Abstract
This review is focused on the role of the ankyrin (A) transient receptor potential (TRP) channel TRPA1 in vascular regulation. TRPA1 is activated by environmental irritants, pungent compounds found in foods such as garlic, mustard and cinnamon, as well as metabolites produced during oxidative stress. The structure of the channel is distinguished by the ∼14–19 ankyrin repeat (AR) domains present in the intracellular amino terminus. TRPA1 has a large unitary conductance (98 pS) and slight selectivity for Ca2+ versus Na+ ions (PCa/PNa ≍ 7.9). TRPA1 is involved in numerous important physiological processes, including nociception, mechanotransduction, and thermal and oxygen sensing. TRPA1 agonists cause arterial dilation through two distinctive pathways. TRPA1 channels present in perivascular nerves mediate vasodilatation of peripheral arteries in response to chemical agonists through a mechanism requiring release of calcitonin gene-related peptide. In the cerebral circulation, TRPA1 channels are present in the endothelium, concentrated within myoendothelial junction sites. Activation of TRPA1 channels in this vascular bed causes endothelium-dependent smooth muscle cell hyperpolarization and vasodilatation that requires the activity of small and intermediate conductance Ca2+-activated K+ channels. Systemic administration of TRPA1 agonists causes transient depressor responses, followed by sustained increases in heart rate and blood pressure that may result from elevated sympathetic nervous activity. These findings indicate that TRPA1 activity influences vascular function, but the precise role and significance of the channel in the cardiovascular system remains to be determined.
Keywords: TRP channels, TRPA1, cation channel, vasculature, artery, vasodilatation, smooth muscle cell, endothelial cell, endothelium-dependent dilation
Introduction: a family of one
The mammalian transient receptor potential (TRP) superfamily of cation channels comprises 28 members assigned to six subfamilies based on sequence homology. The ankyrin (A) subfamily is the smallest and is composed of only a single member, TRPA1 (originally designated as ANKTM1). Despite being one of the last TRP channels to be discovered (Story et al., 2003), TRPA1 has garnered a great deal of recent attention. The essential properties and structure of TRPA1 have been evolutionarily conserved for more than 500 million years (Kang et al., 2010). The channel likely evolved as a sensor of electrophilic toxicity (Kang et al., 2010) before divergent specialized functions developed in different species (Story et al., 2003; Rosenzweig et al., 2005; Cordero-Morales et al., 2011; Geng et al., 2011). TRPA1, like all TRP channels, is expressed as six-transmembrane domain polypeptide subunits, a motif common to many types of ion channels. Functional TRPA1 channels are formed from four of these subunits. Assembled TRPA1 channels are thought to have a homomeric structure (composed of four identical subunits), as there is currently no evidence that heteromultimeric channels involving other TRP channel subunits can form. The channel is distinguished structurally by, and named for, the ∼14–19 ankyrin repeat (AR) domains forming a portion of the protein's intracellular N-terminus. In general, AR domains mediate protein–protein interactions and provide mechanical elasticity (Sedgwick and Smerdon, 1999), although a recent study suggests that particular TRPA1 AR domains can regulate agonist- and heat-induced channel activity (Cordero-Morales et al., 2011). TRPA1 was originally described as a non-selective cation channel that is equally permeable to Na+ versus Ca2+ ions (PCa/PNa reported as 0.84–3.28) (Story et al., 2003; Wang et al., 2008), although Karashima et al. found that during agonist stimulation, PCa/PNa = 7.91 ± 0.60 and the fractional Ca2+ current under these conditions is 17.9–22.3% (Karashima et al., 2010). The unitary conductance of the channel is large (98 pS, when physiological ionic gradients are maintained) (Nagata et al., 2005), indicating that TRPA1 channels can support consequential levels of Ca2+ influx. Predictably, TRPA1 has been shown to influence a broad range of physiological processes that involve Ca2+-dependent signalling pathways, including nociception, mechanotransduction, thermal and oxygen sensing, and responses to environmental irritants and pungent compounds. This manuscript focuses on the role of TRPA1 channels in vascular regulation. The relevant pharmacology is discussed, and studies investigating the consequences of TRPA1 activity on local and integrative control of the vasculature are reviewed.
TRPA1 pharmacology: activators
TRPA1 channels are activated by a large, and still growing, list of diverse compounds, including acrolein (Bautista et al., 2006), diesel exhaust (Hazari et al., 2011), local anaesthetics (Leffler et al., 2011), the non-steroidal anti-inflammatory analgesic acetaminophen (paracetamol) (Nassini et al., 2010; Andersson et al., 2011) and hydrogen sulfide (Streng et al., 2008; Krueger et al., 2010; Miyamoto et al., 2011). In addition to these chemical stimuli, TRPA1 channels in various species are activated by physical factors, such as heat (Rosenzweig et al., 2005; Cordero-Morales et al., 2011), cold (Story et al., 2003) and mechanical stress (Corey et al., 2004; Kwan et al., 2006; Vilceanu and Stucky, 2010). The role of TRPA1 channels in the sensation of noxious cold, recently reviewed in detail (Caspani and Heppenstall, 2009), remains controversial, with approximately equal numbers of studies for and against. For the purposes of the current review, TRPA1 activators present in plants used for food or traditional medicine, and those related to oxidative stress, are discussed in more detail because of their demonstrated or potential roles in vascular regulation.
