Abstract
BACKGROUND AND PURPOSE
Exposure to an acute stress inhibits gastric emptying and stimulates colonic transit via central neuropeptide Y (NPY) pathways; however, peripheral involvement is uncertain. The anxiogenic phenotype of NPY−/− mice is gender-dependent, raising the possibility that stress-induced gastrointestinal (GI) responses are female-dominant through NPY. The aim of this study was to determine GI transit rates, corticosterone levels and food intake after acute restraint (AR) or novel environment (NE) stress in male and female NPY−/− and WT mice.
EXPERIMENTAL APPROACH
Upper gastrointestinal transit (UGIT) (established 30 min after oral gavage) and corticosterone levels were determined under basal or restrained conditions (30 min) and after treatment i.p. with Y1 antagonist BIBO3304 or Y2 antagonist BIIE0246. Faecal pellet output (FPO) was established after AR and treatment i.p. with NPY in the NE, as were colonic bead expulsion rates.
KEY RESULTS
UGIT and FPO were similar in unrestrained male and female mice. NPY−/− females displayed significantly slower UGIT than NPY−/− males after AR, but both genders displayed significantly higher FPO and reduced food intake relative to WT counterparts. Peripheral NPY treatment increased bead expulsion time in WT mice. AR male NPY−/− mice had higher levels of corticosterone than male WT mice; whilst in AR WT mice, after peripheral Y1 and Y2 receptor antagonism in males, and Y2 antagonism in females, corticosterone was significantly elevated.
CONCLUSIONS AND IMPLICATIONS
NPY possesses a role in the gender-dependent susceptibility to stress-induced GI responses. Furthermore, NPY inhibits GI motility through Y2 receptors and corticosterone release via peripheral Y1 and Y2 receptors.
Keywords: neuropeptide Y, stress, gender, Y2 receptor, Y1 receptor, upper gastrointestinal motility, faecal pellet output, bead propulsion, corticosterone
Introduction
Individuals are continuously exposed to different forms of psychological, social and physical stressors; and this chronic or repeated exposure is widely believed to play a major role in the pathogenesis, or exacerbation of symptoms, of many diseases (Heraclides et al., 2011). Often stress-associated disorders, such as functional gastrointestinal (GI) diseases (Maunder and Levenstein, 2008) or eating disorders (Blehar, 1995) are more prevalent among women than men but the mechanisms underlying this gender differentiation are largely unknown.
The 36-amino-acid neuropeptide Y (NPY) is one of the most abundantly expressed neuropeptides in the central and peripheral nervous systems (Dumont et al., 1998) and a key mediator in the responses to both acute and chronic stress. Many experimental stressors induce NPY release (Thorsell et al., 1999) and up-regulate both NPY mRNA and its receptors' mRNA (Y1, Y2 and Y5; nomenclature follows Alexander et al., 2011), which are responsible for the physiological actions of NPY in the periphery and brain (Michel et al., 1998). Acute stress up-regulates NPY in the hypothalamic arcuate (ARC) (Kas et al., 2005) and paraventricular nuclei (PVN) (Dube et al., 1992), where metabolic and stress-related signals are integrated and appropriate feeding, neuroendocrine and visceral responses are initiated. In the PVN, NPY activates the hypothalamic–pituitary–adrenal (HPA) axis and modulates the visceral stress responses mediated through corticotrophin-releasing hormone (CRH) pathways (Dimitrov et al., 2007). Additionally, NPY is potently anxiolytic (Karl et al., 2008), acting through Y1 receptors in the amygdala to inhibit CRH signalling and terminate the behavioural stress and anxiety responses (Kask et al., 2001). In humans, haplotype-driven NPY expression is able to predict responses to stress challenges and is inversely correlated with trait anxiety levels (Zhou et al., 2008). Furthermore, NPY concentrations in the cerebrospinal fluid of patients with post-traumatic stress disorder are low (Sah et al., 2009). Ablation of the NPY gene from mice results in a gender-dependent anxiogenic phenotype, whereby males and females display different anxiogenic responses in behavioural tests, indicating NPY has a sexually dimorphic role in behavioural stress responses (Painsipp et al., 2011). Additionally, GI inflammation, which is known to enhance anxiety in a gender-dependent manner, produces different behavioural responses to stress challenges in female and male NPY−/− mice (Painsipp et al., 2011).
An inhibition of gastric emptying and a stimulation of colonic motility are hallmark GI responses to an acute stress, demonstrated by a number of experimental stressors including exposure to painful stimuli and anger in humans (Rao et al., 1998) or restraint stress in experimental animals (Martinez et al., 2004). PVN Y1 receptors and CRHR2 receptors have been implicated in the central component of stress-induced upper GI motor alterations (Tache et al., 1987; Chen et al., 1997; Martinez et al., 2004), in addition to peripheral sympathetic pathways involving α-adrenoceptors (Nakade et al., 2005), whilst PVN Y1 and downstream CRHR1 receptors are implicated in stress-stimulated colonic motility (Monnikes et al., 2000; Tebbe et al., 2005). In support of these findings, centrally administered NPY or CRH inhibits gastric emptying and stimulates colonic transit in conscious rodents (Matsuda et al., 1993; Monnikes et al., 2000; Martinez et al., 2004). However, NPY is also involved in the peripheral modulation of GI function, where it may counterbalance its central stress-induced stimulating effects on colonic motility (Tough et al., 2011). NPY is found within the enteric nervous system (ENS) in inhibitory secretomotor submucosal nerves co-localized with vasoactive intestinal polypeptide (VIP; Fantaguzzi et al., 2009) and in inhibitory myenteric motor neurons (Sang and Young, 1996). Endogenous NPY inhibits electrolyte secretion (a combination of Y1- and Y2-mediated effects; Tough et al., 2011) and contracts longitudinal smooth muscle in vitro (Hyland et al., 2003), in addition to inhibiting colonic motility in vivo by activating neuronal Y2 receptors (Wang et al., 2010; Tough et al., 2011).
