Abstract
BACKGROUND AND PURPOSE
Phospho-ibuprofen (MDC-917) and phospho-sulindac (OXT-328) are highly effective in cancer and arthritis treatment in preclinical models. Here, we investigated their metabolism by major human cytochrome P450s (CYPs) and flavin monooxygenases (FMOs).
EXPERIMENTAL APPROACH
The CYP/FMO-catalysed metabolism of phospho-ibuprofen and phospho-sulindac was studied by using in silico prediction modelling and a direct experimental approach.
KEY RESULTS
The CYP isoforms catalyse the oxidation of non-steroidal anti-inflammatory drugs (NSAIDs) and phospho-NSAIDs, with distinct activity and regioselectivity. CYP1A2, 2C19, 2D6 and 3A4 oxidize phospho-ibuprofen, but not ibuprofen; whereas CYP2C9 oxidizes ibuprofen, but not phospho-ibuprofen. All CYPs tested oxidize phospho-sulindac, but not sulindac. Among the five CYPs evaluated, CYP3A4 and 2D6 are the most active in the oxidation of phospho-ibuprofen and phospho-sulindac respectively. FMOs oxidized phospho-sulindac and sulindac, but not phospho-ibuprofen or ibuprofen. FMOs were more active towards phospho-sulindac than sulindac, indicating that phospho-sulindac is a preferred substrate of FMOs. The susceptibility of phospho-NSAIDs to CYP/FMO-mediated metabolism was also reflected in their rapid oxidation by human and mouse liver microsomes, which contain a full complement of CYPs and FMOs. Compared with conventional NSAIDs, the higher activity of CYPs towards phospho-ibuprofen and phospho-sulindac may be due to their greater lipophilicity, a key parameter for CYP binding.
CONCLUSIONS AND IMPLICATIONS
CYPs and FMOs play an important role in the metabolism of phospho-NSAIDs, resulting in differential pharmacokinetic profiles between phospho-NSAIDs and NSAIDs in vivo. The consequently more rapid detoxification of phospho-NSAIDs is likely to contribute to their greater safety.
Keywords: phospho-NSAIDs, NSAIDs, cytochrome P450, regioselective oxidation, anticancer drugs, flavin monooxygenase
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used as anti-inflammatory, analgesic and antipyretic agents to treat a number of diseases (Suleyman et al., 2010). NSAIDs have also been shown to be effective in reducing the incidence of colon and other cancers (Gravitz, 2011). However, chronic use of conventional NSAIDs induces significant gastrointestinal and renal toxicities (Vonkeman and van de Laar, 2010). To address this issue, we developed novel phospho-derivatives of NSAIDs, such as phospho-ibuprofen (MDC-917) and phospho-sulindac (OXT-328), which exhibit greater efficacy and are safer than their respective parent NSAIDs in preclinical models of cancer and arthritis (Huang et al., 2010; 2011; Mackenzie et al., 2010).
Prompted by these promising results, we studied the metabolism and pharmacokinetics of phospho-ibuprofen (Xie et al., 2011) and phospho-sulindac (Xie et al., 2012) in mice. Interestingly, we observed that the phospho-modification has a significant impact on the regioselectivity and the extent of NSAID oxidation in vitro and in vivo. Phospho-ibuprofen was oxidized at the 1- and 3-positions of its isobutyl group to form 1-OH-phospho-ibuprofen and 3-OH-phospho-ibuprofen, respectively, the latter leading to carboxy-phospho-ibuprofen; whereas, ibuprofen was oxidized at 2- and 3-positions to give 2-OH-ibuprofen and 3-OH-ibuprofen, respectively, the latter leading to carboxy-ibuprofen (Figure 1A) (Xie et al., 2011). On the other hand, phospho-sulindac and sulindac can be oxidized at their sulfoxide groups to generate phospho-sulindac sulfone and sulindac sulfone respectively (Figure 1B). Phospho-sulindac and sulindac have markedly different metabolite profiles in mice: while sulindac sulfide (the reduced form of sulindac) is the predominant metabolite of sulindac, phospho-sulindac is preferentially oxidized to form phospho-sulindac sulfone, which upon hydrolysis gives sulindac sulfone as the major metabolite (Xie et al., 2012). These findings suggest that cytochrome P450s (CYPs), the key enzymes involved in oxidative transformation of xenobiotics, have different activity and regioselectivity towards NSAIDs and phospho-NSAIDs.
Figure 1.
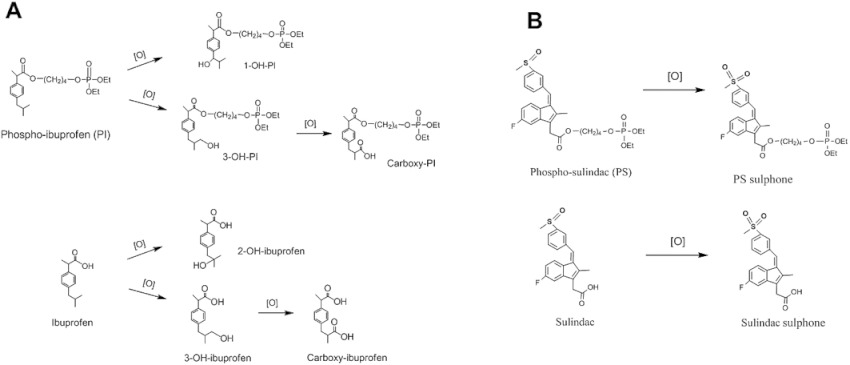
Oxidation pathway of phospho-NSAIDs and NSAIDs. The oxidation pathway of phospho-ibuprofen versus ibuprofen (A) and that of phospho-sulindac versus sulindac (B) in vitro and in vivo are shown.
