Abstract
BACKGROUND AND PURPOSE
Impulsivity is a core symptom in many neuropsychiatric disorders. The main objective of this study was to evaluate the effects of topiramate and pregabalin on the modulation of different impulsivity dimensions in DBA/2 mice.
EXPERIMENTAL APPROACH
The effects of acute and chronic administration of pregabalin (10, 20 and 40 mg·kg−1) and topiramate (12.5, 25 and 50 mg·kg−1) were evaluated in the light–dark box (LDB), hole board test (HBT) and delayed reinforcement task (DRT). α2A-Adrenoceptor, D2-receptor and TH gene expression were evaluated by real-time PCR in the prefrontal cortex (PFC), accumbens (ACC) and ventral tegmental area (VTA), respectively.
KEY RESULTS
Acute pregabalin administration showed a clear anxiolytic-like effect (LDB) but did not modify novelty-seeking behaviour (HBT). In contrast, topiramate produced an anxiolytic effect only at the highest dose, whereas it reduced novelty seeking at all doses tested. In the DRT, acute pregabalin had no effect, whereas topiramate only reduced motor impulsivity. Chronically, pregabalin significantly increased motor impulsivity and topiramate diminished cognitive impulsivity. Pregabalin decreased α2A-adrenoceptor and D2-receptor gene expression in the PFC and ACC, respectively, and increased TH in the VTA. In contrast, chronic administration of topiramate increased α2A-adrenoceptor and D2-receptor gene expression in the PFC and ACC, respectively, and also increased TH in the VTA.
CONCLUSIONS AND IMPLICATIONS
These results suggest that the usefulness of pregabalin in impulsivity-related disorders is related to its anxiolytic properties, whereas topiramate modulates impulsivity. These differences could be linked to their opposite effects on α2A-adrenoceptor and D2-receptor gene expression in the PFC and ACC, respectively.
Keywords: impulsivity, drug abuse, pregabalin, topiramate, dopamine, adrenaline, gene expression, DBA/2 mice
Introduction
Impulsivity constitutes a complex and multidimensional personality trait (Evenden, 1999a,b) that can be studied in both humans and animals by a wide range of methods (Winstanley et al., 2006). Behavioural disinhibition (motor impulsivity), manifested by poor inhibitory control of pre-potent responses, and impulsive choice (cognitive impulsivity), which refers to the preference for smaller immediate rewards over larger delayed rewards, are the most representative dimensions (Dougherty et al., 2003; Otobe and Makino, 2004; Adriani et al., 2010). In addition, there are other behavioural dimensions such as novelty-seeking behaviour closely related to impulsivity (Petry, 2001; James et al., 2007; Evren et al., 2012). Although impulsivity is a normal behaviour in healthy humans allowing adaptation to uncertainty (Marazziti et al., 2010), there are several neuropsychiatric disorders such as ADHD (attention-deficit hyperactivity disorder), drug abuse, pathological gambling, bipolar disorder, obsessive compulsive disorder, aggression, anorexia/bulimia nervosa, suicide, trichotillomania, intermittent explosive disorder, self-injurious behaviour or kleptomania, presenting a high level of impulsivity as a core symptom (Rapport et al., 1985; August and Garfinkel, 1989; Jensen et al., 1990; Fahy and Eisler, 1993). Therefore, novel pharmacological strategies that alleviate impulsive behaviours could be very helpful in the management of these disorders.
In recent years, there has been an increase in the use of anticonvulsant drugs in the treatment of distinct neuropsychiatric disorders characterized by impulse control problems. Carbamazepine was one of the first to be used and it enabled a reduced dose of other antipsychotic drugs to be effective in the treatment of agitation and disruptive behaviours, such as aggressiveness, impulsivity, perversity or suicidal attempts (Vogelaer, 1981). Valproate has been widely used in the management of personality disorders, improving some symptoms like aggression, irritability and high impulsivity (Wilcox, 1994; 1995; Kavoussi and Coccaro, 1998).
Some of the so-called new anticonvulsants that appeared in the 1990s (Bourgeois, 1996; Wilson and Brodie, 1996) have demonstrated efficacy in the treatment of drug abuse disorders by alleviating withdrawal symptoms (Zullino et al., 2004), reducing craving (urge to consume) (Furieri and Nakamura-Palacios, 2007; Vengeliene et al., 2007; Miranda et al., 2008; Reis et al., 2008) or attenuating the pleasurable effects of drug intake, thus avoiding relapse (Bisaga et al., 2006; Martinotti et al., 2007). Among these new antiepileptic drugs, topiramate stands out in substance abuse intervention (mainly alcohol dependence) due to its ability to reduce consumption and relapse (Kampman et al., 2004; Cubells, 2006; Nguyen et al., 2007; Kenna et al., 2009; Johnson and Ait-Daoud, 2010). Topiramate has a complex and not well known mechanism of action, but its main effects include the modulation of voltage-gated sodium channels (Zona et al., 1997; Taverna et al., 1999), an increase in GABA neurotransmission (White et al., 1997; 2000) and the blockade of α-amino-3-hydroxy-5-methylisoxozole-proprionic acid (AMPA)/kainate receptors (Gibbs et al., 2000; Poulsen et al., 2004). Although it has been hypothesized that topiramate's usefulness in the management of drug abuse may be related to its anti-craving effect diminishing the pleasurable effects of drugs mediated by modulation of the dopaminergic mesolimbic pathways (Johnson et al., 2003; Johnson, 2004b), it has been proposed that topiramate could also modulate impulsive behaviours (Smathers et al., 2003; Dolengevich Segal et al., 2006; Rubio et al., 2009).
Another anticonvulsant, pregabalin, which is indicated for the treatment of generalized anxiety disorders and neuropathic pain, is emerging as a potential therapeutic tool in the field of alcoholism. This drug ameliorates alcohol withdrawal symptoms (Martinotti et al., 2008; Di Nicola et al., 2010; Oulis and Konstantakopoulos, 2010) and relapse through a mechanism less related to alcohol craving and more associated with the treatment of the comorbid psychiatric symptomatology such as an increased anxiety level (Martinotti et al., 2010). In addition, a very recent study shows for the first time that pregabalin is able to reduce alcohol consumption (Stopponi et al., 2011). Pregabalin acts as a presynaptic inhibitor of the release of excessive levels of excitatory neurotransmitters by selectively binding to the α2-δ subunit of voltage-gated calcium channels (Stahl, 2004). Through this mechanism, it has been proposed that pregabalin reduces the increase in dopamine in the nucleus accumbens resulting from acute morphine administration (Andrews et al., 2001).
The efficacy of pregabalin or topiramate in impulsive-related disorders (mainly drug abuse) remains poorly understood. In the present study, we evaluated anxiety-like behaviour [light–dark box (LDB)], novelty seeking [hole board test (HBT)] and cognitive and motor impulsivity [delayed reinforcement task (DRT)] in DBA/2 mice, a strain with a high endogenous impulsivity level (Helms et al., 2006; Patel et al., 2006; Navarrete et al., 2012). Dopaminergic and adrenergic key targets gene expression analyses were focused in brain regions from the mesolimbic and mesocortical pathways [ventral tegmental area (VTA), nucleus accumbens (ACC) and prefrontal cortex (PFC)] due to their involvement in impulsive behaviour (Wang et al., 2002; Basar et al., 2010; Kim and Lee, 2010). Tyrosine hydroxylase (TH) and the type 2 dopamine receptor (D2-receptor) were analysed in dopaminergic cell bodies (VTA) and in terminals (ACC), respectively. On the other hand, the α2A-adrenoceptor was studied in the PFC. The main purpose of this study was to elucidate if topiramate and/or pregabalin regulate certain impulsivity dimensions (novelty seeking or intolerance to delay) and if this regulation involves changes in dopaminergic and/or adrenergic pathways. Furthermore, the effects of both anticonvulsants on the high anxiety-like behaviour expressed by DBA/2 mice were evaluated in order to make a better distinction between pregabalin and topiramate.
