Abstract
BACKGROUND AND PURPOSE
Rosiglitazone is an anti-diabetic drug acting as an insulin sensitizer. We recently found that rosiglitazone also inhibits the vascular isoform of ATP-sensitive K+ channels and compromises vasodilatory effects of β-adrenoceptor activation and pinacidil. As its potency for the channel inhibition is in the micromolar range, rosiglitazone may be used as an effective KATP channel inhibitor for research and therapeutic purposes. Therefore, we performed experiments to determine whether other isoforms of KATP channels are also sensitive to rosiglitazone and what their sensitivities are.
EXPERIMENTAL APPROACH
KIR6.1/SUR2B, KIR6.2/SUR1, KIR6.2/SUR2A, KIR6.2/SUR2B and KIR6.2ΔC36 channels were expressed in HEK293 cells and were studied using patch-clamp techniques.
KEY RESULTS
Rosiglitazone inhibited all isoforms of KATP channels in excised patches and in the whole-cell configuration. Its IC50 was 10 µmol·L−1 for the KIR6.1/SUR2B channel and ∼45 µmol·L−1 for KIR6.2/SURx channels. Rosiglitazone also inhibited KIR6.2ΔC36 channels in the absence of the sulphonylurea receptor (SUR) subunit, with potency (IC50= 45 µmol·L−1) almost identical to that for KIR6.2/SURx channels. Single-channel kinetic analysis showed that the channel inhibition was mediated by augmentation of the long-lasting closures without affecting the channel open state and unitary conductance. In contrast, rosiglitazone had no effect on KIR1.1, KIR2.1 and KIR4.1 channels, suggesting that the channel inhibitory effect is selective for KIR6.x channels.
CONCLUSIONS AND IMPLICATIONS
These results suggest a novel KATP channel inhibitor that acts on the pore-forming KIR6.x subunit, affecting the channel gating.
LINKED ARTICLE
This article is commented on by Dart, pp. 23–25 of this issue. To view this commentary visit http://dx.doi.org/10.1111/j.1476-5381.2012.01990.x
Keywords: thiazolidinedione, KATP channel, antagonist, KIR subunit, sulphonylurea receptor
Introduction
Rosiglitazone is a potent anti-diabetic drug. By activating the nuclear transcriptional factor PPAR-γ, rosiglitazone affects the pathogenic processes of type 2 diabetes and its complications. Rosiglitazone is known to interfere with the foam cell formation and inflammatory response, reduce lipid deposition in the vessel wall, and attenuate the development of atherosclerosis (Barnett, 2009). By enhancing insulin sensitivity, it helps glycaemic control. Also, rosiglitazone regulates adipocyte proliferation and lipid storage, thus improving the lipid profile. As remarkable as these beneficial effects are, recent clinical studies suggest that rosiglitazone increases the risk of myocardial infarction (Nissen and Wolski, 2007), suggesting that the pharmacological targets of rosiglitazone may be more complicated than PPAR-γ activation.
Our recent studies (Yu et al., 2011) indicate that rosiglitazone acts on the vascular wall via the inhibition of the KIR6.1/SUR2B isoform of ATP-sensitive K+ (KATP) channels (nomenclature follows Alexander et al., 2011). The KIR6.1/SUR2B channel is expressed in vascular smooth muscle (VSM) and targeted by various vasodilators and vasoconstrictors (Quayle et al., 1997; Ashcroft, 2006; Nichols, 2006; Shi et al., 2007a, b; Yang et al., 2008). The potency of rosiglitazone for vascular KATP channel inhibition is much higher than that of tolbutamide and slightly lower than glibenclamide. Therefore, rosiglitazone may be a novel KATP channel inhibitor if it also inhibits other isoforms of KATP channels. To address this issue, we performed these studies. Our results showed that rosiglitazone inhibited all the isoforms of KATP channels. Rosiglitazone appeared to act mostly on the KIR6.x subunit, although the KATP channel inhibition was moderately enhanced by the SURx subunit. To demonstrate the specificity of the channel inhibition, three inwards rectifier K channels (KIR1.1, KIR2.1 and KIR4.1) were studied, which are expressed in the heart, kidney and nervous system. We found that rosiglitazone did not have any inhibitory effects on these K+ channels. In several patch configurations, we showed evidence for the biophysical basis of the channel inhibition and the potential location of the targeted protein domain. These results, therefore, demonstrate a novel KATP channel inhibitor that acts on the pore-forming KIR6.x subunit selectively, without affecting KIR1.1, KIR2.1 and KIR4.1 channels.
Methods
Expression of KATP channels and other KIR channels in HEK293 cells
HEK293 cells were used to express KIR channels. The cells were cultured in the DMEM/F12 medium at 37°C with 5% CO2 with 10% fetal bovine serum and penicillin/streptomycin. The cells were transfected with cDNAs that were cloned to the eukaryotic expression vector pcDNA3.1. KIR6.1 (GenBank accession #D42145) or KIR6.2 (mBIR, #D50581) was transfected together with SUR2B (#D86038, mRNA isoform #NM_011511), SUR1 (#L40623) or SUR2A (#D83598). Homomeric KIR6.2ΔC36, a KIR6.2 with 36 amino acids truncated at the C-terminal, was used to express functional KATP currents without the SUR subunit (Tucker et al., 1997; Piao et al., 2001). KIR4.1 (#X83585), KIR1.1 (ROMK1, #X72341) and KIR2.1 (#X73052) were expressed in HEK293 cells individually. Green fluorescent protein (GFP) cDNA (0.4 µg, pEGFP-N2, Clontech, Palo Alto, CA) was co-transfected to facilitate the identification of positively transfected cells. Cells were split and transferred to coverslips 12–18 h after transfection. Experiments were performed on the cells in the following 12–48 h.
Electrophysiology
Patch clamp was performed using a bath solution containing the following (in mmol·L−1): 10 KCl, 105 potassium gluconate (KC6H11O7), 5 KF, 5 potassium pyrophosphate (K4P2O7), 0.1 sodium vanadate (NaVO3), 5 EGTA, 5 glucose and 10 HEPES at pH 7.4. The pipette was filled with the same solution when KIR6.2/SURx channels were recorded (Wang et al., 2005), while K2ATP (1 mmol·L−1) and KADP (0.5 mmol·L−1) were added into the pipette solution when KIR6.1/SUR2B channels were studied (Beech et al., 1993). Spermine (10 µmol·L−1) was added to the pipette solution for the KIR1.1, KIR2.1 and KIR4.1 studies (Oliver et al., 1998; Xu et al., 2000). The same internal and external solutions were used for inside-out patches and outside-out patches. Pyrophosphate and vanadate are widely used to alleviate channel rundown. In millimolar concentrations, they do not have evident effects on other channel activities (Nakashima et al., 1993; Wang et al., 2005). With the solution, we found that there was only modest or no channel rundown in 10 min when recordings were done in most patches. In a few patches in which the channel rundown was shown at the end of the experiment, the data were rejected for further analysis. To avoid nucleotide degradation, all internal solutions were freshly made and used within 4 h. Rosiglitazone (Cayman Chemical Company, Ann Arbor, MI, USA), pinacidil and glibenclamide (Sigma Chemicals, St. Louis, MO, USA) were dissolved in DMSO, which had no effect on channel activity in the final concentration (∼0.5%).
