Abstract
Objective. The role of the adaptive immune system has not been explored in detail compared with the innate immune system in systemic JIA (sJIA) pathogenesis. The aim of this study was to examine the phenotype of circulating peripheral blood CD4+ T-cell subpopulations in a cross-sectional study of sJIA patients during disease remission on medication and during acute flare of the disease.
Methods. Flow cytometry was used to examine the phenotype and cytokine production of IFNγ-, IL-4- and IL-17-producing CD4+ T cells in the peripheral blood of 10 sJIA patients with active disease, 9 sJIA with inactive disease, 14 JIA patients with oligoarticular onset, 10 adult control subjects and 10 age-matched control subjects. In parallel, we examined the proportion of FoxP3+ Tregs.
Results. IFNγ- and IL-17-producing CD4+ T cells and IL-17-producing CD3+CD4− T cells were present at higher proportions in the peripheral blood of sJIA patients, irrespective of their disease status. Our data also confirm the known increase of the proportions of IFNγ-producing Th1 cells with increasing age and suggest an increase with age in the IL-17-producing CD4+ T-cell population.
Conclusion. This study is the first to describe significantly higher proportions of Th1 and Th17 T helper cell subsets in the peripheral blood of sJIA patients. These proinflammatory cells may play a pathogenic role in sJIA. Our data also emphasize the importance of using paediatric age-matched control subjects when evaluating the T-cell cytokine profile in JIA.
Keywords: interferon-gamma, interleukin-17, systemic JIA, peripheral blood, flow cytometry
Introduction
JIA is a clinically heterogeneous condition. There is strong evidence that dysregulation in cytokines that mediate innate immune responses plays a major role in the pathogenesis of the systemic JIA subtype (sJIA). For example, the cytokine IL-1 has an important role in sJIA. Pascual et al. [1] reported spontaneous expression of IL-1β in peripheral blood mononuclear cells (PBMCs) of sJIA patients and also an IL-1 signature in gene expression studies. IL-6 production is markedly increased in the serum of sJIA patients, and IL-6 levels have been shown to correlate with disease activity, fever pattern and platelet count [2, 3]. The weak association of HLA genes with sJIA as compared with the strong HLA class II associations reported in the other JIA subtypes [4], and the fact that treatment of sJIA patients with biologic agents blocking IL-1β and IL-6 signalling is highly effective in a substantial proportion of patients resistant to conventional therapies [1, 5–7], lend support to the view that the pathology of sJIA is mainly within the innate immune system. However, the cytokine milieu has a significant influence on the polarization of naive T cells. For example, IL-1 and IL-6 are cytokines that differentiate T cells to IL-17-secreting cells (Th17), which are implicated in autoimmune diseases [8, 9]. Moreover, in addition, Th17 T cells can acquire a Th1 phenotype (Th17/1) and secrete IFNγ [10], contributing to the proinflammatory state.
sJIA is a systemic disease, and increased circulating cytokines have been detected during active disease [1–3]. Therefore the proportions of different phenotypes of T lymphocytes may be altered in the peripheral blood. So far, few studies have examined circulating T cells in children with sJIA. Moreover, the findings have been inconsistent, with reports showing a decreased number of Th2 cells or a mixed Th1/Th2 cytokine profile [11, 12]. In this study we examined the phenotype of circulating peripheral blood T-cell subpopulations in a cross-sectional study of sJIA patients during disease remission on medication and during acute flare of the disease.
Materials and methods
Patients and samples
sJIA patients were divided into two groups: an inactive disease group with no systemic features, no arthritis and normal CRP/ESR, referred to as quiescent in this article. The second group consisted of children with active disease; all have arthritis with or without systemic features and raised CRP/ESR, referred to as flare or active in this article (Table 1). For comparison, age-matched healthy control subjects and oligoarticular onset JIA patients were recruited. Peripheral blood samples of 10 sJIA patients with active disease, 9 with quiescent disease, 14 JIA patients with oligoarticular JIA (9 with extended oligoarthritis and 5 with persistent oligoarthritis), 10 healthy adult control subjects and 10 healthy age-matched control subjects were included in this study. Data from seven of the oligoarticular JIA patients cited in this study were previously published data [13].
