Abstract
Microparticles (MPs) are small membrane-bound vesicles that arise from activated and dying cells and enter the blood to display pro-inflammatory and pro-thrombotic activities. MPs are 0.1–1.0 μm in size and incorporate nuclear, cytoplasmic and membrane molecules as they detach from cells. This process can occur with cell activation as well as cell death, with particles likely corresponding to blebs that form on the cell surface during apoptosis. To measure particle expression, flow cytometry allows determination of particle numbers based on size as well as surface markers that denote the cell of origin; platelet MPs are usually the most abundant type in blood. As shown in in vitro and in vivo systems, MPs can promote inflammation and thrombosis resulting from their content of cytokines like IL-1 and pro-coagulant molecules like tissue factor. Certain particle types can be anti-inflammatory, however, suggesting a range of immunomodulatory activities depending on the cell of origin. Studies on patients with a wide range of rheumatic disease show increased MP numbers in blood, with platelet and endothelial particles associated with vascular manifestations; increased numbers of particles also occur in the joint fluid where they may drive cytokine production and activate synoviocytes. In autoimmune diseases such as SLE and RA, MPs may also contribute to disease pathogenesis by the formation of immune complexes. MPs thus represent novel subcellular structures that can impact on the pathogenesis of rheumatic disease and serve as biomarkers of underlying cellular disturbances.
Keywords: microparticles, inflammation, thrombosis, rheumatic disease, apoptosis, autoimmunity, DNA, RNA
Introduction
Microparticles (MPs) are small membrane-bound vesicles that display potent biological activities that can have an impact on normal physiology as well as the pathogenesis of immune-mediated diseases. These particles range in size from 0.1 to 1.0 μm and arise from activated or dying cells. Although the majority of MPs in the blood originate from platelets, virtually all eukaryotic cells, including immune cells, appear to be able to release MPs. Particle formation thus represents a fundamental cellular response that affects the particle-producing cell as well as the target cells with which the particle interacts [1–4]. Originally considered as inert debris, MPs are now known to display diverse pro-inflammatory and pro-thrombotic activities that can influence the course of rheumatic and other immune-mediated diseases [5]. As such, the assay of MPs as biomarkers may illuminate the operation of various pathogenetic mechanisms and their impact on different tissues and organs.
As shown in studies in in vivo and in vitro systems, intercellular communication involves signalling elements whose dimensions vary enormously, extending from small molecules (e.g. prostanoids, catecholamines and cytokines) to large structures such as cells. Although cells display surface proteins and glycoproteins that can stimulate receptors, they can also form intimate contacts with other cells, including synapses, bridges and conduits, to induce signalling and mediate information transfer. MPs occupy an important place along this spectrum since they are small enough to carry cytokines but nevertheless large enough to bind other cells to transfer material and to present a surface for a facsimile of cell–cell interaction.
Mechanisms of MP generation
MPs are important components of the extracellular milieu, including the blood. Although MPs have features of cells (i.e. membrane-bound structure), they are much smaller in size and have an incomplete array of the various ‘omes’ (e.g. proteome) that comprise a cell [6]. These particles can arise from cells undergoing activation or apoptosis, detaching from the membrane as formed structures. In addition to MPs, another particle type called exosomes can be released from cells [7]. Exosomes are smaller than MPs (50–100 nm) and are produced through exocytosis of multivesicular bodies. Whereas exosomes are generated internally, MPs form at the cell surface; these particle types can therefore differ in their constituent molecules (e.g. tetraspanin proteins for exosomes). MPs have also been called microvesicles and ectosomes.
While cell death is an important setting for particle release, MP formation appears distinct from the generation of apoptotic bodies. Apoptotic bodies are the collapsed remains of apoptotic cells as well as large subcellular fragments that detach from cells as death proceeds. Apoptotic bodies are much larger than MPs and have a significant content of nuclear material. Whereas apoptotic bodies form during the late stages of apoptosis, MPs can be released during the early stages of this process [4, 8]. Despite differences in their size and time course of production, MPs and apoptotic bodies both arise from processes that disassemble a cell to facilitate phagocytosis and safe removal of any immunostimulatory material that may be produced during cell death. In contrast to the large dimensions of apoptotic bodies, the small size of MPs may allow them to avoid phagocytes and to translocate into the extracellular milieu to mediate signalling at local and distant sites.
As shown in in vitro systems, MPs may originate during apoptosis from a membrane bud or bleb that detaches from the cell following stalk fission [9–10]. The formation of a bleb, which can resemble a bubble, involves nucleation, expansion and retraction steps that are mediated by phosphorylation of the myosin light chain [11, 12]. The function of blebbing (and subsequent particle release) is not understood, although this process may regulate cell volume during apoptotic shrinkage; particle formation may also help detoxify cells as deleterious substances (including chemotherapeutics or activated caspases) are packaged for removal [13, 14]. Importantly, while hundreds of blebs form on the cell surface during apoptosis, only a few MPs are released by each cell. In addition to cell death, particle release occurs during cell activation. Platelet activation leads to abundant particle production, although platelet MPs in the blood may also originate from megakaryocytes. Among triggers of platelet activation, elements of the extracellular matrix can interact with the collagen receptor glycoprotein VI, a process that can occur in the synovium [15, 16].
In the context of immune-mediated disease, stimulation by TLR ligands and other activating signals can generate particle formation. Thus, as shown in in vitro systems using RAW264.7 or THP-1 cells as models for macrophages, stimulation of TLR 4 by lipopolysaccharide (LPS) can induce particle production; for RAW264.7 cells, this process depends on nitric oxide [17, 18]. Since particles emanating from stimulated macrophages resemble MPs from apoptotic cells in properties, their origin may reflect a similar mechanism despite the seemingly disparate setting (i.e. activation and apoptosis). As now recognized, TLR stimulation can lead to apoptosis via activation-induced cell death, a process that may be important to host defence. Thus cell death can abort infection by destroying the reservoir for replication of infective agents; cell death can also promote immune system activation via the generation of danger molecules and immunostimulatory MPs (see further). In this scenario, particle release may result from those TLR-stimulated cells that are undergoing cell death, suggesting that the mechanisms of particle release would be similar to those operating during apoptosis. Fig. 1 illustrates mechanisms for particle release from nucleated cells.