Recordings were made with the Axopatch 200B amplifier (Molecular Devices, Union City, CA). The data were low-pass filtered (1 kHz, Bessel four-pole filter, −3 dB) and digitized (10 kHz, 16-bit resolution) with Clampex 9 (Molecular Devices). Single-channel currents were recorded from inside-out or outside-out patches with a membrane potential of −60 mV. Higher sampling rate (20 kHz) was chosen for single-channel studies when fast openings and closures were studied. In some patches, command ramp potentials from −100 to 100 mV were applied at a holding potential of 0 mV. Whole-cell currents were recorded in voltage clamp at a holding potential 0 mV and step to −80 mV for 1 s, and the protocol was repeated in every 3 s.
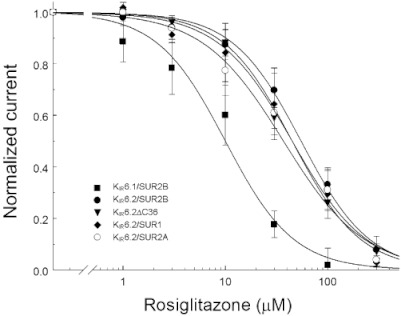
Single-channel conductance was measured with slope command potentials from 100 to −100 mV. The open-state probability (Popen) was calculated by first measuring the time, tj, spent at current levels corresponding to j= 0, 1, 2, … N channels open, based on all evident openings during the entire period of record (Zhu et al., 1999; Yang et al., 2000). The Popen was then obtained as 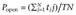 (Equation 1), where N was the number of channels active in the patch, and T was the duration of recordings. Popen values were calculated from a single stretch of recording using the Fetchex 6.0 software (Molecular Devices) in a duration of 40–70 s. Open and closed times were measured from records in which only a single active channel was observed. The open-time and closed-time distributions were fitted using the Marquardt-LSQ method in the Pstat6 software (Molecular Devices). Open/closed events smaller than 0.2 ms were ignored, as a result of the use of 1000 Hz offline filter (Yang et al., 2000). The open dwell-time histograms were fit with one exponential. In some patches, the current amplitude was described using Gaussian distributions, and the difference between two adjacent peaks was taken as the unitary current amplitude.
(Equation 1), where N was the number of channels active in the patch, and T was the duration of recordings. Popen values were calculated from a single stretch of recording using the Fetchex 6.0 software (Molecular Devices) in a duration of 40–70 s. Open and closed times were measured from records in which only a single active channel was observed. The open-time and closed-time distributions were fitted using the Marquardt-LSQ method in the Pstat6 software (Molecular Devices). Open/closed events smaller than 0.2 ms were ignored, as a result of the use of 1000 Hz offline filter (Yang et al., 2000). The open dwell-time histograms were fit with one exponential. In some patches, the current amplitude was described using Gaussian distributions, and the difference between two adjacent peaks was taken as the unitary current amplitude.
Data analysis
Data are presented as the means ± SE. Differences in means were tested with the Student's t-test for two groups and anova for three groups or more. The differences were accepted as significant if P≤ 0.05. The relationship of rosiglitazone dose with channel activity was expressed using the Hill equation: y= 1 / [1 + (x / IC50)h] (Equation 2), where y is normalized channel activity, x is ligand concentration, h is the Hill coefficient and IC50 is half maximal inhibitory concentration.
Results
Rosiglitazone inhibited all isoforms of KATP channels
KIR6.x/SURx channels were expressed in HEK293 cells. Channel activity was studied in inside-out patches with symmetric concentrations of K+ (145 mM) applied to both sides of patch membranes. Under these conditions, inward currents were analysed with the membrane potential held at −60 mV. The KIR6.1/SUR2B channel showed small basal currents. The channel was strongly activated by the KATP channel activator pinacidil (10 µmol·L−1). Following the channel activation by pinacidil, the KIR6.1/SUR2B channel was inhibited dose-dependently by rosiglitazone (Figure 1A) as shown previously (Yu et al., 2011). The channel was inhibited by 11% with rosiglitazone (1 µmol·L−1) applied to the internal solution. Rosiglitazone (100 µmol·L−1) inhibited the channel activity by 98%. The channel showed very weak inward rectification at physiological pH, without polyamine. Measured with slope command potential (−100 to 100 mV), the unitary conductance was 35 ± 0 pS (n= 7) in the control condition (Figure 1B) and remained the same in the presence of 10 µmol·L−1 rosiglitazone (Figure 1C). These results indicated that the effect of rosiglitazone was mediated by suppression of the Popen without affecting the unitary conductance.
Figure 1.
Inhibition of KIR6.1/SUR2B channel by rosiglitazone (RSG) in an inside-out patch. (A) An HEK cell was co-transfected with KIR6.1 and SUR2B. The holding potential for the patch was −60 mV. The channels were activated by 10 µM pinacidil (Pin) and then dose-dependently inhibited by rosiglitazone. Washout led to complete recovery. (B) The conductance of KIR6.1/SUR2B channel (35 pS) was not changed after a treatment with 10 µM rosiglitazone (C, 35 pS).
