Abstract
BACKGROUND AND PURPOSE
Small (KCa2) and intermediate (KCa3.1) conductance calcium-activated potassium channels (KCa) may contribute to both epithelium- and endothelium-dependent relaxations, but this has not been established in human pulmonary arteries and bronchioles. Therefore, we investigated the expression of KCa2.3 and KCa3.1 channels, and hypothesized that activation of these channels would produce relaxation of human bronchioles and pulmonary arteries.
EXPERIMENTAL APPROACH
Channel expression and functional studies were conducted in human isolated small pulmonary arteries and bronchioles. KCa2 and KCa3.1 currents were examined in human small airways epithelial (HSAEpi) cells by whole-cell patch clamp techniques.
RESULTS
While KCa2.3 expression was similar, KCa3.1 protein was more highly expressed in pulmonary arteries than bronchioles. Immunoreactive KCa2.3 and KCa3.1 proteins were found in both endothelium and epithelium. KCa currents were present in HSAEpi cells and sensitive to the KCa2.3 blocker UCL1684 and the KCa3.1 blocker TRAM-34. In pulmonary arteries contracted by U46619 and in bronchioles contracted by histamine, the KCa2.3/ KCa3.1 activator, NS309, induced concentration-dependent relaxations. NS309 was equally potent in relaxing pulmonary arteries, but less potent in bronchioles, than salbutamol. NS309 relaxations were blocked by the KCa2 channel blocker apamin, while the KCa3.1 channel blocker, charybdotoxin failed to reduce relaxation to NS309 (0.01–1 µM).
CONCLUSIONS AND IMPLICATIONS
KCa2.3 and KCa3.1 channels are expressed in the endothelium of human pulmonary arteries and epithelium of bronchioles. KCa2.3 channels contributed to endo- and epithelium-dependent relaxations suggesting that these channels are potential targets for treatment of pulmonary hypertension and chronic obstructive pulmonary disease.
Keywords: calcium-activated potassium channels, pulmonary arteries, bronchioles, KCa3.1, KCa2.3, NS309
Introduction
The respiratory epithelium and the pulmonary arterial endothelium release factors that are thought to be important in the regulation of pulmonary arterial pressure and bronchial tone (Flavahan et al., 1985; Morrison et al., 1990). In the pulmonary arterial system, endothelium-derived NO (Ignarro et al., 1987; Feletou et al., 1995), prostacyclin (Frantz et al., 1989) and an endothelium-derived hyperpolarising factor (EDHF) (Feletou et al., 1995; Zhang et al., 2006) appear to be involved in regulating vascular tone. Activation of calcium-activated potassium channels of small conductance (KCa2, subtype KCa2.3) and intermediate conductance (KCa3.1) are required for initiation of EDHF-type relaxations in several vascular beds including human arteries (Edwards et al., 1998; Buus et al., 2000; Grgic et al., 2009; Chadha et al., 2011; channel nomenclature follows Alexander et al., 2011). In the rat pulmonary circulation, we have recently found that the KCa3.1 channel and the Na+/K+-ATPase are involved in this EDHF-type relaxation (Kroigaard et al., 2010), but the contribution of KCa2.3 and KCa3.1 channels for EDHF-type relaxation in human pulmonary arteries is not known.
Even less is known about the contribution of KCa2.3 and KCa3.1 channels to epithelium-derived relaxation, and it is so far unknown whether an epithelium-derived hyperpolarizing factor (EpDHF) relaxation mechanism involving KCa2.3 and KCa3.1 channels exists. Since the pioneering work of Paul Vanhoutte and colleagues in the mid-eighties, it is well established that the epithelium can release factors producing bronchial dilation (Stuart-Smith and Vanhoutte, 1987; 1988; Vanhoutte, 1987; Morrison et al., 1990). Indeed, removal of the epithelium in larger airways enhances the effect of contractile agonists and reduces the relaxing responses in smaller airways (Stuart-Smith and Vanhoutte, 1987; Kroigaard et al., 2010). Both NO and PGE2 serve as epithelium-derived relaxing factors in human airways (Folkerts and Nijkamp, 1998). In addition, epoxyeicosatrienoic acids were proposed to evoke non-NO, non-prostanoid EpDHF-type relaxation in guinea pig airways (Benoit et al., 2001). Recently, we found that both KCa2.3 and KCa3.1 channels were involved in an EpDHF-type relaxation in rat bronchioles, also associated with activation of Na+/K+-ATPase (Kroigaard et al., 2010). However, it is unclear whether KCa2.3 and KCa3.1 channels are involved in EpDHF-type relaxation in human bronchioles.
The objective of this study was to investigate the expression, cellular localization and function of KCa2.3 and KCa3.1 channels in human pulmonary arteries and bronchioles. We hypothesized that activation of these calcium-activated potassium (KCa) channels by a KCa2.3 and KCa3.1 channel opener NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) (Strobaek et al., 2004), leads to relaxation in human pulmonary arteries and bronchioles. To address the hypothesis, human small pulmonary arteries and bronchioles were obtained from patients undergoing lung surgery. Expression of KCa2.3 and KCa3.1 channels was examined by quantitative PCR and immunoblotting and cellular localization by immunohistochemistry. Functional expression of KCa2.3 and KCa3.1 channels was examined in human small airway epithelial (HSAEpi) cells by patch clamp techniques and use of a compound, naphtho[1,2-d]thiazol-2-ylamine (SKA-31), structurally related to NS309 (Sankaranarayanan et al., 2009). Finally, the relaxant effects of NS309 were investigated in isolated human bronchioles and pulmonary arteries.
Methods
Human tissue
The research was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association and the Local Ethics Committee approved the study (Permission number: 200440154). Each patient was informed about the purpose and nature of the project and gave informed consent. Experiments were performed with human pulmonary arteries and bronchiole branches from 30 patients, who had undergone surgery for lung carcinoma at the Department of Thoracic Surgery, Aarhus University Hospital, Skejby, Denmark. Mean age of the patients examined was 67 years (55–76 years), and all individuals were smokers or former smokers. Bronchioles and small pulmonary arteries were carefully dissected under a microscope by removing the surrounding tissue.
Cell culture
Human small airway epithelial cells (HSAEpi, Sciencell, third to sixth passage) were cultured in poly-l-lysine-coated flasks containing small airway epithelial cell medium (Sciencell), epithelial cell growth supplement (EpiCGS), penicillin and streptomycin. For patch-clamp experiments, cells were trypsinized and seeded on coverslips.