Feeding behaviour is also affected by stress, and although the feeding response often depends on the type of stress delivered, an acute stress such as restraint consistently suppresses food intake experimentally (Kas et al., 2005). Signalling within the feeding circuitry of the ARC includes the orexigenic NPY neurons and affects eating behaviour under adverse conditions. Here, NPY potently stimulates food intake and induces weight gain through the PVN Y1 and Y5 receptors (Chaudhri et al., 2006).
The sexually dimorphic role of NPY in behavioural stress responses revealed in NPY−/− mice (Painsipp et al., 2011) raises the possibility that physiological stress responses mediated through NPY pathways may also differ between males and females. Given the critical role of NPY in both GI motility (Tough et al., 2011) and food intake in addition to stress reactivity, it is conceivable that a gender specific role for NPY could contribute to the marked female predominance of stress-associated diseases. The present investigation therefore set out to establish if there were differences in stress-induced upper and lower GI motor function and food intake in male and female NPY−/− mice. In addition, the role of peripheral NPY and the Y1 and Y2 receptors in the modulation of stress-induced changes in GI transit was established, with previous evidence indicating Y2 receptors play a role in inhibiting transit (Wang et al., 2010). Another objective was to further elucidate the role of peripheral NPY in stress-induced corticosterone release, which Y receptors were involved, and to determine whether there were any gender differences at this level.
Methods
Targeted deletion of NPY
NPY−/− mice without the entire coding sequence of NPY, including the initiation start codon, were generated by homologous recombination in embryonic stem cells as described previously (Karl et al., 2008). These mice exhibited no obvious abnormalities and appeared healthy (Edelsbrunner et al., 2009). WT and NPY−/− were on a mixed 129/SvJ/ C57BL/6 background and housed under controlled conditions (12:12 h light/dark cycle, lights on 07:00 h, 22 ± 2°C) and provided with standard chow and water ad libitum except during experimentation. All mice were aged 10–16 weeks and weight-matched within genders where possible (mean weight of WT female mice: 24.0 ± 0.4 g, WT male: 27.3 ± 0.5 g, NPY−/− female: 21.2 ± 0.3 g and NPY−/− male mice: 26.4 ± 0.6 g, n= 26–30). The results of all studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All experiments were performed in accordance with the Animals (Scientific procedures) Act 1986. Upper GI transit (UGIT), bead propulsion experiments and blood sampling for corticosterone levels were conducted between 10:00 and 13:00 h, whilst faecal pellet output (FPO) experiments were conducted between 10:00 and 14:00 h, to minimize any influence of circadian rhythm.
Upper GI under non-restrained and restrained conditions
Mice were deprived of food for 16 h prior to experimentation, although water was provided ad libitum. UGIT was determined by identifying the leading front of an intragastrically administered charcoal meal marker (10% plant charcoal in 5% gum acacia) in the small intestine as described by Pol et al. (2005). Mice were then either restrained (see below) or placed back in the home cage. After 30 min, animals were killed by dislocation of the neck, and the small intestine was isolated by cutting at the pyloric and ileocaecal junctions. The length of the small intestine was measured, and the distance travelled by the charcoal meal was determined. For each mouse, UGIT was calculated as the % of the distance travelled by the charcoal, relative to the total length of the small intestine.
Acute restraint model
Mice were restrained for 30 min in a capped and ventilated plastic centrifuge tube, which allowed for very limited movement. To establish UGIT or corticosterone levels from trunk blood, the animals were removed from the restraining tubes and killed immediately by dislocation of the neck. In WT mice, BIIE0246 (2 mg·kg−1), BIBO3304 (100 µL of 0.4 mM) or vehicle (100 µL of 10% DMSO), as delivered previously by Tough et al. (2011), were administered i.p. 15 min prior to restraint and therefore 45 min prior to death, to determine UGIT or corticosterone levels. In another experimental group, the number of pellets produced during 30 min restraint was counted, and the mice were removed and placed in a cage to monitor food intake for the subsequent 4 h. Normal food intake after acclimatization in the cages for 3 days was also recorded.
Novel environment stress model
Naive mice, or WT and NPY−/− mice administered i.p. with vehicle (100 µL saline) or NPY (8 nmol·kg−1) 10 min previously were taken out of home cages (group housed) and placed individually in rat cages with a grid bottom. FPO was measured after 15 min and 4 h in this environment. Weighed food pellets were provided throughout and then re-weighed after 4 h to establish food intake during this time.
Corticosterone analysis
Blood was collected and allowed to clot for 30 min at room temperature, the blood serum was extracted after 20 min centrifuging at 562×g and samples were stored at −20° until required. A commercial RIA kit with sensitivity of 7.5 ng·mL−1 was used with intra- and the inter-assay variations of 10.4% and 14.2% respectively.
Bead propulsion
Mice were deprived of food for 16 h prior to experimentation, although water was provided ad libitum. Distal colonic propulsion was measured according to the methods described by Koslo et al. (1986). Ten minutes after administration of vehicle (100 µL saline) or NPY (8 nmol·kg−1), mice were placed under isoflurane anaesthesia; and a 2 mm bead was inserted 2 cm into the distal colon of each mouse using blunt tubing (Portex, 1.7 × 0.4 mm). The mouse was subsequently placed into a grid bottom cage to which it had been acclimatized and the time to expulsion measured.
Statistical analyses
For UGIT, FPO and bead propulsion measurements, single comparisons between data groups were performed using Student's unpaired t-test, whereas multiple comparisons used one-way anova with Bonferoni's post test, to compare data within a genotype. Two-way anova with Bonferoni's post test was used to compare each gender and genotype or WT response to vehicle and agonist/antagonist treatments, or control/stress treatments. P-values ≤ 0.05 were statistically significant.
Materials
BIBO3304 and BIIE0246 were gifts from Boehringer-Ingelheim Pharma KG (Biberach an der Riss, Germany), and stock solutions were dissolved in 10% dimethyl sulfoxide (DMSO) and stored at −20°C. NPY was purchased from Bachem (St. Helens, UK). A commercial corticosterone RIA kit was purchased from MP Biomedicals.