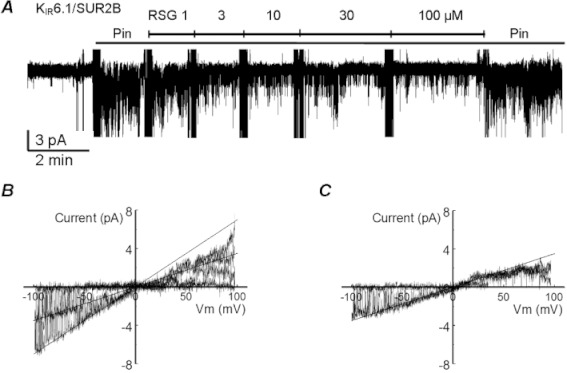
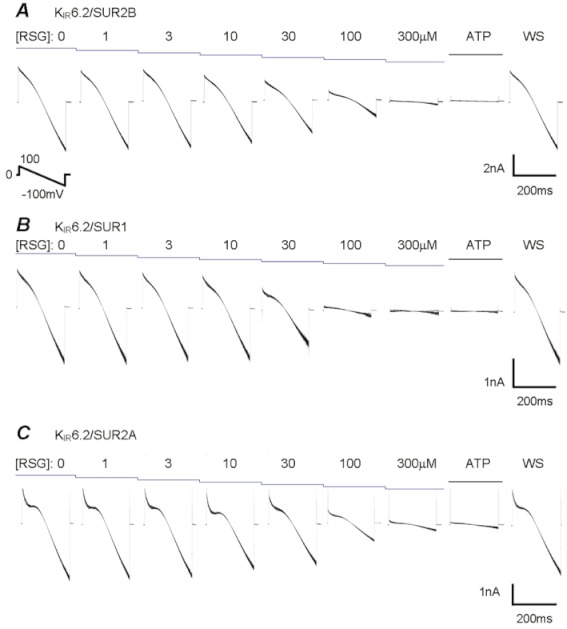
Also, rosiglitazone strongly inhibited KIR6.2/SUR1, KIR6.2/SUR2A and KIR6.2/SUR2B channels known to be expressed in the pancreatic beta cells, the striated muscle and the myocardium, respectively (Figure 2). Since the KIR6.2-containing channels are mostly open without ATP, KATP channel openers were not used to activate these channels. With ramp commands from 100 to −100 mV at a holding potential of 0 mV, the KIR6.2-containing channels showed weak inward rectification. When the patches were exposed to rosiglitazone, concentration-dependent inhibition of these KIR6.2-containing channels was clearly seen (Figure 3). The relationship of channel activity versus rosiglitazone concentrations was described using Equation 2. The IC50 was 10 µmol·L−1 (h 1.3) for the KIR6.1/SUR2B channel, 45 µmol·L−1 for the KIR6.2/SUR1 (h 1.2), 37 µmol·L−1 (h 1.1) for the KIR6.2/SUR2A and 50 µmol·L−1 (h 1.2) for the KIR6.2/SUR2B.
Figure 2.
Concentration-dependent inhibition of three KIR6.2 channel isoforms by rosiglitazone (RSG). At baseline, all KIR6.2-containing channels were active without ATP and KATP channel opener. Exposure to rosiglitazone produced dose-dependent inhibition of the KIR6.2/SUR2B (A), KIR6.2/SUR1 (B) and KIR6.2/SUR2A (C). Complete channel inhibition was seen with 1 mM ATP. The channel inhibition was reversible, and the current amplitudes almost returned to baseline levels after washout (WS). Note that 8 superimposed traces are shown in each panel.
Figure 3.
The relationship of channel activity with rosiglitazone (RSG) concentration was described using Equation 2. All combinations of KIR6.x and SURx subunits showed clear concentration dependence. The IC50 was 10 µM for KIR6.1/SUR2B (h 1.3, n= 10 patches), 45 µM for KIR6.2/SUR1 (h 1.2, n= 5), 37 µM for KIR6.2/SUR2A (h 1.1, n= 5), 50 µM for KIR6.2/SUR2B (h 1.2, n= 6–7) and 45 µM for KIR6.2ΔC36 (h 1.3, n= 5–8). See the text for the h values.
The KIR6.x subunit was likely to be the target of rosiglitazone
Because these KATP channels consist of KIR6.x and SURx subunits, we were interested in knowing whether rosiglitazone acted on the KIR6.x or the SURx subunit. Therefore, we studied the KIR6.2ΔC36 channel, known to express functional KATP currents without the SUR subunit (Tucker et al., 1997; Piao et al., 2001). Under the same experimental condition as for other KIR6.2-containing channels, the KIR6.2ΔC36 channel was inhibited by rosiglitazone (Figure 4A). The IC50 of rosiglitazone was 45 µmol·L−1 (h 1.3, Figure 3). The similar sensitivity to rosiglitazone found for the KIR6.2ΔC36 and other KIR6.2/SURx combinations strongly suggest that the KIR6.x subunit is likely to be the target of the rosiglitazone, although the SUR subunit enhances rosiglitazone sensitivity modestly. The currents with inward rectification had unitary conductance of 74 ± 0 pS (n= 15) and 74 ± 0 pS (n= 14) in the absence and presence of 100 µmol·L−1 rosiglitazone respectively (Figure 4B).
Figure 4.
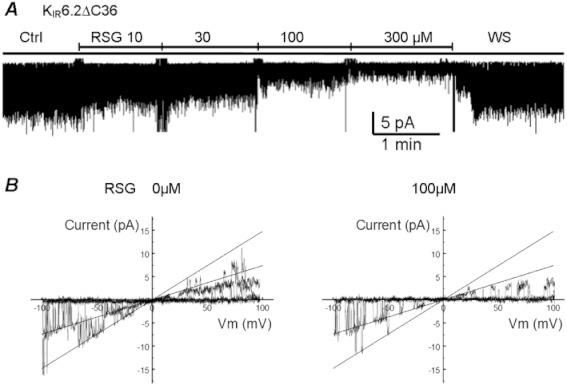
KIR6.2ΔC36 also showed dose-dependent inhibition by rosiglitazone (RSG) (A) The channel inhibition was reversible with washout (WS). Also, the unitary conductance was not changed with 100 µM rosiglitazone treatment, which was 74 pS with or without RSG (B).
Rosiglitazone did not have any effect on KIR1.1, KIR2.1 and KIR4.1 channels
Several other inward rectifying K+ channels (i.e. KIR1.1, KIR2.1 and KIR4.1) were studied. All these channels have high basal activity (Tucker et al., 1997; Zhu et al., 2000; Rojas et al., 2007). Exposures to different concentrations of rosiglitazone (30, 100, 300 µmol·L−1) did not produce any detectable channel inhibition (Figure 5), suggesting that the effect of rosiglitazone is rather specific for KATP channels.
Figure 5.
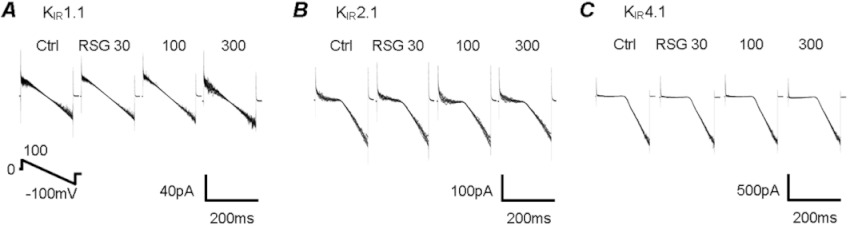
Rosiglitazone (RSG) had no inhibitory effect on KIR1.1, KIR2.1 and KIR4.1 channels. These KIR channels were expressed in HEK cells and studied in the inside-out patches under the same condition in Figure 2. None of these channels were inhibited by rosiglitazone at 30, 100 and 300 µM.