CYPs, consisting of 43 subfamilies and 57 individual enzymes in humans, are involved in endogenous cellular functions, such as hormone biosynthesis (Martignoni et al., 2006). However, CYPs are best recognized as the major drug-metabolizing enzymes that account for ∼75% of the metabolism of clinically used drugs and other xenobiotics (Sweeney and Bromilow, 2006). In addition, CYP1A2, 2C9, 2C19, 2D6 and 3A4 are the major human CYP isoforms accounting for 95% of CYP-mediated drug metabolism (Lamb et al., 2007). Flavin monooxygenases (FMOs), comprising five isoforms (FMO1 to FMO5), also play important roles in detoxifying xenobiotics. While CYPs can oxidize non-nucleophilic substrates at their C-H bond, FMOs oxidize substrates containing a ‘soft nucleophile’ such as nitrogen and sulfur (Krueger and Williams, 2005). Both CYPs and FMOs are primarily expressed in the liver and, to a lesser extent, in the gastrointestinal tract. In general, CYP/FMO-mediated oxidation leads to detoxification and increased water solubility of drugs, which facilitates their excretion from the body.
Here, we used ADMET (absorption, distribution, metabolism, excretion and toxicity) modelling to predict the CYP isoforms involved in the oxidation of phospho-NSAIDs and NSAIDs, and experimentally characterized the activity and regioselectivity of oxidation by human recombinant CYPs/FMOs and liver microsomes. We found that phospho-ibuprofen and phospho-sulindac were preferentially oxidized by CYPs/FMOs relative to ibuprofen and sulindac, respectively, leading to their rapid oxidation by liver microsomes. These findings largely explain the characteristic pharmacokinetic parameters of phospho-NSAIDs compared to conventional NSAIDs, which may contribute to their improved safety profile.
Methods
In silico simulations of drug metabolism by human CYPs
Predictions of the metabolism of phospho-NSAIDs by the major human CYP isoforms (CYP1A2, 2C9, 2C19, 2D6 and 3A4) were performed using ADMET Predictor version 5.5 (Simulations Plus Inc., Lancaster, CA) based on Accelrys metabolite database and Drugbank database, as well as published datasets of drug metabolism and general review articles. The possibility of being a metabolic site was indicated by a score ranging from 0 to 1, with higher scores indicating a greater likelihood, and the highest scoring atom is highlighted with a red hashed circle (Figure 2).
Figure 2.
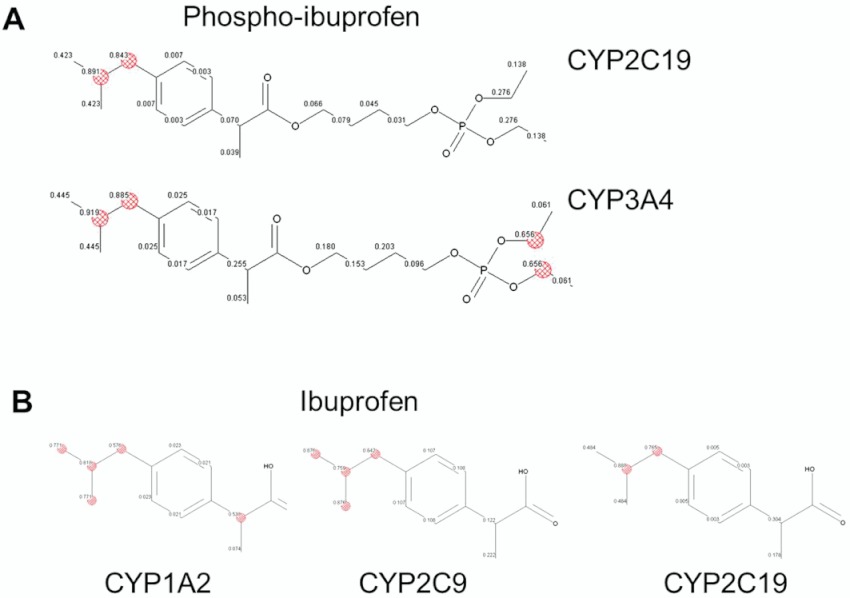
In silico prediction of drug metabolism by CYP isoforms using ADMET modelling. A. Phospho-ibuprofen (PI) is predicted to be oxidized by CYP2C19 and 3A4. The scores ranging from 0 to 1 indicate the possibility of being a metabolic site, and the highest scoring atom is highlighted with a red hashed circle. B. Ibuprofen is predicted to be oxidized by CYP1A2, 2C9 and 2C19.
HPLC-UV analysis
The HPLC system comprised a Waters Alliance 2695 Separations Module equipped with a Waters 2998 photodiode array detector (220 and 328 nm) and a Thermo BDS Hypersil C18 column (150 × 4.6 mm, particle size 3 µm). The mobile phase consisted of a gradient between aqueous solvent A [trifluroacetic acid, acetonitrile, H2O (0.1:4.9:95 v/v/v)] and organic solvent B (acetonitrile) at a flow rate of 1 mL·min−1 at 30°C. We applied gradient elution from 0% to 100% B from 0 to 15 min, and it was maintained at 100% B until 18 min.
Isolation and LC-MS/MS analysis of HPLC peaks
The HPLC peaks corresponding to 3-OH-phospho-ibuprofen and α-OH-phospho-ibuprofen were collected, concentrated under vacuum and subjected to LC-MS/MS analysis. The LC-MS/MS system consisted of Thermo TSQ Quantum Access (Thermo-Fisher, San Jose, CA, USA) triple quadrupole mass spectrometer interfaced by an electrospray ionization probe with an Ultimate 3000 HPLC system (Dionex Corporation, Sunnyvale, CA). Chromatographic separations were achieved on a Luna C18 column (150 × 2 mm), and the mobile phase consisted of a gradient from 10% to 95% acetonitrile.
Oxidation of phospho-NSAIDs and NSAIDs by human CYP and FMO isoforms
Phospho-NSAIDs or conventional NSAIDs (150 µM for phospho-ibuprofen or ibuprofen; 100 µM for phospho-sulindac or sulindac) were pre-incubated at 37°C for 5 min with an NADPH-regenerating solution (1.3 mM NADP, 3.3 mM d-glucose 6-phosphate, 3.3 mM MgCl2 and 0.4 U·mL−1 glucose-6-phosphate dehydrogenase) in 0.1 M potassium phosphate buffer (pH 7.4). Reaction was initiated by the addition of individual recombinant human CYP isoforms (25 pmol·mL−1) or human FMO isoforms (0.125 mg protein mL−1) in a total volume of 1 mL, and samples were maintained at 37°C for various time periods. At each designated time point, an aliquot was mixed with twofold volume of acetonitrile, vortexed and then centrifuged for 10 min at 13 000 ×g. The supernatants were analysed by HPLC.