Table 1.
Demographic and clinical characteristics of the sJIA patients at the time of sampling for this study
| Patient demographics | Flare (n = 10) | Quiescent (n = 9) |
|---|---|---|
| Sex, M/F | 4/6 | 6/3 |
| Age years, median (range) | 10 (4–17) | 14 (10–15) |
| Age at disease onset years, median (range) | 5.5 (2–13) | 5 (2–13) |
| Disease duration years, median (range) | 3 (1–9) | 9 (2–12) |
| CRP mg/l, median (range) | 13.6 (4–86) | <5 (<5–<5) |
| ESR mm/h, median (range) | 42 (2–95) | 4 (<1–<10) |
| Fever ± rash | 6/10 | 0/9 |
| Number of active joints, median (range) | 5.5 (0–17) | 0 (0–0) |
| Number of joints with a limitation of movement, median (range) | 6 (0–17) | 0 (0–0) |
| VAS of disease activity (physician’s), median (range), mm | 36 (1–70) | 0 (0–10) |
| VAS of overall well-being (parent’s), median (range), mm | 33.5 (0–70) | 1 (0–3) |
| Functional ability (CHAQ), median (range) | 0.66 (0–2) | 0.08 (0–0.13) |
| Pred | 2/10 | NR |
| Pred + MTX | 6/10 | NR |
| Pred + TOC | 1/10 | 2/9 |
| MTX + anakinra | 1/10 | NR |
| MTX + TOC | NR | 3/9 |
| Pred + MTX + TOC | NR | 2/9 |
M/F: male/female; VAS: visual analogue scale; CHAQ: Childhood Health Assessment Questionnaire; Pred: prednisolone; TOC: tocilizumab; NR: not reported.
Clinical assessment at the time of sampling was performed using the core set of variables for measurement of JIA disease activity as defined by Giannini et al. [14]. All patients attended the Great Ormond Street Hospital for children in London. Ethics committee approval from Great Ormond Street Hospital for Children NHS Trust and Institute of Child Health Research Ethics Committee and full written informed consent from the parents, adult control subjects and parents of the age-matched control subjects were obtained. PBMCs were isolated using standard Ficoll-Hypaque density-gradient centrifugation and cryopreserved until tested.
Analysis of cytokine production
For stimulation to analyse cytokine production, PBMCs were cultured for 3 h in the presence of 50 ng/ml of phobol myristate acetate, 500 ng/ml of ionomycin and 5 µg/ml of Brefeldin A.
Standard five-colour flow cytometry was performed. For surface markers, the following specific anti-human mAbs were used: peridinin chlorophyll A protein or Qdot605-conjugated CD4 (clones SK3, S3.5), fluorescein isothiocyanate-conjugated anti-CD4 (clone Q4120), phycoerythrin (PE)-Cy7 or peridinin chlorophyll A protein-Cy5.5-conjugated anti-CD3 (clones UCHT1, OKT3) and PE-conjugated anti-CD25 (clone ACT-1).
For intracellular cytokine staining we used fluorescein isothiocyanate or V500-conjugated IFNγ (clone 25723.11, B27), Alexa Fluor 647 or V450-conjugated IL-17 (clone eBio64CAP17, N49-653) and PE-conjugated IL-4 (clone 3010.211). FoxP3 was detected in unstimulated cells using allophycocyanin-conjugated anti-FoxP3 (clone PCH101) antibody. Data were collected on a FACSCalibur or LSRII flow cytometer (both from Becton Dickinson and Co., Franklin Lakes, NJ, USA) and analysed using FlowJo software (Tree Star, Ashland, OR, USA).