The rosiglitazone interaction site appeared to be located on the cytosolic side
In outside-out patches, the inhibitory effect of rosiglitazone was much less potent in comparison with that seen in inside-out patches (Figure 6A). In KIR6.2/SUR2B channels, the IC50 of rosiglitazone was increased to 350 from 50 µmol·L−1 in inside-out patches (Figure 6B). A similar effect was found in KIR6.1/SUR2B channels, with IC50 of 150 µmol·L−1 with outside exposure, compared with 10 µmol·L−1 with inside exposure (Figure 6C). The final concentration (∼0.5%) of the solvent DMSO had no effect on channel activity from either inside or outside the membrane.
Figure 6.
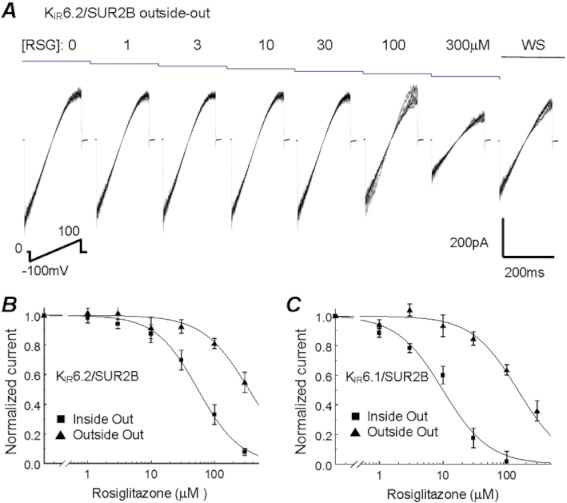
Comparison of channel inhibition between outside-out and inside-out patches. (A) In an outside-out patch, the KIR6.2/SUR2B channel was partially inhibited with external exposure to rosiglitazone (RSG) up to 300 µM. (B) With the external exposure of rosiglitazone, the relationship of KIR6.2/SUR2B channel activity with rosiglitazone concentration was shifted by ∼7-fold toward the higher concentration level, where the IC50 was 350 µM (n= 4), and 50 µM with internal exposure. (C) Similar effects were seen in the KIR6.1/SUR2B channel inhibition where the IC50 was 150 µM with external exposure (n= 15) and 10 µM with internal exposure.
Consistent with these data, whole-cell currents of KIR6.1/SUR2B (Yu et al., 2011), KIR6.2/SUR2B (Figure 7A) and KIR6.2ΔC36 channels were suppressed by rosiglitazone. As rosiglitazone acted on KIR6.1 and KIR6.2 channels with different potencies, 30 and 100 µmol·L−1 rosiglitazone was tested on KIR6.1- and KIR6.2-containing channels respectively. The inhibitory effect of rosiglitazone was less in the whole-cell configuration than in inside-out patches in all three combinations of KIR6.x channels (Figure 7B–D). These data suggest that rosiglitazone is likely to act on the cytosolic side of the channel protein, although they do not rule out the possibility of the existence of an additional low-affinity extracellular interaction site.
Figure 7.
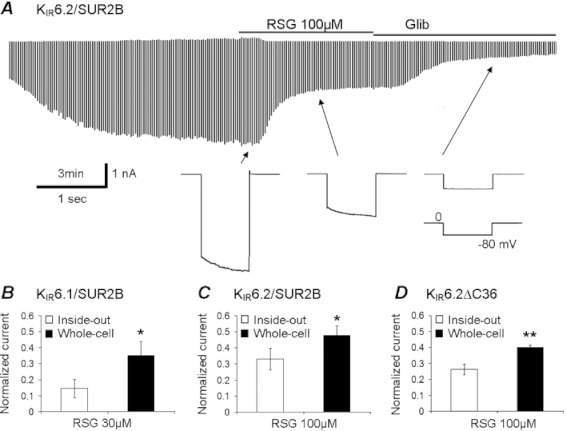
The currents were recorded in the whole-cell configuration with a high concentration (145 mM) of K+ applied to either side of the plasma membrane. The membrane potential was held at 0 mV and stepped to −80 mV every 3 s as shown in the lower panel of A. The KIR6.2/SUR2B channel spontaneously opened without activator application and without ATP in the pipette solution; the channel activation was inhibited by 100 µM rosiglitazone (RSG) and further suppressed by 10 µM glibenclamide (Glib). Whole-cell currents of KIR6.1/SUR2B (B, n= 8), KIR6.2/SUR2B (C, n= 4) and KIR6.2ΔC36 channels (D, n= 5) were inhibited by 30, 100 and 100 µM rosiglitazone respectively. The current inhibition was significantly smaller than that seen in inside-out patches.
Specific single-channel properties were targeted by rosiglitazone
Single-channel activity was analysed on the KIR6.2ΔC36 channel in inside-out patches (Figure 8A,B). In the control condition, the mean open time (TO) of the currents averaged about 2 ms and the mean closed time (TC) was about 17 ms (Table 1, n= 5 patches). In the presence of 100 µmol·L−1 rosiglitazone, the TO did not change, while the TC was doubled (Table 1).
Figure 8.
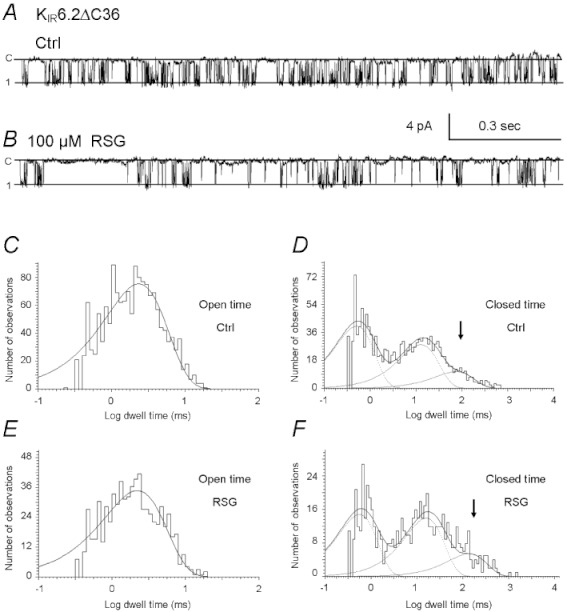
Single-channel kinetic analysis. Single-channel activity of the KIR6.2ΔC36 channel was studied in inside-out patches. (A) KIR6.2ΔC36 single-channel current at baseline. (B) The KIR6.2ΔC36 channel was inhibited by 100 µM rosiglitazone (RSG). (C) The dwell-time histogram of channel openings was described by a single-exponential with the constant τO= 2.3 ms. (D) The dwell-time histogram for channel closures contained three components of time constants: τC1= 0.5 ms, τC2= 12.7 ms, τC3= 74.6 ms. (E,F) With 100 µM rosiglitazone treatment, the dwell-time histograms of the channel openings and closures did not show marked changes (τO= 2.2 ms, τC1= 0.6 ms, and τC2= 15.9 ms) except τC3= 136.4 ms.