Quantitative PCR of KCa2.3and KCa3.1mRNA in human lung
Human intrapulmonary arteries and bronchioles were isolated in calcium free physiological saline solution (PSS) and transferred to RNA-later (Ambion, Foster City, CA). Purification of total RNA was achieved by using the RNeasy Mini Fibrous Tissue Kit from Qiagen (Hilden, Germany). Furthermore, removal of genomic DNA was achieved using a DNase I digestion step (Qiagen). The concentration of the RNA was estimated by OD measurements at 260 nm. Total RNA (100 µg·mL−1) was reverse transcribed using Oligo-dT primer and Superscript™ SIII reverse transcriptase (Invitrogen, Hercules, CA, USA). Expression of KCa channels was assessed by TaqMan quantitative PCR (QPCR). 200 ng RNA was used in a 25 µL reaction and quantitative PCR conducted using Ex Taq™ (TaKaRa, Shiga, Japan). The following cycles were run: 1 cycle at 95°C for 2 min, 40 cycles at 95°C for 15 s, 55°C for 1 min and 70°C for 15 s on a Stratagene Mx3000P machine (La Jolla, CA). Primers were designed from the National Center for Biotechnical Information (NCBI) using PerlPrimer and ordered at MWG-Biotech AG (Ebersberg, Germany): KCa2.3, F: GATTGACCATGCCAAAGTGAG; KCa2.3, R: ACATGACATTCTGCATCTTGG; KCa3.1, F: GTTCTACAAACATACTCGCAGGA; KCa3.1, R: GCGTGTCAATCTGTTTCTCAA. The fluorogenic probe contained a reporter dye, 6-carboxyfluorescein (FAM), at the 5′-end and a Blackhole Quencher 1 dye at the 3′-end: KCa2.3: TCCTCCAAGCTATCCACCAGTTGAG; KCa3.1: TCAACGCGTTCCGCCAGGTGCGGCTGAAA. The amount of cDNA was normalized to the reference gene by using the threshold Ct values: ΔCt = Ct(target) − Ct(GAPDH), and the results were analysed as a ratio to GAPDH expression: Ratio = 2–ΔCt and expressed as a percentage.
Reverse transcriptase-PCR of HSAEpi cells
Reverse transcriptase-PCR (RT-PCR) was done using RNeasy Mini Kit (Qiagen) for RNA purification, iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) for cDNA synthesis and Taq DNA polymerase (Invitrogen) for PCR. A standard PCR protocol was used with initial denaturing at 95°C for 3 min and subsequently 40 cycles of denaturing at 95°C for 25 s, annealing at 57°C for 20 s and extending at 72°C for 40 s. A final step of extension was done at 72°C for 3 min. PCR-products were analysed by gel electrophoresis using 1.5% agarose in TBE buffer and staining with GelRed™ (Biotium Hayward, CA, USA). Specific RT-PCR primers were designed to span intronic sequences. The expected product lengths were 159bp for KCa3.1, 333 bp for KCa2.3, 395 bp for KCa2.2 and 391 bp for KCa2.1. Primer sequences: hKCa3.1 F, 5′-CATCACATTCCTGACCATCG; hKCa3.1 R, 5′-ACGTGCTTCTCTGCCTTGTT; hKCa2.3 F,5′GCTCTCTTGGGGTTTGTACTCAA; hKCa2.3 R, 5′-CGCAGGAACATGGGGATAGA; hKCa2.2 F, 5′-TCTAAGCCCGAGCACAACAA; hKCa2.2 R, 5′-TTGTCCACCATGAACAACTGTA; hKCa2.1 F, 5′-GGGCCTCCAGGTGGTAGT; hKCa2.1 R, 5′-CACCATGAACAGCTGGATCTC.
Immunohistochemistry of KCa2.3and KCa3.1 protein in human lung
Evaluation of the distribution of KCa3.1and KCa2.3 channels was achieved using immunohistochemistry with primary antibodies against KCa2.3 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) and KCa3.1 (1:100 or 1:500; Cell Applications, San Diego, CA). Segments of isolated pulmonary arteries and bronchioles were fixed in 4% formalin. The tissue was processed through increasing alcohol percentages to paraffin wax blocks. Sections of 3 µm were cut with a microtome, collected on glass slides and heated in an oven (80°C). The sections were de-paraffinized, processed in decreasing alcohol percentages, transferred to distilled water, incubated with 0.3% hydrogen peroxide for 20 min and rinsed in PBS. Antigen retrieval was achieved by heating sections in a microwave in TEG-buffer for 2 × 5 min. They were then quenched in 0.2% Triton X in PBS for 15 min. Blocking of non-specific antibody binding was done with 10% fetal calf serum in 1% BSA in PBS for 20 min. Incubation with the primary antibody in PBS with 1% BSA was overnight at 4°C. To ensure that there was no non-specific staining of primary antibodies, sections were incubated with isotype-matched antibodies (rabbit IgG; Abcam, Cambridge, UK) instead of primary antibodies. The sections were rinsed in PBS and incubated for 1 h at room temperature with secondary antibody, goat anti-rabbit IgG coupled to HRP (1:2000; Invitrogen) in PBS containing 1% BSA followed by rinsing in PBS. Finally, DAB was applied for 5 min, the sections rinsed and for histology, and sections were stained with haematoxylin and 0.1% w/v eosin, and dehydrated in increasing concentrations of ethanol. Background staining was controlled by incubating sections only with secondary antibody. The sections were coverslipped and analysed by light microscopy.
Immunoblotting of KCa2.3and KCa3.1 protein in human lung
Human pulmonary arteries and bronchioles were carefully dissected from the surrounding tissue, frozen in liquid nitrogen and transferred to −80°C. Protein was extracted in lysis buffer, and samples were placed in an ultrasonic bath for 45 s and centrifuged for 15 min at 13 250 g at 4°C. The supernatant was frozen at −80°C. Total protein was quantified using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Protein lysate was mixed with sample buffer and incubated at 99°C for 10 min. Samples and a pre-stain marker (Bio-Rad, Hercules, CA) were loaded onto a 4–12% Criterion XT Bis-Tris gel (Bio-Rad Hercules, CA) and separated by SDS-PAGE at 200 V. Proteins were transferred to membrane for 1 h at 100 V. The membrane was washed in TBS-T and blocked in 5% skimmed milk in TBS-T for 2 h. Incubation with primary antibody (β-actin (1:20 000), KCa2.3 (1:200, Santa Cruz Biotechnology) and KCa3.1 (1:200, Cell Applications, San Diego, CA) and in 5% skimmed milk in TBS-T was done overnight at 4°C. The membrane was washed in 5% skimmed milk in TBS-T before incubation with secondary antibody: goat anti-rabbit IgG conjugated to HRP (1:4000; Santa-Cruz Biotechnology), β-actin: goat anti-mouse IgG conjugated to HRP (1:4000; Sigma, St. Louis, MO). The membrane was developed by using the ECL-Plus kit (GE Healthcare, Copenhagen, Denmark). The blot was placed in an X-ray film cassette and developed in photographic developer. Amounts of KCa2.3 and KCa3.1 protein were normalized to β-actin, which was equally expressed in human pulmonary arteries and bronchioles.
