Abstract
Objective
To estimate the prevalence of Bartholin gland cysts in asymptomatic women serving as control participants who underwent pelvic magnetic resonance imaging (MRI) as part of research studies. The secondary aim was to investigate potential demographic characteristics associated with Bartholin gland cysts.
Methods
Pelvic MRIs from 430 control participants enrolled in five research projects were evaluated. All images were evaluated by at least two authors. The presence, laterality, and size of Bartholin gland cysts were recorded. Demographic information for each participant was obtained at the time of enrollment in the respective parent study.
Results
Approximately 3% of the participants had visible Bartholin gland cysts in MRI scans. Fifty-percent of the cysts were identified on the right side, 42.9% were seen on the left side, and 7.1% were bilateral. The cysts were, on average, 1.3 × 1.2 × 1.3 cm, with dimensions ranging from 0.5 – 2.7 cm. There were no demographic differences between women with and without visible Bartholin gland cysts.
Conclusion
Bartholin gland cysts occur in 3% of adult women. The cysts affect women of broad ranges of age and parity. Women with visible Bartholin gland cysts are demographically similar to women without cysts on pelvic imaging.
INTRODUCTION
The Bartholin, or greater vestibular, glands are a pair of mucus-secreting glands in the vulvar vestibule. They are located slightly lateral to and below the vaginal introitus.1 The glands are approximately 1 cm in diameter and drain through a narrow duct that is approximately 2.5 cm in length.2 It is felt that the function of these glands is to provide lubrication for the vulva, particularly during sexual intercourse.3
Blockage of the ducts draining the Bartholin glands can lead to the development of cystic masses. Bartholin gland cysts result in 2% of annual gynecologic visits.1 The prevalence of these cysts is similarly reported at 2%, although the source of this figure is not well known.4 The goal of this study is to therefore estimate the frequency of Bartholin gland cysts seen in a population of healthy volunteers. We secondarily sought to investigate potential demographic characteristics associated with Bartholin gland cysts.
MATERIALS AND METHODS
This is a secondary analysis of five University of Michigan Medical School Institutional Review Board-approved case-control studies of pelvic floor function (IRBMED 2001–0475, HUM00043445, HUM00043944, HUM00043876 and HUM00042901). The participants included in this analysis were all healthy, asymptomatic volunteers serving as control participants and were recruited through community advertisements (newspaper advertisements and posters) in the Ann Arbor, Michigan area. Participants were recruited to be of similar age, race, parity and hysterectomy status (when appropriate) as cases from the respective studies. All participants were informed during the recruitment process that they would be paid for participating in the studies, including completion of questionnaires and undergoing pelvic magnetic resonance imaging (MRI).
Full details of the MRI acquisitions have been previously published. Briefly, multiplanar two-dimensional proton-density fast-spin images were obtained with an echo time of 15 ms and a repetition time of 4 seconds using a 1.5 or 3 T superconducting magnet. The slice thicknesses were 4 mm, with slice spacing of 1 mm.5–7
All participants for whom digital MRIs were available were included in this analysis. Demographics were self-reported by the participants. MR scans were assessed by three authors (M.B.B., N.K., and C.B.) to identify visible Bartholin gland cysts. Images were viewed in the axial, coronal and sagittal planes to verify the presence of cysts (Figures 1a, 1b and 1c). Data were collected on laterality, size, and appearance of the cysts. All measurements were made using ImageJ 1.42q software (National Institutes of Health, Bethesda, MD, USA). Images from all participants found to have Bartholin gland cysts were reviewed by two authors (M.B.B. and C.B.) to ensure agreement about the presence and/or characteristics of the cysts. In the event of discrepancy of opinion, final adjudication was made by the review of the images with an expert on pelvic imaging (J.O.D.). The average value of measurements made for the visible Bartholin gland cysts were used for analyses.
Figure 1.