Pungent dietary molecules
TRPA1 is activated by many substances found in commonly consumed foods and plants used in traditional medical practices of several cultures (Table 1). Allyl isothiocyanate (AITC) (Bandell et al., 2004; Jordt et al., 2004), allicin (Bautista et al., 2005; Macpherson et al., 2005) and cinnamaldehyde (Bandell et al., 2004) (CA), derived from mustard oil, garlic and cinnamon, respectively, are the most commonly used TRPA1 activators. Although AITC can undoubtedly activate TRPA1 channels, recent studies suggest that AITC may have effects that are independent of TRPA1 (Capasso et al., 2012; Everaerts et al., 2011), highlighting the importance of selective pharmacology and/or TRPA1 knockout mice to demonstrate response specificity.
Table 1.
TRPA1 activators derived from pungent foods and traditional medicines
| Agonist | Source | EC50 | References |
|---|---|---|---|
| Allyl isothiocyanate (AITC) | Mustard | 11 ± 1 µM (rat) | Bandell et al. (2004); Jordt et al. (2004) |
| 33 µM (mouse) | |||
| Allicin | Garlic | 1.3 µM (mouse) | Bautista et al. (2005); Macpherson et al. (2005) |
| 1.9 µM (human) | |||
| Cinnamaldehyde (CA) | Cinnamon | 100 µM (mouse) | Bandell et al. (2004) |
| Eugenol | Cloves | NR | Bandell et al. (2004) |
| Gingerol | Ginger | NR | Bandell et al. (2004) |
| Nicotine | Tobacco | ∼10 µM (mouse) | Talavera et al. (2009) |
| Umbellulone | Umbellularia californica (headache tree) | 56.6 ± 8.3 µM (rat) | Nassini et al. (2012); Zhong et al. (2011a) |
| Ligustilide | Celery, lovage, Angelica sinensis, Ligusticum chuanxiong, Ligusticum porteri | 44 µM (mouse) | Zhong et al. (2011b) |
NR, not reported.
Activation of TRPA1 by AITC and other electrophilic compounds occurs through a unique mechanism involving covalent modification of cysteine residues present in the intracellular amino (N) terminus. Two studies examining specific residues required for channel activation identified five distinct cysteine potentially involved in covalent modification (Table 2), but only one (C622 in the mouse gene, corresponding to C619 in the human sequence) was identified by both groups (Hinman et al., 2006; Macpherson et al., 2007a). Cysteine residues at C633 and C856 were shown to participate in activation of the human TRPA1 gene by diallyl disulfide (DADS) (Takahashi et al., 2011). In addition to these cysteine residues, a lysine residue (K708 in the human sequence) also contributes to AITC-induced activation of TRPA1 (Hinman et al., 2006). Specific N-terminal AR domains are also involved in the regulation of TRPA1 activity, as demonstrated by an elegant study from the Julius lab using chimeric genes composed of Crotalus atrox (Western diamondback rattlesnake) and human TRPA1 sequences. ARs in the N-terminus of the C. atrox TRPA1 gene (AR3-8 and AR10-15) involved in sensation of heat were identified (Cordero-Morales et al., 2011). In contrast, AR domains in the human gene (AR11-16) were shown to be involved in sensitivity to AITC (Cordero-Morales et al., 2011). Specific amino acid residues and AR domains involved in TRPA1 activation are summarized in Table 2.
Table 2.
Amino acid residues and ankyrin repeat domains involved in TRPA1 activation
| Species | Stimulus | Residue/domain | References |
|---|---|---|---|
| Mouse | AITC | C415 | Macpherson et al. (2007a) |
| AITC | C422 | Macpherson et al. (2007a) | |
| AITC | C622 | Macpherson et al. (2007a) | |
| Human | AITC | C619 | Hinman et al. (2006) |
| AITC | C639 | Hinman et al. (2006) | |
| AITC | C663 | Hinman et al. (2006) | |
| AITC | K708 | Hinman et al. (2006) | |
| Hyperoxia and DADS | C633 | Takahashi et al. (2011) | |
| Hyperoxia and DADS | C856 | Takahashi et al. (2011) | |
| Hypoxia | P394 | Takahashi et al. (2011) | |
| AITC | AR 11–16 | Cordero-Morales et al. (2011) | |
| Crotalus atrox | Heat | AR 3–8; AR 10–15 | Cordero-Morales et al. (2011) |
Products of oxidative stress
A number of recent studies indicate that TRPA1 channels are activated by substances produced at high levels during oxidative stress, such as H2O2 and specific metabolites of lipid peroxidation (Table 3). Macpherson et al. (2007b) and Trevisani et al. (2007) first reported that TRPA1 channels are activated by 4-hydroxy-2-nonenal (4-HNE), an unsaturated aldehyde produced by lipid peroxidation at high levels during oxidative stress. Two similar substances generated during oxidative injury, 4-oxononenal (4-ONE) and 4-hydroxyhexenal (4-HHE), also stimulate TRPA1 channels (Taylor-Clark et al., 2008). 4-ONE is nearly 10-fold more potent than 4-HNE, whereas 4-HHE is approximately fivefold less potent (Taylor-Clark et al., 2008). A study by Andersson et al. systematically investigated the ability of several oxidative stress-related substances to activate TRPA1 and found that in addition to 4-HNE, 4-ONE and 4-HHE, TRPA1 channels are activated by H2O2 as well as the cyclopentenone prostaglandin 15-deoxy-delta(12,14)-prostaglandin J(2) [15d-PGJ(2) ] (Andersson et al., 2008). These findings strongly suggest that oxidative stress metabolites are endogenous activators of TRPA1 channels in mammalian systems, but the physiological significance of this response is uncertain.
Table 3.