Patch-clamp electrophysiology
Whole-cell membrane currents were recorded using an Axopatch patch-clamp amplifier (Axon Instruments, Foster City, CA) and the Clampex 9.2 data acquisition software and were analysed by the Clampfit 9.2 software. For activation of KCa currents, HSAEpi cells were dialysed with a KCl-pipette solution containing 3 µM [Ca2+]free (in mM): 140 KCl, 1 Na2ATP, 1 MgCl2, 2 EGTA, 1.91 CaCl2 and 5 HEPES, pH 7.2. The NaCl bath solution contained (mM): 137 NaCl, 4.5 Na2HPO4, 3 KCl, 1.5 KH2PO4, 0.4 MgCl2, 0.7 CaCl2 and 10 glucose (pH 7.4). For maximal and stable channel activation, the bath solution contained the KCa2/ KCa3.1 channel opener SKA-31 (1 µM). For blocking experiments, the selective KCa2.3 channel blocker UCL1684 (1 µM) and the KCa3.1 channel blocker TRAM-34 (1 µM) were added to the bath. 1 mM stock solution of TRAM-34, UCL1684 and SKA-31 were prepared in DMSO. The final DMSO concentration did not exceed 0.3%.
Wire myography
For isometric tension recordings, small pulmonary arteries and bronchioles with a length of approximately 2 mm were mounted on two 40 µm steel wires in microvascular myographs (DMT, Aarhus, Denmark). The baths were heated to 37°C and equilibrated with 5% CO2 to maintain the desired pH of 7.4. Segments were allowed to equilibrate for 10 min thereafter. For experiments on bronchioles, the organ bath contained calcium-free PSS during mounting and normalization to avoid development of spontaneous tension. For experiments on arteries, the organ bath contained normal PSS.
Segments were stretched to 2.4 kPa for optimal measurements, corresponding to a transmural pressure of 18 mmHg. The viability of the bronchial segments were examined by exposure to a potassium-rich PSS (60 mM KPSS). This was initially done twice and in the end of each experiment once. Viability of arterial segments was tested with a 124 mM KPSS. Preparations in which the final response to KPSS was not comparable to the two initial responses to KPSS were not further considered. After the initial test of viability, vessels were incubated for 30 min in PSS containing the COX inhibitor, indomethacin (3 µM), to block the synthesis of prostaglandins. Indomethacin remained present throughout the entire experiment. Arterial segments were pre-contracted with the thromboxane analogue, U46619 (9,11-dideoxy-9a,11a-epoxymethanoprostaglandin F2α, 10 nM). After reaching a level of stable pre-contraction, we used acetylcholine (10 µM) to ensure preservation of endothelial function. Only segments exhibiting more than 50% relaxation to acetylcholine were included in the study. Bronchioles were pre-contracted with histamine (2 µM).
Concentration–response curves (0.001–10 µM) were constructed for the KCa2.3 and KCa3.1 channel opener NS309 (Neurosearch A/S, Ballerup, Denmark), the ‘classical’ KCa2.3 and KCa3.1 channel opener 1-ethyl-benzimidazolinone (1-EBIO; Sigma Aldrich, St. Louis, MO); a selective activator of KCa2 channels, cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine (CyPPA); a NO donor, 3-(2-chloro-3-methylphenyl)-5-[[(4-methylphenyl)sulphonyl] amino]-hydroxide (GEA 3175; GEA A/S, Copenhagen, Denmark) and the β2-adrenoceptor agonist, salbutamol (Sigma Aldrich).
The involvement of KCa2.3 and KCa3.1 channels in NS309 relaxation was investigated by incubating the segments with a KCa2.3 channel blocker (apamin, 0.05 µM), a KCa3.1 channel blocker (charybdotoxin, 0.07 µM), charybdotoxin and apamin in combination and a large conductance calcium-activated potassium (K KCa1.1) channel blocker (iberiotoxin 0.1 µM) 15 min before contraction and during construction of the concentration–response curves for NS309.
In preparations where the endothelium or epithelium was removed, this was achieved by rubbing the inner layer with a human hair. Endothelial function was evaluated by contracting the pulmonary arteries with U46619 (0.01 µM) followed by addition of ACh (10 µM). Endothelial removal was successful, when relaxation to ACh was below 10%. There is no functional test for evaluating the presence of bronchiolar epithelium; therefore, epithelial removal was evaluated by histology as previously described (Kroigaard et al., 2010).
Data analysis
All pulmonary arteries or bronchioles originated from different patients, where n indicates the number of preparations (one preparation per patient) examined. The results are expressed as mean ± SEM. The responses with or without blockers were analysed by two-way anova. To compare differences in expression, one-way anova was used. For cell culture studies, a paired Student's t-test was used. In all cases, a probability less than 5% (P < 0.05) was considered significant. For statistical analysis, we used the computer software Graph Pad Prism 5.02 (GraphPad Software, San Diego, CA).
Results
KCa3.1 and KCa2.3 channel expression in human pulmonary arteries and bronchioles
In pulmonary arteries and bronchioles, we detected considerable amounts of KCa3.1 mRNA by RT-PCR. Quantitative RT-PCR also revealed that mRNA expression levels were significantly lower in pulmonary arteries than in bronchioles (Figure 1A). Likewise, we detected KCa2.3 mRNA in pulmonary arteries and bronchioles, with expression levels being similar in both tissues (Figure 1B). If compared with KCa3.1 mRNA expression levels, KCa2.3 mRNA expression was found to be approximately 100 times higher in both tissues. Immunoblotting revealed bands for KCa2.3 protein at 70 kDa and KCa3.1 protein at 50 kDa (Figure S1). The KCa3.1 protein was more abundant in pulmonary arteries than in bronchioles (Figure 2A,C). This finding contrasted with the lower levels of mRNA for KCa3.1 protein found in pulmonary arteries and the higher mRNA expression in bronchioles. KCa2.3 protein levels were similar in pulmonary arteries and bronchioles (Figure 2B,D). Moreover, KCa2.3 and KCa3.1 protein levels were similar. These data showed KCa2.3 and KCa3.1 proteins were present in considerable amounts in both pulmonary arteries and bronchioles. In addition, the observed differences in mRNA expression levels, as determined by qRT-PCR, were not predictive of the actual protein levels.
Figure 1.
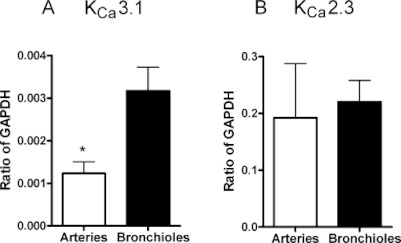
Quantitative RT-PCR analysis of KCa3.1 and KCa2.3 mRNA expression in human pulmonary arteries and bronchioles. Data (ratio of GAPDH) are means ± SEM of n= 8 human pulmonary arteries or bronchioles originating from different individuals; *P < 0.05, one-way anova.