Methods
Animals
DBA/2 OlaHsd mice were purchased from Harlan (Barcelona, Spain). Male mice between 8 and 10 weeks old and 20–25 g in weight were housed in groups of eight per cage (40 × 25 × 22 cm) under controlled conditions (temperature, 23 ± 2°C; relative humidity, 60 ± 10%; 12 h light/dark cycle, lights on from 8:00 to 20:00 h.). Behavioural analyses, initiated after 1 week of acclimatization in the animal room, were performed placing the home cage in the operant-task room 1 h before starting experiments. Standard laboratory chow (commercial diet for rodents A04 Panlab, Barcelona, Spain) and water were available ad libitum in all procedures, except for the DRT in which standard chow was restricted to only 60 min access per day. This food restriction regimen was applied from 3 days before starting and during the operant task (after the end of each daily session) to guarantee mice response to reinforcers. The food restriction schedule produced weight loss in mice of around 15% from their free-feeding weight. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All experiments were in accordance with guidelines established by the European Council Directive (86/609/EEC) and approved by the Institutional Animal Care Committee.
Drugs
Pregabalin (Lyrica® by Pfizer, Madrid, Spain) and topiramate (Topamax® by Janssen-Cilag, Madrid, Spain) were dissolved in distilled water. In all the experiments, pregabalin (10, 20 and 40 mg·kg−1, p.o.) and topiramate (12.5, 25, 50 mg·kg−1, p.o.) were administered in a volume of 10 mL·kg−1. In the LDB and HBT, mice received a unique dose 60 min before the start of the probe. In the DRT, two different administration schedules, acute and chronic, were used. In the acute schedule, mice received the corresponding unique daily dose 60 min before the beginning of each session. This dose was given only during the 10 days of the test phase, in which a delay was imposed (a total of 10 doses). In the chronic schedule, during the 7 day pretreatment the same acute dose was administered twice a day (approximately every 12 h); this preceded the DRT. During testing, the same administration protocol was followed; the morning dose was administered 60 min before each session (27 day treatment for a total of 54 doses). Drugs were freshly prepared each day before testing. A total of seven groups of mice (eight mice per group) were employed for each administration schedule (single, acute and chronic), and therefore, a total of 168 mice were tested in the present study. Although there are no pregabalin or topiramate pharmacokinetic specific data in mice, according to human studies (Shank and Maryanoff, 2008; Bockbrader et al., 2010) and taking into consideration the metabolizing differences between mice and humans (Siefert et al., 1999; Yang and Bankir, 2005), both drugs probably present good gastrointestinal absorption, rapidly reaching maximum and maintained plasma levels in humans. Furthermore, it could be hypothesized that with the acute schedule of administration used for the DRT, there was no drug accumulation since both pregabalin and topiramate would be totally excreted before the subsequent day's dose.
Anxiety-like behaviour – LDB test
The LDB model (Crawley and Goodwin, 1980) consisted of two methacrylate boxes 20 × 20 × 15 cm, one transparent and one black and opaque, linked by an opaque tunnel (4 cm). Light from a 60 W desk lamp located 25 cm above the light box provided room illumination. Mice were individually placed facing the black box and tested in 5 min sessions. The time spent in the lighted area and the number of transitions was recorded. A mouse whose three paws were in the opposite box was considered a transition.
Impulsive-like behaviour
Novelty seeking – HBT
Novelty-seeking behaviour was measured using an apparatus that consisted of a 40 × 40 × 40 cm transparent acrylic square box with a black acrylic board with four equidistant holes placed in each corner and equipped with infrared photocells to detect head dips. First, there was a training phase in which the animals were introduced inside the apparatus for habituation. The number of head dips into each hole was recorded to discard mice with unconditioned preference for any hole. The day after, a small object was introduced into two of the holes at opposite corners to measure preference as the amount of time spent head dipping in holes that had objects divided by the total time spent head dipping (object preference). Mice were individually placed in the centre to initiate a 10 min test.
Delay discounting – DRT
The evaluation of the delay discounting was carried out in eight modular operant chambers (Panlab) placed inside eight soundproof boxes (which have a fan and a light) and equipped with a chamber light, two levers, one feeder device with a magazine to drop food pellets (20 mg Dustless precision rodent pellets, Bio-Serv, Frenchtown, NJ), one stimulus light and a buzzer. In the training phase, each session began with the chamber light on and a lever press switched off. One lever press delivered one food pellet (immediate lever), whereas the other lever delivered three food pellets combined with 0.5 s stimulus light and 0.5 s, 2850 Hz, 85 dB buzzer beep (delayed lever). Following food delivery, a 30 s time out (signalled with the chamber light off) was established, during which additional lever presses of either lever were recorded but without consequence. After the 30 s time out, the chamber light was turned on, indicating the start of the intertrial interval (ITI) in which the next trial is initiated depending on each subject's spontaneous waiting before the lever press. All mice performed one session of 30 min per day. According to the 30 s time out period, the maximal number of trials that an animal could theoretically complete (in the case of response immediately after the end of the timeout) during the training phase (without delays) was 60. The length of the training phase depended on the time to achieve the learning task criteria consisting of (1) reaching >75% of preference for the delayed lever; (2) >10 reinforced trials by session and (3) <20% deviation in the number of reinforced trials, all during 3 days. Once these criteria were reached, mice followed with the test phase where a time delay was introduced between lever pressing in the delayed lever and the delivery of the three pellets. During this period, the stimulus light (not the 0.5 s buzzer beep) was turned on, and additional lever presses of either lever were recorded but without consequence. The delay was fixed for a given daily session and progressively increased over subsequent days (0, 6, 12, 18, 24, 30, 42, 54, 66, 78, 90 s). Change in the percentage of preference for the delayed lever in relation to different delays (cognitive impulsivity) and the number of immediate lever presses during the delay time (motor impulsivity) were analysed. Ineffective responses in the immediate lever increased as a function of the delay time imposed. Each treatment group was tested at the same time, and the treatment group starting order was counterbalanced, placing the treatment group that first initiated a daily session at the end on the following day.
TH, D2–receptor and α2A-adrenoceptor gene expression analysis – real-time PCR
Mice were killed 24 h after the last DRT session (mice were under food restriction), and brains were removed from the skull and frozen over dry ice. Coronal brain sections (500 µm), which were obtained in a cryostat (−10°C), contained the regions of interest according to Paxinos and Franklin (2001) beginning at plates 19–20 (distance from the bregma: 1.42 and 1.34 mm respectively). The PFC, ACC and VTA were microdissected according to the method of Palkovits (Palkovits, 1983). Total RNA was isolated from micropunches of brain tissue using TRI Reagent® (Applied Biosystems, Madrid, Spain) and subsequently retrotranscribed to cDNA. Quantitative analysis of the relative abundance of α2A-adrenoceptors (Mm00845383_s1), D2–receptor (Mm00438541_m1) and TH (Mm00447546_m1) gene expression was performed on the ABI PRISM 7700 Sequence Detector System (Applied Biosystems, Foster City, CA) between the treatment and control groups. All reagents were obtained from Applied Biosystems, and the manufacturers' protocols were followed. The reference gene used was 18S rRNA, detected using Taqman ribosomal RNA control reagents. All primer–probe combinations were optimized and validated for relative quantification of gene expression. Briefly, data for each target gene were normalized to the endogenous reference gene, and the fold change in target gene mRNA abundance was determined using the 2−ΔΔCt method (Livak and Schmittgen, 2001) so that treatment group levels were expressed relative to control group levels. Not all the mice used in the behaviour tests were included in the statistical analyses of real-time PCR studies due to the following reasons: low quantity of total RNA isolated and lack of real-time PCR amplification.
Statistical analysis
Statistical analysis was performed using two-way anova with repeated measures followed by Student–Newman–Keul's test to compare the treatment and control groups at different time points in the DRT. One-way anova followed by Student–Newman–Keul's test was employed when comparing the effects of pregabalin or topiramate on anxiety-like behaviour, novelty seeking or gene expression between the treatment and control groups. Differences were considered significant if the probability of error was less than 5%. SigmaStat v3.11 software (Systat Software Inc., Chicago, IL) was used for all statistical analysis.