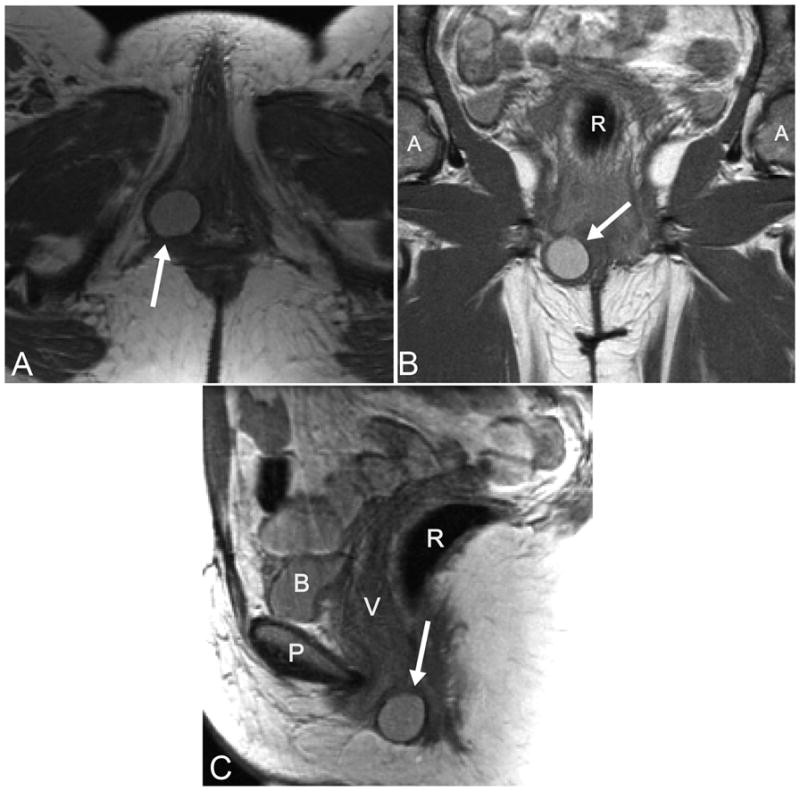
A: Axial slice of a proton-density magnetic resonance imaging (MRI) scan demonstrating a Bartholin gland cyst (arrow).B: Coronal slice of an MRI scan with a visible Bartholin gland cyst (arrow). C: Sagittal slice of an MRI scan with a Bartholin gland cyst identified (arrow). R, rectum; A, acetabulum; P, pubic symphysis; B, bladder; V, vagina.
Continuous variables were compared using Mann-Whitney U-tests, categorical variables with chi-squared or Fisher’s exact tests. PASW version 18.0 (IBM Corporation, Armonk, NY, USA) was used for statistical analyses. P values < 0.05 were considered significant.
RESULTS
430 participants were included in this analysis. The median age was 50.0 years with an interquartile range 37.0–60.0 and total range 20.0–90.0. Median parity was 2 with interquartile range 0–3, total range 0–8. 50.9% of the participants were postmenopausal. 86.7% of the participants were Caucasian, 8.1% African-American, 3.3% Hispanic and 0.5% were Asian or Pacific Islander; the remainder declined to identify race. 12.1% of the participants had undergone hysterectomy prior to participating in the research study for which MRIs were obtained.
Bartholin gland cysts were identified in 3.3% (n = 14, 95% confidence interval 1.6%–4.9%) of the participants. The cysts were identified on the participants’ right side in 50.0% (n = 7), left side in 42.9% (n = 6), and were bilateral in 7.1% (n = 1). Ninety-three percent of the participants had simple cysts (n = 13), and one (the subject with bilateral cysts) had a multicystic-appearing lesion. The dimensions of the cysts (length, width and depth) were (mean ± standard deviation): 1.3 ± 0.6 cm, 1.2 ± 0.6 cm, and 1.3 ± 0.6 cm. The measurements ranged from 0.5–2.7 cm.