Figure 2.
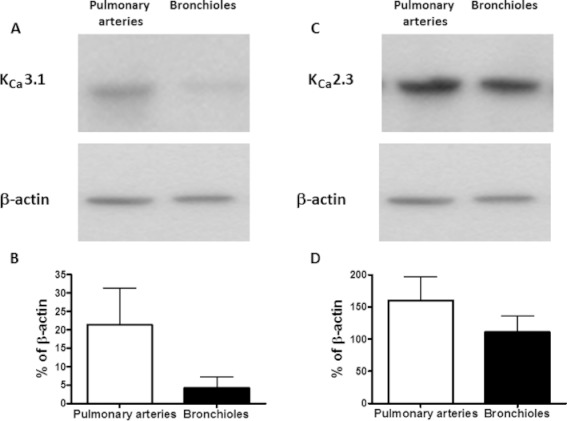
Immunoblotting of KCa3.1 and KCa2.3 protein. Representative immunoblots showing the amount of (A) KCa3.1 (50 kDa) (B) KCa2.3 (70 kDa) and β-actin (42 kDa) protein (lower panels) in human pulmonary arteries and bronchioles. (C and D) Graphs illustrating the protein level based on n= 5 human tissue samples originating from the same five patients. Data are given as means ± SEM.
Cellular localization of KCa2.3 and KCa3.1 proteins in human pulmonary arteries and bronchioles
Immunohistochemistry revealed immunoreactions for both KCa2.3 and KCa3.1 proteins in the epithelium of bronchioles (Figure 3D,E), and the signals obtained with immunoreactions were clearly different from background (Figure 3F). While immunoreactivities for KCa3.1 protein were similar in bronchioles and arteries (Figure 3A,D), immunoreactive KCa2.3 protein was more evident in the epithelium of bronchioles than in arteries (Figure 3B,E) and clearly different from background (Figure 3C). This immunohistochemistry thus showed that KCa2.3 and KCa3.1 channels were located in the human bronchial epithelium as well as in the endothelium of pulmonary arteries.
Figure 3.
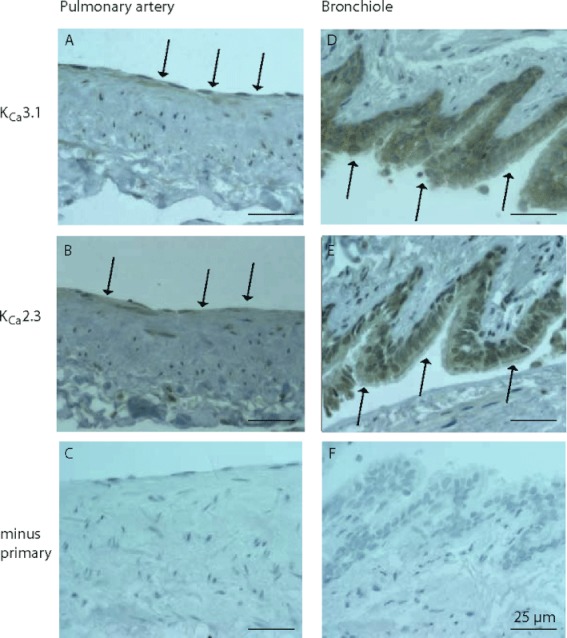
Representative immunostaining of human pulmonary arteries (a–c) and bronchioles (d–f). Immunoreaction for KCa3.1 (A, D), KCa2.3 (B, E) and control (C, F) without primary antibody. Arrows indicate immunoreactions and the scale bar 25 µm.
Patch-clamp electrophysiology
We investigated functional expression of KCa2.3 and KCa3.1 channels in HSAEpi cells by whole-cell patch-clamp analysis. Figure 4A shows representative recordings of composite and voltage-independent KCa2 and KCa3.1 currents elicited by Ca2+ dialysis in combination with the KCa2 / KCa3.1 opener SKA-31 for stable channel activity. Currents were blocked by half by the selective KCa3.1 channel blocker TRAM-34 and were further reduced by the KCa2 channel blocker UCL1684. These data indicated that both channels carry the voltage-independent KCa conductance in HSAEpi cells, with both channels contributing almost equally to the total current (Figure 4B). At the transcriptional level, expression of mRNA for KCa3.1 and KCa2.3 channels, but not for the other KCa2.1 and KCa2.2 channel subtypes was confirmed by RT-PCR as shown in Figure 4C.
Figure 4.
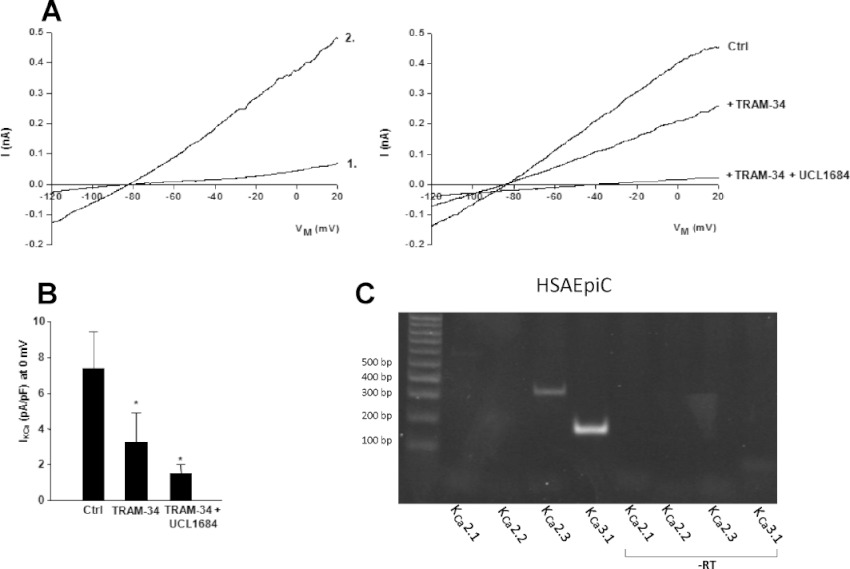
KCa3.1 and KCa2 currents in HSAEpi cells. (A) Upper panel on left illustrates base line currents (1), immediately after membrane rupture (to achieve electrical access) and current–voltage relationship of KCa currents (2; n= 6 experiments) activated during cell dialysis with a pipette solution containing 3 µM [Ca2+]free. To achieve maximal and stable channel activation, the KCa2/KCa3.1 channel opener SKA-31 (1 µM) was added to the bath solution. Upper panel on right: Currents were reduced by the KCa3.1 channel blocker TRAM-34 (1 µM, n= 4 experiments) and further reduced by additional application of the KCa2 channel blocker UCL1684 (1 µM, n= 4 experiments). (B) Summary data for K+-currents at 0 mV. Values are given as mean ± SEM; *P < 0.05, paired Student's t-test. (C) RT-PCR of KCa2 channels on HSAEpi cells and negative controls (non-reverse transcribed mRNA, -RT).