Results
Effects of pregabalin and topiramate on anxiety-like behaviour
Anxiety-like behaviour was evaluated with the LDB paradigm, administering one single dose of each anticonvulsant drug 60 min before the test. Pregabalin fully reduced the high anxiety level expressed by DBA/2 mice, increasing the permanence time in the lighted side at all doses tested (Figure 1A: one-way anova followed by Student–Newman–Keul's method, F(3,31)= 9.549, P < 0.001). In contrast, topiramate decreased anxiety only with the highest dose (Figure 1C: one-way anova followed by Student–Newman–Keul's method, F(3,31)= 4.261, P= 0.013). The number of transitions between compartments was not affected by either pregabalin (Figure 1B: one-way anova followed by Student–Newman–Keul's method, F(3,31)= 0.255, P= 0.857) or topiramate (Figure 1D: one-way anova followed by the Student–Newman–Keul's method, F(3,31)= 0.641, P= 0.595).
Figure 1.
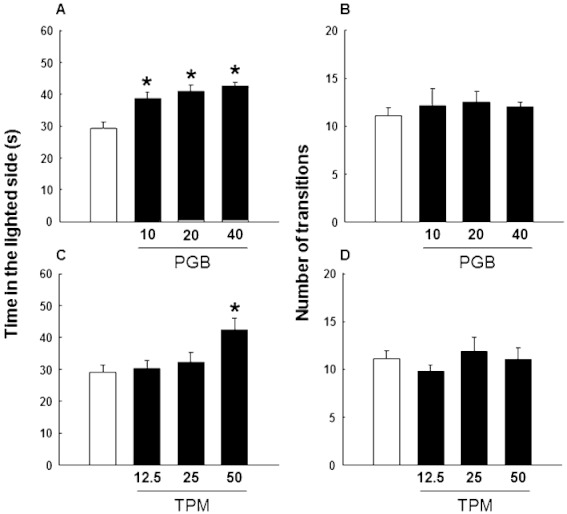
Evaluation of anxiety-like behaviour in DBA/2 mice treated with pregabalin (10, 20 or 40 mg·kg−1, p.o., 1 h before testing) or topiramate (12.5, 25 or 50 mg·kg−1, p.o., 1 h before testing) in the LDB paradigm. Columns represent the means and vertical lines ± SEM of the time spent in the lighted side (A,C) and the number of transitions (B,D). *Values of drug-treated DBA/2 mice that are significantly different (P < 0.05) from its corresponding vehicle group.
Effects of pregabalin and topiramate on impulsive-like behaviours
Novelty seeking
HBT was used to analyse the effects of pregabalin and topiramate, single-dose administration 60 min before the task, on novelty-seeking behaviour. The natural preference for the exploration of two of the four holes with a novel object expressed by DBA/2 mice, was not modified by pregabalin (Figure 2A: one-way anova followed by Student–Newman–Keul's method, F(3,31)= 0.0366, P= 0.990). On the other hand, topiramate significantly and dose-dependently reduced the object preference (Figure 2B: one-way anova followed by Student–Newman–Keul's method, F(3,31)= 6.415, P= 0.002)
Figure 2.
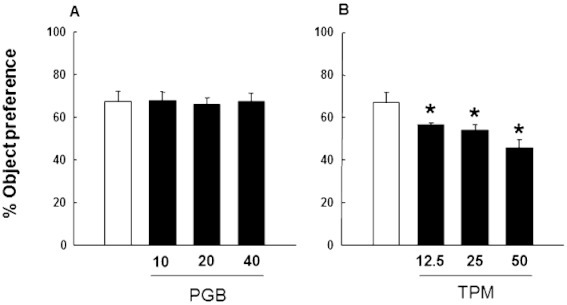
Analysis of novelty-seeking behaviour in DBA/2 mice treated with pregabalin (10, 20 or 40 mg·kg−1, p.o., 1 h prior testing) or topiramate (12.5, 25 or 50 mg·kg−1, p.o., 1 h before testing) on the HBT. Columns represent the means and vertical lines ± SEM of the % preference to explore holes containing an object with pregabalin (A) or topiramate (B). *Values of drug-treated DBA/2 mice that are significantly different (P < 0.05) from its corresponding vehicle group.
Delay discounting
Acute administration schedule
Cognitive impulsivity
In order to evaluate the effects of acute administration of both pregabalin and topiramate on cognitive impulsivity, DBA/2 mice were challenged in the DRT and were given the drug only during the test phase. Statistical analysis of the % preference change along time delays (delay discounting) indicated that neither pregabalin (Figure 3A: two-way anova with RM followed by the Student–Newman–Keul's method, pregabalin dose F(3,319)= 0.645, P= 0.593; delay F(10,319)= 89.305, P < 0.001; pregabalin dose × delay interaction F(30,319)= 0.435, P= 0.996) nor topiramate (Figure 3B: two-way anova with RM followed by the Student–Newman–Keul's method, topiramate dose F(3,319)= 1.054, P= 0.385; delay F(10,319)= 44.541, P < 0.001; topiramate dose ×delay interaction F(30,319)= 1.416, P= 0.081) were able to significantly modify the % preference with respect to the control group at any dose tested. Therefore, when administered acutely only during the test phase, pregabalin and topiramate failed to modulate cognitive impulsivity.
Figure 3.
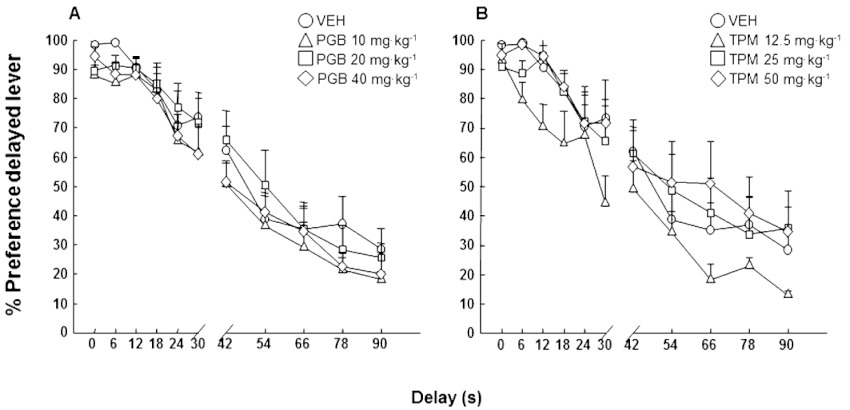
Assessment of cognitive impulsivity (delay discounting) in DBA/2 mice treated with pregabalin (10, 20 or 40 mg·kg−1, p.o., for 10 days and 1 h before testing) or topiramate (12.5, 25 or 50 mg·kg−1, p.o., for 10 days and 1 h before testing) in the DRT. Dots represent the means and vertical lines ± SEM of % preference for delayed reinforcement with pregabalin (A) or topiramate (B) treatment.
Motor impulsivity
The number of ineffective responses during delay onset (not rewarded), reflecting motor dimension of impulsivity, was measured. Pregabalin did not modify the increasing number of immediate lever presses during delays (Figure 4A: two-way anova with RM followed by the Student–Newman–Keul's method, pregabalin dose F(3,319)= 0.829, P= 0.489; delay F(9,319)= 37.147, P < 0.001; pregabalin dose × delay interaction F(27,319)= 1.015, P= 0.448). However, topiramate significantly reduced DBA/2 motor impulsivity, mainly at the highest dose (Figure 4B: two-way anova with RM followed by the Student–Newman–Keul's method, topiramate dose F(3,319)= 5.091, P= 0.006; delay F(9,319)= 57.026, P < 0.001; topiramate dose × delay interaction F(27,319)= 3.954, P < 0.001).
Figure 4.
