Abstract
The beneficial effects of regular exercise for the promotion of health and cure of diseases have been clearly shown. In this review, we would like to postulate the idea that exercise can be considered as a drug. Exercise causes a myriad of beneficial effects for health, including the promotion of health and lifespan, and these are reviewed in the first section of this paper. Then we deal with the dosing of exercise. As with many drugs, dosing is extremely important to get the beneficial effects of exercise. To this end, the organism adapts to exercise. We review the molecular signalling pathways involved in these adaptations because understanding them is of great importance to be able to prescribe exercise in an appropriate manner. Special attention must be paid to the psychological effects of exercise. These are so powerful that we would like to propose that exercise may be considered as a psychoactive drug. In moderate doses, it causes very pronounced relaxing effects on the majority of the population, but some persons may even become addicted to exercise. Finally, there may be some contraindications to exercise that arise when people are severely ill, and these are described in the final section of the review. Our general conclusion is that exercise is so effective that it should be considered as a drug, but that more attention should be paid to the dosing and to individual variations between patients.
Keywords: health, dosing of exercise, contraindications of exercise, sport, training
Exercise, movement and health: definitions
Health promotion is the science and art of helping people change their lifestyle to move towards a state of optimal health (O'Donnell, 1986). The World Health Organization defines health as ‘Physical, mental, and social well-being, not merely the absence of disease and infirmity’. Physical fitness is defined as the physiologic state of well-being that allows one to meet the demands of daily living (health-related physical fitness) or that provides the basis for sport performance (performance-related physical fitness), or both. Although we are aware that there is a clear difference between the terms physical activity (‘any bodily movement’) and exercise (‘a subset of physical activity that is characterized by a planned and purposeful training’) (Caspersen et al., 1985), in this review, we are going to use these two concepts as synonymous because some of the studies to which we will refer use the terms interchangeably.
Historical background
The hypothesis that physical activity promotes health and longevity is not new. As far back as 2500 BC, in ancient China, records of organized exercise for health promotion have been found (Lyons and RJja, 1978; Lee and Skerrett, 2001). In Greco-Roman times, 2500 years ago, Hippocrates (460–370 BC) and later Galen (AD 129–210) recognized the need to promote and prescribe exercise for health-related benefits and the need to provide general medical care for the athletic individual (Speed and Jaques, 2010). In this regard, the philosopher Plato (427–347 BC) said: ‘Lack of activity destroys the good condition of every human being while movement and methodical physical exercise saves and preserves it’ (Fox and Haskell, 1968).
Simple comparisons of men in different occupations provided the first empirical evidence that physical activity was associated with health. The first studies demonstrating a significant inverse relationship between physical activity and coronary heart disease (CHD) were those conducted by Morris et al. (1953b) in London early in the 1950s. These authors found that London bus conductors had only 73% the frequency of CHD that was found in the less active bus drivers. Their later comparison of London postmen and less active postal clerks produced much the same findings (Morris et al., 1953a). These seminal studies were followed by those of Paffenbarger and collaborators in the 1970s, assessing the increase in the relative risk of death from any cause and from specific diseases associated with physical inactivity (Paffenbarger and Hale, 1975; Paffenbarger et al., 1978).
Exercise is beneficial for your health
Exercise is one of the most frequently prescribed therapies both in health and disease. There is irrefutable evidence showing the beneficial effects of exercise both to prevent and to treat several diseases. Researchers have shown that both men and women who report increased levels of physical activity and fitness have reductions in relative risk of death (by about 20%–35%) (Blair et al., 1989; Macera et al., 2003).
Recent research suggests that modest increments in energy expenditure due to physical activity (∼1000 kcal per week) or an increase in physical fitness of 1 MET (metabolic equivalent) is associated with lowering mortality by about 20% (Myers et al., 2004). Physically inactive middle-aged women (engaging in less than 1 h of exercise per week) experience a 52% increase in all-cause mortality, a doubling of cardiovascular-related mortality, and a 29% increase in cancer-related mortality when compared with physically active ones (Hu et al., 2004). Thus, there is clear evidence that regular physical activity produces significant health effects and reduces the risk of premature death from any cause and from cardiovascular disease in particular amongst asymptomatic men and women.
The benefits of physical activity are evident, not only in healthy persons but also in patients. Observational and randomized trials have shown that regular physical activity contributes to the treatment of several chronic diseases (Bouchard et al., 1994; Warburton et al., 2006a). There is evidence for prescribing exercise in the primary and secondary prevention of pulmonary and cardiovascular diseases (CHD, chronic obstructive pulmonary disease, hypertension, intermittent claudication); metabolic disorders (type 2 diabetes, dyslipaemia, obesity, insulin resistance); muscle, bone and joint diseases (rheumatoid arthritis, fibromyalgia, chronic fatigue syndrome, osteoporosis); cancer; and depression (Pedersen and Saltin, 2006; Warburton et al., 2006a). Even if exercise is an effective therapeutic agent for all of these diseases, as with any other medicine, the dosage (volume and intensity of the exercise), frequency of administration (sessions per week), type (aerobic vs. resistance exercise), systemic and psychoactive effects and contraindications and side effects of the exercise must be taken into account to achieve the best clinical outcome. For instance, both resistance and aerobic training have been shown to be of benefit for the control of diabetes; however, resistance training may have greater benefits for glycaemic control than aerobic training (Dunstan et al., 2005).
The dosage of exercise
Dosage is important in clinical medicine and all marketed drugs require data on their efficacy and safety (Lee, 2007). It is known that there is a minimum amount of physical activity for health benefits. These benefits increase with increasing the amount of exercise, but beyond a certain level, adverse effects outweigh benefits (Lee, 2007). Unlike chemical drugs, however, the minimum dose, dose response and maximum safe dose of physical activity are not well understood (Lee, 2007). There is a continuous debate on how much, what type, how often, what intensity and how lengthy physical activity should be. This is important for issuing public health recommendations (Blair et al., 2004). Summarizing available information across studies is difficult because investigators have measured exercise intensity in different ways and classified physical activity according to different dose schemes that are often difficult to compare (Lee, 2007). Over the years, various expert groups, based on the best evidence available, have postulated different physical activity recommendations and guidelines (see Table 1).
Table 1.