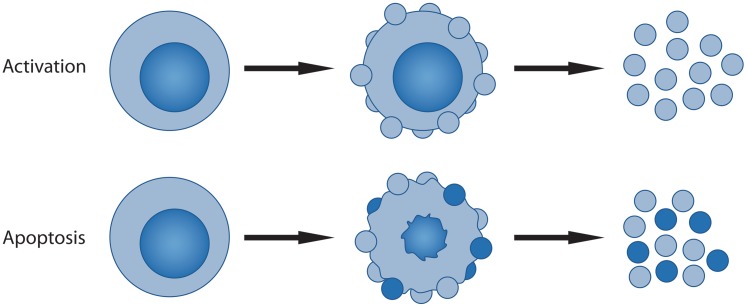
Fig. 1.
Mechanism of MP release by nucleated cells. The figure illustrates the two main mechanisms for the generation and release of MPs from nucleated cells and differences in particle composition. During activation of cells, blebs (shown as small circles) form at the cell surface and undergo release; since the cell is otherwise intact, the released MPs do not contain nuclear components. In contrast, during apoptosis, the cell undergoes drastic changes, including shrinkage, nuclear fragmentation and migration of nuclear constituents into blebs that form at the cell surface. MP release usually occurs during the later stages of apoptosis. As such, MPs can contain nuclear components that are shown in the darker blue. MPs differ from apoptotic bodies that are the collapsed remains of apoptotic cells.
Composition of MPs
Reflecting their cellular origin, MPs contain a wide array of surface, cytoplasmic and nuclear molecules that are incorporated into a membrane-bound structure as the particle leaves the cell; in addition, MPs can contain small-molecule metabolites (e.g. taurine) present in the cell cytoplasm [6, 19–22]. The particle structure may be dynamic, however, with membrane permeability allowing an exchange of molecules between the particle interior and the surrounding milieu; serum proteins can also bind to the MP surface. Thus proteomic studies demonstrate the presence of immunoglobulin as well as complement components in particle preparations. These serum molecules could bind to particles after particle detachment from cells, although they may also deposit on the surface of damaged cells. In this situation, release of membrane regions containing antibody and complement could represent a protective strategy to remove membrane attack complexes that could threaten the cell with lysis [23].
Of MP components, those on the cell surface are the most notable since they allow detection by flow cytometry. As a common feature, particles expose phosphatidylserine (PS), a consequence of membrane flipping. PS normally exists on the inner membrane leaflet, with asymmetry maintained by a trio of enzymes called flippase, floppase and scramblase; during apoptosis, this process is inhibited and PS translocates to the outer membrane leaflet. Not all particles are annexin V-positive, however, suggesting either heterogeneity in the mechanism of production or the presence of PS at concentrations below the limits of detection. The surface of particles also contains membrane molecules from the cell of origin (e.g. CD14 for macrophages, CD61 and CD41 for platelets, and CD62E and CD144 for endothelial cells). Although there is only limited information on the particle proteome, studies on platelets and macrophages suggest heterogeneity in its composition, depending on particle subtype as well as mechanism of production [22]. In addition, since particles may emerge preferentially from regions of the membrane containing lipid rafts, the content of membrane proteins may differ from that of the overall membrane [24].
Among particle components, cytokines are important for their potential role in the induction of immune responses by particles. Thus MPs released by the THP-1 monocyte cell line stimulated by adenosine triphosphate contain very high concentrations of IL-1β [25]. IL-1β also occurs on MPs from dendritic cells as a result of stimulation of the PX27 receptor in a calcium-dependent process [26]; platelet MPs can express both IL-1α and IL-1β [13]. The expression of a cytokine on a particle surface may enhance its action since the cytokine can act in the context of a surface with other receptor ligands [13]. These observations suggest that immune activation in the setting of cell death may originate from complexes, aggregates or particles that concentrate immune mediators, generating powerful signalling by the simultaneous receptor interaction.
MPs have a prominent role in haemostasis, reflecting their display of tissue factor [27–29]. In addition, particles express other molecules that can affect clotting, including von Willebrand factor multimers, which can promote the stability of platelet aggregates, and p-selectin glycoprotein, which interacts with tissue factor and mediates binding to platelets and neutrophils [28]. Among particle types, endothelial MPs express adhesion markers (i.e. e-selectin as well as intercellular adhesion molecule 1), which can lead to binding and activation of leucocytes and monocytes.
Along with membrane and cytoplasmic constituents, MPs contain nuclear molecules, and in this respect resemble apoptotic bodies [30, 31]. In one of the most profound cellular changes during apoptosis, molecules from the cell nucleus transit through the cytoplasm to relocate in blebs [32]. Whereas the mechanism mediating this cellular rearrangement is not known, the end result is the repositioning of nuclear constituents in a form that may be more accessible to the immune system, either on the surface of blebs on the dying cell or on the released MPs. As shown with cell lines undergoing in vitro apoptosis, MPs contain DNA and RNA, including mRNA, rRNA and miRNA [31]. The DNA in the MPs shows a broad size distribution and, while high molecular weight species are present, cleaved DNA with laddering occurs prominently. Of note, the DNA in particles is accessible to antibody binding, reflecting either surface location of this molecule or a very porous structure [31]. Accompanying the DNA, particles can contain histones and other nuclear proteins [30]. The presence of RNA in particles has suggested a role in information transfer, especially with miRNA, which has regulatory activity.