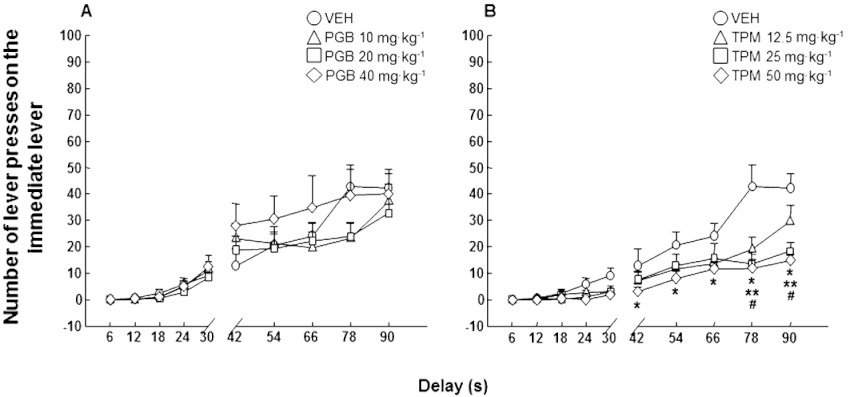
Motor impulsivity evaluation (ineffective responding) in DBA/2 mice treated with pregabalin (10, 20 or 40 mg·kg−1, p.o., for 10 days and 1 h before testing) or topiramate (12.5, 25 or 50 mg·kg−1, p.o., for 10 days and 1 h before testing) in the DRT. Dots represent the means and vertical lines ± SEM of number of lever presses in the immediate lever during delay onset with pregabalin (A) or topiramate (B) treatment. *Values for topiramate 50 mg·kg−1 group that are significantly different (P < 0.05) from its corresponding vehicle group; **values for topiramate 25 mg·kg−1 group that are significantly different (P < 0.05) from its corresponding vehicle group; #values for topiramate 12.5 mg·kg−1 group that are significantly different (P < 0.05) from its corresponding vehicle group.
Chronic administration schedule
Cognitive impulsivity
Cognitive impulsivity was evaluated after chronic treatment twice a day (7 day pretreatment plus treatment during the DRT). Chronic pregabalin did not modify DBA/2 delay discounting at any dose tested (Figure 5A: two-way anova with RM followed by Student–Newman–Keul's method, pregabalin dose F(3,319)= 1.580, P= 0.217; delay F(10,319)= 105.594, P < 0.001; pregabalin dose × delay interaction F(30,319)= 0.894, P= 0.629). On the other hand, topiramate significantly improved DBA/2 cognitive impulsivity, producing a dose-dependent decrease in delay discounting (Figure 5B: two-way anova with RM followed by Student–Newman–Keul's method, topiramate dose F(3,319)= 4.140, P= 0.015; delay F(10,319)= 131.747, P < 0.001; topiramate dose × delay interaction F(30,319)= 1.855, P= 0.006).
Figure 5.
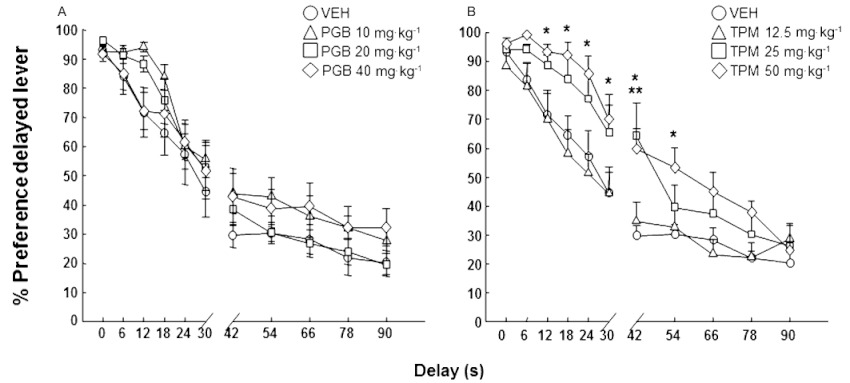
Assessment of cognitive impulsivity (delay discounting) in DBA/2 mice treated with pregabalin (10, 20 or 40 mg·kg−1, p.o., twice a day for 27 days) or topiramate (12.5, 25 or 50 mg·kg−1, p.o., twice a day for 27 days) in the DRT. Dots represent the means and vertical lines ± SEM of % preference for delayed reinforcement with pregabalin (A) or topiramate (B) treatment. *Values from topiramate 50 mg·kg−1 treated mice that are significantly different (P < 0.05) from its corresponding vehicle group; **values from topiramate 25 mg·kg−1 treated mice that are significantly different (P < 0.05) from its corresponding vehicle group.
Motor impulsivity
Surprisingly, the change from acute to chronic administration of pregabalin and topiramate produced an opposite response schedule when evaluating motor impulsivity in the DRT. Pregabalin significantly and dose-dependently increased the number of ineffective responses (Figure 6A: two-way anova with RM followed by Student–Newman–Keul's method, pregabalin dose F(3,319)= 9.055, P < 0.001; delay F(9,319)= 69.405, P < 0.001; pregabalin dose × delay interaction F(27,319)= 1.611, P= 0.032), whereas different topiramate doses were without effects (Figure 6B: two-way anova with RM followed by the Student–Newman–Keul's method, topiramate dose F(3,319)= 0.818, P= 0.495; delay F(9,319)= 61.252, P < 0.001; topiramate dose × delay interaction F(27,319)= 0.624, P= 0.928).
Figure 6.
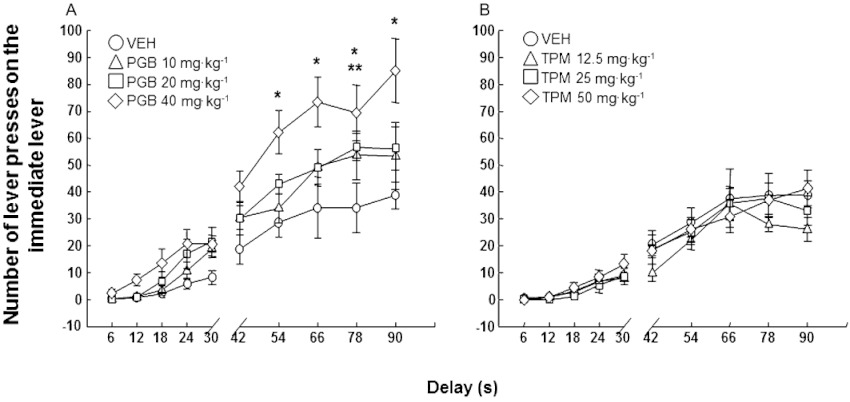
Motor impulsivity evaluation (ineffective responding) in DBA/2 mice treated with pregabalin (10, 20 or 40 mg·kg−1, p.o., twice a day for 27 days) or topiramate (12.5, 25 or 50 mg·kg−1, p.o., twice a day for 27 days) in the DRT. Dots represent the means and vertical lines ± SEM of number of lever presses in the immediate lever during delay onset with pregabalin (A) or topiramate (B) treatment. *Values from pregabalin 40 mg·kg−1 group that are significantly different (P < 0.05) from its corresponding vehicle group; **values from pregabalin 20 mg·kg−1 group that are significantly different (P < 0.05) from its corresponding vehicle group.
Gene expression changes after chronic pregabalin and topiramate administration
Real time-PCR gene expression analyses revealed that the chronic administration of pregabalin significantly reduced α2A-adrenoceptor mRNA levels in the PFC of DBA/2 mice (Figure 7A: one-way anova followed by Student–Newman–Keul's method, F(3,27)= 10.304, P= 0.001) and also decreased D2–receptor gene expression in the ACC (Figure 7B: one-way anova followed by Student–Newman–Keul's method, F(3,31)= 3.091, P= 0.043). Conversely, in the VTA, pregabalin dose-dependently increased TH gene expression (Figure 7C: one-way anova followed by Student–Newman–Keul's method, F(3,31)= 3.894, P= 0.019). On the other hand, chronic topiramate up-regulated α2A-adrenoceptors in the PFC (Figure 7D: one-way anova followed by Student–Newman–Keul's method, F(3,28)= 4.192, P= 0.016) and D2-receptors in the ACC (Figure 7E: one-way anova followed by Student–Newman–Keul's method, F(3,31)= 3.153, P= 0.040). In addition, topiramate increased TH gene expression in the VTA (Figure 7F: one-way anova followed by the Student–Newman–Keul's method, F(3,30)= 6.005, P= 0.003).
Figure 7.
