Abstract
Background
Moderate consumption of alcohol, particularly red wine, has been shown to decrease cardiac risk. We used a hypercholesterolemic swine model of chronic ischemia to examine the effects of two alcoholic beverages on the heart.
Methods and Results
Yorkshire swine fed a high-cholesterol diet underwent left circumflex ameroid constrictor placement to induce chronic ischemia at 8 weeks of age. One group (HCC, n=9) continued on the diet alone, the second (HCW, n=8) was supplemented with red wine (pinot noir, 12.5% alcohol, 375 mL daily), and the third (HCV, n=9) was supplemented with vodka (40% alcohol, 112 mL daily). After 7 weeks, cardiac function was measured, and ischemic myocardium was harvested for analysis of perfusion, myocardial fibrosis, vessel function, protein expression, oxidative stress, and capillary density. Platelet function was measured by aggregometry. Perfusion to the ischemic territory as measured by microsphere injection was significantly increased in both HCW and HCV compared to HCC at rest, but in only the HCW group under ventricular pacing. Microvessel relaxation response to adenosine 5’-diphosphate was improved in the HCW group alone, as was regional contractility in the ischemic territory, though myocardial fibrosis was decreased in both HCW and HCV. Expression of pro-angiogenic proteins phospho-eNOS and VEGF was increased in both HCW and HCV, while phospho-mTOR was increased only in the HCV group. Expression of Sirt-1 and downstream antioxidant phospho-FoxO1 was increased only in the HCW group. Protein oxidative stress was decreased in the HCW group alone, while capillary density was increased only in the HCV group. There was no significant difference in platelet function between groups.
Conclusion
Moderate consumption of red wine and vodka may reduce cardiovascular risk by improving collateral-dependent perfusion via different mechanisms. Red wine may offer increased cardioprotection related to its antioxidant properties.
Keywords: Ischemia, myocardial perfusion, antioxidants, alcohol, animal model
INTRODUCTION
An intriguing paradox in cardiovascular health is the repeatedly validated finding that moderate alcohol consumption decreases cardiovascular risk1. Many studies have demonstrated a “J-shaped” dose-dependent relationship between alcohol consumption and cardiovascular mortality, wherein moderate alcohol intake of 20–30 g/day, equivalent to that found in 2–3 glasses of wine, is associated with maximal cardiovascular protection2. Numerous mechanisms have been suggested to explain the cardioprotective effect of alcohol, including increased HDL cholesterol3, reduced plasma viscosity and fibrinogen concentration4, improved endothelial function5, and decreased inflammation6. Nonetheless, a definitive explanation for this phenomenon remains elusive.
Beer, wine, and spirits have all been associated with reduced cardiovascular risk, suggesting that ethanol itself likely exerts some protective effect on the cardiovascular system7. However, some head-to-head comparisons between wine and other alcoholic beverages have shown that wine drinkers have even lower risk of coronary artery disease and cardiac death compared to beer and spirit drinkers who avoided wine8,9. In particular, several substances unique to red wine have been investigated for their antioxidant, pro-angiogenic, and anti-inflammatory properties. The most popular of these compounds is resveratrol (3,5,4’-trihydroxystilbene), a plant polyphenol that has been shown to have anti-oxidant, anti-cancer, and anti-aging effects. Resveratrol has been shown to activate Sirtuins, a family of deacetylases known to act on numerous cellular pathways10. Among these is the Forkhead class O (FOXO) pathway, which regulates the cellular response to oxidative stress. Alcendor et al demonstrated that hearts of transgenic mice overexpressing Sirt-1 were protected from oxidative stress through the activation of FOXO111, and resveratrol has been shown to upregulate both Sirt-1 and FOXO1 in rat hearts12. Thus, red wine may have additional cardioprotective effects due to the antioxidant properties of resveratrol.
Not surprisingly, interventional studies looking at the cardiovascular effects of alcohol treatment in humans are scarce, and observational studies are limited by patient variation and a high degree of bias. We designed a controlled animal study using a swine model of hypercholesterolemia and chronic ischemia to investigate the effects of resveratrol-containing red wine and resveratrol-free vodka on the heart. We hypothesized that these alcoholic beverages would have beneficial effects on perfusion, cardiovascular function, and oxidative stress in ischemic myocardium.