Results
UGIT under basal and restrained conditions
The role of endogenous NPY in UGIT was determined 30 min after the oral gavage of a non-nutritive charcoal meal in male and female WT and NPY−/− mice, under basal and acute restraint conditions. Restraint for 30 min did not affect the rate of UGIT in female WT mice compared with male WT mice, although there was a tendency for slower transit after restraint in both WT genders (Figure 1). In contrast, restraint stress significantly slowed UGIT in female NPY−/− mice compared with unrestrained females, whilst males showed a non-significant slowing of transit. Furthermore, restraint significantly slowed the rate of UGIT in female NPY−/− mice compared with male NPY−/− mice UGIT (Figure 1).
Figure 1.
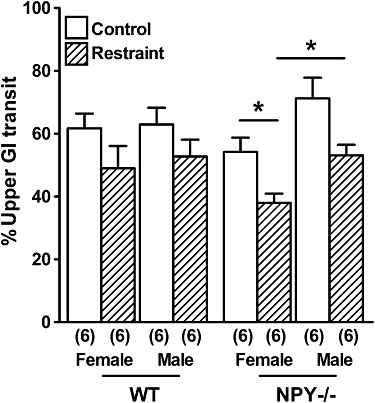
The rates of UGIT in female and male WT mice and NPY−/− mice under normal conditions or after 30 min restraint stress. UGIT was significantly slower in female NPY−/− mice after restraint stress and compared with restrained male NPY−/− mice (*P < 0.05). Each column is the mean + 1 SEM from the number of observations shown in parenthesis.
Effect of restraint on FPO and food intake
During restraint stress, female and male NPY−/− mice produced more faecal pellets than WT mice of the same gender, although this difference was only significant in females (Figure 2A). Furthermore, female NPY−/− mice ate significantly less than female WT mice in the 4 h after restraint stress (Figure 2B); however, male WT mice ate a similar amount as male NPY−/− mice (Figure 2B). Female and male NPY−/− mice ate significantly less in the 4 h after restraint compared with respective non-restrained food intake (Figure 2B).
Figure 2.
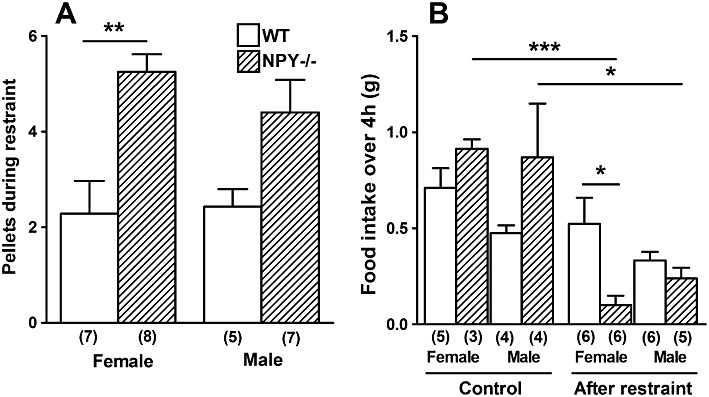
The effect of 30 min restraint stress on (A) the number of faecal pellets produced during restraint and (B) food intake in 4 h under non-stressed conditions (controls) and in the 4 h immediately following restraint in both male and female WT and NPY−/− mice. In (A), NPY−/− females produced significantly more pellets during restraint stress than WT females (**P < 0.01), and in (B), NPY−/− females ate significantly less than WT females (*P < 0.05), NPY−/− females also ate significantly less than when not restrained and acclimatized to the cages (***P < 0.001), as did NPY−/− males (*P < 0.05). Each column is the mean + 1 SEM and the number of observations in parenthesis.
Effect of restraint and either a Y1 or Y2 antagonist i.p. on UGIT
Under normal and restrained conditions, the effect of vehicle, a Y1 (BIBO3304) or a Y2 (BIIE0246) antagonist was determined on UGIT in female and male WT mice. In both female and male WT mice, BIBO3304 and BIIE0246 non-significantly increased UGIT; however, after acute restraint, BIBO3304 significantly reduced UGIT compared with unrestrained UGIT in both genders (Figure 3). In contrast, after an acute restraint stress, BIIE0246 administration increased UGIT compared with vehicle-treated mice; although this difference was only significant in females.
Figure 3.
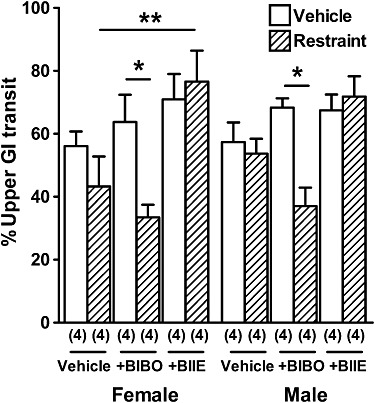
The effect of 30 min restraint on UGIT in the absence and presence of BIBO3304 and BIIE0246. Female and male WT mice displayed significantly slower UGIT after BIBO3304 administration before 30 min restraint compared with normal UGIT after BIBO3304 (*P < 0.05), and female WT mice had significantly faster UGIT after BIIE0246 administration before 30 min restraint compared to vehicle-administered WT mice exposed to 30 min restraint (**P < 0.01). Each column is the mean + 1 SEM from the number of observations shown in parenthesis.
Effect of restraint and either Y1 or Y2 antagonist i.p. on plasma corticosterone levels
Plasma corticosterone levels were also determined in all groups under unrestrained conditions and immediately after acute restraint. WT mice had higher plasma corticosterone after restraint than under normal conditions, and this was significant in females (Figure 4A), whilst both genders of restrained NPY−/− mice displayed significantly higher plasma corticosterone than unrestrained NPY−/− mice of the same gender (Figure 4A). After restraint, NPY−/− males had significantly higher plasma corticosterone than WT males, while female NPY−/− and WT mice displayed similar steroid levels after restraint (Figure 4A).
Figure 4.