Table 1.
Effects of rosiglitazone on mean open and closed times of KATP channels
| Control | Rosiglitazone | ||
|---|---|---|---|
| KIR6.1/SUR2B | TO | 4.98 ± 1.41 ms | 2.97 ± 0.48 ms |
| TC | 8.08 ± 1.69 ms | 34.89 ± 8.09 ms** | |
| KIR6.2ΔC36 | TO | 2.33 ± 0.17 ms | 2.17 ± 0.26 ms |
| TC | 16.92 ± 4.19 ms | 34.18 ± 7.46 ms** |
P < 0.01 (paired Student's t-test; n = 5–6 patches).
In these patches (n= 5), the dwell-time histograms of channel openings of the KIR6.2ΔC36 channel were described with a single exponential equation with the time constant τO= 1.9 ± 0.1 ms (Figure 8C, Table 2). We tried to model the dwell-time histograms for closure with two and three exponentials. Our results showed that the data were well described with three, but not two, exponentials (τC1, τC2, τC3 ) (Figure 8D; Table 2; Supplementary Figure S1). In the presence of 100 µmol·L−1 rosiglitazone, τC3 was increased (Figure 8E,F), while none of the other time constants showed significant changes. Thus, the results are consistent with the rosiglitazone effect on the TC shown above and indicate that rosiglitazone selectively enhances long-lasting closure.
Table 2.
Effects of rosiglitazone on open and closed time constants of KATP channels
| τO1 | τO2 | τC1 | τC2 | τC3 | ||
|---|---|---|---|---|---|---|
| KIR6.1/SUR2B | CTL | 1.12 ± 0.02 ms | 5.71 ± 1.96 ms | 1.46 ± 0.82 ms | 11.96 ± 4.44 ms | |
| (0.38 ± 0.07) | (0.62 ± 0.07) | (0.72 ± 0.14) | (0.29 ± 0.14) | |||
| RSG | 1.41 ± 0.27 ms | 4.86 ± 0.77 ms | 0.77 ± 0.12 ms | 84.08 ± 14.83 ms** | ||
| (0.60 ± 0.16) | (0.40 ± 0.16) | (0.72 ± 0.15) | (0.28 ± 0.15) | |||
| KIR6.2ΔC36 | CTL | 1.90 ± 0.11 ms | 0.60 ± 0.15 ms | 9.81 ± 2.05 ms | 41.73 ± 1.15 ms | |
| (1.00 ± 0.00) | (0.56 ± 0.09) | (0.32 ± 0.08) | (0.12 ± 0.02) | |||
| RSG | 1.91 ± 0.14 ms | 0.47 ± 0.08 ms | 10.72 ± 1.56 ms | 84.20 ± 20.97 ms** | ||
| (1.00 ± 0.00) | (0.53 ± 0.09) | (0.31 ± 0.07) | (0.16 ± 0.04) |
CTL, control; RSG, rosiglitazone. Numbers in parentheses are proportions of the time constant above.
P < 0.01 (paired Student's t-test, n= 5–6 patches).
Similar results were obtained for the KIR6.1/SUR2B channel, where in the presence of 10 µmol·L−1 rosiglitazone, the TC was increased and TO was not changed (Table 1, n= 6 patches). The dwell-time histograms of KIR6.1/SUR2B channel openings were described with a two exponential equation (Table 2), and the dwell-time histograms for closures also contained only two exponential components. After 10 µmol·L−1 rosiglitazone treatment, only τC2 showed significant change. Therefore, the single-channel analysis suggested that rosiglitazone augmented the long-lasting closures of the KATP channels without affecting the open state and shorter closed states.
Discussion and conclusions
Our results suggest that rosiglitazone is a novel KATP channel inhibitor, which was surprising as rosiglitazone is better known as a PPAR-γ activator and has been widely used for the treatment of type 2 diabetes. We have found that rosiglitazone at micromolar concentrations inhibits all isoforms of KATP channels in cell-free isolated membrane patches. Interestingly, rosiglitazone seemed to inhibit the KATP channels by acting on the pore-forming KIR6.x subunit, as it had similar potencies for KIR6.2-containing channels with or without the SUR subunit. Its potency is much higher for the KIR6.1/SUR2B channel than KIR6.2-containing channels. The KATP channel inhibition is specific as rosiglitazone has no effect on KIR1.1, KIR2.1 and KIR4.1 channels.
In the treatment of type 2 diabetes, rosiglitazone has several beneficial cardiovascular effects, which are likely to derive from the improvement of the metabolic profile and VSM remodelling (Wang et al., 2006; How et al., 2007; Lu et al., 2008b; Kanda et al., 2009; Savoia et al., 2010; Torres Tda et al., 2010; Yu et al., 2010). Despite these beneficial outcomes, recent clinical studies have raised the issue of the potential cardiovascular risks in users of rosiglitazone (Zinn et al., 2008; Kaul et al., 2010). The ischemic cardiovascular effects of rosiglitazone have also been studied in animal models, and results of the studies are inconsistent with the reports from patients with type 2 diabetes (Knock et al., 1999; Khandoudi et al., 2002; Abe et al., 2008; Kilter et al., 2009; Potenza et al., 2009; CX Wang et al., 2009; Y Wang et al., 2010).
Potential involvement of ion channels in the effects of rosiglitazone has been examined previously by several research groups (Knock et al., 1999; Mishra and Aaronson, 1999; Eto et al., 2001; Lu et al., 2008a; Chang et al., 2009). Rosiglitazone inhibited Ca2+ currents and voltage-activated K+ currents that play a role in cAMP-mediated vasodilation (Eto et al., 2001; Li et al., 2003). Rosiglitazone also activated Ca2+-activated K+ currents in acutely dissociated mesenteric VSM cells (Eto et al., 2001; Lu et al., 2008a), although it did not produce vasorelaxation in human subcutaneous small arterial rings (Walker et al., 1998). The glibenclamide-sensitive K+ currents of freshly isolated aortic myocytes were inhibited by rosiglitazone (Chang et al., 2009). Moreover, rosiglitazone has been shown to stimulate insulin secretion in pancreatic beta cells via phosphorylation of the KIR6.2 channel by AMP-dependent protein kinase (Chang et al., 2009). Rosiglitazone blocked cardiac KATP channels and promoted the onset of ventricular fibrillation during severe ischaemia (Mishra and Aaronson, 1999).