Activators of TRPA1 related to oxidative stress
| Compound | EC50 | Reference |
|---|---|---|
| 4-hydroxy-2-nonenal (4-HNE) | 10–27 µM | Trevisani et al. (2007); Macpherson et al. (2007b); Andersson et al. (2008); Taylor-Clark et al. (2008) |
| 4-oxo-nonenal (4-ONE) | 1.5–1.9 µM | Andersson et al. (2008); Taylor-Clark et al. (2008) |
| 4-hydroxyhexenal (4-HHE) | 39–50 µM | Andersson et al. (2008); Taylor-Clark et al. (2008) |
| Hydrogen peroxide (H2O2) | 230 µM | Andersson et al. (2008) |
| 15-deoxy-delta(12,14)-prostaglandin J(2) [15d-PGJ(2) ] | 5.6 µM | Andersson et al. (2008) |
In addition to sensing substances produced during oxidative stress, a recent study indicates that TRPA1 channels directly detect molecular oxygen and become active during hyperoxic and hypoxic conditions through distinct molecular pathways (Takahashi et al., 2011). This report demonstrates that under normoxic or slightly hyperoxic conditions [partial pressure of O2 (PO2) = 100–150 mmHg], basal activity of human TRPA1 channels expressed in HEK cells is low. Elevated PO2 (maximal response at PO2 ≍ 250 mmHg) increases TRPA1 activity by a mechanism that requires oxidation of specific cysteine residues (C633 and C856). Takahashi and co-workers also demonstrated that moderate levels of hypoxia (maximal response at PO2 ≍ 70 mmHg) activate human TRPA1 channels in an HEK expression system. Hypoxia-induced activation of TRPA1 may result from diminished activity of prolyl hydroxylase (PHD) enzymes. PHD requires O2 as a co-factor for enzymatic activity, and a reduction in PO2 results in diminished hydroxylation of proline residues at specific recognition sites. Block of PDH activity stimulates TRPA1 activity, and hypoxia-induced increases in TRPA1 activity are associated with decreased hydroxylation of proline residue 394 (P394). In addition, TRPA1 protein levels at the cell surface are increased after ∼10–15 min exposure to hypoxia. These findings suggest that decreased PO2 inhibits PHD activity, leading to decreased hydroxylation of P394, which promotes translocation of TRPA1 channel protein to the cell surface. However, it is unclear if TRPA1 channels that are newly inserted into the plasma membrane become spontaneously active or if these new channels are recruited to activity by agonists produced endogenously under hypoxic conditions. Additional work is needed to determine if TRPA1 channels mediate vascular responses to hypoxia.
TRPA1 pharmacology: selective inhibitors
Many substances such as camphor (Macpherson et al., 2006), menthol (Macpherson et al., 2006), ruthenium red (Nagata et al., 2005) and the trivalent ion Gd3+ (Nagata et al., 2005) block TRPA1 channels, but are not clinically or experimentally useful because they lack specificity. Fortunately, a number of small molecule inhibitors with excellent selectivity for TRPA1 have recently become available (Table 4). HC-030031 is the most widely used TRPA1 blocker (McNamara et al., 2007), and non-specific effects of the compound have not been reported. In addition, one study demonstrates oral bioavailability of HC-030031 (Eid et al., 2008). Chembridge-5861528 is a conger of HC-030031 (Wei et al., 2009; 2010) with similar potency and specificity, and reportedly better solubility in aqueous solutions. AP-18 acts as a partial TRPA1 agonist, leading to desensitization rather than inhibition (Petrus et al., 2007; Defalco et al., 2010). A-967079 is the most potent of the TRPA1 inhibitors currently available but demonstrates species-specific differences for inhibition of human versus rodent TRPA1 channels (McGaraughty et al., 2010). The potency and selectivity of certain tricyclic 3,4-dihydropyrimidine-2-thione derivatives are quite promising (Gijsen et al., 2012), but these compounds are not yet generally available.
Table 4.
Selective TRPA1 inhibitors
| Antagonist | IC50 | Reference |
|---|---|---|
| AP-18 | 3.1 µM (human) | Petrus et al. (2007); Defalco et al. (2010) |
| 4.5 µM (mouse) | ||
| A-967079 | 67 nM (human) | McGaraughty et al. (2010) |
| 289 nM (rat) | ||
| HC-030031 | 5.3 ± 0.2–6.2 ± 0.2 µM (human) | McNamara et al. (2007) |
| Chembridge-5861528 | 14.3 ± 0.7–18.7 ± 0.3 µM (human) | Wei et al. (2009, 2010) |
| Tricyclic 3,4-dihydropyrimidine-2-thione derivatives | As low as ∼10 nM (rat and human) | Gijsen et al. (2012) |
TRPA1 in the vasculature
Nerve-evoked vasodilatation
The first evidence demonstrating a role for TRPA1 channels in vascular tone regulation was reported by Bautista et al., who found that TRPA1 channels are present in adventitial nerve fibres in rat mesenteric arteries, and that AITC, raw garlic extracts, purified allicin and DADS caused relaxation of mesenteric artery rings preconstricted with phenylephrine (Bautista et al., 2005). This response was largely blocked when the tissue was pretreated with ruthenium red, capsaicin or with the calcitonin gene-related peptide (CGRP) receptor agonist CGRP8–37, but was not altered by the TRPV1 blocker capsazepine. These findings were later confirmed by Pozgai et al., who reported that CA dilates preconstricted mouse mesenteric artery rings (Pozsgai et al., 2010). The later study demonstrated that the CA-induced dilation was only partially attenuated by removal of the endothelium and was greatly diminished in TRPA1 knockout mice (Pozsgai et al., 2010). Further evidence supporting a role for TRPA1 in local vascular control was provided by a study demonstrating that increases in hind paw blood flow in response to injections of 4-ONE were absent in TRPA1 and CGRP knockout mice, but did not differ between TRPV1 knockout mice and controls (Graepel et al., 2011). A study by Kunkler et al. investigated the role of TRPA1 channels in meningeal vasodilatation and reported that AITC, CA and acrolein stimulated the release of CGRP from cultured rat trigeminal neurons. This response was blocked by HC-030031 (Kunkler et al., 2011). Nasal administration of AITC and acrolein stimulated transient increases in meningeal blood flow in rats that was inhibited by CGRP8–37 and HC-030031. These studies provide strong evidence supporting the pathway proposed by Bautista et al., who suggested that stimulation of TRPA1 channels present in primary sensory neurons with chemical agonists causes Ca2+ influx, leading to localized release of CGRP at perivascular varicosities present on the walls of arteries (Bautista et al., 2005). CGRP binds G protein-coupled receptors on underlying smooth muscle cells to cause membrane hyperpolarization (Hogestatt et al., 2000), myocyte relaxation and vasodilatation (Figure 1). TRPV1 channels, which are commonly co-expressed with TRPA1 in primary sensory neurons (Story et al., 2003), do not appear to be necessary for this response.