Statistical analysis
Data were analysed with GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Comparisons between groups were made using Mann–Whitney U test. P ≤ 0.05 was considered significant.
Results
Enrichment of Th1 cells in the peripheral blood of sJIA patients regardless of disease status
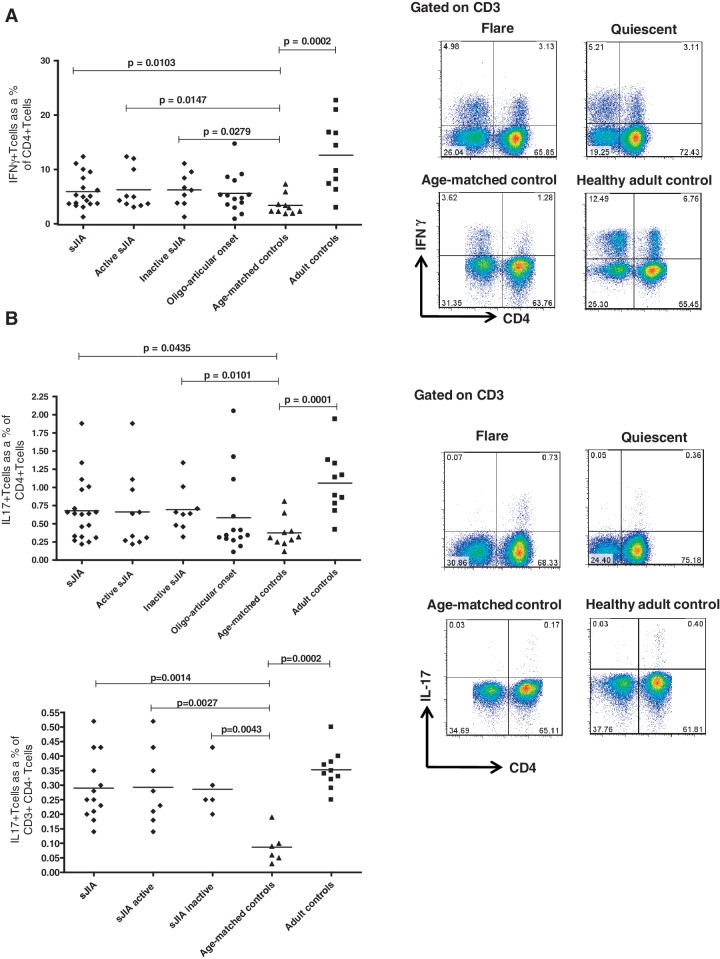
We examined the proportion of IFNγ-producing CD4+ T cells in the peripheral blood of 10 systemic patients with active disease and in 9 patients with quiescent disease. The disease activity measures, demographics and medications taken at the time of sampling are shown in Table 1. There was a significantly higher proportion of IFNγ-producing CD4+ T cells in PBMCs of all sJIA patients regardless of the disease status [median 5.03%; interquartile range (IQR) 3.7–8.99] as compared with PBMCs of paediatric age-matched control subjects (median 2.65%; IQR 2.13–4.74; P = 0.0103) (Fig. 1A). The same trend was observed in the two subgroups of systemic patients with active and inactive disease when analysed separately (P = 0.0147 and 0.0279, respectively).
Fig. 1.
Enrichment of Th1 cells and proportion of IL-17-producing CD4+ T cells and CD3+CD4− T cells in the peripheral blood of sJIA patients.
Proportions of (A) IFNγ- and (B, top left panel) IL-17-producing CD4+ T cells in PBMCs from sJIA patients, oligoarticular JIA patients, age-matched paediatric control subjects and healthy adult control subjects. Horizontal bars show median values. Shown in the right panel (A and B) are representative dot plots from PBMCs of sJIA patients at disease flare and remission (quiescent), from age-matched paediatric controls and healthy adult controls. The cells were gated on CD3. The bottom left of Figure 1B shows the proportion of IL-17-producing CD3+CD4− T cells in PBMCs from sJIA patients and age-matched paediatric control subjects; IL-17-producing CD3+CD4− T cells in PBMCs from oligoarticular JIA patients are not available for comparison.