Historical evolution in physical activity recommendations and guidelines
| Physical activity recommendations | ||||
|---|---|---|---|---|
| Intensity | Minutes | Frequency | Reference | |
| 1970s–1980s | Vigorous exercise (e.g. running) | 20 min·day−1 | 3 times·week−1 | (American College of Sports Medicine, 1978) |
| 1990s | Moderate exercise (e.g. brisk walking) | 30 min·day−1 | Most days of the week | (Pate et al., 1995; Physical activity and cardiovascular health, 1996) |
| 2000s | Moderate exercise | 60 min·day−1 | 3 times·week−1 | (Lee, 2007) |
| 2010 (healthy adults ages 18–45) | Moderate exercise | 30 min·day−1 (150 min week−1) | Most days of the week (5 days·week−1) | (O'Donovan et al., 2010) |
| Vigorous exercise | 75 min·week−1 | (O'Donovan et al., 2010) | ||
Intensity levels of physical activity can be expressed relative to oxygen consumption (VO2) or to heart rate (Warburton et al., 2006b). Moderate-intensity activities are those in which heart rate and breathing are raised; but, still, it is possible to speak comfortably. This occurs around 4–6 METs and brisk walking at 3.0 mph (80.4 m·min−1) is one such activity. Vigorous-intensity activities are that in which heart rate is higher, breathing is heavier and conversation is harder (about 6–8 METs) (Warburton et al., 2006b), for instance jogging. It has been shown that exercising at even 50% of the recommended levels (72 min of moderate exercise a week) appears sufficient to provide some improvement in fitness. However, at this low exercise dosage, cardiovascular risk factors (blood pressure, lipid profile and weight) do not improve (Church et al., 2007). In fact, for many individuals, up to 60 min of daily physical activity are more appropriate if weight control is the primary goal (Lee, 2007). Thus, dose–response relations between physical activity and different health outcomes are different. The evaluation of the minimum amount of physical activity (lower dose) necessary to achieve its beneficial effects has been the object of intense research. Wen et al. (2011) have recently found that 15 min a day or 90 min a week of moderate-intensity exercise is of benefit in terms of life expectancy, even for subjects with cardiovascular risks.
Exhaustive exercise and longevity
Although the health benefits of leisure-time physical activity are well documented, the association between vigorous exercise training and mortality or longevity of elite athletes is not fully understood (Teramoto and Bungum, 2010). For centuries, the general belief has been that exhaustive, competitive exercise is harmful and decreases life expectancy (Ruiz et al., 2010). For instance, Moorstein (1968) stated that all members of the 1948 Harvard rowing crew had died early from cardiac diseases. In contrast, it has been shown that participation in endurance competitive sports increases life expectancy. It was found that the life expectancy of oarsmen was higher than that of their non-athletic controls (Hartley and Llewellyn, 1939; Prout, 1972). Karvonen and co-workers found that Finnish champion skiers (born between 1845 and 1910) lived 2.8–4.3 years longer than general male population in Finland. In contrast with most studies of that time, Polednak (1972) reported evidence against the beneficial effects of strenuous exercise. He found differences in longevity and cardiovascular mortality related to the extent of participation in college athletics. Moreover, in a recent animal study, it has been found that long-term vigorous endurance exercise training may in some cases promote adverse cardiac remodelling and produce a substrate for cardiac arrhythmias (Benito et al., 2011). The incidence of sudden cardiac death (SCD) amongst young athletes (estimated to be 1–3 per 100 000 person-years) is higher than in non-athletes and may possibly still be underestimated (Drezner, 2008). However, it has been shown that the most common cause of SCD in young athletes is underlying inherited cardiac disease, such as cardiomyopathies, congenital coronary anomalies and ion channelopathies (Maron et al., 2009). To clarify this apparent contradiction, we determined the longevity of the participants of the Tour de France and compared it with that of the general population born between 1892 and 1942. The Tour de France is amongst the most gruelling sport events in the world. We found an 11% increase in average longevity in Tour de France participants when compared with the general population (Sanchis-Gomar et al., 2011b) (see Figure 1). Thus, the majority of data in human studies support the notion that prescription of regular, vigorous aerobic exercise might be a useful tool, with a dose–effect response to improve the overall health status and longevity of the general population (Ruiz et al., 2010; Teramoto and Bungum, 2010). In our opinion, physicians, health professionals and general population should not be under the impression that strenuous exercise and/or high-level aerobic competitive sports are bad for one's health and shorten one's life. Thus, a dose–response relation appears to exist, such that people who have the highest levels of physical activity and fitness are at lowest risk of premature death (Warburton et al., 2006a).
Figure 1.
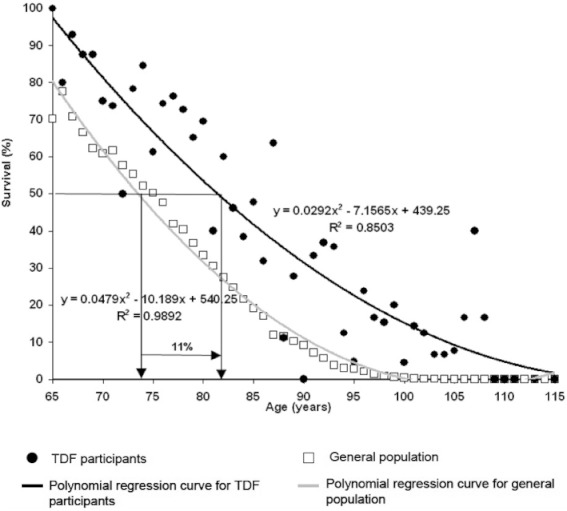
Percentage of survival related to age in Tour de France (TdF) participants and in the general population. Persons born between 1892 and 1942 have been studied. Average life span of Tour de France participants is higher (P= 0.004; 17.5%) than the general population of the same country in which the cyclists were born. The age at which 50% of the general population died was 73.5 years, compared with 81.5 years in Tour de France participants (i.e. 11% increase).
Training status is a very relevant factor in the prescription of the exercise ‘dose’. Increasing the doses of exercise has positive consequences for health in trained individuals (Ruiz et al., 2010; Sanchis-Gomar et al., 2011b), whereas heavy physical exertion can trigger the onset of acute myocardial infarction, particularly in people who are habitually sedentary (Mittleman et al. 1993). Results from the same group showed that less active men participating in vigorous activity were more likely to have a myocardial infarction during exercise than the most active men (Thompson et al., 2007).
In the pharmacological treatment of many conditions, physicians typically start with a dose of a drug believed to be the minimum effective dose. If the patient does not respond, this initial dose may then be titrated upwards to a maximum dose, beyond which the adverse effects of the drug are unacceptable for treatment (Lee, 2007). Thus, the intensity of aerobic training may be also titrated in healthy people (Warburton et al., 2006b). Unfit people can get significant improvements in physical fitness with a low training intensity, while those with a higher fitness level need a greater level of exercise intensity to achieve further improvements in fitness (Shephard, 2001). Thus, these fit individuals who have met the physical activity levels recommended for all healthy adults for at least 6 months may obtain additional health benefits by engaging in 300 min or more of moderate-intensity aerobic activity per week, or 150 min or more of vigorous-intensity aerobic activity each week, or equivalent combinations of moderate- and vigorous-intensity aerobic activities (Lee and Skerrett, 2001; O'Donovan et al., 2010). These relatively low doses are, obviously, not applicable to high level professional athletes who perform exercise at much higher doses.