Figure 1.
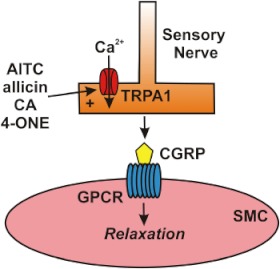
Activation of TRPA1 channels in sensory nerves causes arterial dilation. Allyl isothiocyanate (AITC), allicin, cinnamaldehyde (CA) and 4-oxo-2-nonenal (4-ONE) activate Ca2+ influx via TRPA1 channels in sensory nerves, causing release of calcitonin gene-related peptide (CGRP) from perivascular terminals. CGRP binds to its G protein-coupled receptor (GPCR) on the plasma membrane of vascular smooth muscle cells (SMCs) to cause membrane hyperpolarization and myocyte relaxation.
TRPA1 channels present in primary sensory neurons may play an important role in the vascular component of neurogenic inflammation. This possibility was first reported by Trevisani et al., who showed that the TRPA1 agonist 4-HNE caused oedema when injected into the hind paws of rats (Trevisani et al., 2007). Additional evidence for an inflammatory role for TRPA1 was provided by a study demonstrating that in guinea pigs, tracheal plasma extravasation resulting from cigarette smoke inhalation was attenuated by inhibition of TRPA1 with HC-030031 (Andre et al., 2008). Furthermore, increases in capillary permeability were absent in TRPA1 knockout mice administered an aqueous extract of cigarette smoke (Andre et al., 2008). N-acetyl-p-benzoquinone imine (NAPQI), a metabolite produced when large doses of acetaminophen are administered, activates TRPA1 channels and causes tracheal plasma extravasation in rats and mice that is sensitive to HC-030031 and TRPA1 knockout (Nassini et al., 2010). NAPQI application also increased plasma protein extravasation in the skin conjunctiva of rats and mice that was attenuated by TRPA1 blockade or gene knockout (Nassini et al., 2010). These findings suggest that inhibitors of TRPA1 may be useful for treating increased capillary permeability and oedema associated with certain inflammatory conditions.
Endothelium-dependent vasodilatation
My laboratory reported that TRPA1 channels are present in the endothelium of rat cerebral and cerebellar pial arteries. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Earley et al., 2009; McGrath et al., 2010). More interestingly, expression of TRPA1 channel protein in this tissue is most abundant in projections of the endothelial cell plasma membrane that penetrates the internal elastic lamina and terminates proximal to vascular smooth muscle cells. These structures are called myoendothelial junctions (MEJs) and have recently been shown to house signalling complexes mediating endothelium-dependent hyperpolarization and vasodilatation of resistance vessels (Sandow et al., 2006; Ledoux et al., 2008). The detailed structure of MEJ signalling complexes was first reported by Sandow et al., who demonstrated that expressions of intermediate conductance Ca2+-activated K+ channels (KCa3.1) and connexin proteins 40 and 37 are enriched in the MEJs of rat mesenteric arteries (Sandow et al., 2006). Gap junctions between endothelial and smooth muscle cells (myoendothelial gap junctions) are present in MEJs, consistent with enriched expression of connexin proteins in these structures. A number of earlier studies demonstrate the importance of myoendothelial gap junctions in endothelium-dependent hyperpolarization (EDH) and vasodilation (Yamamoto et al., 1998, 1999; Sandow and Hill, 2000; Griffith et al., 2002; Sandow et al., 2002). Work from Ledoux et al. confirmed that KCa3.1 expression is enriched in MEJs of mouse mesenteric arteries and also showed that segments of the endothelial cell endoplasmic reticulum (ER) are present in these structures (Ledoux et al., 2008). Furthermore, this study showed that inositol trisphosphate receptor (IP3R) expression is enhanced in MEJs. More interestingly, MEJs are the sites of transient, spontaneous, subcellular Ca2+ signals in the endothelium (Ca2+ pulsars) that are generated by Ca2+ released from the ER via IP3Rs. These Ca2+ signals hyperpolarize the endothelial cell plasma membrane by activating proximal KCa3.1 channels present in MEJs. Ledoux et al. proposed that membrane hyperpolarization is conducted to underlying smooth muscle cells via myoendothelial gap junctions to elicit EDH of the sarcolemma and, ultimately, endothelium-dependent vasodilatation (Ledoux et al., 2008). My laboratory added to this mechanistic concept when we reported that both TRPA1 and KCa3.1 channels are concentrated in MEJs of rat cerebral resistance arteries (Earley et al., 2009). Our study also shows that AITC elicits smooth muscle cell hyperpolarization and