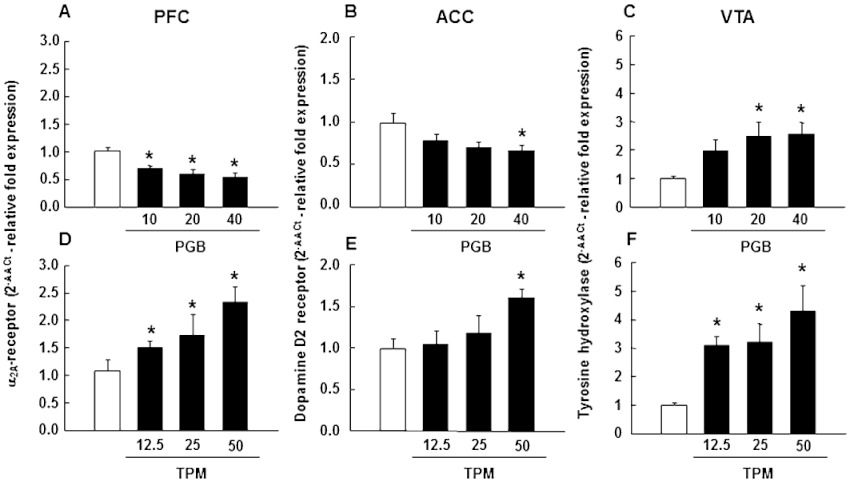
α2A-adrenoceptor, D2-receptor and TH relative gene expressions evaluation in the PFC, ACC and VTA, respectively, of pregabalin (10, 20 or 40 mg·kg−1, p.o., twice a day for 27 days)- or topiramate (12.5, 25 or 50 mg·kg−1, p.o., twice a day for 27 days)-treated mice by real time-PCR. Columns represent the means and vertical lines ± SEM of relative (2−ΔΔCt method) α2A-adrenoceptor gene expression in PFC (A,D), D2-receptor gene expression in ACC (B,E) and TH gene expression in VTA (C,F) of DBA/2 treated mice. *Values of drug-treated DBA/2 mice that are significantly different (P < 0.05) from its corresponding vehicle group.
Discussion
To our knowledge, the results of the present study provide information for the first time about the differential effects of pregabalin and topiramate on anxiety- and impulsive-like behaviours employing different administration schedules. Topiramate reduced novelty seeking and as expected, according to previous clinical studies by our group (Rubio et al., 2009), acutely modulated motor impulsivity and chronically modulated cognitive impulsivity in the DBA/2 strain of mice with a high-impulsive basal level (Navarrete et al., 2012). On the other hand, pregabalin did not have any effect on either object preference or acutely in the DRT, whereas when administered chronically, it exacerbated motor impulsivity levels in DBA/2 mice. In addition, anxiety-like behaviour evaluation showed that pregabalin has a clear anxiolytic profile in comparison with topiramate, suggesting that the therapeutic usefulness of pregabalin in drug dependence management is more related to this emotional aspect. Furthermore, real-time PCR analyses clearly showed that both drugs modulated α2A-adrenoceptors, D2-receptors and TH gene expressions differently in the cortico-mesolimbic pathway, providing novel insight about the neurochemical modulatory effects of pregabalin and topiramate and their possible relationship with impulsivity regulation.
When administered acutely, none of the doses of pregabalin tested (10, 20 and 40 mg·kg−1) or topiramate (12.5, 25 and 50 mg·kg−1) modified the delay discounting progression of DBA/2 mice compared to the corresponding vehicle group. This lack of effect suggests that this schedule of administration (each session during the delay phase of the task) was not adequate to produce any effect on cognitive impulsivity. On the other hand, when motor impulsivity was evaluated by counting the number of immediate lever presses during the delay onset, pregabalin did not produce any effect, but topiramate clearly enhanced behavioural inhibition, mainly at the highest dose (50 mg·kg−1). A possible explanation for this discrepancy may be related to differences in their mechanisms of action. Pregabalin modulates voltage-gated calcium channels binding to the α2-δ subunit mainly in hyperexcitability states (Taylor et al., 2007), whereas topiramate acts through several mechanisms leading to a potent inhibitory state (White et al., 1997; Zona et al., 1997; Reis et al., 2002; Braga et al., 2009) independent of neuronal excitability. This fact may contribute to a more efficacious behavioural inhibition in DBA/2 mice, lowering the number of ineffective responses during the time delay. In the DRT, mice consistently learn to make a response (lever press) to achieve a reward (food). Development of such automatic processes seems to depend on glutamatergic neurotransmission through the activation of N-methyl d-aspartate receptors (Kelley et al., 1997) and the activation of AMPA receptors is needed for their expression (Backstrom and Hyytia, 2003). Topiramate, acting as an AMPA receptor antagonist may improve the ability to these mice to wait, so reducing the number of ineffective (not rewarded) responses. In the same way, this mechanism could also explain the significant reduction in novelty-seeking behaviour of DBA/2 mice that was not achieved with pregabalin. Novelty seeking has been associated with drug abuse (Lange et al., 2010; Cummings et al., 2011). Hence, topiramate's ability to reduce novelty exploration behaviour may account for its usefulness as a drug-dependence treatment.
Since acute administration of either drug did not alleviate the high cognitive impulsivity level in DBA/2 mice, it was hypothesized that chronic administration with a pretreatment phase before the beginning of the DRT and the administration of the drug twice a day would be appropriate to identify whether pregabalin or topiramate is able to modulate delay discounting. Although chronic administration of topiramate was without effect on motor impulsivity, the medium (25 mg·kg−1) and highest (50 mg·kg−1) doses of topiramate significantly reduced delay discounting in DBA/2-treated mice. The percentage of preference for the delayed lever was maintained significantly higher than in the control group from 12 s until 54 s of delay. This effect was not present in the final stages of the experiment, probably due to a tolerance effect. These results suggest that the schedule of dosing and duration of the treatment play a crucial role in the modulatory effect of topiramate on impulsive choice. Indeed, depending on the administration schedule, this drug modulated either motor or cognitive impulsivity behaviours. In contrast, pregabalin failed to alter the preference for the delayed lever and even significantly increased motor impulsivity when administered chronically at a 40 mg·kg−1 dose. This effect could be related to the anxiolytic effect of pregabalin (Lauria-Horner and Pohl, 2003; Frampton and Foster, 2006). A decrease in the anxiety level in spontaneously anxious DBA/2 mice (Griebel et al., 2000; Ohl et al., 2003; Yilmazer-Hanke et al., 2003) may be responsible for behavioural disinhibition, leading to an increase in the number of immediate lever presses. The inability of pregabalin to diminish cognitive or motor impulsivity seems to indicate that its potential beneficial effects on drug abuse may be due to the regulation of other behavioural mechanisms such as co-morbid psychiatric symptomatology (Martinotti et al., 2010). Data shown in Figure 1 clearly indicate that pregabalin presents a potent anxiolytic effect, increasing the time spent in the lighted and open side at all doses tested, supporting the previous hypothesis. Indeed, recent data from a study by our group demonstrated that pregabalin reduces the increase in the anxiety level produced by spontaneous cannabinoid withdrawal in mice (Aracil-Fernandez et al., 2011).
It is important to note that the measurement of motor impulsivity in the DRT is different from the evaluation in the five-choice serial reaction time or Go/NoGo tasks. The former evaluates the inability to wait until the reinforcement is delivered (a response that does not have consequences) and the latter the inability to withhold a prepotent response (a response that has negative consequences). The analysis of motor impulsivity in animal experimental models has been classically developed in tasks in which the animal has to refrain from responding to achieve a goal (reward). In the present study, the number of lever presses during the delay onset would determine the level of restlessness in mice. As stated by other authors, this behavioural parameter also takes part in the definition of motor impulsivity (Dellu-Hagedorn, 2006; Boes et al., 2009). Indeed, the Barratt Impulsiveness Scale (BIS-11), a widely used and well-validated tool to measure human impulsivity, considers motor impulsiveness as ‘acting without thinking and restlessness’ (Patton et al., 1995).