The guidelines discussed above are generally appropriate for young to middle-aged adults. But, as with medicines, special considerations should be taken when prescribing exercise for people with special needs such as elderly, children, pregnant women, overweight or obese patients and patients with chronic diseases (Warburton et al., 2006b). For instance, it has been shown that vigorous activities are not essential for the reduction of cardiovascular risk in men over 60. Regular physical activity is enough to achieve a significant decrease in mortality in this population. Thus, the greatest benefit to health is gained from sustained moderate exercise, above which there appears to be no further benefit to health in older men (Hakim et al., 1998; Wannamethee et al., 1998).
Regarding the ‘dosage’ of exercise, whether it should be performed in either one continuous or two or more accumulated bouts, the available evidence suggest that at least for fitness, accumulated and continuous patterns of exercise training of the same total duration confer similar benefits (Murphy et al., 2009). For instance, it has been shown that five to eight 2 min bouts of stair climbing accumulated over the course of a day confer health benefits, including increases in cardiovascular fitness, compared with non-exercising controls (Boreham et al., 2005).
Although physical activity is beneficial to health with or without weight loss, adults who find it difficult to maintain a normal weight and adults with increased risk of cardiovascular disease or type 2 diabetes, in particular, may benefit from going beyond the levels of activity recommended for all healthy adults and gradually progressing towards meeting the recommendations for conditioned individuals (O'Donovan et al., 2010).
Systemic adaptations to exercise
The exercise-induced adaptations are especially evident in the cardiorespiratory and musculoskeletal systems, and body composition and metabolism (Warburton et al., 2006a; Lee et al., 2010). But the documented health benefits of exercise also include diminished symptoms of depression and anxiety (Kujala, 2011).
Skeletal muscle is the main target of exercise training. Modifications in skeletal muscle are crucial for enhancing endurance and metabolic efficiency (Matsakas and Narkar, 2010). Muscle fibres are commonly classified as type I slow-twitch or oxidative fibres, with a high mitochondrial content, and type II fast-twitch or glycolytic fibres, which have fewer mitochondria. Endurance exercise induces an increase in mitochondriogenesis, a shift in fibre distribution from glycolytic to oxidative and an increase in fatty acid oxidation that ultimately leads to an increase in aerobic capacity and retards diseases such as obesity, type 2 diabetes and cardiovascular diseases (Holloszy and Coyle, 1984; Mootha et al., 2003).
It has been shown that regular exercise can reduce abdominal adiposity and improve weight control (Warburton et al., 2006a), enhance lipoprotein profiles (e.g. reduce triglyceride levels, increase high density lipoprotein and decrease low-density lipoprotein levels), improve glucose homeostasis and insulin sensitivity, reduce blood pressure, improve autonomic tone, reduce systemic inflammation; decrease blood coagulation, improve coronary blood flow, augment cardiac function and enhance endothelial function (Warburton et al., 2006a).
Regular physical activity is also associated with improved psychological well-being (e.g. through reduced stress, anxiety and depression) (Dunn et al., 2001). The beneficial effects of exercise on cognitive function are well known (Neeper et al., 1995). The mechanism behind this is not fully understood, but it seems to be associated with an increased expression of neurotrophic factors in some brain areas. Increased expression of these factors is related to better memory and improved cognitive function. Brain-derived neurotrophic factor (BDNF) can enhance the survival and differentiation of neurons, and voluntary exercise has been shown to increase it (Neeper et al., 1996). Psychological well-being is particularly important for the prevention and management of cardiovascular disease, but it also has important implications for the prevention and management of other chronic diseases such as diabetes, osteoporosis, hypertension, obesity, cancer and depression (Warburton et al., 2006a). It has been shown that physical activity results in specific adaptations that affect individual states in all of these diseases. For instance, adaptations that affect glucose homeostasis, in type 2 diabetes, are of great importance. Several changes occur as a result of regular physical activity, including increased glycogen synthase and hexokinase activities, increased mRNA and protein expression of the glucose transporter GLUT-4 and improved muscle capillary density (resulting improved glucose delivery to the muscle) (Mandroukas et al., 1984).
Exercise causes a significant reduction in cancer rates (specifically colon and breast cancer) (Shephard and Futcher, 1997; Pedersen and Saltin, 2006). Possible explanations include reductions in fat stores, increased energy expenditure offsetting a high fat diet, activity-related changes in sex hormone levels, immune function, insulin and insulin-like growth factors, free radical generation and direct effects on the tumour cell biology (Westerlind, 2003).
The majority of the proposed mechanisms have been discussed in the context of chronic adaptations by regular physical activity. However, it has been shown that isolated exercise sessions (separate doses of exercise) also elicit transient, but still beneficial, changes in risk factors for chronic diseases (Thompson et al., 2001). Many of the training adaptations derive from a single exercise bout that elicits cellular changes at the gene level leading to cumulative effects of training. The acute effect of exercise results in transient reductions in triglyceride levels, increases in HDL cholesterol level, decreases in blood pressure, reductions in insulin resistance and improvements in glucose control (Thompson et al., 2001). These acute changes underpin the important role that individual exercise sessions have on health status. Thus, single doses of exercise have also a relevant impact on health. Figure 2 summarizes the favourable effects of exercise.
Figure 2.
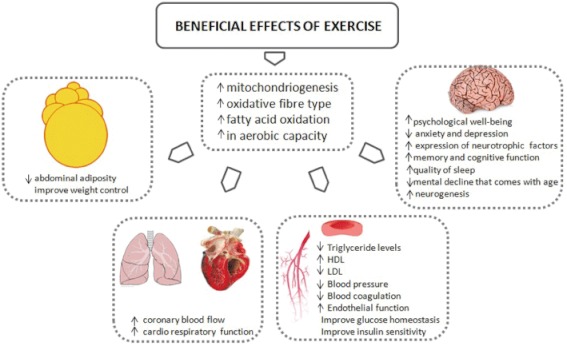
Health benefits of exercise in tissues and organs.
Signalling pathways regulated by exercise in skeletal muscle
The regulation of the cellular functions with exercise are dependent on many stimuli: alterations in metabolite concentrations, a shift in the ATP : ADP ratio, changes in the intracellular concentration of Ca2+, in the intracellular pH, and activations of the oxidative stress-sensitive signalling pathways (Sakamoto and Goodyear, 2002; Ji et al., 2004). To elucidate the molecular signalling mechanisms that enable skeletal muscle to respond to the contractile stimulus and that mediate the adaptations to exercise is of great importance (Sakamoto and Goodyear, 2002). It has been clearly established that physical exercise can activate MAPK signalling, including the ERK1/2 (Goodyear et al., 1996), p38 (Gomez-Cabrera et al., 2005) and JNK pathways (Goodyear et al., 1996). It can also increase the activity of the AMP-activated protein kinase (AMPK), Akt and the p70 S6 kinase (Sakamoto and Goodyear, 2002). In skeletal muscle Ca2+signalling is extensive. In addition to triggering muscle contraction through the troponin system, Ca2+ is also involved in the regulation of relevant intracellular proteins such as PKC, calcineurin and calmodulin kinase that mediate cellular signal transduction (Berchtold et al., 2000).