Our recent study indicates that the KIR6.1/SUR2B channel is a target of rosiglitazone (Yu et al., 2011). KIR6.1/SUR2B channel inhibition leads to an impairment of the coronary vasodilator response as it is a common target of both vasodilating and vasoconstricting hormones (and neurotransmitters) that activate and inhibit the channel by distinct protein phosphorylation respectively (Ashcroft, 2006; Shi et al., 2007a, b; Yang et al., 2008; Orie et al., 2009). The basal level of KATP channel activity is low under physiological conditions (Quayle et al., 1997; Nichols, 2006). When the channels are mostly closed, they cannot significantly contribute to the membrane potential, and further inhibition of these channels may not allow sufficient depolarization to cause muscle contraction. Therefore, rosiglitazone does not have significant effects on basal vascular tones as suggested in several previous studies (Walker et al., 1998; Irat et al., 2006).
Our current studies suggest that rosiglitazone is a selective and potent KATP channel inhibitor. It inhibits all KIR6.x-containing channels without affecting KIR1.1, KIR2.1 and KIR4.1. The IC50 of rosiglitazone is 10 µmol·L−1 for the KIR6.1/SUR2B channels and ∼45 µmol·L−1 for the KIR6.2/SURx channels. Although the IC50 values are slightly higher than the therapeutic concentrations for the treatment of type 2 diabetes, where the plasma concentration was around 3 µM (Cox et al., 2000), the IC50 is greatly reduced in the presence of a therapeutic concentration of glibenclamide, as shown previously (Coppack et al., 1990; Cox et al., 2000; Yu et al., 2011). Therefore, it is possible that KATP channels in various tissues may be partially inhibited when rosiglitazone is used for therapeutic purposes, especially in combination with a sulphonylurea.
A remarkable finding of the study is that rosiglitazone inhibited the KATP channels independently of the SUR subunit. The potency of rosiglitazone (IC50∼45 µmol·L−1) for the KIR6.2ΔC36 channel is the same as for KIR6.2/SURx channels, suggesting that the SUR subunit plays a rather small role in the channel inhibition. Currently, there are two inhibitors for the pore-forming KIR6.x subunit (i.e. Ba2+ and PNU-37883A, as well as its derivatives) (Hill, 1992; Takano and Ashcroft, 1996). Ba2+ blocks KIR currents more effectively from the extracellular side, suppressing inward rectification of the channels. Since Ba2+ is a non-selective KIR channel inhibitor, it cannot be used to inhibit the KATP channels in vivo where many other KIR channels may also be inhibited. PNU-37883A was originally synthesized and tested as a diuretic agent (Perricone et al., 1994; Humphrey et al., 1995). It acts on the pore-forming subunit of KATP channels with similar potencies to rosiglitazone. Also, the vascular KATP channel preference between rosiglitazone and PNU-37883A is similar (Cui et al., 2003; Teramoto, 2006). Both inhibit the KIR6.1/SUR2B channel more potently than KIR6.2/SURx channels. However, unlike PNU-37883A, rosiglitazone is a practical therapeutic agent and has been used clinically for over 10 years. During that period, rosiglitazone has been extensively tested clinically, and its beneficial and adverse effects have been well documented. Thus, knowledge of the rosiglitazone effect on KATP channels, as shown in the present studyn may help drug design by avoiding or deliberately acting on these novel targets of rosiglitazone. A previous autoradiographic study showed that the sulphonylurea glibenclamide may interact with the KIR6.2 subunit in the COS cell line, although whether such interaction has a functional consequence is still unknown (Gros et al., 1999),
Rosiglitazone appeared to act on the intracellular domains of KIR6.x subunits. The potency of rosiglitazone was over 10 times lower when the drug was used extracellularly. Such a weak extracellular effect may result from the relatively high hydrophobicity of the drug, allowing it to pass through the membrane and act on the intracellular domain of the channel protein after being diluted by the cytoplasm or intracellular solution. Consistent with this idea, similar extracellular exposure to rosiglitazone produced less KATP channel inhibition in the whole-cell configuration where rosiglitazone was diluted by the cytoplasm. Despite this, our data cannot rule out the possibility that there is an extracellular site in the channel protein interacting with less affinity to rosiglitazone. Clearly, further studies are needed to understand the structure-function relationship for the rosiglitazone-channel interaction.
Our analysis of single-channel properties indicated that rosiglitazone suppressed the Popen rather than the unitary conductance. This would explain the selective augmentation of the long-lasting closures by rosiglitazone, without affecting the open state time constant and the mean open time. These results suggest that rosiglitazone acts on the gating mechanisms of the KIR6.x subunit, which is located intracellularly, consistent with our observation of the potential interaction site at an intracellular location.
In conclusion, rosiglitazone was a potent inhibitor of all isoforms of KATP channels. It inhibited these K+ channels in a membrane-delimited manner by a direct interaction with the KIR6.x subunit. The interaction site appeared to be on the intracellular domain of the KIR6.x involved in channel gating. The channel inhibition was specific for KIR6.x channels. The biophysical basis of the channel inhibition was the selective augmentation of long-lasting closure, without affecting the open state. Therefore, our results have demonstrated a novel KATP channel inhibitor that could be used for experimental intervention. As the drug is already used clinically, our reesluts also suggest new therapeutic uses to manipulate membrane excitability and metabolic state by targeting KATP channel function in various tissues.
Acknowledgments
This work was supported by the NIH (HD060959, CJ), the American Heart Association (09GRNT2010037, CJ) and the National Natural Science Foundation of China (No.31071007, DZ). LY was partially supported by a scholarship of Harbin Medical University. YY is a Brains & Behavior fellow of Georgia State University. The authors thank Mr Max F Oginsky for his comments on the manuscript.