concentration-dependent dilation of cerebral arteries that is blocked by disruption of the endothelium, inhibition of TRPA1 with HC-030031, as well as antagonists of KCa3.1, small conductance Ca2+-activated K+ channels (KCa2.3) and inwardly-rectifying K+ (KIR) channels. More interestingly, we found that much higher concentrations of AITC are required to provoke global increases in intracellular Ca2+ in native, acutely isolated cerebral artery endothelial cells (EC50 = 400 µM) compared with the levels of AITC required to cause endothelium-dependent vasodilatation (EC50 = 16.4 µM) (Earley et al., 2009; 2010). Hence, we proposed a mechanism in which AITC-induced Ca2+ influx via TRPA1 stimulates Ca2+ pulsar activity within MEJs by Ca2+-induced Ca2+-release, thereby increasing the activity of proximal KCa3.1 channels to hyperpolarize the endothelial cell plasma membrane (Figure 2). This response is presumably transmitted to underlying arterial myocytes via myoendothelial gap junctions to elicit hyperpolarization. KIR channels in smooth muscle cells likely participate in this response by amplifying the initial smooth muscle cell hyperpolarizing stimulus (Smith et al., 2008). The role of KCa2.3 channels is not completely clear, but these channels may play a secondary role to KCa3.1 channels, or may be important for setting the resting membrane potential of the endothelium (Taylor et al., 2003). This proposed mechanism is supported by recent preliminary results demonstrating increased dynamic Ca2+ signalling activity in the endothelium of intact cerebral arteries in response to AITC (Taylor et al., 2012).
Figure 2.
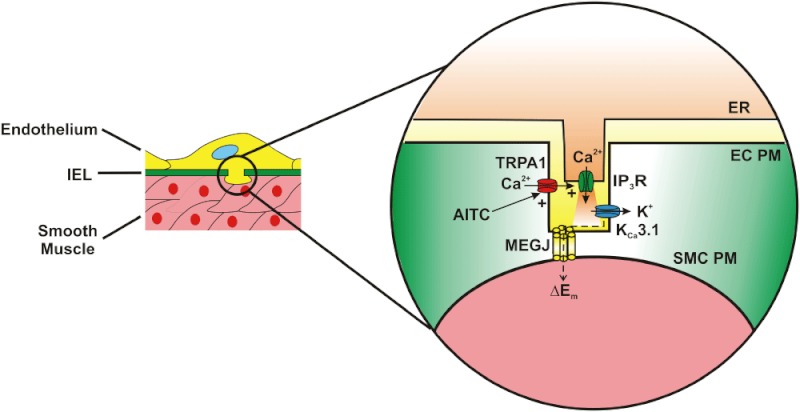
Activation of TRPA1 in cerebral arteries causes endothelium-dependent vasodilation. Allyl isothiocyanate (AITC) activates Ca2+ influx via TRPA1 channels present in myoendothelial junctions in cerebral arteries. TRPA1-mediated Ca2+ influx stimulates Ca2+ release from the endoplasmic reticulum (ER) via inositol trisphosphate receptors (IP3R). The resulting Ca2+ signal (i.e. Ca2+ pulsar) stimulates proximal intermediate conductance Ca2+-activated K+ channels (KCa3.1), resulting in hyperpolarization of the endothelial cell plasma membrane (EC PM). The change in membrane potential (ΔEm) is conducted via myoendothelial gap junctions (MEGJs) to hyperpolarize the vascular smooth muscle cell plasma membrane (SMC PM), resulting in myocyte relaxation.
Endothelial cell expression of TRPA1 has not been reported outside of the cerebral circulation. Preliminary experiments from my laboratory failed to detect TRPA1 expression in the endothelium of rat mesenteric and renal interlobar arteries (unpublished findings) and investigators at the University of Vermont did not record AITC-induced cation currents in patch clamp experiments using native mouse endothelial cells from mesenteric arteries (M.T. Nelson, pers. comm.). Thus, it appears that TRPA1 channels may only be present and functional in the endothelium of certain vascular beds. The significance of this expression pattern is unclear, but is consistent with the possibility of distinct regulatory roles for TRPA1 channels in different segments of the vasculature.
Smooth muscle cells
There is little evidence indicating that TRPA1 agonists elicit vasodilatation by acting directly on TRPA1 channels in vascular smooth muscle cells. Indirect support for such a response was presented by Yanaga et al. examining the vasorelaxant effects of CA on precontracted rat aortic rings (Yanaga et al., 2006). CA-induced dilation was impaired but not abolished by removal of the endothelium or by inhibition of NOS. The endothelium-independent component of the response is suggestive that CA can cause vasodilatation of conduit arteries through direct effects on smooth muscle cells. However, this study did not examine the effects of TRPA1 inhibition on CA-induced dilation of aortic ring segments and did not demonstrate expression of TRPA1 in this tissue. Studies from my laboratory did not detect smooth muscle expression of TRPA1 in immunolabelled intact rat cerebral resistance arteries or enzymatically isolated cerebral arterial myocytes (unpubl. obs.), nor did we detect Ca2+ influx in native rat cerebral artery smooth muscle cells in response to AITC (Earley et al., 2010). The differences in our findings versus those of Yanaga et al. may reflect differential expression of TRPA1 channels in the aorta versus cerebral resistance arteries, or may be due to TRPA1-independent effects of CA on rat aortic rings.