To investigate whether the observed difference is specific to the systemic subtype of JIA, we examined 14 patients with oligoarticular JIA. In PBMCs from oligoarticular JIA patients, there was a trend for a higher median number of IFNγ-producing CD4+ T cells (4.62%; IQR 3.22–6.62) compared with age-matched control subjects (2.65%; IQR 2.13–7.32), but this was not statistically significant (Fig. 1A).
We also examined the proportion of IFNγ-producing and IL-4-producing cells among CD4+ T cells and the CD3+CD4− T-cell population (which includes CD8 cells and some γδ T cells). These proportions did not differ significantly between sJIA patients and paediatric age-matched controls, even when disease status was taken into consideration (data not shown).
Increased frequency of circulating IL-17-producing CD4+ and CD3+CD4− T cells in sJIA
We investigated whether there was a difference in the proportion of circulating Th17 cells in sJIA patients as compared with healthy paediatric age-matched controls. Children with sJIA showed a higher proportion of IL-17-producing CD4+ T cells (median 0.64%; IQR 0.32–0.97) compared with the control group (median 0.32%; IQR 0.24–0.53), P = 0.0435 (Fig. 1B). Interestingly, patients with inactive disease showed a significantly higher proportion of Th17-producing CD4+ T cells (median 0.64%; IQR 0.47–0.86), P = 0.0101 than the paediatric age-matched control subjects (Fig. 1B). Likewise, the frequency of CD3+CD4− T cells producing IL-17 was significantly different in sJIA patients compared with control subjects (Fig. 1B), although the observed percentages were much smaller than that of CD4+ T cells. We compared these results with the proportion of IL-17-producing CD4+ T cells in the PBMCs of the other 14 patients with oligoarticular JIA, including data from seven patients published in a previous study [13]. No significant difference was observed (Fig. 1B).
Given that we have previously shown that in proinflammatory environments, notably the synovial compartment of JIA, Th17 cells may convert into IFNγ-producing cells, likely via a double-positive Th17/Th1 phenotype, we investigated the frequency of Th17/Th1 double-positive cells in the peripheral blood of sJIA patients. However, we found no significant difference in the proportion of this double-positive population between peripheral blood of sJIA patients (median 0.11%; IQR 0.0–0.14) and age-matched paediatric control subjects (median 0.07%; IQR 0.05–0.10).
No difference in the proportion of FoxP3+ Tregs in PBMCs of sJIA compared with adult or age-matched paediatric control subjects.
We examined the number of circulating regulatory FoxP3+ CD4+ T cells in sJIA patients because of the increased IL-17-producing CD4+ T cells. We have previously found that FoxP3+ Tregs have a reciprocal relationship with IL-17-producing T cells in the SF of JIA patients with oligo- and polyarticular JIA [13], we tested whether this was also the case in the peripheral blood of sJIA patients. No significant difference was observed between the sJIA patients and the paediatric and adult control groups, even when stratifying according to disease activity (data not shown).
Importance of using age-matched control subjects in paediatric studies
We analysed PBMCs from both healthy adult and age-matched paediatric control subjects for the proportion of CD4+ cells secreting IFNγ, IL-17 or IL-4 using flow cytometry. Our data confirm an increase of the proportion of IFNγ-producing CD4+ T cells with increasing age [15]; being significantly higher in the adult control subjects (median 12.09%; IQR 6.83–18.90) as compared with the healthy paediatric control subjects (median 2.65%; IQR 2.13–4.74), P = 0.0002 (Fig. 1A).
Our data also suggest that Th17 cells are present at higher proportions within both the CD4+ and CD3+CD4− T-cell populations in healthy adults compared with healthy children, P = 0.0001 and 0.0002, respectively (Fig. 1B). No significant difference was observed when comparing IL-4-producing CD4+ T cells (Th2) and FoxP3+ Tregs in the age-matched control subjects and adult control subjects (data not shown).