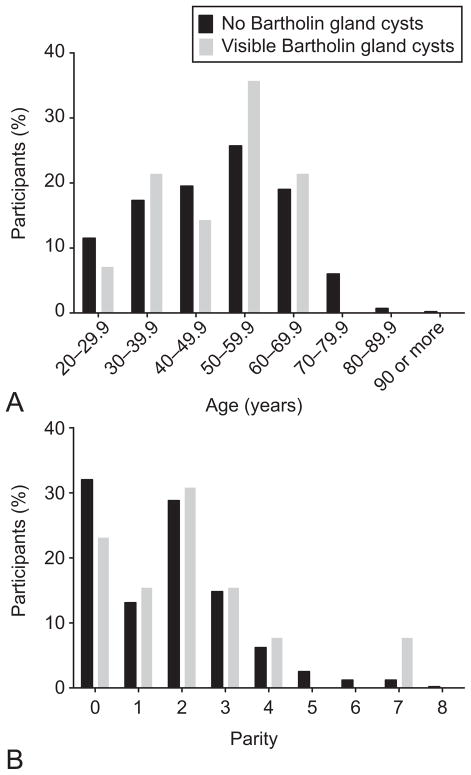
Demographic characteristics of the women with Bartholin gland cysts were similar to those of the participants without visible cysts (Table 1). The age (Figure 2a) and parity (Figure 2b) distributions of women with and without visible Bartholin gland cysts are similar (age: p = 0.96, parity: p = 0.80).
Table 1.
Demographic Characteristics of Women With and Without Visible Bartholin Gland Cysts on Magnetic Resonance Imaging
| Demographic | No Visible Cysts (n = 416) | Bartholin Gland Cysts Visible (n = 14) | P |
|---|---|---|---|
| Age (years) | 50.0 (37.0–60.0, 20.0–90.0) | 50.5 (35.5–56.5, 25.0–69.0) | 0.88 |
| Parity | 2.0 (0–3.0, 0–8.0) | 2.0 (0.5–3.0, 0–7.0) | 0.52 |
| Prior hysterectomy | 12.1 (50/414) | 14.3 (2) | 0.68 |
| Postmenopausal | 51.3 (210/409) | 64.3 (9/14) | 0.42 |
| Race | 0.57 | ||
| Caucasian | 87.0 (362) | 80.0 (11) | |
| African-American | 7.7 (32) | 20.0 (3) | |
| Asian | 0.5 (2) | 0 | |
| Hispanic | 3.4 (14) | 0 | |
| Other or unknown | 0.2 (1) | 0 | |
| Missing or refused | 1.2 (5) | 0 |
Data are median (interquartile range, total range), or percentage (number of participants) unless otherwise specified. Denominators are presented when there are missing data.
Figure 2.
A: Distribution of ages in women without (black) and with (gray) visible Bartholin gland cysts on pelvic magnetic resonance imaging (MRI). B: Distribution of parity in women without (black) and with (gray) visible Bartholin gland cysts on pelvic MRI.
DISCUSSION
The results of our study suggest that in this sample of asymptomatic women volunteering for research studies involving pelvic imaging, approximately 1 in 30 will have a Bartholin gland cyst identified. This is slightly higher than the published prevalence of 2%.4 As our data are from a pooled secondary analysis of case-control studies, rather than results from a population-based cross-sectional study, we cannot assert that the true prevalence of Bartholin gland cysts is 3.3%. Our findings are similar, though, to those of Gousse and colleagues, who identified incidental Bartholin gland cysts in four out of 100 women undergoing pelvic MRI.8 However, the women in the study by Gousse, et al., were having imaging performed for clinical reasons, and so may not be representative of an asymptomatic, healthy population. Given that recent epidemiologic studies have not been published, our data suggest that it may be reasonable to raise the estimate of the occurrence of Bartholin gland cysts.