The big picture: influence of TRPA1 on integrative cardiovascular physiology
It is clear that TRPA1 channel activity can elicit dilation of peripheral arteries through nerve-mediated release of CGRP (Figure 1) or through endothelium-dependent smooth muscle cell hyperpolarization in cerebral arteries (Figure 2). But what effect do these responses have on overall cardiovascular function? Examination of global TRPA1 knockout mice indicates that resting blood mean arterial pressure (MAP) and heart rate (HR) of anaesthetized TRPA1 knockouts do not differ from wild-type controls (Pozsgai et al., 2010), suggesting that mice are able to compensate for lack of TRPA1 activity and maintain basic cardiovascular function in the absence of physiological stressors. However, the effects of TRPA1 knockout on the regulation of MAP and cardiac output in conscious mice at rest or under conditions requiring dynamic cardiovascular regulation have not been reported.
The effects of systemic TRPA1 activation were investigated by Pozagai et al., who reported that in anaesthetized female CD-1 mice, i.v. administration of CA has bimodal effects, causing a short-term fall in MAP and HR (depressor response), followed by a sustained rise in both parameters (pressor response). The depressor response was independent of the CA concentration administered, but the pressor response was concentration dependent (EC50 = 78.6 µM·kg−1 body weight). Further experiments were performed using male and female knockout mice of mixed genetic background. The drop in MAP and HR in response to a moderate dose of CA (80 µM·kg−1 body weight) was diminished in TRPA1 knockout animals compared with controls; however, the pressor response was not observed in the control strain used for these experiments at this concentration. A higher concentration of CA (320 µM·kg−1 body weight) elicited both pressor and depressor responses in wild-type mice, and the pressor, but not depressor, response was blunted in the TRPA1 knockout animals. Using TRPV1 and CGRP knockout mice as well as an inhibitor of substance P, the authors demonstrate lack of involvement of these pathways in both the pressor and the depressor responses, suggesting that sensory nerve-derived vasodilators are not involved in systemic responses to i.v. CA administration. Cholinergic receptor blockade (atropine) reduced CA-induced HR decrease, diminished the depressor response to the lower concentration of CA, but had little effect on the pressor response. Block of α-adrenergic receptors attenuated both pressor and depressor responses, but ganglionic blockade (hexamethonium) combined with atropine did not influence the pressor response. The authors conclude that the depressor response results from vasovagal reflex activation, whereas the pressor response is mediated by peripheral sympathetic activation and release of noradrenalin. It is difficult to draw firm conclusions regarding the depressor effects of i.v. CA administration from this report, as the response was very transient in nature (∼1 min), there was no relationship between the dose of CA administered and the drop in MAP and HR, and, when the higher concentration of CA was administered, the depressor response did not differ between wild-type and TRPA1 knockout mice. Interpretation of CA-induced pressor responses is confounded by the reported differences in sensitivity to CA among the strains of mice that were used. Female CD-1 mice exhibited concentration-dependent increases in blood pressure in response to CA (EC50 ≍ 80 µM·kg−1 body weight), but this concentration did not induce pressor responses in the mixed background TRPA1 knockout mice and littermate controls used for the study. Consequently, a CA concentration (320 µM·kg−1 body weight) that produced maximal pressor responses in CD-1 mice was used for the experiments employing knockout animals. To better understand the significance of the channel in regulation of cardiovascular homeostasis, further experiments examining the systemic effects of TRPA1 agonist administration are warranted. The consequences of longer-term administration of TRPA1 activators and inhibitors on cardiovascular responses of conscious animals will be of particular interest.
A study by Koda et al. measured increases in renal sympathetic nerve activity (RSNA) in response to intra-arterial injection of AITC in decerebrate rats (Koba et al., 2011). This response was prevented by sectioning the sciatic nerve, indicating a muscle-based autonomic reflex response. Maintained (30 s) static contraction of hindlimb muscle induced by electrical stimulation resulted in elevated blood pressure and RSNA activity that was diminished by HC-030031. Injections of metabolic by-products of muscular contraction (arachidonic acid, bradykinin and diprotonated phosphate) resulted in increased RSNA that was diminished by HC-030031. The authors conclude that activation of TRPA1 channels present on muscle afferents by the by-products of muscle contraction contributes to increased sympathetic nervous activity during exercise. This response may contribute to exercise-induced increases in HR and respiration, but additional work is needed to test this hypothesis.