Oxidation of phospho-NSAIDs and NSAIDs by BNPP-treated human and mouse liver microsomes
Human or mouse liver microsomes were pre-incubated with BNPP (final 250 µM) at 37°C for 15 min and were subsequently treated with phospho-NSAIDs or conventional NSAIDs (150 µM for phospho-ibuprofen or ibuprofen; 100 µM for phospho-sulindac or sulindac) at 37°C for up to 9 h with NADPH-regenerating solution in 0.1 M potassium phosphate buffer (pH 7.4) (final 0.5 mg protein mL-1 of liver microsomes) in a total volume of 1 mL. The oxidized products were extracted at various time points and assayed by HPLC.
Determination of reactive oxygen species (ROS)
ROS assay kit (Cell Biolabs, Inc. San Diego, CA) was used in this study according to the protocol provided by the manufacturer. Briefly, equimolar phospho-ibuprofen or ibuprofen at 150 µM was incubated with CYP3A4 (25 pmol·mL−1) or BNPP-treated HLM (0.5 mg protein mL−1) for 1 h, and 50 µL of sample was incubated with 150 µL of dichlorodihydrofluorescein solution for 30 min at room temperature in a black 96-well plate, and the fluorescence of the mixtures was determined on a fluorometric plate reader at 480 nm/530 nm.
Data analysis
Data are shown as means ± SEM unless otherwise indicated. Results were analysed using the Student's t-test; P≤ 0.05 was considered statistically significant.
Materials
Phospho-ibuprofen and phospho-sulindac were provided by Medicon Pharmaceuticals, Inc (Stony Brook, NY). Ibuprofen, 3-OH-ibuprofen, bis(4-nitro phenyl)-phosphate (BNPP), sulindac, porcine liver esterase and acetonitrile were purchased from Sigma-Aldrich (St. Louis, MO). 2-OH-ibuprofen was purchased from US Biological (Swampscott, MA). α-OH-ibuprofen, ketoconazole and quinidine were purchased from Toronto Research Chemicals (North York, ON, Canada). cDNA-expressed human CYPs (CYP1A2, 2C9, 2C19, 2D6 and 3A4) and FMOs (FMO1, FMO3 and FMO5), human and mouse liver microsomes, and NADPH regenerating solution were purchased from BD Biosciences (San Jose, CA).
Results
In silico prediction of the oxidation of phospho-NSAIDs and NSAIDs by CYPs
Predictions of the metabolism of phospho-NSAIDs by the major human CYP isoforms (CYP1A2, 2C9, 2C19, 2D6 and 3A4) were obtained using the ADMET Predictor. It was predicted that phospho-NSAIDs and conventional NSAIDs would be oxidized by distinct CYPs. Thus, 2C19 and 3A4 would oxidize phospho-ibuprofen; while 1A2, 2C9 and 2C19 oxidize ibuprofen (Figure 2). 3A4 would oxidize phospho-sulindac, while 1A2 and 2C9 would oxidize sulindac (Table 1). Moreover, the isobutyl group of phospho-ibuprofen or ibuprofen, as well as the sulfoxide group of phospho-sulindac or sulindac were predicted to be the most likely sites of oxidation by CYPs, which we have independently demonstrated (Xie et al., 2011; 2012).
Table 1.
Lipophilicity (LogP), modelling prediction and experimental results on the metabolism of phospho-ibuprofen, phospho-sulindac and the parent NSAIDs by human CYPs
| Human CYP isoforms | |||
|---|---|---|---|
| Compound | LogP | Prediction | Experimental |
| Phospho-ibuprofen | 5.4 | 2C19, 3A4 | 1A2, 2C19, 2D6, 3A4 |
| Ibuprofen | 3.8 | 1A2, 2C9, 2C19 | 2C9 |
| Phospho-sulindac | 3.9 | 3A4 | 1A2, 2C9, 2C19, 2D6, 3A4 |
| Sulindac | 2.3 | 1A2, 2C9 | None |
LogP (P is octanol–water partition coefficient) is determined using ChemDraw 7.0.
Regioselective oxidation of phospho-ibuprofen and ibuprofen by CYPs
To evaluate the in silico predictions experimentally, we examined the metabolism of phospho-ibuprofen and ibuprofen by the recombinant major human CYPs. As shown in Figure 3A, CYP1A2, 2C19, 2D6 and 3A4 catalyzed the oxidation of phospho-ibuprofen, with 3A4 being the most active. In contrast, CYP2C9 was inactive towards phospho-ibuprofen (data not shown). Interestingly, the four CYPs oxidized phospho-ibuprofen with differential regioselectivity. CYP1A2 oxidized phospho-ibuprofen primarily at the 1-position of the isobutyl group to produce 1-OH-phospho-ibuprofen, whereas 2C19 and 2D6 oxidized this substrate exclusively at the 3-position to form 3-OH-phospho-ibuprofen. On the other hand, 3A4 generated both 1-OH- and 3-OH-phospho-ibuprofen at similar rates and uniquely oxidized phospho-ibuprofen at the α-position of the ibuprofen moiety to give α-OH-phospho-ibuprofen.
Figure 3.
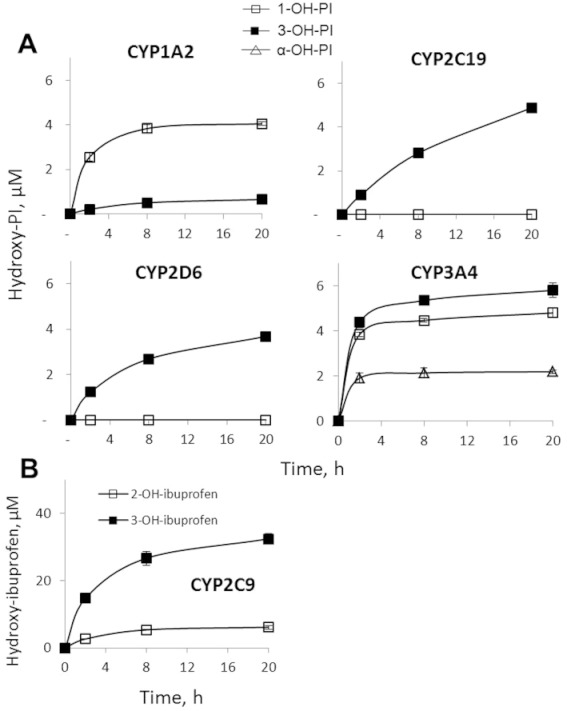
Kinetics of the oxidation of phospho-ibuprofen (PI) or ibuprofen by CYPs. Equimolar phospho-ibuprofen (A) or ibuprofen (B) at 150 µM was incubated with individual human CYP isoforms and NADPH-regenerating solution at 37°C for up to 20 h. The oxidized products were extracted at the designated time points and assayed by HPLC. Values are mean ± SEM.