Assay of particles
MPs represent unique biomarkers since they provide information related to the activation and death of specific cell populations. Importantly, particles in the blood can originate from tissues that would otherwise be difficult to sample without biopsy; particle expression in the blood can also be readily sampled on multiple occasions to assess events in pathogenesis over time. For this purpose, flow cytometry [fluorescence-activated cell sorting (FACS)] provides a sensitive and flexible platform to measure particles, although biochemical determinations of molecular components (i.e. protein, PS, nucleic acid) can allow enumeration in isolated preparations. Assays of functional activity (i.e. tissue factor) have also been used for certain particle subpopulations [33–35].
FACS analysis is very informative, although the small size of particles can present technical challenges. Indeed, since particles can be 10–100 times smaller in diameter than a cell, their surface area can be up to 104 times less, limiting detection of surface markers. Interestingly, immune complexes can appear as particles by flow cytometry, but the formation of particles of this size range may depend on the nature of the antigen and stoichiometry of immune reactants [36].
For FACS analysis, MP measurement from biological samples (e.g. blood, effusions) usually involves centrifugation steps to remove cells (including platelets) and debris. Whereas particles can be analysed in such preparations by establishing gates to delimit objects of particle size, high-speed centrifugation can pellet the MPs for more direct analysis. Important issues in particle analysis concern conditions for blood drawing (e.g. the anti-coagulant) and subsequent handling and storage (e.g. duration and temperature); importantly, freezing–thawing may alter MP counts and phenotype [34, 37]. At present there is no single procedure accepted as a standard for the preparation of MP analysis [4]. Fig. 2 depicts a FACS profile of a culture supernatant of cells undergoing apoptosis, indicating the size distribution of MP cells compared with marker beads.
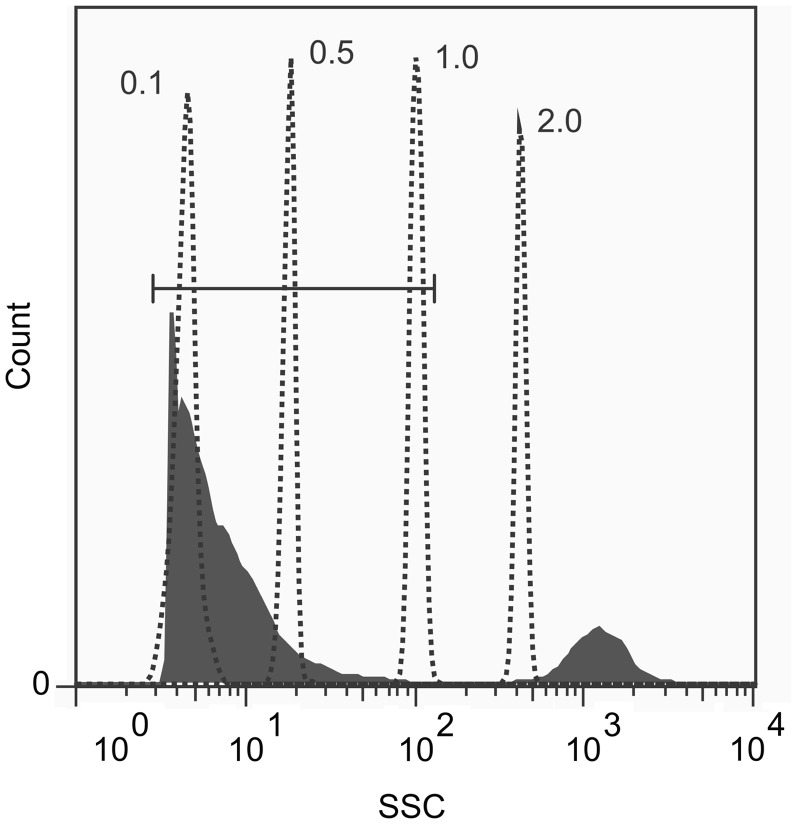
Fig. 2.
Size distribution of MPs by flow cytometry. Jurkat cells were treated with 1 μM staurosporine for 18 h to induce apoptosis. The culture supernatant was analysed by flow cytometry by side scatter (SSC), as depicted in the filled profile. The size of the MPs and cells (indicated in the filled peaks) was determined by reference to fluorescent microspheres (Fluorospheres, Size Kit #2, Invitrogen, Carlsbad, CA, USA) of 0.1, 0.5, 1.0 and 2.0 μm sizes (dotted peaks). The first filled peak, comprising the MP fraction, is indicated by the horizontal gate. The second filled peak beyond the horizontal gate comprises the Jurkat cells. The FACS instrument was calibrated using diluent alone to ensure that no background events were detected by side scatter.
Total MPs can be measured in a sample by light scattering, although standard flow cytometers have a detection limit of ∼0.2 μm; as such, instruments may not be able to discriminate smaller MPs from the background noise. Furthermore, instruments can differ in size resolution [38], although defined size beads can serve as reference points for measurement. As in the case of cells, the measurement of cell surface markers forms the mainstay of FACS analysis. Of reagents for these markers, fluorescently labelled annexin V can bind PS on the MP surface [39]; annexin V staining, however, may also result from the loss of particle membrane permeability that permits access of annexin V to internal sources of PS. Whereas annexin V binding is often used to identify particles, a significant proportion of the MPs in a sample may be annexin V negative [40, 41]. For determination of particle subpopulations, cell surface markers allow identification and quantitation, although marker density must be sufficient to allow detection.
The presence of nucleic acids provides another means to measure MPs by cytometry using dyes (e.g. SYTO13) that interact with DNA and RNA [31, 42]. This approach works best for MPs from nucleated cells and can allow detection of particles smaller than those seen by light scatter alone. The use of nucleic acid-binding dyes can provide information on the cell of origin of particles since those from nucleated cells (e.g. lymphocytes or monocytes) have much greater nucleic acid content than those from platelets.