Concluding remarks
Investigation of TRPA1 channels in the vasculature is in an early stage and its definitive role and significance in cardiovascular regulation remains uncertain. It is clear that TRPA1 channels are present in the vascular wall and activation with chemical agonists causes vasodilatation. However, further investigation is needed to determine what physiological and/or pathophysiological situations lead to activation of TRPA1 channels in the vasculature. Activation of TRPA1 channels by substances found in garlic and mustard oil has prompted speculation that this response mediates putative cardioprotective benefits of these foods (Bautista et al., 2005; Earley et al., 2009), but this has not been demonstrated experimentally. In any case, it is unlikely that sensation of dietary molecules is the primary function of TRPA1 channels in the vasculature. It seems much more likely that oxidative stress and hypoxia are the endogenous regulators of TRPA1 channels in resistance arteries. For example, TRPA1 channels in the endothelium of the cerebral circulation may directly sense and mediate arterial dilation in response to changes in PaO2 associated with impaired oxygenation, but this has not been demonstrated. It is also conceivable that substances such as 4-HNE and 4-ONE that are produced during oxidative stress associated with reperfusion following ischaemia and other conditions cause arterial dilation through activation of TRPA1 channels in the endothelium. However, this putative response requires generation of highly reactive superoxide anions (O2-) responsible for lipid peroxidation proximal to TRPA1 channels in the vascular wall by an unknown mechanism. Thus, considerable additional investigation is necessary to determine endogenous signalling pathways and physiological roles of TRPA1 in healthy and disease arteries. In addition, the significance of the channel's differential pattern of expression (perivascular nerves vs. MEJs) in various vascular beds also awaits discovery. Such studies will likely require tissue-specific and/or inducible TRPA1 knockout models. These are critically important issues, as TRPA1 channels are under investigation for pharmaceutical development, particularly for the treatment of chronic pain, and the long-term effects of TRPA1-modifying agents on the cardiovascular system in humans are unknown. Most importantly, it is not known if TRPA1 channels are potential targets for the development of new therapies for the treatment or prevention of cardiovascular disease.
Acknowledgments
I thank Dr Brett Kirby, Dr Albert L. Gonzales and Ms Michelle N. Sullivan for critically reading the manuscript. This work was supported by HL091905 (SE).
Glossary
- 15d-PGJ(2)
15-deoxy-delta(12,14)-prostaglandin J(2)
- 4-HHE
4-hydroxyhexenal
- 4-HNE
4-hydroxy-2-nonenal
- 4-ONE
4-oxo-nonenal
- AITC
allyl isothiocyanate
- AR
ankyrin repeat
- CA
cinnamaldehyde
- CGRP
calcitonin gene-related peptide
- DADS
diallyl disulfide
- ER
endoplasmic reticulum
- HR
heart rate
- IP3R
inositol trisphosphate receptor
- KCa2.3
small conductance Ca2+-activated K+ channel
- KCa3.1
intermediate conductance Ca2+-activated K+ channel
- KIR
inwardly-rectifying K+ channel
- MAP
mean arterial pressure
- MEJ
myoendothelial junction
- N
amino terminus
- NR
not reported
- RSNA
renal sympathetic nerve activity
- TRP
transient receptor potential
Conflict of interest
No conflicts.
References
- Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid delta(9)-tetrahydrocannabiorcol. Nat Commun. 2011;2:551. doi: 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Capasso R, Gabriella A, Barbara R, Borrelli F, De Petrocellis L, Di Marzo V, et al. Modulation of mouse gastrointestinal motility by allyl isothiocyanate, a constituent of cruciferous vegetables: evidence for TRPA1-independent effects. Br J Pharmacol. 2012;165:1966–1977. doi: 10.1111/j.1476-5381.2011.01703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspani O, Heppenstall PA. TRPA1 and cold transduction: an unresolved issue? J Gen Physiol. 2009;133:245–249. doi: 10.1085/jgp.200810136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci U S A. 2011;108:E1184–E1191. doi: 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- Defalco J, Steiger D, Gustafson A, Emerling DE, Kelly MG, Duncton MA. Oxime derivatives related to AP18: agonists and antagonists of the TRPA1 receptor. Bioorg Med Chem Lett. 2010;20:276–279. doi: 10.1016/j.bmcl.2009.10.113. [DOI] [PubMed] [Google Scholar]
- Earley S, Gonzales AL, Crnich R. Endothelium-dependent cerebral artery dilation mediated by TRPA1 and Ca2+-Activated K+ channels. Circ Res. 2009;104:987–994. doi: 10.1161/CIRCRESAHA.108.189530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Gonzales AL, Garcia ZI. A dietary agonist of transient receptor potential cation channel V3 elicits endothelium-dependent vasodilation. Mol Pharmacol. 2010;77:612–620. doi: 10.1124/mol.109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HA, Choong KC, Dima S, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, et al. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol. 2011;21:316–321. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Geng J, Liang D, Jiang K, Zhang P. Molecular evolution of the infrared sensory gene TRPA1 in snakes and implications for functional studies. PLoS ONE. 2011;6:e28644. doi: 10.1371/journal.pone.0028644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijsen HJ, Berthelot D, De Cleyn MA, Geuens I, Brone B, Mercken M. Tricyclic 3,4-dihydropyrimidine-2-thione derivatives as potent TRPA1 antagonists. Bioorg Med Chem Lett. 2012;22:797–800. doi: 10.1016/j.bmcl.2011.12.068. [DOI] [PubMed] [Google Scholar]