CYP3A4 also displayed a unique kinetic behaviour: all the three products reached their peak levels rapidly within ∼2 h and plateaued afterwards (Figure 3A). In contrast, the other three CYPs (1A2, 2C19 and 2D6) exhibited similar kinetic patterns; the levels of their products gradually increased in a time-dependent manner during the entire period of observation.
3-OH-phospho-ibuprofen and α-OH-phospho-ibuprofen were identified by LC-MS/MS analysis. The mass spectrum of the 3-OH-metabolite showed a [M + Na]+ ion at m/z 453.0, which was fragmented to yield ions at m/z 153 and 317 (Figure 4A). Similarly, the mass spectrum of α-OH-phospho-ibuprofen showed a [M + Na]+ ion at m/z 453.0, which was fragmented to produce m/z at 177 and 249 (Figure 4B). The position of the – OH group of the 3-OH- and the α-OH-phospho-ibuprofen was determined by treating them with esterases from porcine liver, which completely hydrolysed these metabolites to release 3-OH-ibuprofen and α-OH-ibuprofen, respectively.
Figure 4.
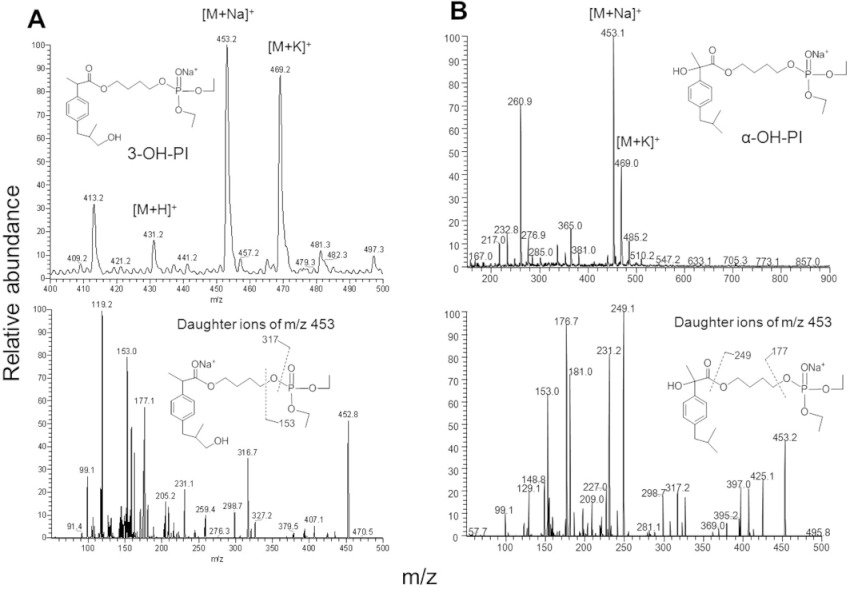
Identification of the metabolites of phospho-ibuprofen (PI) by CYPs. A. MS and MS/MS spectrum of 3-OH-PI fraction collected from HPLC. B. MS and MS/MS spectrum of α-OH-PI fraction collected from HPLC.
We have also examined the oxidation of ibuprofen by individual CYPs. CYP2C9 was highly active towards ibuprofen, generating 2-OH-ibuprofen and 3-OH-ibuprofen (Figure 3B); this regioselectivity was distinct from that of phospho-ibuprofen. In contrast to CYP2C9, none of the other four CYPs appreciably oxidized ibuprofen (data not shown). Thus, the selectivity of CYPs for phospho-ibuprofen and ibuprofen are strikingly different: CYP2C9 exclusively oxidizes ibuprofen, while the other four CYPs exclusively oxidize phospho-ibuprofen. This finding unambiguously explains our previous observation that phospho-ibuprofen and ibuprofen were oxidized with differential regioselectivity (Figure 1A).
Oxidation of phospho-sulindac and sulindac by CYPs and FMOs
We also evaluated the oxidation of phospho-sulindac and sulindac by human CYPs. CYP2D6 was by far the most active in oxidizing phospho-sulindac to form phospho-sulindac sulfone (Figure 5). In comparison, all the other four CYPs modestly oxidized phospho-sulindac with quite low levels of phospho-sulindac sulfone being detected. The metabolism of phospho-sulindac was not a result of non-enzymatic oxidation, as no phospho-sulindac sulfone could be detected in a negative control reaction without NADPH. All five CYPs tested failed to appreciably oxidize sulindac (data not shown). This indicated that phospho-sulindac, but not sulindac, was a substrate for CYPs.
Figure 5.
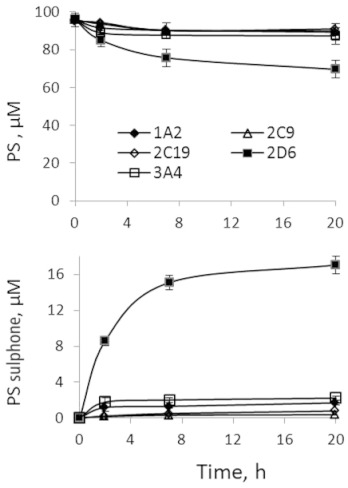
Kinetics of the oxidation of phospho-sulindac (PS) by CYPs. Phospho-sulindac at 100 µM was incubated with individual human CYP isoforms and NADPH-regenerating solution at 37°C for up to 20 h. The oxidized products were extracted at the designated time points and analysed by HPLC. Values are mean ± SEM.