More recently, it has been shown, that low-to-moderate levels of reactive oxygen species (ROS) play multiple regulatory roles in cells such as the control of gene expression, regulation of cell signalling pathways and modulation of skeletal muscle force production (Reid, 2001) (see Figure 3).
Figure 3.
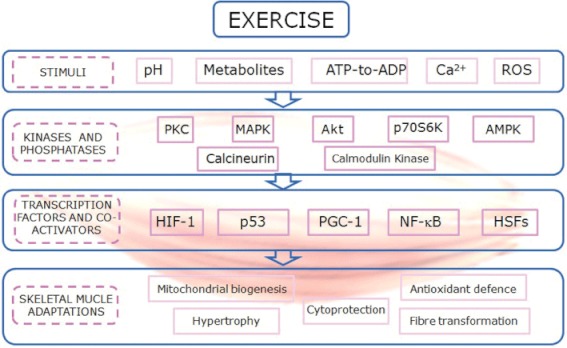
A summary of the signalling pathways regulated by exercise in skeletal muscle.
ROS and exercise: training as an antioxidant intervention
The role of ROS in the exercise-induced adaptations in skeletal muscle has been extensively studied (Salminen and Vihko, 1983; Gomez-Cabrera et al., 2008b). The idea of the deleterious effects of ROS has been firmly entrenched in the minds of scientists during the last 30 years. However, there is growing evidence that the continued presence of low concentrations of free radicals is, in fact, able to induce the expression of antioxidant enzymes and other defence mechanisms. In this scenario, radicals may be seen as beneficial as they act as signals to enhance defences rather than deleterious as they are when cells are exposed to high levels of these radicals. Animals frequently exposed to chronic exercise have shown less oxidative damage after exhaustive exercise than untrained ones (Salminen and Vihko, 1983). This is largely due to the up-regulation of endogenous antioxidant enzymes such as glutathione peroxidase, mitochondrial superoxide dismutase (MnSOD) and γ-glutamylcysteine synthetase (Salminen and Vihko, 1983). A major conclusion that can be drawn from these results is that exercise itself acts as an antioxidant, because training increases the expression of antioxidant enzymes (Gomez-Cabrera et al., 2008b). Subsequently, we and others have shown that antioxidant supplements prevent the induction of mitochondrial biogenesis, molecular regulators of insulin sensitivity and endogenous antioxidant defence by physical exercise (Gomez-Cabrera et al., 2008a; Ristow et al., 2009). Thus, ROS act as signals in exercise because decreasing their formation prevents activation of important signalling pathways, which cause useful adaptations in cells.
Because of the widespread implications of ROS in almost all important biological functions, it is difficult to define all the pathways and gene targets that are affected by redox signalling during exercise. The following are some of the most relevant signalling pathways modulated by exercise: the PPAR-γ coactivator-1α and β (PGC-1α and PGC-1β) (Gomez-Cabrera et al., 2008a; Ristow et al., 2009), p53 (Borras et al., 2011), hypoxia-inducible factor 1 (HIF-1) (Huang et al., 1996), heat shock factor (HSF) (Palomero et al., 2008), NF-κB and MAPK signalling pathways (Ji et al., 2004; Gomez-Cabrera et al., 2005). Several important adaptations in skeletal muscle such as mitochondrial biogenesis, antioxidant defence, hypertrophy, cytoprotection and fibre transformation are regulated primarily by these pathways. Thus, its regulation should be tightly controlled (Gomez-Cabrera et al., 2009) (see Figure 3).
Exercise, a psychoactive drug
The effects of exercise training on brain function have received much attention. In the early ‘80s, exercise was shown to increase β-endorphin in peripheral blood in humans (Bortz et al., 1981; Carr et al., 1981). Elevated serum β-endorphin concentrations induced by exercise have since been linked to a variety of psychological and physiological changes, including mood state changes and ‘exercise-induced euphoria’, altered pain perception and responses to numerous stress hormones (growth hormone, ACTH, prolactin, catecholamines and cortisol) (Harber and Sutton, 1984).
Exercise training can favourably influence cognitive function (Dishman et al., 2006; Vaynman and Gomez-Pinilla, 2006). Exercise improves learning and memory (van Praag et al., 1999), improves the quality of sleep, counteracts the mental decline that comes with age (Laurin et al., 2001) and facilitates functional recovery from brain injury (Grealy et al., 1999) and depression (Siuciak et al., 1996; Shirayama et al., 2002). Exercise is a very powerful stimulus to the induction of neurogenesis in the adult dentate gyrus (van Praag et al., 1999) that can contribute to remodelling hippocampal synaptic circuits and to enhance cognitive function.
Exercise training can also mitigate the consequences of acute exposure to different types of psychological stress (Dishman et al., 2006) and exercise-induced alterations in the 5-hydroxytryptaminergic and the noradrenergic systems can explain these responses (Dishman et al., 2006). Most of the positive effects of exercise, as mentioned previously, have been related to the induction, in different brain areas, of neurotrophic proteins, including BDNF, glial cell-derived neurotrophic factor (GDNF) and insulin growth factor (IGF). Whether brain metabolic responses to acute physical activity extend beyond regions specifically involved with motor, sensory or cardiovascular autonomic control is not as yet clear (Dishman et al., 2006). Transient increases in local cerebral glucose use and in cerebral blood flow have been reported in the different brain areas in response to acute strenuous treadmill running in rats and in humans (Vissing et al., 1996). Also, the discharge rate of a select pool of hippocampal pyramidal cells increased as running velocity increased (Czurko et al., 1999). Moreover, exercise increased metabolic capacity in the motor cortex and striatum (McCloskey et al., 2001).
The psychoactive effects of exercise that we have just mentioned are not free from risks. Pathological patterns of behaviour in gym clients have been reported (Lejoyeux et al., 2008). As observed in patients with eating disorders, active individuals usually worry about their body shape, put special attention on their eating patterns, show exercise addiction and have a perfectionism personality trait (Freimuth et al., 2011). This body image disorder has been addressed as reverse anorexia, vigorexia or muscle dysmorphia (Lejoyeux et al., 2008). Based on a review of a wide range of studies on exercise addiction, it has been estimated that its prevalence in the general population is close to 3%. Amongst certain groups such as ultra-marathon runners, body builders and sport science students, the percentage is even higher (Freimuth et al., 2011; Sussman and Sussman, 2011).
Contraindications for exercise
The purpose of this section is to discuss why under some circumstances physical exercise does not increase the quality of life.