Biological properties of MPs
MPs display important biological properties that can impact on many different diseases, serving as potentially important mediators of disease pathogenesis; indeed, in their use as biomarkers, MPs can provide important information about ongoing pathogenetic processes that would be valuable clinically for assessing disease activity and predicting the likelihood of certain events. In many respects, MPs are like alarmins or death- or damage-associated molecular patterns (DAMPs). Alarmins are a diverse group of molecules, both large and small, that emanate from dead and dying cells to induce inflammation, promote chemotaxis and potentiate immune responses [43]. Although certain preformed cytokines can be classified as alarmins, DAMPs represent molecules whose immunological activity results from degradation, post-translation modification (including redox state) or exposure during cell death. A close relationship between the activity of DAMPs and MPs is likely since they are produced concomitantly by dead and dying cells and, furthermore, MPs can contain DAMPs.
Whereas the activity of DAMPs and alarmins is usually conceptualized in terms of single molecules, there is increasing evidence that the activity of molecules like HMGB1 reflects its formation of complexes with cytokines (e.g. IL-1), other DAMPs (e.g. DNA) or pathogen-associated molecular patterns (e.g. LPS or endotoxin). As such, the immune activity of some alarmins may result from either their capacity for assembly into complexes or their disposition in a structure like MPs that can contain cytokines and other DAMPs [44–47]. In this model, MPs provide a framework or nanostructure to intensify or even unmask the activity of the component molecules. At present, most studies of MPs use preparations that have not been rigorously analysed in terms of components like cytokines or DAMPs that may contribute to immune activity.
Studies on the immune properties of MPs have generally utilized preparations obtained from cells or cell lines undergoing in vitro activation or apoptosis, although some studies have employed MPs isolated from blood or tissue lesions (e.g. atherosclerotic plaques). These studies have demonstrated that MPs can, in an autocrine and paracrine way, stimulate a wide array of cell types, with endothelial cells, macrophages, dendritic cells and synovial fibroblasts among others responding to this type of activation. Importantly, this stimulation can lead to the generation of cytokines and chemokines, which can further intensify inflammation [48–58].
In general, these responses are pro-inflammatory in nature and are consistent with the function of MPs as a mega-DAMP that can promote host defence, on the one hand, or potentiate autoimmunity or inflammation, on the other. These responses involve activation of NF-κB and downstream signalling pathways such as the mitogen activated protein (MAP) kinases. In general, stimulation requires only a few particles per responding cell, highlighting the potency of this form of stimulation. It is important to note that, depending on the cell of origin and inducing triggers, MPs can also have anti-inflammatory activity, suggesting that the component molecules influence the activity of the overall structure [59]. Table 1 lists the activities of MPs.
Table 1.
Biological activities of MPs
| Activation of NF-κB and MAP kinases |
| Induction of cytokines and chemokines |
| Up-regulation of adhesion molecules |
| Display of tissue factor |
| Information transfer (receptors, mediators and nucleic acids) |
Given their origin and composition, MPs can stimulate cells by diverse mechanisms, including the transfer of molecules such as receptors or mediators such as arachidonic acid that have intrinsic immune-activating properties [60–62]. The process of this transfer is not well understood, although MPs may interact with target cells either by fusion or engulfment. The molecules mediating these contact events can vary depending on particle and cell type and can include complement components on the particles that can bind to the CR2 receptor [63]. In other settings, the transfer of molecules between immune cells can occur by a process called trogocytosis (from the Greek trogo, to gnaw) in which cells accept membrane and cytosolic material from other cells [64, 65]. Such a transfer can alter the properties of the accepting cell, for example, by increasing the concentration of a downstream mediator of signal transduction, a regulatory RNA or even a new cell surface receptor. Whereas trogocytosis has usually been considered as a form of cell–cell interaction, it may also occur with MPs and account for some of their actions. In addition to stimulating inflammation, MPs can promote thrombosis because of their display of tissue factor as well as a membrane structure that allows assembly of pro-coagulant complexes.
As described above, MPs have a significant content of nucleic acid and represent an important source of extracellular nucleic acids [31, 42]. Whereas DNA in the blood exists in both a particulate and non-particulate form, most of the RNA circulates in the form of particle, likely because the membrane structure protects the RNA from nuclease activity. The presence of nucleic acids in particles suggests two distinct possible activities for these structures. The first is stimulation of cells via internal nucleic acid sensors, including the Toll-like receptors (TLRs). These TLRs include TLR3 for dsRNA, TLR7 for ssRNA and TLR9 for DNA. Since nucleic acids in particles may have access to these receptors following transfer from MPs, they could potentially activate programs for innate immune responses. Interestingly, the presence of nucleic acids in liposomes or other transfection agents can dramatically boost their immune activity, although an analogous activity of nucleic acids in particles has not been established [66]. Finally, MPs may affect the function of the target cell with which it interacts by transfer of informational nucleic acids, with mRNA and miRNA both present in particles and capable of inducing new genomic responses [67]. Transfer of informational RNA occurs with exosomes and seems plausible with MPs. Table 2 presents activities of MPs relevant to pathogenesis of rheumatic disease.
Table 2.
Role of MPs in rheumatic disease
| Modulate inflammation |
| Endothelial cell activation |
| Fibroblast activation |
| Coagulation |
| Immune complex formation |
The role of MPs in rheumatic disease
Studies on patients with a wide variety of rheumatic and non-rheumatic diseases have demonstrated significant elevations in particle numbers compared with those in control populations, thus supporting the use of MPs as biomarkers. These elevations are most notable with conditions with a strong vascular component and primarily involve platelet MPs, although elevations of MPs from endothelium, among other cell types, have also been documented [68–78]. The association of elevations of MP numbers with diseases of the vasculature is consistent with the origin of particles from activation of platelet and endothelium as well as the ability of particles to promote thrombosis and activate endothelium [79]. MPs can also affect angiogenesis and circulating endothelial cell precursors, extending particle effects on the vasculature [80]. In some situations (e.g. infection or systemic autoimmune disease), elevations of MPs from lymphocytes, monocytes and granulocytes also occur, likely reflecting the activation of these cell populations during inflammation and innate immune responses. There have been few studies on the clearance of particles from the blood, although, as noted, serum proteins can bind to particles to promote phagocytosis while the enzyme phospholipase A2 may degrade particles [73]. It is not clear therefore whether elevation of particle numbers results from increased production or impaired removal.