Gene expression analyses were focused on dopaminergic and adrenergic neurotransmission systems. There is much evidence for the critical involvement of dopamine in impulsive behaviour (van Gaalen et al., 2006; Buckholtz et al., 2010) and special attention has been paid to the role of D2-receptors in this effect (Dalley et al., 2007; Hamidovic et al., 2009; Lee et al., 2009). On the other hand, PFC adrenergic circuit involvement in decision making is well known (Dalley et al., 2008; Kim and Lee, 2010). Agonists of α2A-adrenoceptors have been shown to be useful in the treatment of inattention, hyperactivity and impulsiveness in ADHD (Scahill, 2009); and, recently, the α2A-adrenoceptor agonist guanfacine was found to ameliorate impulsive choice behaviours in primates (Kim et al., 2011). For these reasons, in the present study we investigated whether the effects of pregabalin and topiramate on impulsivity dimensions are related to their modulation of α2A-adrenoceptor, D2-receptor and TH gene expression. These studies were carried out in the mesolimbic–mesocortical pathways for three reasons: (1) the critical involvement of this pathway in the regulation of impulsive behaviours (van Gaalen et al., 2006; Dalley et al., 2007; Lee et al., 2009; Basar et al., 2010; Buckholtz et al., 2010; Kim and Lee, 2010); (2) its crucial role in reinforcement effects of drugs of abuse (Phillips and Fibiger, 1973; Leshner and Koob, 1999; Hyman and Malenka, 2001); and (3) dopaminergic and adrenergic tone are both modulated by pregabalin (Andrews et al., 2001; Gajraj, 2005; Takeuchi et al., 2007) and topiramate (Johnson, 2004a,b). The neuropharmacological action of topiramate includes facilitation of GABA-mediated neurotransmission and blockade of AMPA/kainate glutamate receptors. According to Johnson's hypothesis (Johnson et al., 2003), because mesocorticolimbic dopamine release is under tonic inhibitory control via GABAergic neurons and excitatory control via glutamatergic neurons, topiramate may inhibit dopamine release and consequently reduce receptor activation. Maintenance of this effect with chronic administration could produce a compensatory effect. Real-time PCR results support this hypothesis since topiramate dramatically increased TH gene expression in the VTA and also up-regulated D2-receptors in the ACC. Furthermore, it is widely accepted that low D2-receptor availability in the brains of animals or humans is related with a high impulsivity level (Dalley et al., 2007; Lee et al., 2009), probably due to a high basal dopaminergic tone. Indeed, it has been reported that pharmacological modulation by D2-receptor antagonists induced impulsive choice, suggesting that these receptors normally promote choice of the delayed reinforcement (Wade et al., 2000). DBA/2 mice present low D2-receptor gene expression in comparison with a low-impulsive strain (Navarrete et al., 2012). Therefore, it seems that the enhancement of D2-receptor expression in the ACC, achieved with the chronic administration of topiramate, could be closely associated with the cognitive impulsivity modulation. On the other hand, pregabalin showed no effect on cognitive impulsivity, a fact that could be partially explained by a distinct dopaminergic modulation that entails an opposite effect on D2-receptor gene expression and a smaller increase in TH in the VTA in comparison with topiramate.
Interestingly, the administration of topiramate up-regulated the α2A-adrenoceptor gene expression in the PFC dose-dependently, which would fit with a direct/indirect adrenergic blockade not previously described in the literature for this drug. Genetic variants of the α2A-adrenoceptor are involved in drug abuse (Feng et al., 1998; Prestes et al., 2007) and ADHD (Xu et al., 2001; Schmitz et al., 2006). Furthermore, α2A-adrenoceptor gene expression in the PFC has been inversely correlated with lever pressing to obtain a reward (Pickering et al., 2007), suggesting that animals with a low responding rate present higher α2A-adrenoceptor gene expression levels. This finding seems to agree with the chronic pregabalin effect on motor impulsivity since the dose-dependent increase in the number of ineffective responses is associated with a dose-dependent decrease in α2A-adrenoceptor gene expression in the PFC. In the same way, it could be hypothesized that the lack of effect of chronic topiramate on behavioural inhibition in comparison with the acute schedule may be related to the significant increase in α2A-adrenoceptor mRNA levels in the PFC.
In conclusion, the present study demonstrates that the chronic administration of topiramate regulated cognitive impulsivity, whereas acute drug treatment regulated motor impulsivity expressed by DBA/2 mice. These results point out the relevance of the administration schedule to regulate distinct dimensions of impulsive behaviour. In addition, topiramate reduced novelty-seeking behaviour, which is closely associated with drug abuse vulnerability. These findings suggest that the therapeutic utility of topiramate in addictive behaviours, such as alcohol-dependence, may be due to its ability to control impulsive-like behaviours. The impulsivity modulation showed by topiramate seems to be associated with differential gene expression changes in mesolimbic–mesocortical dopaminergic and adrenergic neurotransmission. The present results suggest that the up-regulation of D2-receptor gene expression induced by topiramate could be the main mechanism responsible for the reduction in novelty seeking and cognitive impulsivity in DBA/2 mice. On the other hand, the therapeutic utility of pregabalin in impulsive-related disorders appears to be more associated with its ability to regulate other behavioural aspects such as anxiety, since no beneficial effects were achieved in either the HBT or in the DRT.
Acknowledgments
This study was supported by grant 2007/061 from ‘Plan Nacional Sobre Drogas’ (PNSD, Spanish Ministry of Health) to JM and by ‘Red Temática de Investigación Cooperativa en Salud’ (RETICS, Instituto de Salud Carlos III, MICINN and FEDER, Madrid, Spain, ‘Red de Trastornos Adictivos’, RD06/0001/1004) to JM. FN is a pre-doctoral fellow supported by MICINN. JMP-O is a postdoctoral fellow supported by FISCAM (Fundación para la investigación sanitaria en Castilla La Mancha). We thank Patricia Rodríguez and Analía Rico for excellent technical assistance. AR and PR are technicians supported by RETICS and FISCAM, respectively.
Glossary
- ACC
accumbens nucleus
- ADHD
attention-deficit hyperactivity disorder
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid
- D2-receptor
D2 dopamine receptor
- DRT
delayed reinforcement task
- HBT
hole board test
- LDB
light–dark box
- PFC
prefrontal cortex
- VTA
ventral tegmental area
Conflicts of interest
All authors report no biomedical financial interests or potential conflicts of interest.
References
- Adriani W, Zoratto F, Romano E, Laviola G. Cognitive impulsivity in animal models: role of response time and reinforcing rate in delay intolerance with two-choice operant tasks. Neuropharmacology. 2010;58:694–701. doi: 10.1016/j.neuropharm.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Andrews N, Loomis S, Blake R, Ferrigan L, Singh L, McKnight AT. Effect of gabapentin-like compounds on development and maintenance of morphine-induced conditioned place preference. Psychopharmacology (Berl) 2001;157:381–387. doi: 10.1007/s002130100839. [DOI] [PubMed] [Google Scholar]
- Aracil-Fernandez A, Almela P, Manzanares J. Pregabalin and topiramate regulate behavioural and brain gene transcription changes induced by spontaneous cannabinoid withdrawal in mice. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00406.x. doi: 10.1111/j.1369-1600.2011.00406.x. [DOI] [PubMed] [Google Scholar]
- August GJ, Garfinkel BD. Behavioral and cognitive subtypes of ADHD. J Am Acad Child Adolesc Psychiatry. 1989;28:739–748. doi: 10.1097/00004583-198909000-00016. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Attenuation of cocaine-seeking behaviour by the AMPA/kainate receptor antagonist CNQX in rats. Psychopharmacology (Berl) 2003;166:69–76. doi: 10.1007/s00213-002-1312-y. [DOI] [PubMed] [Google Scholar]
- Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81:267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bockbrader HN, Radulovic LL, Posvar EL, Strand JC, Alvey CW, Busch JA, et al. Clinical pharmacokinetics of pregabalin in healthy volunteers. J Clin Pharmacol. 2010;50:941–950. doi: 10.1177/0091270009352087. [DOI] [PubMed] [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: a neuroanatomical correlate of impulse control in boys. Soc Cogn Affect Neurosci. 2009;4:1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois BF. New antiepileptic drugs. Curr Opin Pediatr. 1996;8:543–548. doi: 10.1097/00008480-199612000-00002. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Li H, Rogawski MA. Topiramate reduces excitability in the basolateral amygdala by selectively inhibiting GluK1 (GluR5) kainate receptors on interneurons and positively modulating GABAA receptors on principal neurons. J Pharmacol Exp Ther. 2009;330:558–566. doi: 10.1124/jpet.109.153908. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Cubells JF. Topiramate for cocaine dependence. Curr Psychiatry Rep. 2006;8:130–131. doi: 10.1007/s11920-006-0011-5. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Gowl BA, Westenbroek C, Clinton SM, Akil H, Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2:1–10. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Dellu-Hagedorn F. Relationship between impulsivity, hyperactivity and working memory: a differential analysis in the rat. Behav Brain Funct. 2006;2:10. doi: 10.1186/1744-9081-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nicola M, Martinotti G, Tedeschi D, Frustaci A, Mazza M, Sarchiapone M, et al. Pregabalin in outpatient detoxification of subjects with mild-to-moderate alcohol withdrawal syndrome. Hum Psychopharmacol. 2010;25:268–275. doi: 10.1002/hup.1098. [DOI] [PubMed] [Google Scholar]
- Dolengevich Segal H, Rodriguez Salgado B, Conejo Garcia A, San Sebastian Cabases J. Efficacy of topiramate in children and adolescent with problems in impulse control: preliminary results. Actas Esp Psiquiatr. 2006;34:280–282. [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Marsh DM, Moeller FG, Mathias CW, et al. Behavioral impulsivity paradigms: a comparison in hospitalized adolescents with disruptive behavior disorders. J Child Psychol Psychiatry. 2003;44:1145–1157. doi: 10.1111/1469-7610.00197. [DOI] [PubMed] [Google Scholar]
- Evenden J. Impulsivity: a discussion of clinical and experimental findings. J Psychopharmacol. 1999a;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999b;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evren C, Durkaya M, Evren B, Dalbudak E, Cetin R. Relationship of relapse with impulsivity, novelty seeking and craving in male alcohol-dependent inpatients. Drug Alcohol Rev. 2012;31:81–90. doi: 10.1111/j.1465-3362.2011.00303.x. [DOI] [PubMed] [Google Scholar]
- Fahy T, Eisler I. Impulsivity and eating disorders. Br J Psychiatry. 1993;162:193–197. doi: 10.1192/bjp.162.2.193. [DOI] [PubMed] [Google Scholar]
- Feng J, Sobell JL, Heston LL, Goldman D, Cook E, Jr, Kranzler HR, et al. Variants in the alpha2A AR adrenergic receptor gene in psychiatric patients. Am J Med Genet. 1998;81:405–410. doi: 10.1002/(sici)1096-8628(19980907)81:5<405::aid-ajmg9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Frampton JE, Foster RH. Pregabalin: in the treatment of generalised anxiety disorder. CNS Drugs. 2006;20:685–693. doi: 10.2165/00023210-200620080-00010. discussion 694-5. [DOI] [PubMed] [Google Scholar]
- Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68:1691–1700. doi: 10.4088/jcp.v68n1108. [DOI] [PubMed] [Google Scholar]
- Gajraj NM. Pregabalin for pain management. Pain Pract. 2005;5:95–102. doi: 10.1111/j.1533-2500.2005.05205.x. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(Suppl. 1):S10–S16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Griebel G, Belzung C, Perrault G, Sanger DJ. Differences in anxiety-related behaviours and in sensitivity to diazepam in inbred and outbred strains of mice. Psychopharmacology (Berl) 2000;148:164–170. doi: 10.1007/s002130050038. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Dlugos A, Skol A, Palmer AA, de Wit H. Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: an exploratory study with d-amphetamine in healthy participants. Exp Clin Psychopharmacol. 2009;17:374–383. doi: 10.1037/a0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms CM, Reeves JM, Mitchell SH. Impact of strain and d-amphetamine on impulsivity (delay discounting) in inbred mice. Psychopharmacology (Berl) 2006;188:144–151. doi: 10.1007/s00213-006-0478-0. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- James AS, Groman SM, Seu E, Jorgensen M, Fairbanks LA, Jentsch JD. Dimensions of impulsivity are associated with poor spatial working memory performance in monkeys. J Neurosci. 2007;27:14358–14364. doi: 10.1523/JNEUROSCI.4508-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JM, Pettinati HM, Evans BD, Meyers K, Valliere VN. Impulsivity and substance abusers: changes with treatment and recovery. NIDA Res Monogr. 1990;105:287–288. [PubMed] [Google Scholar]
- Johnson BA. Progress in the development of topiramate for treating alcohol dependence: from a hypothesis to a proof-of-concept study. Alcohol Clin Exp Res. 2004a;28:1137–1144. doi: 10.1097/01.alc.0000134533.96915.08. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Topiramate-induced neuromodulation of cortico-mesolimbic dopamine function: a new vista for the treatment of comorbid alcohol and nicotine dependence? Addict Behav. 2004b;29:1465–1479. doi: 10.1016/j.addbeh.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N. Topiramate in the new generation of drugs: efficacy in the treatment of alcoholic patients. Curr Pharm Des. 2010;16:2103–2112. doi: 10.2174/138161210791516404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kavoussi RJ, Coccaro EF. Divalproex sodium for impulsive aggressive behavior in patients with personality disorder. J Clin Psychiatry. 1998;59:676–680. doi: 10.4088/jcp.v59n1206. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci USA. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Lomastro TL, Schiesl A, Leggio L, Swift RM. Review of topiramate: an antiepileptic for the treatment of alcohol dependence. Curr Drug Abuse Rev. 2009;2:135–142. doi: 10.2174/1874473710902020135. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee D. Prefrontal cortex and impulsive decision making. Biol Psychiatry. 2010;69:1140–1146. doi: 10.1016/j.biopsych.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bobeica I, Gamo NJ, Arnsten AF, Lee D. Effects of alpha-2A adrenergic receptor agonist on time and risk preference in primates. Psychopharmacology (Berl) 2011;219:363–375. doi: 10.1007/s00213-011-2520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange LA, Kampov-Polevoy AB, Garbutt JC. Sweet liking and high novelty seeking: independent phenotypes associated with alcohol-related problems. Alcohol Alcohol. 2010;45:431–436. doi: 10.1093/alcalc/agq040. [DOI] [PubMed] [Google Scholar]
- Lauria-Horner BA, Pohl RB. Pregabalin: a new anxiolytic. Expert Opin Investig Drugs. 2003;12:663–672. doi: 10.1517/13543784.12.4.663. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, et al. Striatal Dopamine D2/D3 Receptor Availability Is Reduced in Methamphetamine Dependence and Is Linked to Impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Masala I, Golia F, Consoli G, Massimetti G, et al. Impulsivity, gender, and the platelet serotonin transporter in healthy subjects. Neuropsychiatr Dis Treat. 2010;6:9–15. [PMC free article] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Romanelli R, Andreoli S, Pozzi G, Moroni N, et al. High and low dosage oxcarbazepine versus naltrexone for the prevention of relapse in alcohol-dependent patients. Hum Psychopharmacol. 2007;22:149–156. doi: 10.1002/hup.833. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Tedeschi D, Mazza M, Janiri L, Bria P. Efficacy and safety of pregabalin in alcohol dependence. Adv Ther. 2008;25:608–618. doi: 10.1007/s12325-008-0066-2. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Tedeschi D, Andreoli S, Reina D, Pomponi M, et al. Pregabalin versus naltrexone in alcohol dependence: a randomised, double-blind, comparison trial. J Psychopharmacol. 2010;24:1367–1374. doi: 10.1177/0269881109102623. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, et al. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Navarrete F, Perez-Ortiz JM, Manzanares J. Cannabinoid CB(2) receptor-mediated regulation of impulsive-like behaviour in DBA/2 mice. Br J Pharmacol. 2012;165:260–273. doi: 10.1111/j.1476-5381.2011.01542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen SA, Malcolm R, Middaugh LD. Topiramate reduces ethanol consumption by C57BL/6 mice. Synapse. 2007;61:150–156. doi: 10.1002/syn.20350. [DOI] [PubMed] [Google Scholar]