We next evaluated the oxidation of phospho-sulindac and sulindac by major human FMO isoforms (FMO1, FMO3 and FMO5). All the FMOs oxidized phospho-sulindac far more rapidly and extensively than sulindac (Figure 6), indicating that phospho-sulindac was a preferred substrate of FMOs. On the other hand, FMOs were inactive towards phospho-ibuprofen or ibuprofen (data not shown). Thus, phospho-sulindac was oxidized by both CYPs and FMOs, while phospho-ibuprofen was oxidized exclusively by CYPs.
Figure 6.
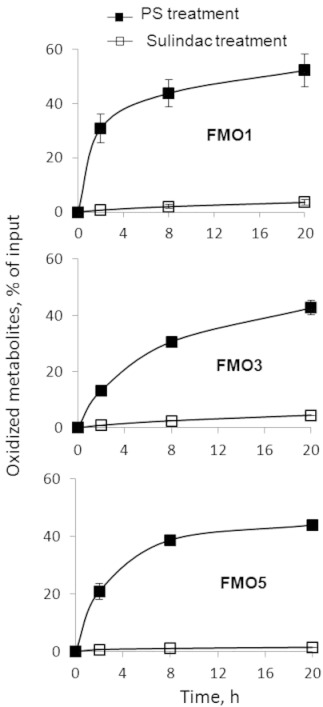
Kinetics of the oxidation of phospho-sulindac (PS) or sulindac by FMOs. Equimolar phospho-sulindac or sulindac at 100 µM was incubated with individual human FMOs (FMO1, FMO3 and FMO5) and NADPH-regenerating solution at 37°C for up to 20 h. The oxidized products were extracted at the designated time points and analysed by HPLC. Results were expressed as % of input drug. Values are mean ± SEM.
Comparison of the oxidation of phospho-ibuprofen and ibuprofen by liver microsomes
We next compared the oxidation of phospho-ibuprofen and ibuprofen by human liver microsomes (HLM), which contain a full complement of CYPs. Carboxylesterases are abundant in HLM, leading to significant hydrolysis of phospho-ibuprofen (Xie et al., 2011). To inhibit the hydrolysis of phospho-ibuprofen, we pre-treated HLM with BNPP, a non-specific esterase inhibitor, and then evaluated the oxidation of phospho-ibuprofen or ibuprofen by the BNPP-treated HLM (Figure 7A). Half an hour after the initiation of the reaction, 2% of ibuprofen and 20% of phospho-ibuprofen were oxidized, indicating that phospho-ibuprofen was more rapidly and extensively oxidized by HLM than ibuprofen.
Figure 7.
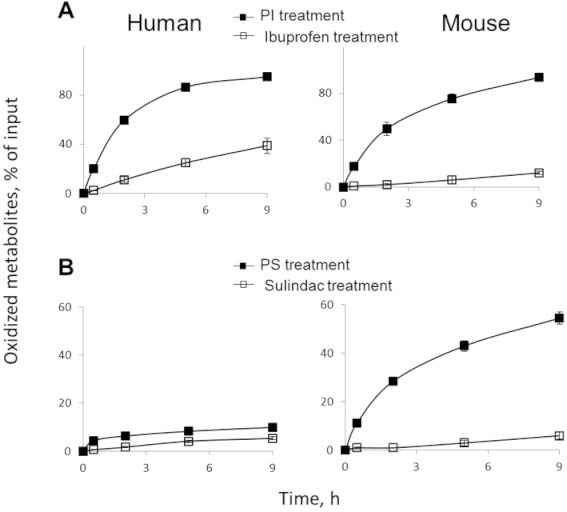
Kinetics of the oxidation of phospho-NSAIDs or NSAIDs by BNPP-treated human and mouse liver microsomes. Human or mouse liver microsomes were pre-incubated with BNPP (final 250 µM) at 37°C for 15 min and subsequently treated with phospho-ibuprofen (PI) or ibuprofen (A) at 150 µM or phospho-sulindac (PS) or sulindac (B) at 100 µM at 37°C for up to 9 h. The oxidized products extracted at the designated time points and assayed by HPLC. Results were expressed as % of input drug. Values are mean ± SEM.
Our data in Figure 3A suggested that CYP3A4, the most abundant isoform in human liver, played a major role in the oxidation of phospho-ibuprofen. To test this, we assessed the effect of ketoconazole (CYP3A4 inhibitor) and quinidine (CYP2D6 inhibitor) on the oxidation of phospho-ibuprofen. We observed that ketoconazole (0.5 µM) completely inhibited CYP3A4 activity and quinidine (10 µM) inhibited CYP2D6 activity by 90%. We then evaluated the effects of these inhibitors on the oxidation of phospho-ibuprofen by BNPP-treated HLM. Ketoconazole (0.5 µM) inhibited the oxidation of phospho-ibuprofen in HLM by 84%, whereas quinidine (10 µM) had no appreciable effect on phospho-ibuprofen oxidation. This result indicated that CYP3A4 plays a major role in the oxidation of phospho-ibuprofen by HLM.
We have recently determined the pharmacokinetic parameters of phospho-ibuprofen and ibuprofen in mice, but mice differ from humans in composition and expression of CYP isoforms (Martignoni et al., 2006). Therefore, we also compared the oxidation of phospho-ibuprofen and ibuprofen by BNPP-treated mouse liver microsomes (MLM) (Figure 7A). Phospho-ibuprofen was far more rapidly oxidized by the MLM than ibuprofen; the oxidation of phospho-ibuprofen was essentially complete after a 9 h incubation, while only 12% of ibuprofen was oxidized over the same time period (Figure 7A).
Comparison of the oxidation of phospho-sulindac and sulindac by liver microsomes
We next examined and compared the oxidation of phospho-sulindac and sulindac by BNPP-treated liver microsomes, which contain both CYPs and FMOs. Phospho-sulindac was oxidized more rapidly than sulindac by HLM and to a much larger extent by MLM (Figure 7B). These data further substantiate our findings that phospho-sulindac is more susceptible to CYP/FMO-mediated oxidation than sulindac, leading to enhanced clearance in vivo.