Although both the heart and the lung benefit significantly from physical activity, there are some contraindications when exercise is performed by patients suffering from heart and pulmonary diseases. Pedersen and Saltin (2006) reviewed the possible contraindications of exercise in most of the diseases in which exercise have shown beneficial effects. For instance in patients with CHD, exercise is contraindicated until the condition has been stable for at least 5 days; dyspnea at rest, aortic stenosis, pericarditis, myocarditis, endocarditis, fever and severe hypertension all are contraindications to exercise (Pedersen and Saltin, 2006). Black et al. (1975) were amongst the first to find that strenuous exercise can cause acute injury to coronary plaques, leading to occlusion of coronary arteries. However, years later, it was found that although the risk of primary cardiac arrest was transiently increased during a single bout of vigorous exercise, habitual vigorous exercise was associated with an overall decrease in this risk (Siscovick et al., 1984; Albert et al., 2000). There are no absolute contraindications to very moderate exercise in chronic obstructive pulmonary disease patients (Pedersen and Saltin, 2006). However, in patients with asthma, a pause in training is recommended when an acute exacerbation occurs. In cases of infection, a pause in training is recommended until the patient has been asymptomatic for a day, where after training can be slowly resumed (Pedersen and Saltin, 2006).
Regarding muscle, bone and joint diseases, for instance, osteoarthritis and rheumatoid arthritis, exercise is contraindicated in cases of acute joint inflammation, if pain worsens after training and in cases of pericarditis and pleuritis (Pedersen and Saltin, 2006). The training of patients with osteoporosis should include activities with a low risk of falling (Pedersen and Saltin, 2006).
In cancer patients being treated with chemotherapy or radiotherapy, exercise is contraindicated when leukocyte concentrations fall below 0.5 × 109 cells L−1, haemoglobin below 100 g·L−1, thrombocyte concentration below 20 × 109 cells L−1 and temperature above 38°C. Patients with bone metastases should not perform strength conditioning at high load. In cases of infection, a pause in training is recommended until the patient has been asymptomatic for a day, where after training can be slowly resumed (Pedersen and Saltin, 2006). A major concern is whether exercise training influences the anticancer effects of conventional cytotoxic therapy. The potential interaction between exercise and chemotherapy efficacy is biologically plausible. Indeed, earlier preclinical studies have reported both an inhibitory (Baracos, 1989) and augmentary (Thompson et al., 1989) effect of endurance exercise training on mammary tumour growth and progression, although others have reported no association (Jones et al., 2005).
In diabetic patients (both types I and II), exercise should be postponed if blood glucose is >2.5 g·L−1 together with ketonuria and >3.0 g·L−1 even without ketonuria, in both cases, before it is corrected. In patients with hypertension and active proliferative retinopathy, high-intensity training or training involving Valsalva-like manoeuvres should be avoided. Patients with neuropathy and incipient foot ulcers should refrain from activities entailing the bearing of the patient's own body weight.
In metabolic syndrome-related disorders, such as insulin resistance, dyslipaemia and obesity, there are no general contraindications; but training should take into account any comorbidities (Pedersen and Saltin, 2006). Finally, hypertensive patients with a blood pressure >180/105 should begin pharmacotherapy before regular physical activity is initiated (relative contraindication) (Pescatello et al., 2004). There is no evidence for an enhanced risk of sudden death or stroke in physically active persons with hypertension (Tipton, 1999). The American College of Sports Medicine (ACSM) recommends caution when performing very intensive dynamic exercise or strength conditioning with very heavy weights. Patients with left-sided cardiac hypertrophy should be particularly cautious about heavy strength conditioning. Patients with CHD should refrain from short intensive exercise situations.
It is well known that eccentric muscle contractions cause structural damage to muscle cells or inflammatory reactions within the muscles, as shown by an increase in the plasma activity of cytosolic enzymes and sarcolemma and Z-line disruption (Armstrong et al., 1983). The severity of the damage and the extent of discomfort are exacerbated over time and can last for several days. The damaging effects of eccentric contractions can affect subsequent exercise sessions due to residual muscle pain, restriction of movement and reduced capacity to exercise at an intensity that may be beneficial for the exerciser (Howatson and van Someren, 2008). Thus, caution should be paid in exercise programs which include eccentric contractions especially in recreational or old practitioners.
Exercise mimetics
Despite the clear evidence showing the powerful influence of exercise on health, physical inactivity remains a pressing public health issue. Technology and economic incentives tend to discourage activity: technology by reducing the energy needed for activities of daily living and economics by paying more for sedentary than for physically active work (Haskell et al., 2007). Moreover, endurance exercise can also be unapproachable for most people in whom it might be impractical because of physical limitations or, as mentioned in the previous section, side effects. This fact stimulates the search for exercise mimetics (or ‘exercise pills’) that mimic exercise and, therefore, it has been the focus of important research over the past decades (Goodyear, 2008).
As mentioned in a previous section, exercise improves performance by activating several pathways that induce genetic changes, particularly in skeletal muscle, to increase aerobic metabolism and vascularisation, to ultimately enhance performance (Narkar et al., 2011). AMPK is activated by exercise and is essential for the exercise-mediated switch to aerobic myofibres in skeletal muscle (Wojtaszewski et al., 2000). AMPK stimulates catabolic and suppresses anabolic pathways in an effort to restore cellular ATP levels and is activated robustly in skeletal muscle by acute as well as by chronic exercise (Winder et al., 2006; Matsakas and Narkar, 2010). Recently, it was reported that 5-aminoimidazole-4- carboxamide-1-β-d-ribofuranoside (AICAR) can mimic the effects of exercise by increasing GLUT-4 protein, hexokinase activity, resting glycogen content and muscle mitochondria (Narkar et al., 2008). Mitochondrial biogenesis is activated by AMPK (Jorgensen et al., 2007). This may be explained because AMPK is present in a transcriptional complex with PPAR-δ, where it can potentiate receptor activity via direct protein–protein interaction and/or by phosphorylating and activating coactivators such as PGC-1α (Jager et al., 2007). Puigserver et al. (1998) identified PGC-1α as the master regulator of mitochondriogenesis and fuel homeostasis in mammalian tissues and Koves et al. (2005) observed that PGC-1α mediates metabolic remodelling of skeletal myocytes, mimics exercise and reverses lipid-induced mitochondrial inefficiency. Thus, exercise training and oxidative fibre type are associated with increased mRNA expression of PGC-1α (Lin et al., 2002). Over-expression of PGC-1α in mice induces dramatic changes in skeletal muscle such as increased mitochondrial biogenesis and fibre remodelling (Lin et al., 2005). Moreover, PGC-1α may be activated by the Ca2+-signalling pathway involving both calcineurin and Ca2+/calmodulin-dependent kinase and by p38 MAPK (Schiaffino et al., 2007).
Recently, we have found an age-associated lack of reactivity of PGC-1α in response to exercise (Derbréet al., 2012), as aged rats had the same response as mice with genetic deletion (knockout) of PGC-1α. Our results highlight the role of PGC-1α in the loss of mitochondriogenesis associated with aging and point to this important transcriptional co-activator as a target for pharmacological interventions to prevent age-associated sarcopenia (Derbréet al., 2012). Modulation of PGC-1α levels in skeletal muscle is crucial for the prevention and treatment of age-related disorders (Sandri et al., 2006). Accordingly, we recently proposed several pharmacological, non-hormonal, interventions to prevent the loss of muscle mass and sarcopenia such as PGC-1α activators, angiotensin II receptor antagonists and allopurinol (Sanchis-Gomar et al., 2011a).