Functional studies in human pulmonary arteries and bronchioles
The diameter of the pulmonary arteries averaged 649 ± 44 µm (n= 45), whereas the diameters of accompanying stretched bronchioles were larger and averaged 1132 ± 85 µm (n= 45). In pulmonary arteries, contraction induced by U46619 (10 nM) was 5.3 ± 0.5 N m−1 and in bronchioles contraction to histamine (2 µM) was 1.4 ± 0.2 N m−1. We next tested the efficacy of either the KCa2.3/KCa3.1 channel openers, NS309 and 1-EBIO, the opener of ATP-sensitive potassium (KATP) channels, levcromakalim, the β2-adrenoceptor agonist, salbutamol or the NO donor, GEA 3175, to produce relaxation of pulmonary arteries and bronchioles. The results are shown in Figure 5A–D.
Figure 5.
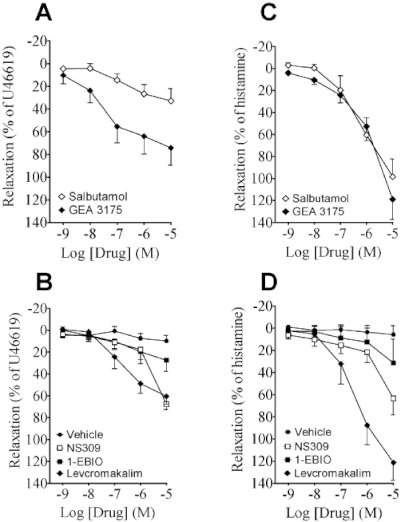
The effect of vehicle, NS309, 1-EBIO, levcromakalim, salbutamol and GEA 3175 in (A, B) human pulmonary arteries and (C, D) human bronchioles. Data points represent means ± SEM, with n= 4–12 per data point.
As expected the NO donor, GEA 3175, induced potent concentration-dependent relaxations in pulmonary arteries, starting at 10 nM (approx. 25%); and the greatest relaxation was observed at the highest concentration tested (approx. 70% at 10 µM; Figure 5A). Salbutamol was less effective in pulmonary arteries (Figure 5A). In pulmonary arteries, levcromakalim induced concentration-dependent relaxations, and the maximum relaxation was observed at 10 µM (Figure 5B). In pulmonary arteries, NS309 induced concentration-dependent relaxations, reaching 70% at the highest concentration of NS309 (Figure 5B). 1-EBIO produced similar relaxations (Figure 5B), with the exception that relaxations were smaller at 10 µM, than those obtained with 10 µM NS309. Thus, if compared with direct stimulation of smooth muscle by the endothelium-independent vasorelaxing agent, GEA 3175, NS309, 1-EBIO and salbutamol relaxed human pulmonary arteries with substantially comparable effects at ≤1 µM.
In bronchioles, salbutamol was more efficient in producing relaxation, approximately 55% vs. 25% in pulmonary arteries at 1 µM (Figure 5C). As expected, GEA 3175 evoked strong relaxations (approx. 120% at 10 µM). Interestingly, these were apparently smaller at concentrations in the submicromolar range but larger at 10 µM when compared with pulmonary arteries. In bronchioles (Figure 5D), levcromakalim produced potent concentration-dependent relaxations, which reached 120% at 10 µM. NS309 also induced concentration-dependent relaxations in this tissue (approx. 65% at 10 µM), similar to that seen in the pulmonary arteries. The other dual channel opener, 1-EBIO, also relaxed bronchioles, although it was less potent at the highest dose tested (approx. 30% at 10 µM; Figure 5D). Together, NS309 and 1-EBIO were found to be fairly potent bronchodilating agents in human bronchioles, relative to the established bronchodilating efficacy of salbutamol (approx. 100%) and of a NO donor, here GEA3175.
The KCa3.1 channel blocker charybdotoxin reduced the response to 10 µM NS309 in pulmonary arteries but failed to reduce the relaxations to 100 nM and 1 µM NS309 (Figure 6A,B). In contrast, the KCa2.3 channel blocker apamin clearly reduced relaxation to NS309 at 100 nM, 1 µM and 10 µM in pulmonary arteries and in bronchioles (Figure 6A,B); and a combination of charybdotoxin and apamin did not reduce this relaxation further. In human pulmonary arteries, the specific KCa2.2 and KCa2.3 channel activator, CyPPA, induced concentration-dependent relaxations, which were reduced by apamin (Figure S3A). In bronchioles, only 100 µM CyPPA induced relaxations which were reduced in the presence of apamin (Figure S3B). In both bronchioles and pulmonary arteries, blocking the KCa1.1 channels with iberiotoxin did not change NS309 relaxation (n= 4; data in Figure S2). This indicated a major contribution of KCa2.3 channels in the relaxation response. Notably, the marked relaxation caused by 10 µM NS309 was only reduced by the combination of apamin and charybdotoxin, indicating other effects of NS309 at this high concentration.
Figure 6.
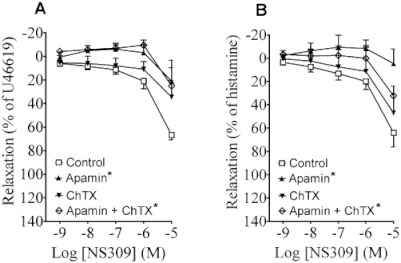
The effect of NS309 in (A) human pulmonary arteries and (B) human bronchioles in the presence and absence of charybdotoxin (ChTX), apamin or a combination. Data points represent means ± SEM, with n= 4–12 per data point. The concentration–response curves were compared by two-way anova. *P < 0.05, versus control curve.
The relaxation induced by NS309 was reduced in pulmonary arteries without endothelium (Figure 7A). The relaxation to salbutamol was also blunted by removing the endothelium (Figure 7B), whereas relaxation to the NO-donor GEA3175 was unaffected by removing the endothelium (Figure 7C). In the bronchioles, removing the epithelium reduced the relaxation to NS309 to a similar degree as in the pulmonary arteries. Salbutamol-evoked relaxation was also blunted in bronchioles without epithelium (Figure 7E), whereas GEA3175-induced relaxations were unaltered in preparations without epithelium (Figure 7F).
Figure 7.