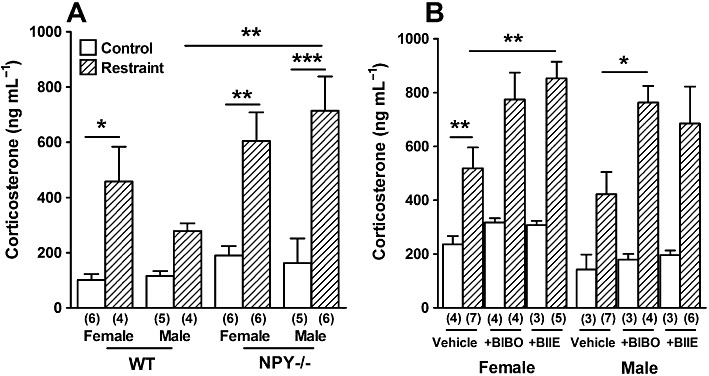
The effect of 30 min restraint on (A) plasma corticosterone in female and male WT and NPY−/− mice compared with normal conditions (B) plasma corticosterone levels in female and male WT mice administered i.p. with vehicle, BIBO3304 or BIIE0246. In (A), male NPY−/− mice had significantly higher plasma corticosterone after 30 min restraint (***P < 0.001) and compared with male WT mice after 30 min restraint (**P < 0.01). Female WT and NPY−/− mice had significantly higher corticosterone after restraint (*P < 0.05 and **P < 0.01 respectively). In (B), BIBO3304-treated WT male mice and BIIE0246-treated female WT mice had significantly higher corticosterone levels than controls after restraint (*P < 0.05 and **P < 0.01 respectively), whilst BIBO3304 and BIIE0246 had no effect on corticosterone in unrestrained mice of either gender (**P < 0.01, *P < 0.05, respectively). On comparing non-stressed vehicle controls (in B) with control WT mice (in A), it was found only female mice had significantly higher corticosterone levels after vehicle treatment (**P < 0.01, asterisk not shown). Each column is the mean + 1 SEM from the number of observations shown in parenthesis.
After administration of a Y1 or Y2 receptor antagonist, there was no change in plasma corticosterone under normal conditions in either gender of WT mice. However, administration of BIIE0246 significantly increased plasma corticosterone in restrained female WT mice and non-significantly in male WT mice compared with vehicle controls (Figure 4B), whilst BIBO3304 also increased corticosterone compared with vehicle in both genders of restrained WT mice; this was only significant in males (Figure 4B). These data indicate that acute restraint stress is indeed an effective stressor. Interestingly, vehicle treatment in non-stressed female WT mice significantly increased plasma corticosterone compared with basal corticosterone levels (Figure 4A and B).
Effect of novel environment stress on FPO and food intake
The effect of individual housing in a novel environment on FPO and food intake was determined in NPY−/− and WT mice of either gender after 15 min and up to 4 h. After 15 min, female and male NPY−/− mice displayed significantly higher FPO than WT counterparts (Figure 5A). FPO at 15 min and 4 h in a novel environment resulted in similar numbers of faecal pellets produced by female and male mice of the same genotype (Figure 5A and B). In contrast, after 4 h in the novel environment female NPY−/− mice exhibited significantly greater FPO than female WT mice (Figure 5B). There was no significant difference in food intake between the genders of each genotype over 4 h (Figure 5C). However, female and male NPY−/− mice displayed a significant reduction in food intake during this stress compared with their respective WT counterparts (Figure 5C). Furthermore, both female and male NPY−/− mice ate significantly less in a novel environment than under normal non-stressed conditions (non-stressed food intake shown in Figure 2B).
Figure 5.
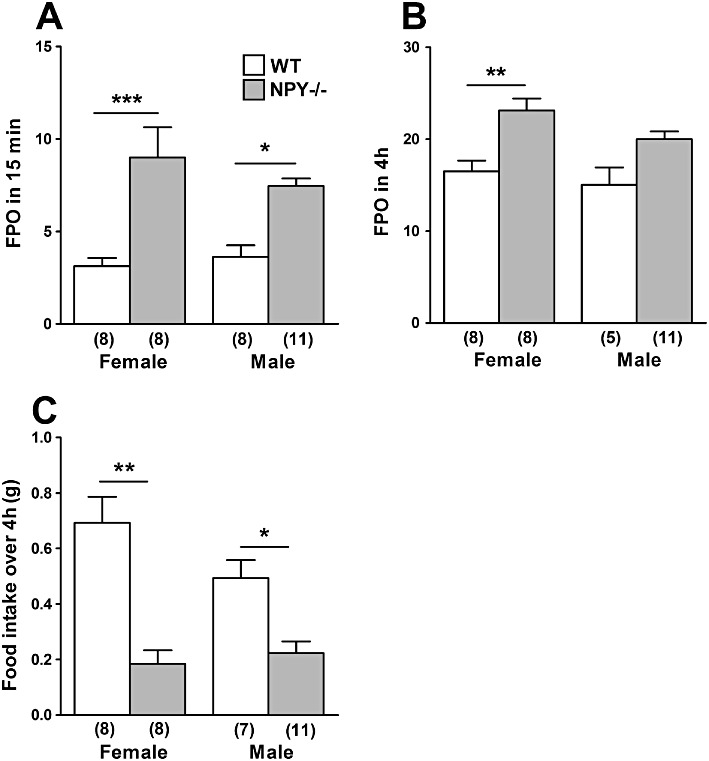
Effect of a novel environment stress in male and female WT and NPY−/− mice on faecal pellet output over (A) 15 min and (B) 4 h, and (C) on food intake in 4 h in the novel environment. In (A), female NPY−/− mice produced significantly more pellets after 15 min in a novel environment than female WT mice (***P < 0.001), and male NPY−/− mice produced more pellets than male WT mice (*P < 0.05). In (B), female NPY−/− mice produced more pellets than female WT over 4 h (**P < 0.01). In (C), female NPY−/− mice ate significantly less than female WT mice (**P < 0.01), whilst male NPY−/− mice ate significantly less than male WT mice over 4 h (*P < 0.05). Also, on comparing data in (C) with the respective control data in Figure 2B, NPY−/− females were found to eat significantly less in 4 h in the novel environment than when acclimatised to the cages (***P < 0.001, asterisk not shown), as did NPY−/− males (*P < 0.05, asterisk not shown). Each column is the mean + 1 SEM from the number of observations shown in parenthesis.