Imaging is increasingly being used as part of diagnostic evaluations.9, 10 There are therefore reports of several gynecologic findings which are becoming more frequently identified incidentally, such as adnexal masses, endometrial fluid collections, and other endometrial abnormalities in asymptomatic postmenopausal women.11–16 Bartholin gland cysts are readily identifiable with routine imaging modalities.1, 17–20 We therefore predict that the incidental detection of Bartholin gland cysts, like the gynecologic conditions noted above, will occur with growing frequency. Radiologists and gynecologic providers must be informed that these cysts are relatively common and generally benign.21
It is commonly taught that Bartholin gland cysts in postmenopausal women are abnormal and should raise a higher index of suspicion for malignancy.22 By contrast, we find that the age distribution and self-reported menopausal status of participants with Bartholin gland cysts are similar to that of women without visible cysts. Furthermore, our population of women with visible cysts can be almost equally stratified into groups older than 50 years of age and younger than 50 years old. Considerations regarding potential malignancy would therefore be better if based on the changing occurrence of the disease with age. We endorse the notion that clinical evaluation may be more meaningful than strict algorithms based solely on patients’ age and/or menopausal status.23
There are several strengths to this study, including the wide range of ages, use of asymptomatic volunteers as research participants, inclusion of information on parity, and the use of high resolution MRI. We must also acknowledge several limitations, including the relative racial homogeneity of our participants which may limit generalizability, the lack of data about whether the participants were aware of and/or symptomatic from their cysts, as well as the lack of long-term follow-up data about the participants. Although these participants were recruited from the community as healthy volunteers, it is possible that gynecologic symptoms, including those from Bartholin gland cysts, may have motivated some of these women to volunteer, leading to a selection bias. As described earlier, our study design also precludes calculation of the true population prevalence.
In conclusion, Bartholin gland cysts may be visualized on pelvic magnetic resonance imaging with reasonably high frequency. We must stress, however, that the incidentally-identified Bartholin gland cyst is a relatively new gynecologic entity. Given our lack of knowledge about the natural history of these lesions, further research is necessary to determine how they should be managed clinically.
Acknowledgments
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grants R01 HD38665 and R01 DK51405, and ORWH grant P50 HD44406.
Footnotes
Financial Disclosure: The Pelvic Floor Research Group, of which Dr. DeLancey is the director, receives research support from American Medical Systems, Johnson & Johnson, Kimberly Clark, and Proctor & Gamble through the University of Michigan. The other authors did not report any potential conflicts of interest.
References
- 1.Marzano DA, Haefner HK. The bartholin gland cyst: past, present, and future. J Low Genit Tract Dis. 2004 Jul;8(3):195–204. doi: 10.1097/00128360-200407000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Bora SA, Condous G. Bartholin’s, vulval and perineal abscesses. Best Pract Res Clin Obstet Gynaecol. 2009 Oct;23(5):661–6. doi: 10.1016/j.bpobgyn.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Patil S, Sultan AH, Thakar R. Bartholin’s cysts and abscesses. J Obstet Gynaecol. 2007 Apr;27(3):241–5. doi: 10.1080/01443610701194762. [DOI] [PubMed] [Google Scholar]
- 4.Wechter ME, Wu JM, Marzano D, Haefner H. Management of Bartholin duct cysts and abscesses: a systematic review. Obstet Gynecol Surv. 2009 Jun;64(6):395–404. doi: 10.1097/OGX.0b013e31819f9c76. [DOI] [PubMed] [Google Scholar]