As both CYPs and FMOs can oxidize phospho-sulindac, we determined which enzyme primarily oxidizes phospho-sulindac in HLM. The activities of CYPs, but not of FMOs, are abolished at alkaline pH (Cashman, 2005). We observed complete inhibition of CYPs at pH 9.5, while the activities of FMOs were essentially the same at pH 7.4 and pH 9.5 (data not shown). We next compared the oxidation of phospho-NSAIDs by HLM at pH 7.4 and pH 9.5. HLM was inactive towards phospho-ibuprofen at pH 9.5, supporting the notion that CYPs exclusively oxidize phospho-ibuprofen. On the other hand, the activity of HLM towards phospho-sulindac was reduced by 88% at pH 9.5 compared with pH 7.4, suggesting that CYPs, rather than FMOs, predominantly oxidize phospho-sulindac in HLM.
Induction of ROS by phospho-ibuprofen or ibuprofen
As CYP-mediated oxidation of various substrates can generate ROS (Puntarulo and Cederbaum, 1998), we measured and compared the ROS level generated by the CYP-mediated oxidation of phospho-ibuprofen and ibuprofen. Equimolar concentrations of phospho-ibuprofen or ibuprofen were incubated with CYP3A4 for 1 h, and ROS were determined using dichlorodihydrofluorescein, a general ROS probe. Ibuprofen generated a negligible level of ROS during its incubation with CYP3A4, whereas phospho-ibuprofen markedly increased ROS levels during CYP3A4-mediated oxidation, with their difference being statistically significant (P < 0.01) (Figure 8A). Likewise, ibuprofen generated a minimal level of ROS in BNPP-treated HLM, whereas phospho-ibuprofen greatly enhanced the ROS level, with their difference being statistically significant (P < 0.01) (Figure 8B).
Figure 8.
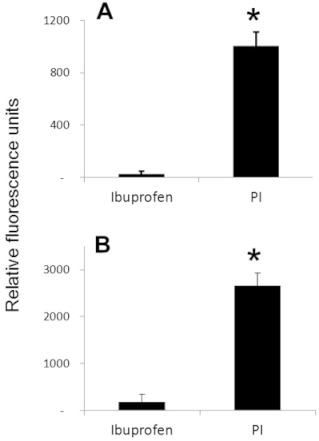
Induction of ROS by phospho-ibuprofen (PI) or ibuprofen. Equimolar phospho-ibuprofen or ibuprofen at 150 µM was incubated with CYP3A4 (A) or BNPP-treated HLM (B) for 1 h, and ROS were determined as described in Methods. Values are mean ± SEM. *, P < 0.01.
Discussion and conclusions
Here, we report the unique activity and regioselectivity of the oxidation of phospho-NSAIDs by human CYPs and FMOs. Our data establish that these enzymes are highly active towards phospho-NSAIDs compared with their parent NSAIDs, leading to the rapid oxidation of phospho-NSAIDs by liver microsomes. The CYP/FMO-mediated clearance of phospho-NSAIDs results in their unique pharmacokinetic profiles, which potentially contribute to their improved safety.
Phospho-NSAIDs were preferentially oxidized by human CYPs compared to conventional NSAIDs. Phospho-ibuprofen was oxidized by CYP1A2, 2C9, 2D6 and 3A4; while phospho-sulindac was a substrate for all five CYPs. On the contrary, the CYPs tested did not appreciably catalyze the oxidation of ibuprofen and sulindac except for CYP2C9, which specifically oxidizes ibuprofen. With the use of the in silico modelling program, we were able to identify the potential site(s) of attack by CYPs. However, the in silico predictions tend to underestimate the range of CYP isoforms that can act on phospho-sulindac or phospho-ibuprofen. The disparity may arise from the fact that in silico prediction is based upon an existing substrate database, which may not be sufficiently comprehensive to take into account the structural features of the novel phospho-NSAIDs.
The interaction of CYPs with drugs depends on several factors, such as molecular size, lipophilicity (LogP) and ionization constant (pKa) (Lewis, 2000). Substrate lipophilicity is highly correlated with their binding affinity with CYPs (Lewis et al., 2004), as the lower desolvation energy of lipophilic substrates contributes to their high binding affinity to CYPs (Jacobsen et al., 2000). Phospho-ibuprofen and phospho-sulindac are more lipophilic than ibuprofen and sulindac, respectively, as shown by their higher LogP values (Table 1).
Another critical factor to their binding by CYPs is the altered pKa of phospho-NSAIDs. The major human CYPs, 1A2, 2C9, 2D6 and 3A4, all prefer neutral or basic substrates (e.g. phospho-ibuprofen and phospho-sulindac). An exception is CYP2C9, which primarily oxidizes acidic or anionic substrates such as ibuprofen, with its positively charged Arg108 residue contributing to high-affinity binding (Dickmann et al., 2004; Locuson et al., 2004). Thus, the phospho-modification of conventional NSAIDs at their – COOH group enhances lipophilicity and masks their acid group, thus promoting their binding by CYPs.
CYP3A4 displays three unique features in the oxidation of phospho-ibuprofen. First, CYP3A4 displays a broad regioselectivity, uniquely generating all the three hydroxy-phospho-ibuprofen metabolites (1-OH-, 3-OH- and α-OH-phospho-ibuprofen); whereas other CYPs generate either the 1-OH- or 3-OH-metabolite. Second, CYP3A4 generated the highest levels of each product during the entire period of observation. Third, ketoconazole, the CYP3A4 inhibitor, inhibited the oxidation of phospho-ibuprofen by 84%. Together, these observations suggest that CYP3A4, the most abundant isoform in human liver, is the major isoform contributing to the oxidation of phospho-ibuprofen. Indeed, CYP3A4 accounts for 45–60% of the metabolism of all drugs currently used (Hustert et al., 2001). Substrate binding to CYP3A4 induces large increase in the volume of its active site (Ekroos and Sjogren, 2006), which could account for its preference for bulky substrates such as phospho-ibuprofen. In addition, the structure of CYP3A4–ligand complex is remarkably flexible (Ekroos and Sjogren, 2006; Bonn et al., 2010), which may lead to its broad regioselectivity in phospho-ibuprofen oxidation.