Effect of exogenous NPY on FPO and colonic bead expulsion
The effect of novel environment stress on FPO was determined in NPY−/− and WT mice of both genders administered vehicle or NPY i.p. After administration of NPY, WT and NPY−/− mice produced fewer stress-stimulated pellets within 15 min than their vehicle-treated counterparts, although this difference was not statistically significant (Figure 6A).
Figure 6.
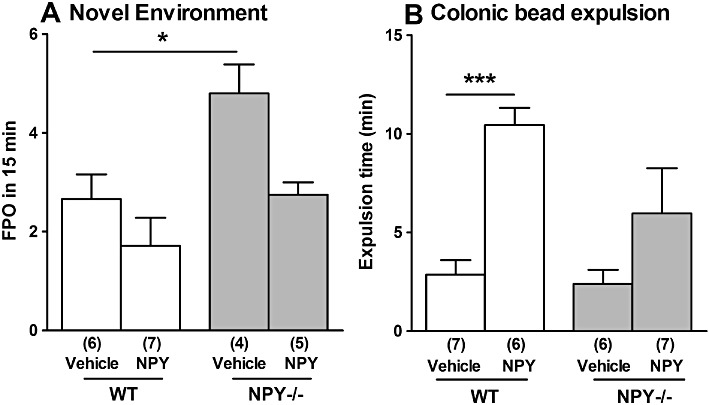
The effect of i.p. administration of NPY (8 nmol·kg−1) on (A) novel environment stress-induced faecal pellet output and (B) on the time taken for expulsion of a 2 mm bead, in WT and NPY−/− mice and compared with vehicle treatment (100 µL saline). In (A), NPY−/− mice produced significantly more pellets than WT mice after vehicle treatment (*P < 0.05). In (B), WT mice treated with NPY had a significantly increased time until bead expulsion compared with vehicle controls (***P < 0.001). Each column is the mean + 1 SEM from the numbers of observations shown in parenthesis.
In WT males, exogenous NPY administration significantly increased the time to expulsion of a bead inserted 2 cm into the distal colon compared with vehicle (Figure 6B). Removal of NPY had no significant effect on bead expulsion time after vehicle administration, although there was increased expulsion time after exogenous NPY treatment, but this was not statistically significant (Figure 6B).
Discussion
The present results show NPY−/− mice display the greatest inhibition of UGIT and elevated plasma corticosterone levels after acute restraint, and the largest FPO and inhibition of food intake when placed in a novel environment, all corroborating the well-established anxiogenic phenotype of NPY null mice (Karl et al., 2008; Painsipp et al., 2011). Importantly, this study also shows for the first time that male and female NPY−/− mice display a different susceptibility to stress-induced GI and feeding responses, whereby females are consistently more vulnerable.
After an acute restraint stress, WT mice (both genders) showed only a non-significant slowing of UGIT compared to normal UGIT rates, whilst restrained female NPY−/− mice had significantly slower UGIT. Previous evidence has suggested a role for NPY in the stress-induced inhibition of gastric emptying (Chen et al., 1997); however, our findings point to a redundancy in the central signalling pathway in the PVN, whereby NPY is not required for an inhibition of gastric emptying during stress. Under normal conditions, NPY−/− males displayed faster UGIT, whilst NPY−/− females exhibited slower UGIT than gender-matched WT mice. Taken together, these results suggest endogenous NPY does not contribute significantly to the inhibition of gastric emptying or activation of the ileal brake, a feedback mechanism in the GI tract stimulated by the presence of nutrients in the ileum (Van Citters and Lin, 1999), that is more likely to be mediated by PYY (Lin et al., 1996). These observations are supported by recent findings from our group showing that UGIT was unaffected by NPY removal but significantly faster in PYY null mice (Tough et al., 2011). It follows that NPY is not involved in inhibiting UGIT under normal conditions as NPY is a potent stimulator of feeding in the ARC and induces motor activity in the duodenum which mirrors that of the fasted state (Fujimiya et al., 2000). Nevertheless, female NPY−/− mice displayed slower UGIT than male NPY−/− mice after restraint, indicating stress-induced pathways leading to slower UGIT, known to be at least partially mediated through central CRF receptors (Martinez et al., 2004), are increased in females. It is possible these gender-differences are due to the influence of sex hormones and the oestrus cycle on PVN CRF2 expression (Iwasaki-Sekino et al., 2009). Additionally, an acute stress did not inhibit UGIT significantly in WT mice of either gender as expected; therefore, a more severe stress may be required to reveal this effect, whilst in anxiogenic NPY−/− mice, this stress was sufficient to reveal the inhibited UGIT, in addition to gender differences.
The present study has highlighted opposing roles of peripheral Y1 and Y2 receptors in modulating UGIT under acute stress. In unrestrained male and female WT mice, both peripherally acting, competitive antagonists BIBO3304 and BIIE0246 slightly, but non-significantly, increased UGIT. However, during restraint, BIBO3304 administration significantly reduced UGIT in both genders, whilst BIIE0246 led to a significant increase in UGIT in females and to a lesser degree in males. This is similar to the differential roles we revealed for the Y1 and Y2 receptors in colonic motility in vitro (Tough et al., 2011). As Y1 receptors are present on the cell bodies of nitrergic myenteric neurons (Peaire et al., 1997), most of which are motor nerves innervating the circular smooth muscle (Sang and Young, 1996), it is possible that the increased NPY released during stress will promote the activation of Y1 receptors to hyperpolarize these tonically active inhibitory neurons and reduce their activity. The presence of a Y1 antagonist would therefore prevent this tonic inhibition, inducing relaxation and slowing transit as we observed for post-restraint UGIT in the presence of the Y1 antagonist BIBO3304. In contrast, Y2-mediated stress mechanisms have the opposite effect upon UGIT. Y2 antagonism with BIIE0246 increased UGIT during stress, particularly in female mice, indicating that Y2 receptors are involved in a different tonically active intramural mechanism that inhibits UGIT under stressed conditions.