Elevation of particle numbers in blood occurs in RA, SLE, APL syndrome, vasculitis and progressive SSc (PSSc), all conditions characterized by both immune system activation as well as vascular abnormalities including thrombosis. The expression of MPs can also occur in SF; since MP levels can far exceed those of blood, these findings suggest intense local generation in the synovial microenvironment or even preferential localization or trapping of circulating MPs [16]. The particles in SF may have an important role in synovitis by promoting coagulation; particles can drive synovial fibroblasts to produce MMPs as well as chemokines by a mechanism dependent on NF-κB [52, 53].
In addition to their role as pro-inflammatory and pro-thrombotic mediators, MPs may contribute to the pathogenesis of rheumatic disease by the formation of immune complexes. Thus particles from patients with RA and SLE may have increased concentrations of IgG on their surface, with studies on particles in RA also showing increased levels of complement components [81, 82]. Furthermore, studies using both mAbs as well as patient plasma indicate that anti-nucleosomal antibodies can bind to particles generated in vitro by apoptotic cells (Fig. 3). Since these particles contain DNA and other nuclear antigens, these findings suggest that particles can serve as a nidus for immune complex formation by binding of ANAs; the specificity of the antibodies from RA blood binding to particles is not known. It is possible, however, that the presence of antibodies on the particle surface may result from interaction with Fc receptors on certain particles rather than binding to exposed nuclear antigens. The impact of antibodies on particle trafficking as well as their deposition in sites such as the kidney is not known, although complexes built with particles as opposed to soluble antigen could have distinct activities in terms of pathogenicity or nephritogenicity.
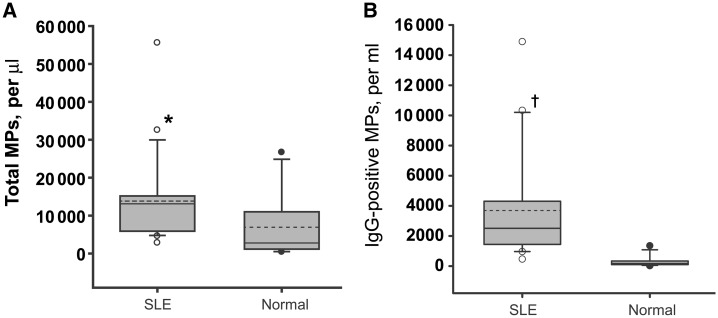
Fig. 3.
Total and IgG-bound MPs in the plasma of SLE patients. MPs were isolated from 21 SLE patient (○) and normal control (•) plasmas by differential centrifugation and analysed by FACS by side scatter and binding of a phycoerythrin-labelled goat anti-human IgG antibody. (A) Total MP numbers determined by side scatter. (B) The number of particles with IgG. The distribution of MP counts is indicated by box plots. The grey box indicates samples within the range of the 25th and 75th percentiles. The broken horizontal line indicates the population means and the solid line indicates the population median values. Although the total number of MPs in SLE plasmas did not differ significantly from those of normal controls (*), the numbers of IgG-positive MPs in SLE patient plasmas was significantly higher compared with 12 normal controls (†P = 0.00038). Adapted from [82], with permission from Elsevier.
Although the correlation of increased particle numbers with disease suggests a role in immunopathogenesis, elucidating this role is difficult at present. In this regard, studies have suggested that increases in particle numbers are not invariable among rheumatic diseases, and furthermore, may show a paradoxical relationship to disease activity. Thus, in patients with PSSc, particle numbers were inversely correlated with skin thickness, whereas, in a study of patients with SLE, overall particle numbers were decreased compared with controls [72, 77]. These discrepancies could occur if particles in the blood or tissue were bound to other cells or even to each other to prevent detection by flow cytometry. Furthermore, as noted above, there is evidence that certain MP subpopulations may have anti-inflammatory activity, with the overall mix of particles determining clinical outcome.
Delineating a specific role of MPs in immunopathogenesis can be complicated by their concomitant expression with other pro-inflammatory and pro-thrombotic molecules, such as the DAMPs and alarmins. Since approaches that would block particle production (e.g. caspase inhibitors, kinase inhibitors) would also block the production of these other immune activators, distinguishing the contributions of MPs in an animal disease model, for example, may not be possible. Interestingly, studies in animal models of malaria have suggested that certain agents can block particle production and clinical disease, although interpretation of these studies is difficult given the potentially broad activity of these agents [83, 84]. Finally, although an agent like anti-IL-1β may work by blocking the activity of particle IL-1β, it could also inhibit IL-1β in a non-particulate form, preventing a more precise delineation of the specific contribution of the particle. In this regard, the activity of cytokines like IL-1β on the surface of a particle may be more difficult to inhibit than when the cytokine is free, limiting certain anti-cytokine therapies.
The role of MPs as biomarkers
As these considerations suggest, MPs may represent novel biomarkers whose measurement can reveal the state of tissues involved in disease pathogenesis. While elevations of either total MP numbers or those of different MP subtypes may not be specific for different diagnoses, nevertheless, MP measurement may help delineate the state of target tissues in disease, especially the vasculature, by simple and non-invasive blood tests; in addition, MP measurement may provide insight into events (e.g. immune cell activation) that may involve only a limited population of cells in the blood or the tissue. Such data may be useful in longitudinal patient assessment and the evaluation of new and existing therapies that may potentially affect multiple cell types. Due to technical issues with current analytic approaches, MP measurement is not yet routinely performed in the clinical setting, although, with improvements in assays, MP measurement may be incorporated into a portfolio of assays to characterize events in pathogenesis that may impact on disease course, treatment response and prognosis.