Among the CYPs, CYP2D6 is the most active in mediating the oxidation of phospho-sulindac. The CYP2D6 substrate binding uniquely relies on an ion pair between the basic moiety (e.g. amine group) of the substrates and the negatively charged residues of CYP2D6 including Asp301 (Ellis et al., 1995) and Glu216 (Guengerich et al., 2003). It is probable that the partially positively charged sulfur and phosphorus atoms of phospho-sulindac may interact with the Asp301 and Glu216 residues of CYP2D6, thereby facilitating its binding and favourable orientation in the active site.
FMOs oxidized phospho-sulindac and sulindac, but not phospho-ibuprofen or ibuprofen. This result was entirely consistent with the notion that FMOs act on substrates containing soft nucleophiles such as nitrogen and sulfur. FMOs oxidized phospho-sulindac far more rapidly than sulindac. Unlike the case of CYPs, substrate binding does not affect the reaction rate of FMO-mediated oxidation (Krueger and Williams, 2005). The difference in the oxidation rate of phospho-sulindac and sulindac by FMOs can be explained by two reasons. First, compounds containing a negative charge (e.g. sulindac) are generally poor substrates of FMOs (Krueger and Williams, 2005). Second, phospho-sulindac is much larger than sulindac (Figure 1B), and the rate of FMO-mediated reactions increases with increasing size of the substrate (Nagata et al., 1990).
In agreement with the CYP activity profiles, liver microsomes oxidized phospho-NSAIDs more rapidly than their corresponding parent NSAIDs. However, we observed significant differences in their metabolism between mouse and human liver microsomes. While phospho-ibuprofen was oxidized at similar rates in HLM and MLM, ibuprofen was oxidized much more rapidly in HLM. This occurs probably because CYP2C9, the isoform efficiently oxidizing ibuprofen, is abundant in humans but absent in mice (Martignoni et al., 2006). While sulindac was oxidized at similar rates in HLM and MLM, phospho-sulindac was oxidized more rapidly in MLM. This is not surprising since in humans only CYP2D6 can significantly oxidize phospho-sulindac. Whether other CYP isoforms in mouse can efficiently oxidize phospho-sulindac remains to be determined.
The rapid and extensive oxidation of phospho-NSAIDs by liver microsomes has important implications for their distinct pharmacokinetic properties and safety profiles in animal models. The rapid clearance of phospho-NSAIDs by CYPs/FMOs leads to their lower peak concentration (Cmax), shorter elimination half-life (t1/2) and lower AUC0–24 h compared with those of their parent compounds (Table 2). Thus, phospho-NSAIDs reduce the systemic exposure of drugs in blood, which is a favourable factor in terms of safety. It is conceivable that phospho-NSAIDs may also be metabolized and eliminated rapidly in humans, leading to their better safety profile. In the case of phospho-sulindac, preferential oxidation to form sulindac sulfone as the major metabolite, rather than reduction to form sulindac sulfide, further contributes to its improved safety. Sulindac sulfone does not inhibit COX activity and is therefore safer than sulindac sulfide (Glavin and Sitar, 1986; Piazza et al., 2009). On the other hand, the rapid clearance of phospho-NSAIDs in vivo may affect drug efficacy, which could be addressed by optimizing the dosing regimen. Importantly, we have demonstrated that phospho-ibuprofen (Xie et al., 2011) and phospho-sulindac (Mackenzie et al., 2010) are more efficacious in cancer treatment in mice than ibuprofen and sulindac respectively.
Table 2.
Pharmacokinetic parameters of phospho-ibuprofen, phospho-sulindac and their parent compounds
| Drug administered | Dose (mmol kg−1) | Cmax (µM) | Elimination t1/2 (h) | AUC0-24h(µM h) | Reference |
|---|---|---|---|---|---|
| Phospho-ibuprofen | 1.0 | 530 | 7.7 | 1816 | Xie et al. (2011) |
| Ibuprofen | 1.0 | 932 | 12.8 | 4146 | |
| Phospho-sulindac | 0.28 | 234 | 3.5 | 707 | Xie et al. (2012) |
| Sulindac | 0.28 | 261 | 5.4 | 1476 |
Phospho-NSAIDs inhibit cancer cell growth much more potently than their parent compounds (Xie et al., 2011). A unique feature of phospho-NSAID-induced apoptosis in cancer cells is a significant elevation of intracellular ROS levels (Sun et al., 2011). This may be explained on the basis of their greater susceptibility to oxidation by CYPs/FMOs. It has been shown that CYPs (Puntarulo and Cederbaum, 1998) and FMOs (Krueger and Williams, 2005) generate significant ROS levels while oxidizing various substrates. Indeed, phospho-ibuprofen (in contrast to ibuprofen) generated significant levels of ROS during its oxidation by CYP3A4 or HLM. It is thus conceivable that ROS generation via CYP/FMO-mediated oxidation of phospho-NSAIDs may potentially contribute to excess oxidative stress, leading to apoptosis and inhibition of tumour growth.
In conclusion, phospho-NSAIDs are preferred substrates of CYPs/FMOs, leading to their rapid oxidation by liver microsomes. These findings largely explain the characteristic pharmacokinetic features of phospho-NSAIDs that may account for their improved safety.
Acknowledgments
This work was supported by the National Institute of Health Grant R01CA139454 and Department of Defense grant W81XWH1010873. We thank R Rieger and T Koller, Stony Brook University, for the expert LC-MS/MS analysis of our samples and the shared instrumentation grant NIH/NCRR 1 S10 RR023680-1.
Glossary
- BNPP
bis(4-nitro phenyl)-phosphate
- CYP
cytochrome P450
- FMO
flavin monooxygenases
- HLM
human liver microsomes
- MLM
mouse liver microsomes
- NSAIDs
non-steroidal anti-inflammatory drugs
- ROS
reactive oxygen species
Conflicts of interest
The authors have nothing to disclose except for BR, who has an equity position in Medicon Pharmaceuticals, Inc.