Exposure to a novel environment is an established rodent stressor, which induces colonic motor responses (Wang et al., 2010). In the novel environment, NPY−/− females produced significantly more pellets over 15 min compared with WT females and this difference lasted for up to 4 h. NPY−/− males had higher FPO than WT males within 15 min in a novel environment; however, this difference did not last 4 h. In addition, female NPY−/− mice produced more faecal pellets during 30 min restraint than female WT mice; and the same, although non-significant, trend was observed in males. These data point to a moderate gender-dependent colonic stress response involving NPY. In addition, the greater vulnerability to stress-induced FPO and faster colonic bead expulsion rates in NPY−/− mice in vivo, and the ability of i.p. administered NPY to prevent these defecation responses after NPY removal indicates peripheral NPY has a predominantly inhibitory influence on colonic motility during stress that may be more pronounced in females. We have previously reported that the pellet propulsion in the isolated colon tends to be faster in those from NPY−/− mice (Tough et al., 2011). This all corroborates findings by Wang et al. (2010), whereby i.p. administered NPY inhibited FPO induced by a novel environment, in addition to bethanechol-induced diarrhoea, but opposed the central stimulatant effect NPY exerted on colonic motor function through Y1 and CRH pathways (Monnikes et al., 2000; Tebbe et al., 2005). Nevertheless, peripheral NPY is a neuromodulator via neurogenic Y2 receptors within both intramural plexi of the colon (Wang et al., 2010), as well as inhibiting upper GI inhibitory motor mechanisms in vivo and colonic motility in vitro via Y1 receptors (see above).
Not completely congruent with the orexigenic role for NPY, previous studies have shown that under basal conditions NPY−/− mice exhibit no changes in food intake or body weight, although they do exhibit blunted hyperphagia and weight gain after fasting (Patel et al., 2006). In this study, both genders of NPY−/− mice ate significantly less than WT mice in a novel environment and after an acute restraint stress, and compared with their respective food intake after acclimatization; thus, it is possible the metabolic consequences of losing NPY becomes more evident physiologically under stressed conditions. Additionally, NPY−/− females showed a greater, although non-significant reduction in feeding compared with NPY−/− males during stress, indicating females may be more susceptible to the stress-induced suppression of feeding. This is supported by the larger reduction in food intake and greater weight loss reported during chronic single housing of female NPY−/− mice compared with WT females and NPY−/− males (Edelsbrunner et al., 2009). Interestingly, male WT mice ate less than female WT mice throughout this study, despite having a higher body weight, although this was not statistically significant.
Another important finding of the present study is that after 30 min restraint, male NPY−/− mice had significantly higher levels of plasma corticosterone than restrained WT counterparts, indicating that endogenous NPY inhibits corticosterone secretion and thus the stress responses. An abundance of data has shown that centrally administered NPY dose-dependently activates the HPA-axis and thus increases corticosterone release (Dimitrov et al., 2007); however, reports concerning the effect of peripheral NPY on regulating corticosterone/cortisol secretion have been contradictory and inconclusive (Renshaw et al., 2000). Despite a lack of significant difference in basal corticosterone levels between any groups in this study, higher basal levels have been reported in female NPY−/− mice (Painsipp et al., 2011). However, as high corticosterone levels have been observed in both genders of NPY−/− mice after the forced swim test, which were similar to the levels observed after restraint stress here, it is possible that stress-induced NPY release may induce the inhibition of corticosterone release (Painsipp et al., 2011). Furthermore, these data point to an inhibitory role for NPY acting via both peripheral Y1 and Y2 receptors on corticosterone release during acute stress, as peripherally administered antagonists BIBO3304 and BIIE0246 in restrained WT males, and BIIE0246 in restrained WT females, significantly increased corticosterone levels compared with vehicle. However, this increase in plasma corticosterone was not observed after administration of the antagonists under normal conditions in either gender. Although elevations in corticosterone may be dependent on the type of stressor, support is provided by deletion of the Y1 or Y2 receptor from mice leading to high plasma corticosterone concentrations (Cavadas et al., 2006; Painsipp et al., 2008) and high levels previously reported in response to peripheral BIIE0246 administration in WT mice (Kuo et al., 2008). Furthermore, NPY inhibits corticosterone release in vitro both from isolated rat adrenocortical cells (Malendowicz et al., 1990) and from human adrenal cortical H295R cells through the Y1 receptor (Kempna et al., 2010). In humans, NPY administered i.v. inhibits cortisol release (Antonijevic et al., 2000). NPY and the Y1 and Y2 receptors are expressed in the human adrenal gland, with binding studies demonstrating that Y1 receptors are highly expressed in the adrenal cortex (Korner et al., 2004). In the mouse, NPY immunoreactive nerve fibres have been detected around blood vessels and in the cortical cells of the zona glomerulosa (Fernandez-Vivero et al., 1993). Taken together, this indicates a direct Y1- and probably a presynaptic Y2-mediated inhibitory action of peripheral NPY, potentially released from intracortical nerve fibres (Li et al., 1999), upon corticosterone release from adrenal cortical cells. Interestingly, non-stressed WT females had a significant increase in corticosterone after vehicle treatment. These cohorts were treated differently, the vehicle cohort being handled previously and having vehicle administered i.p. 45 min before blood collection rather than immediate blood collection under control conditions, possibly explaining the difference.
In conclusion, the present work has shown gender-dependent responses in UGIT, FPO and food intake induced by both restraint and novel environment stresses in NPY−/− mice. Female NPY−/− mice were more susceptible than male NPY−/− mice to the physiological effects of acute stress, suggesting that NPY plays a moderate sexually dimorphic role in some physiological stress responses. Additionally, this work has provided further support that endogenous NPY in both genders plays an inhibitory role in colonic motility, but not in UGIT under normal conditions; however, it has highlighted opposing roles for the Y1 and Y2 receptors on UGIT during restraint stress. Removal of NPY from mice and pharmacological interventions in WT mice revealed that NPY inhibits the release of corticosterone through both peripheral Y1 and Y2 receptors during stress events. Thus, it is possible that peripheral NPY may activate pathways that contribute to the restoration of GI and colonic homeostasis and inhibit HPA axis activity after a stress event in both genders. These results are significant given the critical role of NPY and its cognate Y receptors in stress/anxiety and energy homeostasis and the increasing prevalence of stress and anxiety-associated diseases, especially in women.