On the other hand, activation or over-expression of the transcription factor PPAR-δ in muscle also results in an increase in mitochondrial biogenesis and in the proportion of oxidative muscle fibres (Narkar et al., 2008). This results in increased running endurance and protection against diet-induced obesity and type 2 diabetes. The contrary is also true: muscle-specific knockout of PPAR-δ results in an age-dependent loss of oxidative muscle fibres, running endurance and insulin sensitivity (Schuler et al., 2006). Thus, several potent and selective PPAR-δ agonists such as GW1516 have been also identified and proposed as mimetic drugs for endurance exercise (Narkar et al., 2008).
Resveratrol has also been considered as an exercise mimetic. High doses of resveratrol improve endurance (Lagouge et al., 2006). The deacetylase enzyme Sir2 and its mammalian homologue SIRT1 have been identified as its putative primary targets. Resveratrol also activates AMPK in cells in culture, and it has been proposed for the prevention of mitochondrial dysfunction (Ungvari et al., 2011) and of the wasting disorders associated with mechanical unloading (Momken et al., 2011).
Remodelling of skeletal muscle by exercise is extremely complex. Many targets are available to mimic muscle adaptations induced by exercise. However, exercise is linked to other multiple physiological adaptations that affect the vast majority of organs. It seems premature to conclude that all these molecules or substances are mimetics of exercise until the effects in other organs have been fully investigated. Applicability of these compounds at this point in time is limited. Studies aimed to find potential drugs that mimic exercise are now being performed.
Concluding remarks
Exercise is so beneficial for health that it should be considered as a drug. As for any other drug, dosing is very important. Otherwise, unfavourable side effects may occur. Some of the favourable effects of exercise apply to the general population. Prominent amongst these are its role in prevention of many diseases and in the promotion of healthy longevity (see Figure 2). But exercise can also be considered as treatment of established diseases. These include commonly occurring conditions such as depression, diabetes or cardiovascular diseases.
Acknowledgments
We thank Mrs Marilyn Noyes for her kind help in reviewing the manuscript. Our work is supported by grants SAF2008-00270, SAF2009-08334 from the Spanish Ministry of Education and Science; PROMETEO/2010/074 from the Consellería de Educación de la Generalitat Valenciana. ISCIII2006-RED13-027 from the ‘Red Temática de Investigación Cooperativa en Envejecimiento y Fragilidad (RETICEF)’, EU Funded COSTB35 and DPS2008- 06968 from Spanish Ministry of Innovation and Science. Our studies have also been co financed by FEDER funds from the European Union.
Glossary
- ACSM
American College of Sports Medicine
- ACTH
adrenocorticotropic hormone
- AICAR
5-aminoimidazole-4- carboxamide-1-β-d-ribofuranoside
- AMPK
AMP-activated protein kinase
- BDNF
brain-derived neurotrophic factor
- CHD
coronary heart disease
- GDNF
glial cell-derived neurotrophic factor
- HIF-1
hypoxia-inducible factor 1
- HSF
heat shock factor
- HSP
heat shock protein
- IGF
insulin growth factor
- MET
metabolic equivalent (estimated oxygen cost of 3.5 mL·min−1·kg−1)
- MnSOD
mitochondrial superoxide dismutase
- PGC-1α
PPAR-γ coactivator-1α
- ROS
reactive oxygen species
- SCD
sudden cardiac death
- VO2
oxygen consumption
Conflict of interest
The authors declare that no conflict of interest exists.
References
- American College of Sports Medicine. Position statement on the recommended quantity and quality of exercise for developing and maintaining fitness in healthy adults. Med Sci Sports Exerc. 1978;10:vii–x. [PubMed] [Google Scholar]
- Albert CM, Mittleman MA, Chae CU, Lee IM, Hennekens CH, Manson JE. Triggering of sudden death from cardiac causes by vigorous exertion. N Engl J Med. 2000;343:1355–1361. doi: 10.1056/NEJM200011093431902. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Baracos VE. Exercise inhibits progressive growth of the Morris hepatoma 7777 in male and female rats. Can J Physiol Pharmacol. 1989;67:864–870. doi: 10.1139/y89-135. [DOI] [PubMed] [Google Scholar]
- Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123:13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000;80:1215–1265. doi: 10.1152/physrev.2000.80.3.1215. [DOI] [PubMed] [Google Scholar]
- Black A, Black MM, Gensini G. Exertion and acute coronary artery injury. Angiology. 1975;26:759–783. doi: 10.1177/000331977502601101. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- Blair SN, LaMonte MJ, Nichaman MZ. The evolution of physical activity recommendations: how much is enough? Am J Clin Nutr. 2004;79:913S–920S. doi: 10.1093/ajcn/79.5.913S. [DOI] [PubMed] [Google Scholar]
- Boreham CA, Kennedy RA, Murphy MH, Tully M, Wallace WF, Young I. Training effects of short bouts of stair climbing on cardiorespiratory fitness, blood lipids, and homocysteine in sedentary young women. Br J Sports Med. 2005;39:590–593. doi: 10.1136/bjsm.2002.001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borras C, Gomez-Cabrera MC, Vina J. The dual role of p53: DNA protection and antioxidant. Free Radic Res. 2011;45:643–652. doi: 10.3109/10715762.2011.571685. [DOI] [PubMed] [Google Scholar]
- Bortz WM, 2nd, Angwin P, Mefford IN, Boarder MR, Noyce N, Barchas JD. Catecholamines, dopamine, and endorphin levels during extreme exercise. N Engl J Med. 1981;305:466–467. [PubMed] [Google Scholar]
- Bouchard C, Shephard RJ, Stephens T. Physical Activity, Fitness, and Health: International Proceedings and Consensus Statement: [Second International Consensus Symposium on Physical Activity, Fitness, and Health, Held May 5 to May 9, 1992, in Toronto, Canada] Champaign, IL; Leeds: Human Kinetics Publishers; 1994. [Google Scholar]
- Carr DB, Bullen BA, Skrinar GS, Arnold MA, Rosenblatt M, Beitins IZ, et al. Physical conditioning facilitates the exercise-induced secretion of beta-endorphin and beta-lipotropin in women. N Engl J Med. 1981;305:560–563. doi: 10.1056/NEJM198109033051006. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- Czurko A, Hirase H, Csicsvari J, Buzsaki G. Sustained activation of hippocampal pyramidal cells by ‘space clamping’ in a running wheel. Eur J Neurosci. 1999;11:344–352. doi: 10.1046/j.1460-9568.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- Derbré F, Gomez-Cabrera MC, Nascimento AL, Sanchis-Gomar F, Martinez-Bello VE, Tresguerres JA, et al. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1alpha to exercise training. Age (Dordr) 2012;34:669–679. doi: 10.1007/s11357-011-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Drezner JA. Contemporary approaches to the identification of athletes at risk for sudden cardiac death. Curr Opin Cardiol. 2008;23:494–501. doi: 10.1097/HCO.0b013e32830b3624. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O'Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;6(Suppl.):S587–S597. doi: 10.1097/00005768-200106001-00027. Discussion 609–610. [DOI] [PubMed] [Google Scholar]