References
- Bonn B, Masimirembwa CM, Castagnoli N. Exploration of catalytic properties of CYP2D6 and CYP3A4 through metabolic studies of levorphanol and levallorphan. Drug Metab Dispos. 2010;38:187–199. doi: 10.1124/dmd.109.028670. [DOI] [PubMed] [Google Scholar]
- Cashman JR. Some distinctions between flavin-containing and cytochrome P450 monooxygenases. Biochem Biophys Res Commun. 2005;338:599–604. doi: 10.1016/j.bbrc.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Dickmann LJ, Locuson CW, Jones JP, Rettie AE. Differential roles of Arg97, Asp293, and Arg108 in enzyme stability and substrate specificity of CYP2C9. Mol Pharmacol. 2004;65:842–850. doi: 10.1124/mol.65.4.842. [DOI] [PubMed] [Google Scholar]
- Ekroos M, Sjogren T. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc Natl Acad Sci USA. 2006;103:13682–13687. doi: 10.1073/pnas.0603236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SW, Hayhurst GP, Smith G, Lightfoot T, Wong MM, Simula AP, et al. Evidence that aspartic acid 301 is a critical substrate-contact residue in the active site of cytochrome P450 2D6. J Biol Chem. 1995;270:29055–29058. doi: 10.1074/jbc.270.49.29055. [DOI] [PubMed] [Google Scholar]
- Glavin GB, Sitar DS. The effects of sulindac and its metabolites on acute stress-induced gastric ulcers in rats. Toxicol Appl Pharmacol. 1986;83:386–389. doi: 10.1016/0041-008x(86)90315-7. [DOI] [PubMed] [Google Scholar]
- Gravitz L. Chemoprevention: first line of defence. Nature. 2011;471:S5–S7. doi: 10.1038/471S5a. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Hanna IH, Martin MV, Gillam EM. Role of glutamic acid 216 in cytochrome P450 2D6 substrate binding and catalysis. Biochemistry. 2003;42:1245–1253. doi: 10.1021/bi027085w. [DOI] [PubMed] [Google Scholar]
- Huang L, Zhu C, Sun Y, Xie G, Mackenzie GG, Qiao G, et al. Phospho-sulindac (OXT-922) inhibits the growth of human colon cancer cell lines: a redox/polyamine-dependent effect. Carcinogenesis. 2010;31:1982–1990. doi: 10.1093/carcin/bgq149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Mackenzie G, Ouyang N, Sun Y, Xie G, Johnson F, et al. The novel phospho-non-steroidal anti-inflammatory drugs, OXT-328, MDC-22 and MDC-917, inhibit adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;162:1521–1533. doi: 10.1111/j.1476-5381.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustert E, Zibat A, Presecan-Siedel E, Eiselt R, Mueller R, Fuss C, et al. Natural protein variants of pregnane X receptor with altered transactivation activity toward CYP3A4. Drug Metab Dispos. 2001;29:1454–1459. [PubMed] [Google Scholar]
- Jacobsen W, Kuhn B, Soldner A, Kirchner G, Sewing KF, Kollman PA, et al. Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab Dispos. 2000;28:1369–1378. [PubMed] [Google Scholar]
- Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Ther. 2005;106:357–387. doi: 10.1016/j.pharmthera.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb DC, Waterman MR, Kelly SL, Guengerich FP. Cytochromes P450 and drug discovery. Curr Opin Biotechnol. 2007;18:504–512. doi: 10.1016/j.copbio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Lewis DF. On the recognition of mammalian microsomal cytochrome P450 substrates and their characteristics: towards the prediction of human p450 substrate specificity and metabolism. Biochem Pharmacol. 2000;60:293–306. doi: 10.1016/s0006-2952(00)00335-x. [DOI] [PubMed] [Google Scholar]
- Lewis DF, Jacobs MN, Dickins M. Compound lipophilicity for substrate binding to human P450s in drug metabolism. Drug Discov Today. 2004;9:530–537. doi: 10.1016/S1359-6446(04)03115-0. [DOI] [PubMed] [Google Scholar]
- Locuson CW, 2nd, Rock DA, Jones JP. Quantitative binding models for CYP2C9 based on benzbromarone analogues. Biochemistry. 2004;43:6948–6958. doi: 10.1021/bi049651o. [DOI] [PubMed] [Google Scholar]
- Mackenzie GG, Sun Y, Huang L, Xie G, Ouyang N, Gupta RC, et al. Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology. 2010;139:1320–1332. doi: 10.1053/j.gastro.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martignoni M, Groothuis GM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- Nagata T, Williams DE, Ziegler DM. Substrate specificities of rabbit lung and porcine liver flavin-containing monooxygenases: differences due to substrate size. Chem Res Toxicol. 1990;3:372–376. doi: 10.1021/tx00016a016. [DOI] [PubMed] [Google Scholar]
- Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila Pa) 2009;2:572–580. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med. 1998;24:1324–1330. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33:224–234. doi: 10.1007/s10753-009-9176-5. [DOI] [PubMed] [Google Scholar]
- Sun Y, Huang L, Mackenzie GG, Rigas B. Oxidative stress mediates through apoptosis the anticancer effect of phospho-nonsteroidal anti-inflammatory drugs: implications for the role of oxidative stress in the action of anticancer agents. J Pharmacol Exp Ther. 2011;338:775–783. doi: 10.1124/jpet.111.183533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney BP, Bromilow J. Liver enzyme induction and inhibition: implications for anaesthesia. Anaesthesia. 2006;61:159–177. doi: 10.1111/j.1365-2044.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- Vonkeman HE, van de Laar MA. Nonsteroidal anti-inflammatory drugs: adverse effects and their prevention. Semin Arthritis Rheum. 2010;39:294–312. doi: 10.1016/j.semarthrit.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Xie G, Sun Y, Nie T, Mackenzie GG, Huang L, Kopelovich L, et al. Phospho-ibuprofen (MDC-917) is a novel agent against colon cancer: efficacy, metabolism, and pharmacokinetics in mouse models. J Pharmacol Exp Ther. 2011;337:876–886. doi: 10.1124/jpet.111.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Nie T, Mackenzie G, Sun Y, Huang L, Ouyang N, et al. The metabolism and pharmacokinetics of phospho-sulindac (OXT-328) and the effect of difluoromethylornithine. Br J Pharmacol. 2012;165:2152–2166. doi: 10.1111/j.1476-5381.2011.01705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