Glossary
- KIR
inward rectifying potassium channel
- SUR
sulphonylurea receptor
- VSM
vascular smooth muscle
Conflict of interest
None of the authors has conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 In both control (A) and 100 µM rosiglitazone (RSG) treatment (B) conditions, the KIR6.2ΔC36 channel dwell-time histograms of channel openings were not fit with two exponentials.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Abe M, Takiguchi Y, Ichimaru S, Kaji S, Tsuchiya K, Wada K. Different effect of acute treatment with rosiglitazone on rat myocardial ischemia/reperfusion injury by administration method. Eur J Pharmacol. 2008;589:215–219. doi: 10.1016/j.ejphar.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. From molecule to malady. Nature. 2006;440:440–447. doi: 10.1038/nature04707. [DOI] [PubMed] [Google Scholar]
- Barnett AH. Redefining the role of thiazolidinediones in the management of type 2 diabetes. Vasc Health Risk Manag. 2009;5:141–151. doi: 10.2147/vhrm.s4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993;110:573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TJ, Chen WP, Yang C, Lu PH, Liang YC, Su MJ, et al. Serine-385 phosphorylation of inwardly rectifying K+ channel subunit (Kir6.2) by AMP-dependent protein kinase plays a key role in rosiglitazone-induced closure of the K(ATP) channel and insulin secretion in rats. Diabetologia. 2009;52:1112–1121. doi: 10.1007/s00125-009-1337-4. [DOI] [PubMed] [Google Scholar]
- Coppack SW, Lant AF, McIntosh CS, Rodgers AV. Pharmacokinetic and pharmacodynamic studies of glibenclamide in non-insulin dependent diabetes mellitus. Br J Clin Pharmacol. 1990;29:673–684. doi: 10.1111/j.1365-2125.1990.tb03688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PJ, Ryan DA, Hollis FJ, Harris AM, Miller AK, Vousden M, et al. Absorption, disposition, and metabolism of rosiglitazone, a potent thiazolidinedione insulin sensitizer, in humans. Drug Metab Dispos. 2000;28:772–780. [PubMed] [Google Scholar]
- Cui Y, Tinker A, Clapp LH. Different molecular sites of action for the KATP channel inhibitors, PNU-99963 and PNU-37883A. Br J Pharmacol. 2003;139:122–128. doi: 10.1038/sj.bjp.0705228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K, Ohya Y, Nakamura Y, Abe I, Fujishima M. Comparative actions of insulin sensitizers on ion channels in vascular smooth muscle. Eur J Pharmacol. 2001;423:1–7. doi: 10.1016/s0014-2999(01)01047-0. [DOI] [PubMed] [Google Scholar]
- Gros L, Virsolvy A, Salazar G, Bataille D, Blache P. Characterization of low-affinity binding sites for glibenclamide on the Kir6.2 subunit of the beta-cell KATP channel. Biochem Biophys Res Commun. 1999;257:766–770. doi: 10.1006/bbrc.1999.0529. [DOI] [PubMed] [Google Scholar]
- Hill A. Measles, mumps, and rubella vaccination. BMJ. 1992;304:779. doi: 10.1136/bmj.304.6829.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How OJ, Larsen TS, Hafstad AD, Khalid A, Myhre ES, Murray AJ, et al. Rosiglitazone treatment improves cardiac efficiency in hearts from diabetic mice. Arch Physiol Biochem. 2007;113:211–220. doi: 10.1080/13813450701783281. [DOI] [PubMed] [Google Scholar]
- Humphrey SJ, Ludens JH, Perricone SC, Skaletzky LL, Graham BE, Zins GR. Diuretic activity of N′-disubstituted morpholinoguanidine analogs of U-37883A in rats and dogs. Methods Find Exp Clin Pharmacol. 1995;17:255–266. [PubMed] [Google Scholar]
- Irat AM, Aslamaci S, Karasu C, Ari N. Alteration of vascular reactivity in diabetic human mammary artery and the effects of thiazolidinediones. J Pharm Pharmacol. 2006;58:1647–1653. doi: 10.1211/jpp.58.12.0012. [DOI] [PubMed] [Google Scholar]
- Kanda T, Brown JD, Orasanu G, Vogel S, Gonzalez FJ, Sartoretto J, et al. PPARgamma in the endothelium regulates metabolic responses to high-fat diet in mice. J Clin Invest. 2009;119:110–124. doi: 10.1172/JCI36233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Bolger AF, Herrington D, Giugliano RP, Eckel RH. Thiazolidinedione drugs and cardiovascular risks: a science advisory from the American Heart Association and American College Of Cardiology Foundation. J Am Coll Cardiol. 2010;55:1885–1894. doi: 10.1016/j.jacc.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Khandoudi N, Delerive P, Berrebi-Bertrand I, Buckingham RE, Staels B, Bril A. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma, inhibits the Jun NH(2)-terminal kinase/activating protein 1 pathway and protects the heart from ischemia/reperfusion injury. Diabetes. 2002;51:1507–1514. doi: 10.2337/diabetes.51.5.1507. [DOI] [PubMed] [Google Scholar]
- Kilter H, Werner M, Roggia C, Reil JC, Schafers HJ, Kintscher U, et al. The PPAR-gamma agonist rosiglitazone facilitates Akt rephosphorylation and inhibits apoptosis in cardiomyocytes during hypoxia/reoxygenation. Diabetes Obes Metab. 2009;11:1060–1067. doi: 10.1111/j.1463-1326.2009.01097.x. [DOI] [PubMed] [Google Scholar]
- Knock GA, Mishra SK, Aaronson PI. Differential effects of insulin-sensitizers troglitazone and rosiglitazone on ion currents in rat vascular myocytes. Eur J Pharmacol. 1999;368:103–109. doi: 10.1016/s0014-2999(99)00020-5. [DOI] [PubMed] [Google Scholar]
- Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285:H1213–H1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- Lu L, Reiter MJ, Xu Y, Chicco A, Greyson CR, Schwartz GG. Thiazolidinedione drugs block cardiac KATP channels and may increase propensity for ischaemic ventricular fibrillation in pigs. Diabetologia. 2008a;51:675–685. doi: 10.1007/s00125-008-0924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YL, Jimbu YM, Chen Y, Zhao JB, Ye TT, Yang H. The effects of rosiglitazione on renal artery endothelium in diabetic rats. Exp Clin Endocrinol Diabetes. 2008b;116:537–540. doi: 10.1055/s-2008-1058087. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Aaronson PI. Differential block by troglitazone and rosiglitazone of glibenclamide-sensitive K(+) current in rat aorta myocytes. Eur J Pharmacol. 1999;386:121–125. doi: 10.1016/s0014-2999(99)00713-x. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Kakei M, Tanaka H. Activation of the ATP-sensitive K+ channel by decavanadate in guinea-pig ventricular myocytes. Eur J Pharmacol. 1993;233:219–226. doi: 10.1016/0014-2999(93)90053-k. [DOI] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Oliver D, Hahn H, Antz C, Ruppersberg JP, Fakler B. Interaction of permeant and blocking ions in cloned inward-rectifier K+ channels. Biophys J. 1998;74:2318–2326. doi: 10.1016/S0006-3495(98)77941-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orie NN, Thomas AM, Perrino BA, Tinker A, Clapp LH. Ca2+/calcineurin regulation of cloned vascular K ATP channels: crosstalk with the protein kinase A pathway. Br J Pharmacol. 2009;157:554–564. doi: 10.1111/j.1476-5381.2009.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone SC, Humphrey SJ, Skaletzky LL, Graham BE, Zandt RA, Zins GR. Synthesis and diuretic activity of alkyl- and arylguanidine analogs of N,N′-dicyclohexyl-4-morpholinecarboxamidine in rats and dogs. J Med Chem. 1994;37:3693–3700. doi: 10.1021/jm00048a005. [DOI] [PubMed] [Google Scholar]