Acknowledgments
SCF is supported by an Integrative Mammalian Biology BBSRC PhD studentship. HH is supported by a Senior Research Fellowship from the NHMRC, Australia. We thank Prof Kevin O'Byrne for critical discussions during these studies.
Glossary
- AR
acute restraint
- ARC
arcuate nucleus
- BIBO3304
(R)-N-[[4-(aminocarbonylaminomethyl)-phenyl]methyl]-N2-(diphenylacetyl)-argininamide trifluoroacetate)
- BIIE0246
(S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6H)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide
- CRH
corticotrophin-releasing hormone
- DMSO
dimethyl sulfoxide
- DPPIV
dipeptidyl peptidase IV
- ENS
enteric nervous system
- FPO
faecal pellet output
- GI
gastrointestinal
- HPA
hypothalamic–pituitary–adrenal
- IBS
irritable bowel syndrome
- NPY
neuropeptide Y
- NPY(3–36)
neuropeptide Y(3–36)
- PVN
paraventricular nucleus
- PYY
peptide YY
- PYY(3–36)
peptide YY(3–36)
- UGIT
upper gastrointestinal transit
- WT
wild type
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition (2011) Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonijevic IA, Murck H, Bohlhalter S, Frieboes RM, Holsboer F, Steiger A. Neuropeptide Y promotes sleep and inhibits ACTH and cortisol release in young men. Neuropharmacology. 2000;39:1474–1481. doi: 10.1016/s0028-3908(00)00057-5. [DOI] [PubMed] [Google Scholar]
- Blehar MC. Gender differences in risk factors for mood and anxiety disorders: implications for clinical treatment research. Psychopharmacol Bull. 1995;31:687–691. [PubMed] [Google Scholar]
- Cavadas C, Cefai D, Rosmaninho-Salgado J, Vieira-Coelho MA, Moura E, Busso N, et al. Deletion of the neuropeptide Y (NPY) Y1 receptor gene reveals a regulatory role of NPY on catecholamine synthesis and secretion. Proc Natl Acad Sci USA. 2006;103:10497–10502. doi: 10.1073/pnas.0600913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187–1209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol Motil. 1997;9:109–116. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- Dimitrov EL, DeJoseph MR, Brownfield MS, Urban JH. Involvement of neuropeptide Y Y1 receptors in the regulation of neuroendocrine corticotropin-releasing hormone neuronal activity. Endocrinology. 2007;148:3666–3673. doi: 10.1210/en.2006-1730. [DOI] [PubMed] [Google Scholar]
- Dube MG, Sahu A, Kalra PS, Kalra SP. Neuropeptide Y release is elevated from the microdissected paraventricular nucleus of Food-deprived rats: an in vitro study. Endocrinology. 1992;131:684–688. doi: 10.1210/endo.131.2.1639015. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Jacques D, Bouchard P, Quiro R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998;402:372–338. [PubMed] [Google Scholar]
- Edelsbrunner ME, Herzog H, Holzer P. Evidence from knockout mice that peptide YY and neuropeptide Y enforce murine locomotion, exploration and ingestive behaviour in a circadian Cycle- and gender-dependent manner. Behav Brain Res. 2009;203:97–107. doi: 10.1016/j.bbr.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantaguzzi CM, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009;336:179–189. doi: 10.1007/s00441-009-0773-2. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vivero J, Rodriguez-Sanchez F, Verastegui C, Cordoba Moriano F, Romero A, de Castro JM. Immunocytochemical distribution of serotonin and neuropeptide Y (NPY) in mouse adrenal gland. Histol Histopathol. 1993;8:509–520. [PubMed] [Google Scholar]
- Fujimiya M, Itoh E, Kihara N, Yamamoto I, Fujimura M, Inui A. Neuropeptide Y induces fasted pattern of duodenal motility via Y(2) receptors in conscious fed rats. Am J Physiol Gastrointest Liver Physiol. 2000;278:32–38. doi: 10.1152/ajpgi.2000.278.1.G32. [DOI] [PubMed] [Google Scholar]
- Heraclides AM, Chandola T, Witte DR, Brunner EJ. 2011. Work stress, obesity and the risk of Type 2 diabetes: gender-specific bidirectional effect in the Whitehall II study. Obesity DOI: 10.1038/oby.2011.95.