Discussion
In this study we have shown for the first time that both IFNγ-producing Th1 cells and IL-17-producing Th17 cells are present in higher proportions in the peripheral blood of sJIA patients compared with paediatric age-matched controls, whereas no significant difference was observed in the proportions of IL-4-producing Th2 cells or FoxP3+ Tregs.
Previous studies of T-cell phenotypes in PBMCs of sJIA have been inconsistent. Raziuddin et al. [12] showed increased secretion of IL-4 and IL-10 with concomitant deficiency of IL-2 and IFNγ in the peripheral blood of sJIA patients with active disease. However, Huang et al. [11] reported a lower number of IL-4-producing Th2 cells, with no significant difference in IFNγ-producing Th1 cells.
The proinflammatory cytokines IL-1 and IL-6 have been implicated in the pathogenesis of sJIA [1–3], with higher plasma levels of IL-1 and IL-6 reported in sJIA patients compared with paediatric age-matched control subjects [16]. Because these cytokines are also involved in the development of Th17 cells [8, 9], it is interesting that we found an increased proportion of IL-17 in both CD4+ T cells and CD3+CD4− T cells in sJIA patients. Our results also show that there is an increased proportion of IFNγ-producing cells in sJIA irrespective of clinical disease status when compared with paediatric age-matched controls, unlike previous publications. These findings may represent an underlying pathology that may lead to Th1 and Th17 cell populations persisting even when there are no detectable markers of inflammation or symptoms. In future, it would be interesting to compare the proportions of Th1 and Th17 in patients early in their disease process at presentation, a time when the pathology could reflect triggering mechanisms leading to chronicity, and in patients in remission off medication to see whether these changes are specific markers of sJIA pathology. Although we have previously published data showing that the Th17 phenotype can evolve into IFNγ-producing T cells within a localized inflammatory environment [10], we did not observe a significant difference in the double-positive population between peripheral blood of sJIA patients and paediatric age-matched controls. In sJIA, the mechanisms for the coexistence of increased proportions of Th1 and Th17 cells in the peripheral blood may be different from previous findings in the SF. The observed increased proportion of IL-17 irrespective of T-cell subpopulation may be owing to the fact that all IL-17+ T cells, including CD4+, CD8+ and CD4−CD8− cells, originate from CD161-expressing T-cell precursors, which upregulate IL-17 secretion in response to IL-1β and IL-23 [17]. Therefore, the enrichment of IL-17+ cells in both CD4+ and CD4− T-cell subsets in sJIA suggests a common mechanism, possibly a result of dendritic cell/antigen presenting cell polarization towards IL-1β/IL-23 secretion.
An increasing role for Th17 cells has been found in other diseases of innate immunity such as cryopyrin-associated diseases [18, 19], with NLRP3 having an important role in Th17 responses [20]. Because sJIA is now considered to have more in common with these autoinflammatory diseases, our finding is consistent with this hypothesis. Therefore the interrelationships between innate immunity mediators and effector T cell function in sJIA merit further investigation. There may be a role for adaptive immunity in a disease that is generally regarded as typically autoinflammatory, especially in the case of sJIA.

Acknowledgements
The authors would like to thank the patients and their parents for donation of samples to the study. In addition, the authors thank the staff of Great Ormond Street Hospital for assistance with the collection of clinical samples, members of the laboratory for help with sample processing and Ms Laura Kassoumeri for help with clinical data collection. E.O. and R.H. were supported by an Arthritis Research UK programme grant (17287); K.N. by an Arthritis Research UK Fellowship (17998); H.M. and S.U. by SPARKS UK (08ICH09) and A.P. by the Nuffield Foundation (Oliver Bird PhD Programme). The work is within the remit of the ARUK programme grant.