- Dunstan DW, Daly RM, Owen N, Jolley D, Vulikh E, Shaw J, et al. Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care. 2005;28:3–9. doi: 10.2337/diacare.28.1.3. [DOI] [PubMed] [Google Scholar]
- Fox SM, 3rd, Haskell WL. Physical activity and the prevention of coronary heart disease. Bull N Y Acad Med. 1968;44:950–967. [PMC free article] [PubMed] [Google Scholar]
- Freimuth M, Moniz S, Kim SR. Clarifying exercise addiction: differential diagnosis, co-occurring disorders, and phases of addiction. Int J Environ Res Public Health. 2011;8:4069–4081. doi: 10.3390/ijerph8104069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008a;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008b;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Gomez-Cabrera MC, Vina J, Ji LL. Interplay of oxidants and antioxidants during exercise: implications for muscle health. Phys Sportsmed. 2009;37:116–123. doi: 10.3810/psm.2009.12.1749. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ. The exercise pill – too good to be true? N Engl J Med. 2008;359:1842–1844. doi: 10.1056/NEJMcibr0806723. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Chang PY, Sherwood DJ, Dufresne SD, Moller DE. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol. 1996;271((2 Pt 1)):E403–E408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- Grealy MA, Johnson DA, Rushton SK. Improving cognitive function after brain injury: the use of exercise and virtual reality. Arch Phys Med Rehabil. 1999;80:661–667. doi: 10.1016/s0003-9993(99)90169-7. [DOI] [PubMed] [Google Scholar]
- Hakim AA, Petrovitch H, Burchfiel CM, Ross GW, Rodriguez BL, White LR, et al. Effects of walking on mortality among nonsmoking retired men. N Engl J Med. 1998;338:94–99. doi: 10.1056/NEJM199801083380204. [DOI] [PubMed] [Google Scholar]
- Harber VJ, Sutton JR. Endorphins and exercise. Sports Med. 1984;1:154–171. doi: 10.2165/00007256-198401020-00004. [DOI] [PubMed] [Google Scholar]
- Hartley PH, Llewellyn GF. Longevity of oarsmen. Br Med J. 1939;1:657–662. doi: 10.1136/bmj.1.4082.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- Howatson G, van Someren KA. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008;38:483–503. doi: 10.2165/00007256-200838060-00004. [DOI] [PubMed] [Google Scholar]
- Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin CS, Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- Jones LW, Eves ND, Courneya KS, Chiu BK, Baracos VE, Hanson J, et al. Effects of exercise training on antitumor efficacy of doxorubicin in MDA-MB-231 breast cancer xenografts. Clin Cancer Res. 2005;11:6695–6698. doi: 10.1158/1078-0432.CCR-05-0844. [DOI] [PubMed] [Google Scholar]
- Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, et al. Role of AMPKalpha2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab. 2007;292:E331–E339. doi: 10.1152/ajpendo.00243.2006. [DOI] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- Kujala UM. Born to be rich, physically active, fit and healthy? Scand J Med Sci Sports. 2011;20:367. doi: 10.1111/j.1600-0838.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol. 2010;24(4) Suppl.:27–35. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IM. Dose-response relation between physical activity and fitness: even a little is good; more is better. JAMA. 2007;297:2137–2139. doi: 10.1001/jama.297.19.2137. [DOI] [PubMed] [Google Scholar]
- Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;33(6) Suppl.:S459–S471. doi: 10.1097/00005768-200106001-00016. Discussion S493–S454. [DOI] [PubMed] [Google Scholar]
- Lejoyeux M, Avril M, Richoux C, Embouazza H, Nivoli F. Prevalence of exercise dependence and other behavioral addictions among clients of a Parisian fitness room. Compr Psychiatry. 2008;49:353–358. doi: 10.1016/j.comppsych.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Lyons AS, RJja P. Medicine: An Illustrated History. New York: H. N. Abrams; 1978. [Google Scholar]
- Macera CA, Hootman JM, Sniezek JE. Major public health benefits of physical activity. Arthritis Rheum. 2003;49:122–128. doi: 10.1002/art.10907. [DOI] [PubMed] [Google Scholar]
- Mandroukas K, Krotkiewski M, Hedberg M, Wroblewski Z, Bjorntorp P, Grimby G. Physical training in obese women. Effects of muscle morphology, biochemistry and function. Eur J Appl Physiol Occup Physiol. 1984;52:355–361. doi: 10.1007/BF00943363. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- Matsakas A, Narkar VA. Endurance exercise mimetics in skeletal muscle. Curr Sports Med Rep. 2010;9:227–232. doi: 10.1249/JSR.0b013e3181e93938. [DOI] [PubMed] [Google Scholar]
- McCloskey DP, Adamo DS, Anderson BJ. Exercise increases metabolic capacity in the motor cortex and striatum, but not in the hippocampus. Brain Res. 2001;891:168–175. doi: 10.1016/s0006-8993(00)03200-5. [DOI] [PubMed] [Google Scholar]
- Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I, et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25:3646–3660. doi: 10.1096/fj.10-177295. [DOI] [PubMed] [Google Scholar]
- Moorstein B. Life expectancy of Ivy League rowing crews. JAMA. 1968;205:106. [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet. 1953a;265:1111–1120. doi: 10.1016/s0140-6736(53)91495-0. concl. [DOI] [PubMed] [Google Scholar]
- Morris JN, Heady JA, Raffle PA, Roberts CG, Parks JW. Coronary heart-disease and physical activity of work. Lancet. 1953b;265:1053–1057. doi: 10.1016/s0140-6736(53)90665-5. contd. [DOI] [PubMed] [Google Scholar]
- Murphy MH, Blair SN, Murtagh EM. Accumulated versus continuous exercise for health benefit: a review of empirical studies. Sports Med. 2009;39:29–43. doi: 10.2165/00007256-200939010-00003. [DOI] [PubMed] [Google Scholar]
- Myers J, Kaykha A, George S, Abella J, Zaheer N, Lear S, et al. Fitness versus physical activity patterns in predicting mortality in men. Am J Med. 2004;117:912–918. doi: 10.1016/j.amjmed.2004.06.047. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, et al. Exercise and PGC-1alpha-independent synchronization of type I muscle metabolism and vasculature by ERRgamma. Cell Metab. 2011;13:283–293. doi: 10.1016/j.cmet.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- O'Donnell MP. Definition of health promotion: Part II: Levels of programs. Am J Health Promot. 1986;1:6–9. doi: 10.4278/0890-1171-1.2.6. [DOI] [PubMed] [Google Scholar]
- O'Donovan G, Blazevich AJ, Boreham C, Cooper AR, Crank H, Ekelund U, et al. The ABC of Physical Activity for Health: a consensus statement from the British Association of Sport and Exercise Sciences. J Sports Sci. 2010;28:573–591. doi: 10.1080/02640411003671212. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Hale WE. Work activity and coronary heart mortality. N Engl J Med. 1975;292:545–550. doi: 10.1056/NEJM197503132921101. [DOI] [PubMed] [Google Scholar]