- Ohl F, Roedel A, Binder E, Holsboer F. Impact of high and low anxiety on cognitive performance in a modified hole board test in C57BL/6 and DBA/2 mice. Eur J Neurosci. 2003;17:128–136. doi: 10.1046/j.1460-9568.2003.02436.x. [DOI] [PubMed] [Google Scholar]
- Otobe T, Makino J. Impulsive choice in inbred strains of mice. Behav Processes. 2004;67:19–26. doi: 10.1016/j.beproc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Oulis P, Konstantakopoulos G. Pregabalin in the treatment of alcohol and benzodiazepines dependence. CNS Neurosci Ther. 2010;16:45–50. doi: 10.1111/j.1755-5949.2009.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M. Punch sampling biopsy technique. Methods Enzymol. 1983;103:368–376. doi: 10.1016/s0076-6879(83)03025-6. [DOI] [PubMed] [Google Scholar]
- Patel S, Stolerman IP, Asherson P, Sluyter F. Attentional performance of C57BL/6 and DBA/2 mice in the 5-choice serial reaction time task. Behav Brain Res. 2006;170:197–203. doi: 10.1016/j.bbr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Academic Press. Harcourt Science and Technology Company; 2001. [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 2001;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. Dopaminergic and noradrenergic substrates of positive reinforcement: differential effects of d- and l-amphetamine. Science. 1973;179:575–577. doi: 10.1126/science.179.4073.575. [DOI] [PubMed] [Google Scholar]
- Pickering C, Avesson L, Lindblom J, Liljequist S, Schioth HB. To press or not to press? Differential receptor expression and response to novelty in rats learning an operant response for reward. Neurobiol Learn Mem. 2007;87:181–191. doi: 10.1016/j.nlm.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Poulsen CF, Simeone TA, Maar TE, Smith-Swintosky V, White HS, Schousboe A. Modulation by topiramate of AMPA and kainate mediated calcium influx in cultured cerebral cortical, hippocampal and cerebellar neurons. Neurochem Res. 2004;29:275–282. doi: 10.1023/b:nere.0000010456.92887.3b. [DOI] [PubMed] [Google Scholar]
- Prestes AP, Marques FZ, Hutz MH, Roman T, Bau CH. Tobacco smoking and the ADRA2A C-1291G polymorphism. J Neural Transm. 2007;114:1503–1506. doi: 10.1007/s00702-007-0769-6. [DOI] [PubMed] [Google Scholar]
- Rapport MD, DuPaul GJ, Stoner G, Birmingham BK, Masse G. Attention deficit disorder with hyperactivity: differential effects of methylphenidate on impulsivity. Pediatrics. 1985;76:938–943. [PubMed] [Google Scholar]
- Reis AD, Castro LA, Faria R, Laranjeira R. Craving decrease with topiramate in outpatient treatment for cocaine dependence: an open label trial. Rev Bras Psiquiatr. 2008;30:132–135. doi: 10.1590/s1516-44462008005000012. [DOI] [PubMed] [Google Scholar]
- Reis J, Tergau F, Hamer HM, Muller HH, Knake S, Fritsch B, et al. Topiramate selectively decreases intracortical excitability in human motor cortex. Epilepsia. 2002;43:1149–1156. doi: 10.1046/j.1528-1157.2002.09902.x. [DOI] [PubMed] [Google Scholar]
- Rubio G, Martinez-Gras I, Manzanares J. Modulation of impulsivity by topiramate: implications for the treatment of alcohol dependence. J Clin Psychopharmacol. 2009;29:584–589. doi: 10.1097/JCP.0b013e3181bfdb79. [DOI] [PubMed] [Google Scholar]
- Scahill L. Alpha-2 adrenergic agonists in children with inattention, hyperactivity and impulsiveness. CNS Drugs. 2009;23(Suppl. 1):43–49. doi: 10.2165/00023210-200923000-00006. [DOI] [PubMed] [Google Scholar]
- Schmitz M, Denardin D, Silva TL, Pianca T, Roman T, Hutz MH, et al. Association between alpha-2a-adrenergic receptor gene and ADHD inattentive type. Biol Psychiatry. 2006;60:1028–1033. doi: 10.1016/j.biopsych.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Shank RP, Maryanoff BE. Molecular pharmacodynamics, clinical therapeutics, and pharmacokinetics of topiramate. CNS Neurosci Ther. 2008;14:120–142. doi: 10.1111/j.1527-3458.2008.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefert HM, Domdey-Bette A, Henninger K, Hucke F, Kohlsdorfer C, Stass HH. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J Antimicrob Chemother. 1999;43(Suppl. B):69–76. doi: 10.1093/jac/43.suppl_2.69. [DOI] [PubMed] [Google Scholar]
- Smathers SA, Wilson JG, Nigro MA. Topiramate effectiveness in Prader-Willi syndrome. Pediatr Neurol. 2003;28:130–133. doi: 10.1016/s0887-8994(02)00490-3. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Mechanism of action of alpha2delta ligands: voltage sensitive calcium channel (VSCC) modulators. J Clin Psychiatry. 2004;65:1033–1034. doi: 10.4088/jcp.v65n0801. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, de Guglielmo G, Kallupi M, Cannella N, et al. Pregabalin reduces alcohol drinking and relapse to alcohol seeking in the rat. Psychopharmacology (Berl) 2011;220:87–96. doi: 10.1007/s00213-011-2457-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y, Takasu K, Ono H, Tanabe M. Pregabalin, S-(+)-3-isobutylgaba, activates the descending noradrenergic system to alleviate neuropathic pain in the mouse partial sciatic nerve ligation model. Neuropharmacology. 2007;53:842–853. doi: 10.1016/j.neuropharm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Taverna S, Sancini G, Mantegazza M, Franceschetti S, Avanzini G. Inhibition of transient and persistent Na+ current fractions by the new anticonvulsant topiramate. J Pharmacol Exp Ther. 1999;288:960–968. [PubMed] [Google Scholar]
- Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73:137–150. doi: 10.1016/j.eplepsyres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Heidbreder CA, Spanagel R. The effects of lamotrigine on alcohol seeking and relapse. Neuropharmacology. 2007;53:951–957. doi: 10.1016/j.neuropharm.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Vogelaer J. Carbamazepine in the treatment of psychotic and behavioral disorders. A pilot study. Acta Psychiatr Belg. 1981;81:532–541. [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wang HD, Takigawa M, Hamada K, Shiratani T, Takenouchi K. A shift in information flow between prefrontal cortex and the ventral tegmental area in methamphetamine-sensitized rats. Int J Psychophysiol. 2002;44:251–259. doi: 10.1016/s0167-8760(02)00010-7. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate enhances GABA-mediated chloride flux and GABA-evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Res. 1997;28:167–179. doi: 10.1016/s0920-1211(97)00045-4. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41(Suppl. 1):S17–S20. [PubMed] [Google Scholar]
- Wilcox J. Divalproex sodium in the treatment of aggressive behavior. Ann Clin Psychiatry. 1994;6:17–20. doi: 10.3109/10401239409148834. [DOI] [PubMed] [Google Scholar]
- Wilcox JA. Divalproex sodium as a treatment for borderline personality disorder. Ann Clin Psychiatry. 1995;7:33–37. doi: 10.3109/10401239509149022. [DOI] [PubMed] [Google Scholar]
- Wilson EA, Brodie MJ. New antiepileptic drugs. Baillieres Clin Neurol. 1996;5:723–747. [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Schachar R, Tannock R, Roberts W, Malone M, Kennedy JL, et al. Linkage study of the alpha2A adrenergic receptor in attention-deficit hyperactivity disorder families. Am J Med Genet. 2001;105:159–162. [PubMed] [Google Scholar]
- Yang B, Bankir L. Urea and urine concentrating ability: new insights from studies in mice. Am J Physiol Renal Physiol. 2005;288:F881–F896. doi: 10.1152/ajprenal.00367.2004. [DOI] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM, Roskoden T, Zilles K, Schwegler H. Anxiety-related behavior and densities of glutamate, GABAA, acetylcholine and serotonin receptors in the amygdala of seven inbred mouse strains. Behav Brain Res. 2003;145:145–159. doi: 10.1016/s0166-4328(03)00107-4. [DOI] [PubMed] [Google Scholar]
- Zona C, Ciotti MT, Avoli M. Topiramate attenuates voltage-gated sodium currents in rat cerebellar granule cells. Neurosci Lett. 1997;231:123–126. doi: 10.1016/s0304-3940(97)00543-0. [DOI] [PubMed] [Google Scholar]
- Zullino DF, Khazaal Y, Hattenschwiler J, Borgeat F, Besson J. Anticonvulsant drugs in the treatment of substance withdrawal. Drugs Today (Barc) 2004;40:603–619. doi: 10.1358/dot.2004.40.7.850478. [DOI] [PubMed] [Google Scholar]