- Hyland NP, Sjöberg F, Tough IR, Herzog H, Cox HM. Functional consequences of neuropeptide Y Y 2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br J Pharmacol. 2003;139:863–871. doi: 10.1038/sj.bjp.0705298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychologicalstress in rats. Psychoneuroendocrinol. 2009;34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. Eur J Neurosci. 2008;28:173–180. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- Kas MJ, Bruijnzeel AW, Haanstra JR, Wiegant VM, Adan RA. Differential regulation of agouti-related protein and neuropeptide y in hypothalamic neurons following a stressful event. J Mol Endocrinol. 2005;35:159–164. doi: 10.1677/jme.1.01819. [DOI] [PubMed] [Google Scholar]
- Kask A, Nguyen HP, Pabst R, Von Horsten S. Neuropeptide Y Y1 receptor-mediated anxiolysis in the dorsocaudal lateral septum: functional antagonism of corticotropin-releasing hormone-induced anxiety. Neuroscience. 2001;104:799–806. doi: 10.1016/s0306-4522(01)00116-6. [DOI] [PubMed] [Google Scholar]
- Kempna P, Korner M, Waser B, Hofer G, Nuoffe JM, Reubi JC, et al. Neuropeptide Y modulates steroid production of human adrenal H295R cells through Y1 receptors. Mol Cell Endocrinol. 2010;314:101–109. doi: 10.1016/j.mce.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner M, Waser B, Reubi JC. High expression of neuropeptide y receptors in tumors of the human adrenal gland and extra-adrenal paraganglia. Clin Cancer Res. 2004;10:8426–8433. doi: 10.1158/1078-0432.CCR-04-0821. [DOI] [PubMed] [Google Scholar]
- Koslo RJ, Burks TF, Porreca F. Centrally administered bombesin affects gastrointestinal transit and colonic bead expulsion through supraspinal mechanisms. Pharmacol Exp Ther. 1986;238:62–67. [PubMed] [Google Scholar]
- Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnansky R, Zukowska Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann N Y Acad Sci. 2008;1148:232–237. doi: 10.1196/annals.1410.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Johansson H, Grimelius L. Innervation of human adrenal gland and adrenal cortical lesions. Virchows Arch. 1999;435:580–589. doi: 10.1007/s004280050444. [DOI] [PubMed] [Google Scholar]
- Lin HC, Zhai XT, Wang L, Wong H. Fat-induced ileal brake in the dog depends on peptide YY. Gastroenterology. 1996;110:1491–1495. doi: 10.1053/gast.1996.v110.pm8613054. [DOI] [PubMed] [Google Scholar]
- Malendowicz LK, Lesniewska B, Miskowiak B. Neuropeptide Y inhibits corticosterone secretion by isolated rat adrenocortical cells. Experientia. 1990;46:721–722. doi: 10.1007/BF01939945. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, Aono M, Moriga M, Okuma M. Centrally administered neuropeptide Y (NPY) inhibits gastric emptying and intestinal transit in the rat. Dig Dis Sci. 1993;38:845–850. doi: 10.1007/BF01295910. [DOI] [PubMed] [Google Scholar]
- Maunder RG, Levenstein S. The role of stress in the development and clinical course of inflammatory bowel disease: epidemiological evidence. Curr Mol Med. 2008;8:247–252. doi: 10.2174/156652408784533832. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, et al. XVI. international union of pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143. [PubMed] [Google Scholar]
- Monnikes H, Tebbe J, Bauer C, Grote C, Arnold R. Neuropeptide Y in the paraventricular nucleus of the hypothalamus stimulates colonic transit by peripheral cholinergic and central CRF pathways. Neurogastroenterol Motil. 2000;12:343–352. doi: 10.1046/j.1365-2982.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takahashi T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:427–432. doi: 10.1152/ajpregu.00499.2004. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Herzog H, Holzer P. Implication of neuropeptide-Y Y2 receptors in the effects of immune stress on emotional, locomotor and social behavior of mice. Neuropharmacology. 2008;55:117–126. doi: 10.1016/j.neuropharm.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Herzog H, Sperk G, Holzer P. Sex-dependent control of murine emotional-affective behaviour in health and colitis by peptide YY and neuropeptide Y. Br J Pharmacol. 2011;163:1302–1314. doi: 10.1111/j.1476-5381.2011.01326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel HR, Qi Y, Hawkins EJ, Hileman SM, Elmquist JK, Imai Y, et al. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes. 2006;55:3091–3098. doi: 10.2337/db05-0624. [DOI] [PubMed] [Google Scholar]
- Peaire AE, Krantis A, Staines WA. Distribution of the NPY receptor subtype Y1 within human colon: evidence for NPY targeting a subpopulation of nitrergic neurons. J Auton Nerv Syst. 1997;67:168–175. doi: 10.1016/s0165-1838(97)00101-x. [DOI] [PubMed] [Google Scholar]
- Pol O, Sasaki M, Jimenez N, Dawson VL, Dawson TM, Puig MM. The involvement of nitric oxide in the enhanced expression of mu-opioid receptors during intestinal inflammation in mice. Br J Pharmacol. 2005;145:758–766. doi: 10.1038/sj.bjp.0706227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SS, Hatfield RA, Suls JM, Chamberlain MJ. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol. 1998;93:985–990. doi: 10.1111/j.1572-0241.1998.00293.x. [DOI] [PubMed] [Google Scholar]
- Renshaw D, Thomson LM, Carroll M, Kapas S, Hinson JP. Actions of neuropeptide Y on the rat adrenal cortex. Endocrinology. 2000;141:169–173. doi: 10.1210/endo.141.1.7251. [DOI] [PubMed] [Google Scholar]
- Sah R, Ekhator NN, Strawn JR, Sallee FR, Baker DG, Horn PS, et al. Low cerebrospinal fluid neuropeptide Y concentrations in posttraumatic stress disorder. Biol Psychiatry. 2009;66:705–707. doi: 10.1016/j.biopsych.2009.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- Tache Y, Maeda-Hagiwara M, Turkelson CM. Central nervous system action of corticotropin-releasing factor to inhibit gastric emptying in rats. Am J Physiol. 1987;253:241–245. doi: 10.1152/ajpgi.1987.253.2.G241. [DOI] [PubMed] [Google Scholar]
- Tebbe JJ, Mronga S, Tebbe CG, Ortmann E, Arnold R, Schafer MK. Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1- and corticotrophin-releasing factor 1 receptor activation. J Neuroendocrinol. 2005;17:570–576. doi: 10.1111/j.1365-2826.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10:3003–3007. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- Tough IR, Forbes S, Tolhurst R, Ellis M, Herzog H, Bornstein JC, et al. Endogenous peptide YY and neuropeptide Y inhibit colonic ion transport, contractility and transit differentially via Y(1) and Y(2) receptors. Br J Pharmacol. 2011;164:471–484. doi: 10.1111/j.1476-5381.2011.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Citters GW, Lin HC. The Ileal brake: a fifteen-year progress report. Curr Gastroenterol Rep. 1999;1:404–409. doi: 10.1007/s11894-999-0022-6. [DOI] [PubMed] [Google Scholar]
- Wang L, Gourcerol G, Yuan PQ, Wu SV, Million M, Larauche M, et al. Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G45–G56. doi: 10.1152/ajpgi.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]