Summary
MPs, originally conceptualized as inert debris from cell death, are now viewed as an essential outcome of a well-regulated process that can generate potentially powerful disease mediators. Importantly, particles appear to act at low numbers, with only a few particles capable of driving responding cells to activation and the production of pro-inflammatory mediators. Although MPs have properties suggesting a key role in the pathogenesis of rheumatic disease, evidence for this possibility comes primarily from model studies as well as observations on increases of particle numbers in the blood of patients. As interest in particles increases, studies in animal models as well as patients should provide more decisive evidence of their pathogenicity in rheumatic diseases and provide new approaches to understand the role of these fascinating biologic structures in normal and aberrant immunity and their utility as biomarkers to reveal key events in immunopathogenesis.
Funding: This work was supported by a VA Merit Review grant; National Institute of Health (AI082402); an Arthritis Foundation Award; and a Kirkland Scholar Award Program from The Hospital for Special Surgery in New York City. The Kirkland Scholar Award is funded exclusively by Rheuminations, Inc., a non-profit foundation dedicated to supporting research leading to the treatment and cure of lupus.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Diamant M, Tushuizen ME, Sturk A, et al. Cellular microparticles: new players in the field of vascular disease? Eur J Clin Invest. 2004;34:392–401. doi: 10.1111/j.1365-2362.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 2.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–57. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 4.György B, Szabó TG, Pasztói M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer C, Pisetsky DS. The role of microparticles in the pathogenesis of rheumatic diseases. Nat Rev Rheumatol. 2010;6:21–9. doi: 10.1038/nrrheum.2009.229. [DOI] [PubMed] [Google Scholar]
- 6.Mayr M, Grainger D, Mayr U, et al. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009;2:379–88. doi: 10.1161/CIRCGENETICS.108.842849. [DOI] [PubMed] [Google Scholar]
- 7.Denzer K, Kleijmeer MJ, Heijnen HFG, et al. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–74. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 8.Morel O, Jesel L, Freyssinet J-M, et al. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31:15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 9.Charras GT. A short history of blebbing. J Microsc. 2008;231:466–78. doi: 10.1111/j.1365-2818.2008.02059.x. [DOI] [PubMed] [Google Scholar]
- 10.Charras GT, Coughlin M, Mitchison TJ, et al. Life and times of a cellular bleb. Biophys J. 2008;94:1836–53. doi: 10.1529/biophysj.107.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills JC, Stone NL, Erhardt J, et al. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–36. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sebbagh M, Renvoizé C, Hamelin J, et al. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–52. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 13.Abid Hussein MN, Böing An, Sturk A, et al. Inhibition of microparticle release triggers endothelial cell apoptosis and detachment. Thromb Haemost. 2007;98:1096–107. doi: 10.1160/th05-04-0231. [DOI] [PubMed] [Google Scholar]
- 14.Goler-Baron V, Assaraf YG. Structure and function of ABCG2-rich extracellular vesicles mediating multidrug resistance. PLoS ONE. 2011;6:e16007. doi: 10.1371/journal.pone.0016007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morel O, Morel N, Freyssinet J-M, et al. Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses. Platelets. 2008;19:9–23. doi: 10.1080/09537100701817232. [DOI] [PubMed] [Google Scholar]
- 16.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–3. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauley J, Pisetsky DS. The release of microparticles by RAW 264.7 macrophage cells stimulated with TLR ligands. J Leukoc Biol. 2010;87:1–9. doi: 10.1189/jlb.0709465. [DOI] [PubMed] [Google Scholar]
- 18.Wang JG, Williams JC, Davis BK, et al. Monocytic microparticles activate endothelial cells in an IL-1β-dependent manner. Blood. 2011;118:2366–74. doi: 10.1182/blood-2011-01-330878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin M, Drwal G, Bourgeois T, et al. Distinct proteome features of plasma microparticles. Proteomics. 2005;5:1940–52. doi: 10.1002/pmic.200401057. [DOI] [PubMed] [Google Scholar]
- 20.Smalley DM, Root KE, Cho H, et al. Proteomic discovery of 21 proteins expressed in human plasma-derived but not platelet-derived microparticles. Thromb Haemost. 2007;97:67–80. [PubMed] [Google Scholar]
- 21.Kolowos W, Gaipl US, Sheriff A, et al. Microparticles shed from different antigen-presenting cells display an individual pattern of surface molecules and a distinct potential of allogeneic T-cell activation. Scand J Immunol. 2005;61:226–33. doi: 10.1111/j.1365-3083.2005.01551.x. [DOI] [PubMed] [Google Scholar]
- 22.Bernimoulin M, Waters EK, Foy M, et al. Differential stimulation of monocytic cells results in distinct populations of microparticles. Thromb Haemost. 2009;7:1019–28. doi: 10.1111/j.1538-7836.2009.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilzer D, Gasser O, Moskovich O, et al. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immun. 2005;27:375–87. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 24.Del Conde I, Shrimpton CN, Thiagarajan P, et al. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106:1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 25.MacKenzie A, Wilson HL, Kiss-Toth E, et al. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;8:825–35. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 26.Pizzirani C, Ferrari D, Chiozzi P, et al. Stimulation of P2 receptors causes release of IL-1β-loaded microvesicles from human dendritic cells. Blood. 2007;109:3856–64. doi: 10.1182/blood-2005-06-031377. [DOI] [PubMed] [Google Scholar]