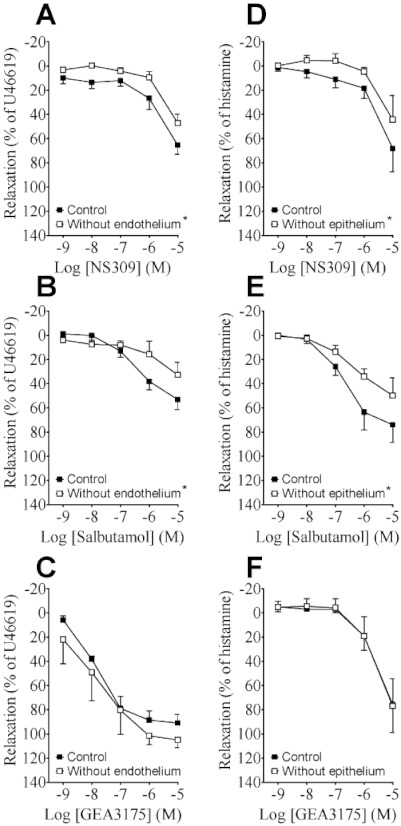
The effect of NS309, salbutamol and GEA3175 in (A–C) human pulmonary arteries with or without endothelium, (D–F) human bronchioles with or without epithelium. Data points represent means ± SEM, with n= 4–7 per data point. The concentration–response curves were compared by two-way anova. *P < 0.05, versus control curve.
Discussion
The main findings of the present study are that KCa2.3 and KCa3.1 channels are localized in the human pulmonary endothelium and epithelium and that the pharmacological activation of these channels by a selective activator, NS309, relaxed both human isolated pulmonary arteries and bronchioles.
Our data on tissue expression of the two channels support previous findings and provides additional insights regarding channel expression in human lung tissues. Thus, in trachea and lung, previous studies suggested a high expression of KCa3.1 channels using RT-PCR, Northern blotting and RNA array (Jensen et al., 2001; MaoXiang et al., 2004). KCa3.1 mRNA expression is well established in systemic arteries (Grgic et al., 2009) and, in particular, human mesenteric endothelium (Kohler et al., 2000; Chadha et al., 2011). Here, we found that the mRNAs for KCa3.1 and KCa2.3 channels were expressed both in human pulmonary arteries and bronchioles. Notably, the expression level of KCa3.1 mRNA was higher in bronchioles than in pulmonary arteries. However, KCa3.1 protein expression was found to be higher in pulmonary arteries than in bronchioles, showing that mRNA expression level did not necessarily correlate with protein levels, as previously reported (Griffin et al., 2002; Minagawa et al., 2008). Nonetheless, the present findings showed that KCa3.1 channels were expressed in considerable amounts in both human pulmonary arteries and bronchioles, and that KCa3.1 channels were likely to be more prominent in human pulmonary arteries.
Previous studies reported that KCa2.3 channels were expressed not only in various tissues especially in brain but also in peripheral tissues, such as the lung (MaoXiang et al., 2004) and in arteries (Grgic et al., 2009). In agreement with these previous reports, our study found expression of mRNA for KCa2.3 protein in human pulmonary arteries and bronchioles. Moreover, protein levels of KCa2.3 in human pulmonary arteries and bronchioles were comparable.
Our immunohistological approaches revealed that KCa3.1 channels were localized to the endothelium and the epithelium of human pulmonary arteries and bronchioles. The KCa2.3 channels were found in human bronchiolar epithelium and arterial endothelium. Thus, these results, together with previous reports by our group (Kroigaard et al., 2010), suggest that the KCa2.3 and KCa3.1 channels are preferentially localised in the epithelial cell layer of the bronchioles and the endothelial cell layer of human pulmonary arteries. That mRNA expression and proteins of both channels led to functional channels at the membrane level in human bronchial epithelium was supported by the detection of KCa currents with the electrophysiological and pharmacological fingerprints of KCa2.3 and KCa3.1 channels in HSAEpi cells.
Concerning endothelium-dependent vasodilatation, our present study demonstrated that NS309 and the classical but less potent channel opener 1-EBIO induced dose-dependent relaxations in human pulmonary arteries. Considering the higher potency of NS309 over 1-EBIO, NS309-induced relaxations were larger than those elicited by 1-EBIO at the highest dose tested. NS309 relaxation was reduced by endothelial cell removal, suggesting that it induced endothelium-dependent relaxations. Relaxations induced by NS309 were indeed mediated by activation of KCa2.3 and KCa3.1 channels, as indicated by the blocking effects of apamin and of charybdotoxin at the highest NS309 concentration tested. However, at high concentrations (10 µM) of NS309, this channel opener caused relaxations in preparations without endothelium and these relaxations were partly insensitive to charybdotoxin and apamin. These relaxations resistant to apamin and charybdotoxin were most likely caused by a blockade of L-type calcium channels (Morimura et al., 2006; Dalsgaard et al., 2009), thus indicating loss of specificity for KCa2.3 and KCa3.1 channels of NS309 at concentrations ≥10 µM. Concerning the relaxations by lower NS309 concentrations, apamin was effective in virtually abolishing the response, while charybdotoxin was ineffective here.
In summary, these results suggest that NS309 mainly activated KCa2.3 channels and, to a lesser extent, KCa3.1 channels to elicit endothelium-dependent relaxations in human pulmonary arteries, irrespective of a similar expression of the two channels. This discrepancy may be explained by considering the mechanism by which NS309 stimulates KCa2.3 and KCa3.1 channels. We found that NS309 increased the open probability of the two channels at a given Ca2+ concentration but did not activate the closed channels. Thus, our finding of a predominant activation of KCa2.3 channels could indicate basal activity of KCa2.3, but not of KCa3.1, channels in this preparation, which was then potentiated by NS309 at concentrations in the submicromolar range. Concerning KCa3.1 channels, the lack of a contribution of these channels to relaxations induced by 0.01–1 µM NS309 is in line with previous studies that showed that the related KCa3.1/KCa2.3 channel activator, SKA-31, per se, was also not able to produce KCa3.1 channel-mediated vasodilatation in murine carotid arteries; while SKA-31 potentiated KCa3.1 channel-mediated vasodilatation to acetylcholine (Sankaranarayanan et al., 2009). Thus, activation of KCa3.1 channels by this class of activators appeared to require first pre-stimulation, for instance, by Ca2+ mobilization following receptor activation. Indeed, the contribution of KCa3.1 channels to vasodilatation was only seen during acetylcholine stimulation and genetic deficiency was shown to impair this vasodilatation, selectively (Brahler et al., 2009). Despite these circumstances, our functional studies suggested that NS309 activation of KCa2 channels (and at high concentration also of KCa3.1 channels) produced relaxation in human pulmonary arteries, highlighting these channels as vasorelaxing effector proteins in the pulmonary circulation. This is furthermore supported by the observation that the selective opener of KCa2 channels, CyPPA, induced apamin-sensitive relaxations in human pulmonary arteries.