- Paffenbarger RS, Jr, Brand RJ, Sholtz RI, Jung DL. Energy expenditure, cigarette smoking, and blood pressure level as related to death from specific diseases. Am J Epidemiol. 1978;108:12–18. [PubMed] [Google Scholar]
- Palomero J, Broome CS, Rasmussen P, Mohr M, Nielsen B, Nybo L, et al. Heat shock factor activation in human muscles following a demanding intermittent exercise protocol is attenuated with hyperthermia. Acta Physiol (Oxf) 2008;193:79–88. doi: 10.1111/j.1748-1716.2007.01774.x. [DOI] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(Suppl. 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- Pescatello LS, Franklin BA, Fagard R, Farquhar WB, Kelley GA, Ray CA. American College of Sports Medicine position stand. Exercise and hypertension. Med Sci Sports Exerc. 2004;36:533–553. doi: 10.1249/01.mss.0000115224.88514.3a. [DOI] [PubMed] [Google Scholar]
- Physical activity and cardiovascular health. NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA. 1996;276:241–246. Review. [PubMed] [Google Scholar]
- Polednak AP. Longevity and cause of death among Harvard College athletes and their classmates. Geriatrics. 1972;27:53–64. [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prout C. Life expectancy of college oarsmen. JAMA. 1972;220:1709–1711. [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Reid MB. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz JR, Moran M, Arenas J, Lucia A. Strenuous endurance exercise improves life expectancy: it's in our genes. Br J Sports Med. 2010;45:159–161. doi: 10.1136/bjsm.2010.075085. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goodyear LJ. Invited review: intracellular signaling in contracting skeletal muscle. J Appl Physiol. 2002;93:369–383. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- Salminen A, Vihko V. Endurance training reduces the susceptibility of mouse skeletal muscle to lipid peroxidation in vitro. Acta Physiol Scand. 1983;117:109–113. doi: 10.1111/j.1748-1716.1983.tb07184.x. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Gomez-Cabrera MC, Vina J. The loss of muscle mass and sarcopenia: non hormonal intervention. Exp Gerontol. 2011a;46:967–969. doi: 10.1016/j.exger.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Sanchis-Gomar F, Olaso-Gonzalez G, Corella D, Gomez-Cabrera MC, Vina J. Increased average longevity among the ‘Tour de France’ cyclists. Int J Sports Med. 2011b;32:644–647. doi: 10.1055/s-0031-1271711. [DOI] [PubMed] [Google Scholar]
- Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 2007;22:269–278. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, et al. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab. 2006;4:407–414. doi: 10.1016/j.cmet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Shephard RJ. Absolute versus relative intensity of physical activity in a dose-response context. Med Sci Sports Exerc. 2001;33(6) Suppl.::S400–S418. doi: 10.1097/00005768-200106001-00008. Discussion S419–S420. [DOI] [PubMed] [Google Scholar]
- Shephard RJ, Futcher R. Physical activity and cancer: how may protection be maximized? Crit Rev Oncog. 1997;8:219–272. doi: 10.1615/critrevoncog.v8.i2-3.40. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. N Engl J Med. 1984;311:874–877. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Boylan C, Fritsche M, Altar CA, Lindsay RM. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996;710:11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- Speed C, Jaques R. High-performance sports medicine: an ancient but evolving field. Br J Sports Med. 2010;45:81–83. doi: 10.1136/bjsm.2010.075325. [DOI] [PubMed] [Google Scholar]
- Sussman S, Sussman AN. Considering the definition of addiction. Int J Environ Res Public Health. 2011;8:4025–4038. doi: 10.3390/ijerph8104025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto M, Bungum TJ. Mortality and longevity of elite athletes. J Sci Med Sport. 2010;13:410–416. doi: 10.1016/j.jsams.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Ronan AM, Ritacco KA, Tagliaferro AR. Effect of type and amount of dietary fat on the enhancement of rat mammary tumorigenesis by exercise. Cancer Res. 1989;49:1904–1908. [PubMed] [Google Scholar]
- Thompson PD, Crouse SF, Goodpaster B, Kelley D, Moyna N, Pescatello L. The acute versus the chronic response to exercise. Med Sci Sports Exerc. 2001;33(6) Suppl.:S438–S445. doi: 10.1097/00005768-200106001-00012. Discussion S452–S433. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Franklin BA, Balady GJ, Blair SN, Corrado D, Estes NA, 3rd, et al. Exercise and acute cardiovascular events placing the risks into perspective: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism and the Council on Clinical Cardiology. Circulation. 2007;115:2358–2368. doi: 10.1161/CIRCULATIONAHA.107.181485. [DOI] [PubMed] [Google Scholar]
- Tipton CM. Exercise training for the treatment of hypertension: a review. Clin J Sport Med. 1999;9:104. doi: 10.1097/00042752-199904000-00016. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Sonntag WE, de Cabo R, Baur JA, Csiszar A. Mitochondrial protection by resveratrol. Exerc Sport Sci Rev. 2011;39:128–132. doi: 10.1097/JES.0b013e3182141f80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. Revenge of the ‘sit’: how lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J Neurosci Res. 2006;84:699–715. doi: 10.1002/jnr.20979. [DOI] [PubMed] [Google Scholar]
- Vissing J, Andersen M, Diemer NH. Exercise-induced changes in local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab. 1996;16:729–736. doi: 10.1097/00004647-199607000-00025. [DOI] [PubMed] [Google Scholar]
- Wannamethee SG, Shaper AG, Walker M. Changes in physical activity, mortality, and incidence of coronary heart disease in older men. Lancet. 1998;351:1603–1608. doi: 10.1016/S0140-6736(97)12355-8. [DOI] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006a;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton DE, Nicol CW, Bredin SS. Prescribing exercise as preventive therapy. CMAJ. 2006b;174:961–974. doi: 10.1503/cmaj.1040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- Westerlind KC. Physical activity and cancer prevention – mechanisms. Med Sci Sports Exerc. 2003;35:1834–1840. doi: 10.1249/01.MSS.0000093619.37805.B7. [DOI] [PubMed] [Google Scholar]
- Winder WW, Taylor EB, Thomson DM. Role of AMP-activated protein kinase in the molecular adaptation to endurance exercise. Med Sci Sports Exerc. 2006;38:1945–1949. doi: 10.1249/01.mss.0000233798.62153.50. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528((Pt 1)):221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