- 27.Müller I, Klocke A, Alex M, et al. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003;17:476–8. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- 28.Jy W, Jimenez JJ, Mauro LM, et al. Endothelial microparticles induce formation of platelet aggregates via a von Willebrand factor/ristocetin dependent pathway, rendering them resistant to dissociation. J Thromb Haemost. 2005;3:1301–8. doi: 10.1111/j.1538-7836.2005.01384.x. [DOI] [PubMed] [Google Scholar]
- 29.Mackman N. The may faces of tissue factor. Thromb Haemost. 2009;7:136–9. doi: 10.1111/j.1538-7836.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiller M, Bekeredijan-Ding I, Heyder P, et al. Autoantigens are translocated into small apoptotic bodies during early states of apoptosis. Cell Death Differ. 2008;15:183–91. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- 31.Reich CF, 3rd, Pisetsky DS. The content of DNA and RNA in microparticles released by Jurkat and HL-60 cells undergoing in vitro apoptosis. Exp Cell Res. 2009;315:760–8. doi: 10.1016/j.yexcr.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jy W, Horstman LL, Jimenez JJ, et al. Measuring circulating cell-derived microparticles. J Thromb Haemost. 2004;2:1842–51. doi: 10.1111/j.1538-7836.2004.00936.x. [DOI] [PubMed] [Google Scholar]
- 34.Shah MD, Bergeron AL, Dong JF, et al. Flow cytometric measurement of microparticles: pitfalls and protocol modifications. Platelets. 2008;19:365–72. doi: 10.1080/09537100802054107. [DOI] [PubMed] [Google Scholar]
- 35.Gelderman MP, Simak J. Flow cytometric analysis of cell membrane microparticles. Methods Mol Biol. 2008;484:79–93. doi: 10.1007/978-1-59745-398-1_6. [DOI] [PubMed] [Google Scholar]
- 36.Gyorgy B, Modos K, Pállinger E, et al. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood. 2011;117:e39–48. doi: 10.1182/blood-2010-09-307595. [DOI] [PubMed] [Google Scholar]
- 37.Mobarrez F, Antovic J, Egberg N, et al. A multicolor flow cytometric assay for measurement of platelet-derived microparticles. Thromb Res. 2010;125:e110–6. doi: 10.1016/j.thromres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Lacroix R, Robert S, Poncelet P, et al. Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative Workshop. J Thromb Haemost. 2010;8:2571–4. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- 39.Dachary-Prigent J, Freyssinet JM, Pasquet JM, et al. Annexin-V as a probe of aminophospholipid exposure and platelet membrane vesiculation – a flow-cytometry study showing a role for free sulfhydryl-groups. Blood. 1993;81:2554–65. [PubMed] [Google Scholar]
- 40.Briede JJ, Heemskerk JW, Hemker HC, et al. Heterogeneity in microparticle formation and exposure of anionic phospholipids at the plasma membrane of single adherent platelets. Biochim Biophys Acta. 1999;1451:163–72. doi: 10.1016/s0167-4889(99)00085-3. [DOI] [PubMed] [Google Scholar]
- 41.Connor DE, Exner T, Ma DD, et al. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein lb. Thromb Haemost. 2010;103:1044–52. doi: 10.1160/TH09-09-0644. [DOI] [PubMed] [Google Scholar]
- 42.Ullal AJ, Pisetsky DS, Reich CF., 3rd Use of SYTO 13, a fluorescent dye binding nucleic acids, for the detection of microparticles in in vitro systems. Cytometry A. 2010;77:294–301. doi: 10.1002/cyto.a.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 44.Pisetsky DS, Erlandsson-Harris H, Andersson U. High-mobility group box protein 1 (HMGB1): an alarmin mediating the pathogenesis of rheumatic disease. Arthritis Res Ther. 2008;10:209. doi: 10.1186/ar2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sha Y, Zmijewski J, Xu Z, et al. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol. 2008;180:2531–7. doi: 10.4049/jimmunol.180.4.2531. [DOI] [PubMed] [Google Scholar]
- 46.Hreggvidsdottir HS, Ostberg T, Wähämaa H, et al. The alarmin HMGB1 acts in synergy with endogenous and exogenous danger signals to promote inflammation. J Leukoc Biol. 2009;86:655–62. doi: 10.1189/jlb.0908548. [DOI] [PubMed] [Google Scholar]
- 47.Pisetsky DS. Cell death in the pathogenesis of immune-mediated diseases: the role of HMGB1 and DAMP-PAMP complexes. Swiss Med Wkly. 2011;141:w13256. doi: 10.4414/smw.2011.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mesri M, Altieri DC. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998;161:4382–7. [PubMed] [Google Scholar]
- 49.Mesri M, Altieri DC. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem. 1999;274:23111–8. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- 50.Nomura S, Tandon NN, Nakamura T, et al. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2000;158:277–87. doi: 10.1016/s0021-9150(01)00433-6. [DOI] [PubMed] [Google Scholar]
- 51.Dalli J, Norling LV, Renshaw D, et al. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–9. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 52.Berckmans RJ, Nieuwland R, Kraan MC, et al. Synovial microparticles from arthritic patients modulate chemokine and cytokine release by synoviocytes. Arthritis Res Ther. 2005;7:R536–44. doi: 10.1186/ar1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Distler JH, Jüngel A, Huber LC, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci USA. 2005;102:2892–7. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cerri C, Chimenti D, Conti I, et al. Monocyte/macrophage-derived microparticles up-regulate inflammatory mediator synthesis by human airway epithelial cells. J Immunol. 2006;177:1975–80. doi: 10.4049/jimmunol.177.3.1975. [DOI] [PubMed] [Google Scholar]
- 55.Scanu A, Molnarfi N, Brandt KJ, et al. Stimulated T cells generate microparticles, which mimic cellular contact activation of human monocytes: differential regulation of pro-and anti-inflammatory cytokine production by high-density lipoproteins. J Leukoc Biol. 2008;83:921–7. doi: 10.1189/jlb.0807551. [DOI] [PubMed] [Google Scholar]
- 56.Aharon A, Tamari T, Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost. 2008;100:878–85. doi: 10.1160/th07-11-0691. [DOI] [PubMed] [Google Scholar]
- 57.Angelot F, Seillès E, Biichlé S, et al. Endothelial cell-derived microparticles induce plasmacytoid dendritic cell maturation: potential implications in inflammatory diseases. Haematologica. 2009;94:1502–12. doi: 10.3324/haematol.2009.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tesse A, Martínez MC, Hugel B, et al. Upregulation of proinflammatory proteins through NF-κB pathway by shed membrane microparticles results in vascular hyporeactivity. Arterioscler Thromb Vasc Biol. 2005;25:2522–7. doi: 10.1161/01.ATV.0000189298.62240.5d. [DOI] [PubMed] [Google Scholar]