The epithelium generates more than one factor, inducing relaxation of the underlying bronchial smooth muscle layer (Stuart-Smith and Vanhoutte, 1987; Benoit et al., 2001). Moreover, activation of the KCa1.1 channel led to relaxation of airway smooth muscle (Benoit et al., 2001), suggesting a role of KCa channels in these responses. Our present study shows that the KCa2.3 and KCa3.1 channel activator NS309 evoked concentration-dependent relaxations in human bronchioles that were greater than those evoked by 1-EBIO, a finding that was explained by the higher potency of NS309, as discussed above. Moreover, NS309 relaxation was blunted by epithelial removal, indicating epithelium-dependent relaxation.
As in pulmonary arteries without endothelium, NS309 at high concentrations also relaxed bronchioles without epithelium, suggesting other mechanisms may also contribute to NS309 relaxations. However, apamin blocked NS309 relaxation to a larger degree than epithelial cell removal (see Figure 6B vs. 7B). Based on histological examinations, it is unlikely that a remainder of the epithelium contributes to NS309 relaxations in bronchioles without epithelium, and these findings suggest that apamin-sensitive channels in other cells in the bronchioles contribute to the NS309 relaxations. KCa2.3 appeared to be the channel involved in the relaxations because apamin was more effective than charybdotoxin in blocking the response. Thus, similar to our findings in the pulmonary arteries, KCa2.3 channels (exhibiting some basal activity) appeared to be predominantly used by NS309 to produce relaxation in human bronchioles. There are relevant species differences, as in rats both apamin- and charybdotoxin-sensitive channels are involved in NS309 relaxation in bronchioles (Kroigaard et al., 2010). KCa3.1 channels are clearly expressed in human bronchioles and rather than a role in tone regulation, these channels may control chloride secretion in bronchial cells to maintain liquid composition and volume (Bardou et al., 2009). Also the choice of spasmogen/constrictor can influence the relaxation of bronchodilators (Hernandez et al., 1998) and we cannot exclude the possibility that using another spasmogen would increase the relaxation to, for instance, NS309 and reveal contributions from KCa3.1 channels. Despite the differential contribution of the channels to epithelium-dependent relaxation, our study demonstrates that pharmacological activation of KCa2.3 channels was able to relax human bronchioles, most likely by mechanisms similar to those of EDHF-induced relaxations in arteries.
In terms of pulmonary disease, KCa3.1 channels were found to inhibit human airway smooth muscle proliferation (Shepherd et al., 2007), and activation of these channels potentiated the degranulation of human lung mast cells (Duffy et al., 2001; 2005). Hence blockade of KCa3.1 channels was proposed as a useful approach to the treatment of asthma (Bradding and Wulff, 2009). The present study on intact human bronchioles and pulmonary arteries suggested that blockade of KCa3.1 channels could reduce receptor-mediated relaxation responses of pulmonary arteries and could thereby add to dysregulation of arterial tone. While pharmacological modulators of KCa3.1 channels could have a Janus face, selective activators of KCa2.3 channels could serve as more potent and safe pharmacological targets to produce pulmonary vasodilatation and bronchodilatation.
In conclusion, pharmacological enhancement of the functions of epithelial and endothelial KCa2.3 channels may represent a novel approach to treat chronic obstructive pulmonary disease and associated pulmonary hypertension.
Acknowledgments
We thank Helle Zibrandtsen, Henriette Gram Johanson and Susie Mogensen for excellent technical assistance. Christel Kroigaard was supported by a grant from the Danish Heart Foundation, while Ulf Simonsen was supported by a grant from the Novo Nordisk foundation.
Glossary
- 1-EBIO
1-ethyl-2-benzimidazolinone
- COPD
chronic obstructive pulmonary disease
- CyPPA
cyclohexyl-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-pyrimidin-4-yl]-amine
- EDHF
endothelium-derived hyperpolarizing factor
- EET
epoxyeicosatrinoic acid
- EpDHF
epithelium-derived hyperpolarizing factor
- GEA
3175, 3-(2-chloro-3-methylphenyl)-5-[[(4-methylphenyl)sulphonyl] amino]-hydroxide
- KATP
channel, ATP-sensitive potassium channel
- KCa1.1
channel, large conductance calcium-activated potassium channel
- KCa2
channel, small conductance calcium-activated potassium channel
- KCa3.1
channel, intermediate conductance calcium-activated potassium channel
- NS309
6,7-dichloro-1H-indole-2,3-dione 3-oxime
- SKA-31
naphtho[1,2-d]thiazol-2-ylamine
- TRAM-34
1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- U46619
9,11-dideoxy-9a,11a-epoxymethanoprostaglandin F2α
- UCL1684
6,12,19,20,25,26-hexahydro-5,27:13,18:21,24-trietheno-11,7-metheno-7H-dibenzo [b,n][1,5,12,16]tetraazacyclotricosine-5,13-diium dibromide
Conflict of interest
The authors state no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Immunoblotting of KCa2.3 and KCa3.1 protein. Representative immunoblots showing the amount of (A) KCa2.3 (70 kDa) and (B) KCa3.1 (50 kDa) protein in human pulmonary arteries (PA) and bronchioles (B).
Figure S2 The effect of NS309 in human pulmonary arteries (A) and human bronchioles (B) in the presence and absence of iberiotoxin (IbTX). Data points represent means ± SEM, with n = 4 per data point. The concentration–response curves were compared by two-way ANOVA, *P < 0.05 versus control curve.
