmural extent of infarction to ΔFFR0.8, with the patient-specific findings indicated in red. The right panels show the corresponding gadolinium contrast-enhanced enhancement MR images, with the late gadolinium enhancement areas outlined in yellow and risk area outlined in blue.
Acknowledgments
Funding:
This work was supported by a grant [2009007] from the Korean Society of Interventional Cardiology. AZF and RJL were supported by the Division of Intramural Research, National Heart Lung and Blood Institute, National Institutes of Health USA [Z01-HL006061-01].
Acronyms and Abbreviations
- FFR
fractional flow reserve
- Δ FFR0.8
0.8 - hyperemic Pd/PAo
- MRI
magnetic resonance imaging
- Pd/PAo
Ratio of distal to aortic pressure
- PCI
Percutaneous coronary intervention
- TEI
Transmural extent of myocardial infarction
Footnotes
Acknowledgements: Disclosures / Relationship with industry
There was no industry involvement in the design, conduct, financial support, or analysis. The authors had full access to the data and assume responsibility for this analysis and report.
References
- 1.Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114(12):1321–1341. doi: 10.1161/CIRCULATIONAHA.106.177276. [DOI] [PubMed] [Google Scholar]
- 2.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 3.De Bruyne B, Pijls N, Bartunek J, Kulecki K, Bech J, De Winter H, Van Crombrugge P, Heyndrickx G, Wijns W. Fractional flow reserve in patients with prior myocardial infarction. Circulation. 2001;104(2):157–162. doi: 10.1161/01.cir.104.2.157. [DOI] [PubMed] [Google Scholar]
- 4.Kern MJ, Samady H. Current concepts of integrated coronary physiology in the catheterization laboratory. J Am Coll Cardiol. 2010;55(3):173–185. doi: 10.1016/j.jacc.2009.06.062. [DOI] [PubMed] [Google Scholar]
- 5.Marques K, Knaapen P, Boellaard R, Lammertsma A, Westerhof N, Visser F. Microvascular function in viable myocardium after chronic infarction does not influence fractional flow reserve measurements. J Nucl Med. 2007;48(12):1987–1992. doi: 10.2967/jnumed.107.044370. [DOI] [PubMed] [Google Scholar]
- 6.Marques K, Knaapen P, Boellaard R, Westerhof N, Lammertsma A, Visser C, Visser F. Hyperaemic microvascular resistance is not increased in viable myocardium after chronic myocardial infarction. Eur Heart J. 2007;28(19):2320–2325. doi: 10.1093/eurheartj/ehm309. [DOI] [PubMed] [Google Scholar]
- 7.Hundley WG, Bluemke D, Bogaert JG, Friedrich MG, Higgins CB, Lawson MA, McConnell MV, Raman SV, van Rossum AC, Flamm S, et al. Society for Cardiovascular Magnetic Resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson. 2009;11:5. doi: 10.1186/1532-429X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Regan D, Ahmed R, Neuwirth C, Tan Y, Durighel G, Hajnal J, Nadra I, Corbett S, Cook S. Cardiac MRI of myocardial salvage at the peri-infarct border zones after primary coronary intervention. Am J Physiol Heart Circ Physiol. 2009;297(1):H340–H346. doi: 10.1152/ajpheart.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, Klocke FJ, Bonow RO, Judd RM. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 10.Fearon W, Aarnoudse W, Pijls N, De Bruyne B, Balsam L, Cooke D, Robbins R, Fitzgerald P, Yeung A, Yock P. Microvascular resistance is not influenced by epicardial coronary artery stenosis severity: experimental validation. Circulation. 2004;109(19):2269–2272. doi: 10.1161/01.CIR.0000128669.99355.CB. [DOI] [PubMed] [Google Scholar]
- 11.Aarnoudse W, Fearon W, Manoharan G, Geven M, van de Vosse F, Rutten M, De Bruyne B, Pijls N. Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation. 2004;110(15):2137–2142. doi: 10.1161/01.CIR.0000143893.18451.0E. [DOI] [PubMed] [Google Scholar]
- 12.Ng M, Yeung A, Fearon W. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation. 2006;113(17):2054–2061. doi: 10.1161/CIRCULATIONAHA.105.603522. [DOI] [PubMed] [Google Scholar]
- 13.Fearon W, Shah M, Ng M, Brinton T, Wilson A, Tremmel J, Schnittger I, Lee D, Vagelos R, Fitzgerald P, et al. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;51(5):560–565. doi: 10.1016/j.jacc.2007.08.062. [DOI] [PubMed] [Google Scholar]
- 14.Lim HS, Yoon MH, Tahk SJ, Yang HM, Choi BJ, Choi SY, Sheen SS, Hwang GS, Kang SJ, Shin JH. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. Eur Heart J. 2009;30(23):2854–2860. doi: 10.1093/eurheartj/ehp313. [DOI] [PubMed] [Google Scholar]
- 15.Gould KL. Coronary artery stenosis and reversing atherosclerosis. ix. London: Oxford University Press; 1999. 689 pp. p. p. [Google Scholar]
- 16.Kocaman S, Sahinarslan A, Arslan U, Timurkaynak T. The delta fractional flow reserve can predict lesion severity and long-term prognosis. Atherosclerosis. 2009;203(1):178–184. doi: 10.1016/j.atherosclerosis.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Akasaka T, Yamamuro A, Kamiyama N, Koyama Y, Akiyama M, Watanabe N, Neishi Y, Takagi T, Shalman E, Barak C, et al. Assessment of coronary flow reserve by coronary pressure measurement: comparison with flow- or velocity-derived coronary flow reserve. J Am Coll Cardiol. 2003;41(9):1554–1560. doi: 10.1016/s0735-1097(03)00258-4. [DOI] [PubMed] [Google Scholar]
- 18.MacCarthy P, Berger A, Manoharan G, Bartunek J, Barbato E, Wijns W, Heyndrickx GR, Pijls NH, De Bruyne B. Pressure-derived measurement of coronary flow reserve. J Am Coll Cardiol. 2005;45(2):216–220. doi: 10.1016/j.jacc.2004.09.063. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Berry C, Kellman P, Mancini C, Chen MY, Bandettini WP, Lowrey T, Hsu LY, Aletras AH, Arai AE. Magnetic resonance imaging delineates the ischemic area at risk and myocardial salvage in patients with acute myocardial infarction. Circ Cardiovasc Imaging. 2010;3(5):527–535. doi: 10.1161/CIRCIMAGING.109.900761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teiger E, Garot J, Aptecar E, Bosio P, Woscoboinik J, Pernes J, Gueret P, Kern M, Dubois-Randé J, Dupouy P. Coronary blood flow reserve and wall motion recovery in patients undergoing angioplasty for myocardial infarction. Eur Heart J. 1999;20(4):285–292. doi: 10.1053/euhj.1998.1195. [DOI] [PubMed] [Google Scholar]
- 22.Tobis J, Azarbal B, Slavin L. Assessment of intermediate severity coronary lesions in the catheterization laboratory. J Am Coll Cardiol. 2007;49(8):839–848. doi: 10.1016/j.jacc.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 23.Tsagalou E, Anastasiou-Nana M, Agapitos E, Gika A, Drakos S, Terrovitis J, Ntalianis A, Nanas J. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2008;52(17):1391–1398. doi: 10.1016/j.jacc.2008.05.064. [DOI] [PubMed] [Google Scholar]