- 59.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–8. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 60.Barry OP, Kazanietz MG, Pratico D, et al. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1999;274:7545–6. doi: 10.1074/jbc.274.11.7545. [DOI] [PubMed] [Google Scholar]
- 61.Barry OP, Pratico D, Lawson JA, et al. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99:2118–27. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jungel A, Distler O, Schulza-Horsel U, et al. Microparticles stimulate the synthesis of prostaglandin E(2) via induction of cyclooxygenase 2 and microsomal prostaglandin E synthase 1. Arthritis Rheum. 2007;56:3564–74. doi: 10.1002/art.22980. [DOI] [PubMed] [Google Scholar]
- 63.Köppler B, Cohen C, Schlöndorff D, et al. Differential mechanisms of microparticle transfer to B cells and monocytes: anti-inflammatory properties of microparticles. Eur J Immunol. 2006;36:648–60. doi: 10.1002/eji.200535435. [DOI] [PubMed] [Google Scholar]
- 64.David DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nature Rev Immunol. 2007;7:238–43. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 65.Waschbisch A, Meuth SG, Herrmann AM, et al. Intercellular exchanges of membrane fragments (trogocytosis) between human muscle cells and immune cells: a potential mechanicsm for the modulation of muscular immune responses. J Neuroimmunol. 2009;209:131–8. doi: 10.1016/j.jneuroim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 66.Jiang W, Reich Cf, III, Pisetsky DS. Mechanisms of activation of the RAW 264.7 macrophage cell line by transfected mammalian DNA. Cell Immunol. 2004;229:31–40. doi: 10.1016/j.cellimm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Ratajczak J, Wysoczynski M, Hayek F, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–95. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 68.Combes V, Simon A-C, Grau G-E, et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brogan PA, Shah V, Brachet C, et al. Endothelial and platelet microparticles in vasculitis of the young. Arthritis Rheum. 2004;50:927–36. doi: 10.1002/art.20199. [DOI] [PubMed] [Google Scholar]
- 70.Dignat-George F, Camoin-Jau L, Sabatier F, et al. Endothelial microparticles: a potential contribution to the thrombotic complications of the antiphospholipid syndrome. Thromb Haemost. 2004;91:667–73. doi: 10.1160/TH03-07-0487. [DOI] [PubMed] [Google Scholar]
- 71.Erdbruegger U, Grossheim M, Hertel B, et al. Diagnostic role of endothelial microparticles in vasculitis. Rheumatology. 2008;47:1820–5. doi: 10.1093/rheumatology/ken373. [DOI] [PubMed] [Google Scholar]
- 72.Guiducci S, Distler JH, Jungel A, et al. The relationship between plasma microparticles and disease manifestations in patients with systemic sclerosis. Arthritis Rheum. 2008;58:2845–53. doi: 10.1002/art.23735. [DOI] [PubMed] [Google Scholar]
- 73.Sellam J, Proulle V, Jüngel A, et al. Increased levels of circulating microparticles in primary Sjögren's syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther. 2008;11:R156. doi: 10.1186/ar2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clarke LA, Hong Y, Eleftheriou D, et al. Endothelial injury and repair in systemic vasculitis of the young. Arthritis Rheum. 2010;62:1770–80. doi: 10.1002/art.27418. [DOI] [PubMed] [Google Scholar]
- 75.Duval A, Helley D, Capron L, et al. Endothelial dysfunction in systemic lupus patients with low disease activity: evaluation by quantification and characterization of circulating endothelial microparticles, role of anti-endothelial cell antibodies. Rheumatology. 2010;49:1049–55. doi: 10.1093/rheumatology/keq041. [DOI] [PubMed] [Google Scholar]
- 76.Baka Z, Senolt L, Vencovsky J, et al. Increased serum concentration of immune cell derived microparticles in polymyositis/dermatomyositis. Immunol Lett. 2010;128:124–30. doi: 10.1016/j.imlet.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 77.Nielsen CT, Ostergaard O, Johnsen C, et al. Distinct features of circulating microparticles and their relationship to clinical manifestations in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3067–77. doi: 10.1002/art.30499. [DOI] [PubMed] [Google Scholar]
- 78.Oyabu C, Morinobu A, Sugiyama D, et al. Plasma platelet-derived microparticles in patients with connective tissue diseases. J Rheumatol. 2011;38:680–4. doi: 10.3899/jrheum.100780. [DOI] [PubMed] [Google Scholar]
- 79.Bucciarelli P, Martinelli I, Artoni A, et al. Circulating microparticles and risk of venous thromboembolism. Thromb Res. 2011 doi: 10.1016/j.thromres.2011.08.020. doi:10.1016/j.thromres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 80.Distler JH, Akhmetshina A, Dees C, et al. Induction of apoptosis in circulating angiogenic cells by microparticles. Arthritis Rheum. 2011;63:2067–77. doi: 10.1002/art.30361. [DOI] [PubMed] [Google Scholar]
- 81.Biro E, Nieuwland R, Tak PP, et al. Activated complement components and complement activator molecules on the surface of cell-derived microparticles in patients with rheumatoid arthritis and healthy individuals. Ann Rheum Dis. 2007;66:1085–92. doi: 10.1136/ard.2006.061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ullal AJ, Reich CF, 3rd, Clowse M, et al. Microparticles as antigenic targets of antibodies to DNA and nucleosomes in systemic lupus erythematosus. J Autoimmun. 2011;36:173–80. doi: 10.1016/j.jaut.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Wassmer SC, Cianciolo GJ, Combes V, et al. Inhibition of endothelial activation: a new way to treat cerebral malaria? PLoS Med. 2005;2:e245. doi: 10.1371/journal.pmed.0020245. doi:10.1371/journal.pmed.0020245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Penet M-F, Abou-Hamdan M, Coltel N, et al. Protection against cerebral malaria by the low-molecular-weight thiol pantethine. Proc Natl Acad Sci USA. 2008;105:1321–6. doi: 10.1073/pnas.0706867105. [DOI] [PMC free article] [PubMed] [Google Scholar]