Funding: This work was supported as part of a programme grant (17287), funded by Arthritis Research UK.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201:1479–86. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rooney M, David J, Symons J, et al. Inflammatory cytokine responses in juvenile chronic arthritis. Br J Rheumatol. 1995;34:454–60. doi: 10.1093/rheumatology/34.5.454. [DOI] [PubMed] [Google Scholar]
- 3.De Benedetti F, Massa M, Robbioni P, et al. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991;34:1158–63. doi: 10.1002/art.1780340912. [DOI] [PubMed] [Google Scholar]
- 4.Thomson W, Barrett JH, Donn R, et al. Juvenile idiopathic arthritis classified by the ILAR criteria: HLA associations in UK patients. Rheumatology. 2002;41:1183–9. doi: 10.1093/rheumatology/41.10.1183. [DOI] [PubMed] [Google Scholar]
- 5.Woo P, Wilkinson N, Prieur AM, et al. Open label phase II trial of single, ascending doses of MRA in Caucasian children with severe systemic juvenile idiopathic arthritis: proof of principle of the efficacy of IL-6 receptor blockade in this type of arthritis and demonstration of prolonged clinical improvement. Arthritis Res Ther. 2005;7:R1281–8. doi: 10.1186/ar1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lequerre T, Quartier P, Rosellini D, et al. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset Still disease: preliminary experience in France. Ann Rheum Dis. 2008;67:302–8. doi: 10.1136/ard.2007.076034. [DOI] [PubMed] [Google Scholar]
- 7.Gattorno M, Piccini A, Lasiglie D, et al. The pattern of response to anti-interleukin-1 treatment distinguishes two subsets of patients with systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2008;58:1505–15. doi: 10.1002/art.23437. [DOI] [PubMed] [Google Scholar]
- 8.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–9. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 10.Nistala K, Adams S, Cambrook H, et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci USA. 2010;107:14751–6. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang JL, Kuo ML, Hung IJ, et al. Lowered IL-4-producing T cells and decreased IL-4 secretion in peripheral blood from subjects with juvenile rheumatoid arthritis. Chang Gung Med J. 2001;24:77–83. [PubMed] [Google Scholar]
- 12.Raziuddin S, Bahabri S, Al Dalaan A, Siraj AK, Al Sedairy S. A mixed Th1/Th2 cell cytokine response predominates in systemic onset juvenile rheumatoid arthritis: immunoregulatory IL-10 function. Clin Immunol Immunopathol. 1998;86:192–8. doi: 10.1006/clin.1997.4457. [DOI] [PubMed] [Google Scholar]
- 13.Nistala K, Moncrieffe H, Newton KR, et al. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58:875–87. doi: 10.1002/art.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannini EH, Ruperto N, Ravelli A, et al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40:1202–9. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Wedderburn LR, Woo P. Type 1 and type 2 immune responses in children: their relevance in juvenile arthritis. Springer Semin Immunopathol. 1999;21:361–74. doi: 10.1007/BF00812262. [DOI] [PubMed] [Google Scholar]
- 16.de Jager W, Hoppenreijs EP, Wulffraat NM, et al. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Ann Rheum Dis. 2007;66:589–98. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maggi L, Santarlasci V, Capone M, et al. CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol. 2010;40:2174–81. doi: 10.1002/eji.200940257. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi A, Iwata H, Ohnishi H, et al. Interleukin-17 expression in the urticarial rash of familial cold autoinflammatory syndrome: a Case Reports. Br J Dermatol. 2010;163:1351–3. doi: 10.1111/j.1365-2133.2010.09978.x. [DOI] [PubMed] [Google Scholar]
- 19.Lasiglie D, Traggiai E, Federici S, et al. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ather JL, Ckless K, Martin R, et al. Serum amyloid A activates the NLRP3 inflammasome and promotes Th17 allergic asthma in mice. J Immunol. 2011;187: 64–73. doi: 10.4049/jimmunol.1100500. [DOI] [PMC free article] [PubMed] [Google Scholar]