- 5.DeLancey JO, Miller JM, Kearney R, Howard D, Reddy P, Umek W, et al. Vaginal birth and de novo stress incontinence: relative contributions of urethral dysfunction and mobility. Obstet Gynecol. 2007 Aug;110(2 Pt 1):354–62. doi: 10.1097/01.AOG.0000270120.60522.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007 Feb;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 7.DeLancey JO, Trowbridge ER, Miller JM, Morgan DM, Guire K, Fenner DE, et al. Stress urinary incontinence: relative importance of urethral support and urethral closure pressure. J Urol. 2008 Jun;179(6):2286–90. doi: 10.1016/j.juro.2008.01.098. discussion 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gousse AE, Barbaric ZL, Safir MH, Madjar S, Marumoto AK, Raz S. Dynamic half Fourier acquisition, single shot turbo spin-echo magnetic resonance imaging for evaluating the female pelvis. J Urol. 2000 Nov;164(5):1606–13. [PubMed] [Google Scholar]
- 9.Maitino AJ, Levin DC, Parker L, Rao VM, Sunshine JH. Nationwide Trends in Rates of Utilization of Noninvasive Diagnostic Imaging among the Medicare Population between 1993 and 19991. Radiology. 2003 Apr 1;227(1):113–7. doi: 10.1148/radiol.2272020617. [DOI] [PubMed] [Google Scholar]
- 10.Rao VM, Levin DC, Parker L, Frangos AJ, Sunshine JH. Trends in Utilization Rates of the Various Imaging Modalities in Emergency Departments: Nationwide Medicare Data From 2000 to 2008. J Am Coll Radiol. 2011;8(10):706–9. doi: 10.1016/j.jacr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.McDonald JM. The incidental postmenopausal adnexal mass. Clin Obstet Gynecol. 2006;49(3):506–16. doi: 10.1097/00003081-200609000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Solnik MJ. Ovarian incidentaloma. Best Pract Res Clin Endocrinol Metab. 2012;26(1):105–16. doi: 10.1016/j.beem.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Vuento MH, Pirhonen JP, Mäkinen JI, Tyrkkö JE, Laippala PJ, Gröroos M, et al. Endometrial fluid accumulation in asymptomatic postmenopausal women. Ultrasound Obstet Gynecol. 1996;8(1):37–41. doi: 10.1046/j.1469-0705.1996.08010037.x. [DOI] [PubMed] [Google Scholar]
- 14.Worley MJ, Jr, Dean KL, Lin SN, Caputo TA, Post RC. The significance of a thickened endometrial echo in asymptomatic postmenopausal patients. Maturitas. 2011;68(2):179–81. doi: 10.1016/j.maturitas.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Menzies R. Significance of abnormal sonographic findings in postmenopausal women with and without bleeding. J Obstet Gynaecol Can. 2011;33(9):944–51. doi: 10.1016/s1701-2163(16)35020-4. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein SR. Significance of incidentally thick endometrial echo on transvaginal ultrasound in postmenopausal women. Menopause. 2011 Apr;18(4):434–6. doi: 10.1097/gme.0b013e31820ad00b. [DOI] [PubMed] [Google Scholar]
- 17.Moulopoulos LA, Varma DG, Charnsangavej C, Wallace S. Magnetic resonance imaging and computed tomography appearance of asymptomatic paravaginal cysts. Clin Imaging. 1993 Apr-Jun;17(2):126–32. doi: 10.1016/0899-7071(93)90052-o. [DOI] [PubMed] [Google Scholar]
- 18.Abulafia O, Sherer DM. Bartholin gland abscess: sonographic findings. J Clin Ultrasound. 1997 Jan;25(1):47–9. doi: 10.1002/(sici)1097-0096(199701)25:1<47::aid-jcu9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Kozawa E, Irisawa M, Heshiki A, Kimura F, Shimizu Y. MR findings of a giant Bartholin’s duct cyst. Magn Reson Med Sci. 2008;7(2):101–3. doi: 10.2463/mrms.7.101. [DOI] [PubMed] [Google Scholar]
- 20.Grant LA, Sala E, Griffin N. Congenital and acquired conditions of the vulva and vagina on magnetic resonance imaging: a pictorial review. Semin Ultrasound CT MR. 2010 Oct;31(5):347–62. doi: 10.1053/j.sult.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Kondi-Pafiti A, Grapsa D, Papakonstantinou K, Kairi-Vassilatou E, Xasiakos D. Vaginal cysts: a common pathologic entity revisited. Clin Exp Obstet Gynecol. 2008;35(1):41–4. [PubMed] [Google Scholar]
- 22.Stehman FB. Invasive Cancer of the Vulva. In: DiSaia PJ, Creasman WT, editors. Clinical Gynecologic Oncology. Philadelphia, PA: Elsevier Health Sciences; 2007. pp. 235–64. [Google Scholar]
- 23.Visco AG, Del Priore G. Postmenopausal bartholin gland enlargement: a hospital-based cancer risk assessment. Obstet Gynecol. 1996 Feb;87(2):286–90. doi: 10.1016/0029-7844(95)00404-1. [DOI] [PubMed] [Google Scholar]