- Piao H, Cui N, Xu H, Mao J, Rojas A, Wang R, et al. Requirement of multiple protein domains and residues for gating K(ATP) channels by intracellular pH. J Biol Chem. 2001;276:36673–36680. doi: 10.1074/jbc.M106123200. [DOI] [PubMed] [Google Scholar]
- Potenza MA, Gagliardi S, De Benedictis L, Zigrino A, Tiravanti E, Colantuono G, et al. Treatment of spontaneously hypertensive rats with rosiglitazone ameliorates cardiovascular pathophysiology via antioxidant mechanisms in the vasculature. Am J Physiol Endocrinol Metab. 2009;297:E685–E694. doi: 10.1152/ajpendo.00291.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Rojas A, Cui N, Su J, Yang L, Muhumuza JP, Jiang C. Protein kinase C dependent inhibition of the heteromeric Kir4.1-Kir5.1 channel. Biochim Biophys Acta. 2007;1768:2030–2042. doi: 10.1016/j.bbamem.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C, Ebrahimian T, Lemarie CA, Paradis P, Iglarz M, Amiri F, et al. Countervailing vascular effects of rosiglitazone in high cardiovascular risk mice: role of oxidative stress and PRMT-1. Clin Sci (Lond) 2010;118:583–592. doi: 10.1042/CS20090289. [DOI] [PubMed] [Google Scholar]
- Shi W, Cui N, Shi Y, Zhang X, Yang Y, Jiang C. Arginine vasopressin inhibits Kir6.1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol. 2007a;293:R191–R199. doi: 10.1152/ajpregu.00047.2007. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, et al. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007b;293:R1205–R1214. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Ashcroft FM. The Ba2+ block of the ATP-sensitive K+ current of mouse pancreatic beta-cells. Pflugers Arch. 1996;431:625–631. doi: 10.1007/BF02191912. [DOI] [PubMed] [Google Scholar]
- Teramoto N. Pharmacological Profile of U-37883A, a Channel Blocker of Smooth Muscle-Type ATP-Sensitive K Channels. Cardiovasc Drug Rev. 2006;24:25–32. doi: 10.1111/j.1527-3466.2006.00025.x. [DOI] [PubMed] [Google Scholar]
- Torres Tda S, Aguila MB, Mandarim-de-Lacerda CA. Rosiglitazone reverses cardiac adverse remodeling (fibrosis and vascularization) in perinatal low protein rat offspring. Pathol Res Pract. 2010;206:642–646. doi: 10.1016/j.prp.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Tucker SJ, Gribble FM, Zhao C, Trapp S, Ashcroft FM. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- Walker AB, Naderali EK, Chattington PD, Buckingham RE, Williams G. Differential vasoactive effects of the insulin sensitizers rosiglitazone (BRL 49653) and troglitazone on human small arteries in vitro. Diabetes. 1998;47:810–814. doi: 10.2337/diabetes.47.5.810. [DOI] [PubMed] [Google Scholar]
- Wang CX, Ding X, Noor R, Pegg C, He C, Shuaib A. Rosiglitazone alone or in combination with tissue plasminogen activator improves ischemic brain injury in an embolic model in rats. J Cereb Blood Flow Metab. 2009;29:1683–1694. doi: 10.1038/jcbfm.2009.87. [DOI] [PubMed] [Google Scholar]
- Wang K, Zhou Z, Zhang M, Fan L, Forudi F, Zhou X, et al. Peroxisome proliferator-activated receptor gamma down-regulates receptor for advanced glycation end products and inhibits smooth muscle cell proliferation in a diabetic and nondiabetic rat carotid artery injury model. J Pharmacol Exp Ther. 2006;317:37–43. doi: 10.1124/jpet.105.095125. [DOI] [PubMed] [Google Scholar]
- Wang R, Rojas A, Wu J, Piao H, Adams CY, Xu H, et al. Determinant role of membrane helices in K ATP channel gating. J Membr Biol. 2005;204:1–10. doi: 10.1007/s00232-005-0741-z. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, et al. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2010;298:E663–E670. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Yang Z, Cui N, Giwa LR, Abdulkadir L, Patel M, et al. Molecular determinants for the distinct pH sensitivity of Kir1.1 and Kir4.1 channels. Am J Physiol Cell Physiol. 2000;279:C1464–C1471. doi: 10.1152/ajpcell.2000.279.5.C1464. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shi Y, Guo S, Zhang S, Cui N, Shi W, et al. PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta. 2008;1778:88–96. doi: 10.1016/j.bbamem.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Xu H, Cui N, Qu Z, Chanchevalap S, Shen W, et al. Biophysical and molecular mechanisms underlying the modulation of heteromeric Kir4.1-Kir5.1 channels by CO2 and pH. J Gen Physiol. 2000;116:33–45. doi: 10.1085/jgp.116.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang Z, Li Z, Feng X, He L, Liu S, et al. Peroxisome proliferator-activated receptor-gamma(PPARgamma) agonist improves coronary artery endothelial function in diabetic patients with coronary artery disease. J Int Med Res. 2010;38:86–94. doi: 10.1177/147323001003800110. [DOI] [PubMed] [Google Scholar]
- Yu L, Jin X, Yang Y, Cui N, Jiang C. Rosiglitazone inhibits vascular K(ATP) channels and coronary vasodilation produced by isoproterenol. Br J Pharmacol. 2011;164:2064–2072. doi: 10.1111/j.1476-5381.2011.01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra- and extracellular acidifications on single channel Kir2.3 currents. J Physiol. 1999;516:699–710. doi: 10.1111/j.1469-7793.1999.0699u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Liu C, Qu Z, Chanchevalap S, Xu H, Jiang C. CO(2) inhibits specific inward rectifier K(+) channels by decreases in intra- and extracellular pH. J Cell Physiol. 2000;183:53–64. doi: 10.1002/(SICI)1097-4652(200004)183:1<53::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Zinn A, Felson S, Fisher E, Schwartzbard A. Reassessing the cardiovascular risks and benefits of thiazolidinediones. Clin Cardiol. 2008;31:397–403. doi: 10.1002/clc.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