Figure S3 The effect of CyPPA in (A) human pulmonary arteries, in which apamin reduced the relaxation to CyPPA, and (B) human bronchioles, in which apamin reduced CyPPA relaxation at the highest concentration applied (100 µM, Bonferroni post test). Data points represent means ± SEM, with n = 3 per data point. The concentration–responsecurves were compared by two-way ANOVA, *P < 0.05 versus control.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (5th Edition) 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou O, Trinh NT, Brochiero E. Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L145–L155. doi: 10.1152/ajplung.90525.2008. [DOI] [PubMed] [Google Scholar]
- Benoit C, Renaudon B, Salvail D, Rousseau E. EETs relax airway smooth muscle via an EpDHF effect: BK(Ca) channel activation and hyperpolarization. Am J Physiol Lung Cell Mol Physiol. 2001;280:L965–L973. doi: 10.1152/ajplung.2001.280.5.L965. [DOI] [PubMed] [Google Scholar]
- Bradding P, Wulff H. The K+ channels K(Ca)3.1 and K(v)1.3 as novel targets for asthma therapy. Br J Pharmacol. 2009;157:1330–1339. doi: 10.1111/j.1476-5381.2009.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahler S, Kaistha A, Schmidt VJ, Wolfle SE, Busch C, Kaistha BP, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation. 2009;119:2323–2332. doi: 10.1161/CIRCULATIONAHA.108.846634. [DOI] [PubMed] [Google Scholar]
- Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol. 2000;129:184–192. doi: 10.1038/sj.bjp.0703041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha PS, Liu L, Rikard-Bell M, Senadheera S, Howitt L, Bertrand RL, et al. Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions intermediate conductance calcium-activated k+ channel and nitric oxide. J Pharmacol Exp Ther. 2011;336:701–708. doi: 10.1124/jpet.110.165795. [DOI] [PubMed] [Google Scholar]
- Dalsgaard T, Kroigaard C, Bek T, Simonsen U. Role of calcium-activated potassium channels with small conductance in bradykinin-induced vasodilation of porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2009;50:3819–3825. doi: 10.1167/iovs.08-3168. [DOI] [PubMed] [Google Scholar]
- Duffy SM, Lawley WJ, Conley EC, Bradding P. Resting and activation-dependent ion channels in human mast cells. J Immunol. 2001;167:4261–4270. doi: 10.4049/jimmunol.167.8.4261. [DOI] [PubMed] [Google Scholar]
- Duffy SM, Cruse G, Lawley WJ, Bradding P. Beta2-Adrenoceptor regulation of the K+ channel iKCa1 in human mast cells. FASEB J. 2005;19:1006–1008. doi: 10.1096/fj.04-3439fje. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Feletou M, Girard V, Canet E. Different involvement of nitric oxide in endothelium-dependent relaxation of porcine pulmonary artery and vein: influence of hypoxia. J Cardiovasc Pharmacol. 1995;25:665–673. doi: 10.1097/00005344-199504000-00022. [DOI] [PubMed] [Google Scholar]
- Flavahan NA, Aarhus LL, Rimele TJ, Vanhoutte PM. Respiratory epithelium inhibits bronchial smooth muscle tone. J Appl Physiol. 1985;58:834–838. doi: 10.1152/jappl.1985.58.3.834. [DOI] [PubMed] [Google Scholar]
- Folkerts G, Nijkamp FP. Airway epithelium: more than just a barrier! Trends Pharmacol Sci. 1998;19:334–341. doi: 10.1016/s0165-6147(98)01232-2. [DOI] [PubMed] [Google Scholar]
- Frantz E, Soifer SJ, Clyman RI, Heymann MA. Bradykinin produces pulmonary vasodilation in fetal lambs: role of prostaglandin production. J Appl Physiol. 1989;67:1512–1517. doi: 10.1152/jappl.1989.67.4.1512. [DOI] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses – relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol. 2009;157:509–526. doi: 10.1111/j.1476-5381.2009.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin TJ, Gygi SP, Ideker T, Rist B, Eng J, Hood L, et al. Complementary profiling of gene expression at the transcriptome and proteome levels in saccharomyces cerevisiae. Mol Cell Proteomics. 2002;1:323–333. doi: 10.1074/mcp.m200001-mcp200. [DOI] [PubMed] [Google Scholar]
- Hernandez M, Elmedal B, Mulvany MJ, Simonsen U. Mechanisms of relaxations of bovine isolated bronchioles by the nitric oxide donor, GEA 3175. Br J Pharmacol. 1998;123:895–905. doi: 10.1038/sj.bjp.0701684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- Jensen BS, Strobaek D, Olesen SP, Christophersen P. The Ca2+-activated K+ channel of intermediate conductance: a molecular target for novel treatments? Curr Drug Targets. 2001;2:401–422. doi: 10.2174/1389450013348173. [DOI] [PubMed] [Google Scholar]
- Kohler R, Degenhardt C, Kuhn M, Runkel N, Paul M, Hoyer J. Expression and function of endothelial Ca2+-activated K+ channels in human mesenteric artery: a single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res. 2000;87:496–503. doi: 10.1161/01.res.87.6.496. [DOI] [PubMed] [Google Scholar]
- Kroigaard C, Dalsgaard T, Simonsen U. Mechanisms underlying epithelium-dependent relaxation in rat bronchioles: analogy to EDHF-type relaxation in rat pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2010;298:L531–L542. doi: 10.1152/ajplung.00220.2009. [DOI] [PubMed] [Google Scholar]
- MaoXiang C, Gorman A, Bill B, Kuljit S, Paul H, Michel C, et al. Small and intermediate conductance Ca 2+-activated K + channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn Schmiedebergs Arch Pharmacol. 2004;V369:602–615. doi: 10.1007/s00210-004-0934-5. [DOI] [PubMed] [Google Scholar]
- Minagawa H, Honda M, Miyazaki K, Tabuse Y, Teramoto R, Yamashita T, et al. Comparative proteomic and transcriptomic profiling of the human hepatocellular carcinoma. Biochem Biophys Res Commun. 2008;366:186–192. doi: 10.1016/j.bbrc.2007.11.101. [DOI] [PubMed] [Google Scholar]
- Morimura K, Yamamura H, Ohya S, Imaizumi Y. Voltage-dependent Ca2+-channel block by openers of intermediate and small conductance Ca2+-activated K+ channels in urinary bladder smooth muscle cells. J Pharmacol Sci. 2006;100:237–241. doi: 10.1254/jphs.sc0060011. [DOI] [PubMed] [Google Scholar]
- Morrison KJ, Gao Y, Vanhoutte PM. Epithelial modulation of airway smooth muscle. Am J Physiol. 1990;258:L254–L262. doi: 10.1152/ajplung.1990.258.6.L254. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, et al. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol. 2009;75:281–295. doi: 10.1124/mol.108.051425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd MC, Duffy SM, Harris T, Cruse G, Schuliga M, Brightling CE, et al. KCa3.1 Ca2+activated K+ channels regulate human airway smooth muscle proliferation. Am J Respir Cell Mol Biol. 2007;37:525–531. doi: 10.1165/rcmb.2006-0358OC. [DOI] [PubMed] [Google Scholar]
- Strobaek D, Teuber L, Jorgensen TD, Ahring PK, Kjaer K, Hansen RS, et al. Activation of human IK and SK Ca2+-activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) Biochim Biophys Acta. 2004;1665:1–5. doi: 10.1016/j.bbamem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Stuart-Smith K, Vanhoutte PM. Heterogeneity in the effects of epithelium removal in the canine bronchial tree. J Appl Physiol. 1987;63:2510–2515. doi: 10.1152/jappl.1987.63.6.2510. [DOI] [PubMed] [Google Scholar]
- Stuart-Smith K, Vanhoutte PM. Airway epithelium modulates the responsiveness of porcine bronchial smooth muscle. J Appl Physiol. 1988;65:721–727. doi: 10.1152/jappl.1988.65.2.721. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Airway epithelium and bronchial reactivity. Can J Physiol Pharmacol. 1987;65:448–450. doi: 10.1139/y87-076. [DOI] [PubMed] [Google Scholar]
- Zhang RZ, Yang Q, Yim APC, Huang Y, He GW. Role of NO and EDHF-mediated endothelial function in the porcine pulmonary circulation: comparison between pulmonary artery and vein. Vascul Pharmacol. 2006;44:183–191. doi: 10.1016/j.vph.2005.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