- Graepel R, Fernandes ES, Aubdool AA, Andersson DA, Bevan S, Brain SD. 4-oxo-2-nonenal (4-ONE): evidence of transient receptor potential ankyrin 1-dependent and -independent nociceptive and vasoactive responses in vivo. J Pharmacol Exp Ther. 2011;337:117–124. doi: 10.1124/jpet.110.172403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith TM, Chaytor AT, Taylor HJ, Giddings BD, Edwards DH. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc Natl Acad Sci U S A. 2002;99:6392–6397. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, et al. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect. 2011;119:951–957. doi: 10.1289/ehp.1003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogestatt ED, Johansson R, Andersson DA, Zygmunt PM. Involvement of sensory nerves in vasodilator responses to acetylcholine and potassium ions in rat hepatic artery. Br J Pharmacol. 2000;130:27–32. doi: 10.1038/sj.bjp.0703258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, et al. Analysis of drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Prenen J, Talavera K, Janssens A, Voets T, Nilius B. Agonist-induced changes in Ca(2+) permeation through the nociceptor cation channel TRPA1. Biophys J. 2010;98:773–783. doi: 10.1016/j.bpj.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koba S, Hayes SG, Sinoway LI. Transient receptor potential A1 channel contributes to activation of the muscle reflex. Am J Physiol Heart Circ Physiol. 2011;300:H201–H213. doi: 10.1152/ajpheart.00547.2009. [DOI] [PubMed] [Google Scholar]
- Krueger D, Foerster M, Mueller K, Zeller F, Slotta-Huspenina J, Donovan J, et al. Signaling mechanisms involved in the intestinal pro-secretory actions of hydrogen sulfide. Neurogastroenterol Motil. 2010;22:1224–1231. doi: 10.1111/j.1365-2982.2010.01571.x. e319–e320. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Ballard CJ, Oxford GS, Hurley JH. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain. 2011;152:38–44. doi: 10.1016/j.pain.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Bonev AD, Nelson MT. Ca2+-activated K+ channels in murine endothelial cells: block by intracellular calcium and magnesium. J Gen Physiol. 2008;131:125–135. doi: 10.1085/jgp.200709875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler A, Lattrell A, Kronewald S, Niedermirtl F, Nau C. Activation of TRPA1 by membrane permeable local anesthetics. Mol Pain. 2011;7:62. doi: 10.1186/1744-8069-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaraughty S, Chu KL, Perner RJ, Didomenico S, Kort ME, Kym PR. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol Pain. 2010;6:14. doi: 10.1186/1744-8069-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007a;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, et al. An ion channel essential for sensing chemical damage. J Neurosci. 2007b;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto R, Otsuguro K, Ito S. Time- and concentration-dependent activation of TRPA1 by hydrogen sulfide in rat DRG neurons. Neurosci Lett. 2011;499:137–142. doi: 10.1016/j.neulet.2011.05.057. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Andre E, Sartiani L, Aldini G, Trevisani M, et al. Acetaminophen, via its reactive metabolite N-acetyl-p-benzo-quinoneimine and transient receptor potential ankyrin-1 stimulation, causes neurogenic inflammation in the airways and other tissues in rodents. FASEB J. 2010;24:4904–4916. doi: 10.1096/fj.10-162438. [DOI] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Vriens J, Prenen J, Benemei S, De Siena G, et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain. 2012;135:376–390. doi: 10.1093/brain/awr272. [DOI] [PubMed] [Google Scholar]
- Petrus M, Peier AM, Bandell M, Hwang SW, Huynh T, Olney N, et al. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol Pain. 2007;3:40. doi: 10.1186/1744-8069-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, Brain SD. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc Res. 2010;87:760–768. doi: 10.1093/cvr/cvq118. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick SG, Smerdon SJ. The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci. 1999;24:311–316. doi: 10.1016/s0968-0004(99)01426-7. [DOI] [PubMed] [Google Scholar]
- Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, et al. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008;586:1147–1160. doi: 10.1113/jphysiol.2007.145474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, et al. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol. 2008;53:391–399. doi: 10.1016/j.eururo.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, et al. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol. 2011;7:701–711. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, et al. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- Taylor MS, Qian X, Francis M, Earley S, Solodushko V. Recruitment of dynamic cerebral artery endothelial Ca2+ signals by the TRPA1 channel activator AITC. FASEB J. 2012;26:853.2. doi: 10.1111/micc.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, et al. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol. 2008;586:3447–3459. doi: 10.1113/jphysiol.2008.153585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilceanu D, Stucky CL. TRPA1 mediates mechanical currents in the plasma membrane of mouse sensory neurons. PLoS ONE. 2010;5:e12177. doi: 10.1371/journal.pone.0012177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Hamalainen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111:147–154. doi: 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- Wei H, Chapman H, Saarnilehto M, Kuokkanen K, Koivisto A, Pertovaara A. Roles of cutaneous versus spinal TRPA1 channels in mechanical hypersensitivity in the diabetic or mustard oil-treated non-diabetic rat. Neuropharmacology. 2010;58:578–584. doi: 10.1016/j.neuropharm.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Fukuta H, Nakahira Y, Suzuki H. Blockade by 18beta-glycyrrhetinic acid of intercellular electrical coupling in guinea-pig arterioles. J Physiol. 1998;511:501–508. doi: 10.1111/j.1469-7793.1998.501bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Imaeda K, Suzuki H. Endothelium-dependent hyperpolarization and intercellular electrical coupling in guinea-pig mesenteric arterioles. J Physiol. 1999;514:505–513. doi: 10.1111/j.1469-7793.1999.505ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaga A, Goto H, Nakagawa T, Hikiami H, Shibahara N, Shimada Y. Cinnamaldehyde induces endothelium-dependent and -independent vasorelaxant action on isolated rat aorta. Biol Pharm Bull. 2006;29:2415–2418. doi: 10.1248/bpb.29.2415. [DOI] [PubMed] [Google Scholar]
- Zhong J, Minassi A, Prenen J, Taglialatela-Scafati O, Appendino G, Nilius B. Umbellulone modulates TRP channels. Pflugers Arch. 2011a;462:861–870. doi: 10.1007/s00424-011-1043-1. [DOI] [PubMed] [Google Scholar]
- Zhong J, Pollastro F, Prenen J, Zhu Z, Appendino G, Nilius B. Ligustilide: a novel TRPA1 modulator. Pflugers Arch. 2011b;462:841–849. doi: 10.1007/s00424-011-1021-7. [DOI] [PubMed] [Google Scholar]