MATERIALS AND METHODS
Animal Model
Twenty-seven intact male Yorkshire swine (Parsons Research, Amherst, MA) were fed a high-cholesterol diet (500g once daily, Sinclair Research, Columbia, MO) starting at four weeks of age and continuing for the duration of the experiment. At 8 weeks age, swine underwent left circumflex (LCx) ameroid placement (Research Instruments SW, Escondido, CA) to induce chronic ischemia, and were then divided into three groups. One group (HCC, n=9) continued on the high-cholesterol diet alone, the second (HCW, n=9) received high-cholesterol diet supplemented with 375 mL of red wine daily (Black Mountain pinot noir, 12.5% alcohol v/v, 0.3–0.5 µg/mL resveratrol, Haro Hills, CA), and the third group (HCV, n=9) received high-cholesterol diet supplemented with 112 mL of vodka daily (Rubinoff vodka, 40% alcohol v/v, Somerville, MA). Resveratrol content in this particular variety of pinot noir was quantified using liquid chromatography-mass spectroscopy. The doses of beverage were selected to provide equal amounts of alcohol to both treated groups, and the beverages were consumed mixed with chow. All three groups were provided with water ad libitum. One animal from the HCW group died prior to the end of the experiment, presumably from cardiac arrhythmia, resulting in a final n=8 for the HCW group. Animals were assigned a unique ID number at the start of the experiment, and all subsequent analyses were carried out using only these identifying numbers to eliminate observational bias.
Surgical Procedures
Anesthesia was induced with intramuscular telazol (4.4 mg/kg) and maintained with 3.0% isoflurane. After intubation, titanium ameroid constrictors (1.75–2.25 mm internal diameter, sized to LCx diameter) were placed around the proximal LCx via left thoracotomy. Aspirin (325 mg/day) was administered one day prior to the procedure and continued for 5 days afterwards to prevent peri-procedural thrombosis. Alcohol supplementation was begun on the first postoperative day.
Two weeks prior to myocardial harvest, six animals from each group were briefly anesthetized with intramuscular telazol (2.2 mg/kg) one hour postprandially, and whole blood was drawn from the external jugular vein for serum alcohol quantification and platelet aggregation studies.
Seven weeks after ameroid placement, all swine were once again anesthetized and intubated. An arterial sheath was placed into the right femoral artery via cutdown, and blood samples were drawn and analyzed for total and HDL cholesterol (Beckman DXC 800 chemistry analyzer, Brea, CA). Coronary angiography was performed. After midline sternotomy, hemodynamic and functional measurements were performed, followed by cardiac harvest. 1-cm thick transverse slices were taken through the LV, and the resulting rings were divided into 8 sections each. Myocardial samples were rapidly frozen in liquid nitrogen (molecular studies), placed in 4 °C Krebs solution (microvessel studies) or dried at 60 degrees (microsphere analysis).
All experiments were approved by the Rhode Island Hospital Institutional Animal Care and Use Committee. Animals were cared for in accordance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ (NIH publication no. 5377-3 1996).
Coronary Angiography
X-ray coronary angiography with iohexol (GE Healthcare, Princeton, NJ) was carried out via femoral artery approach to verify LCx occlusion at the terminal surgery. A 5-French Amplatz R1 catheter (Cordis Corporation, Bridgewater, NJ) was advanced into the right and left coronary artery ostia and 4 mL of contrast injected per side to visualize coronary vessels. The resulting angiograms were read by a blinded cardiologist. Angiographic collateral formation was assessed according to the Rentrop grading system of 0 to 3, depending on the presence and extension of the collateral filling of coronary epicardial vessels. Myocardial perfusion was scored using the blush scoring system (also 0 to 3)13.
Measurement of Global and Regional Myocardial Function
Heart rate (HR), mean arterial pressure (MAP), developed left ventricular pressure (DLVP), first derivative of LV pressure (+dP/dt), and regional myocardial contractility in the ischemic area at risk (AAR) were recorded prior to cardiac harvest using intraventricular and intra-aortic single-sensor pressure catheters (Millar Instruments, Houston, TX) and the Sonometrics system (Sonometrics Corp. London, ON, Canada) as previously described14.
Myocardial Perfusion Analysis
Myocardial perfusion was measured via isotope-labeled microspheres (BioPAL, Worcester, MA). 1.5×107 gold-labeled microspheres were injected during temporary LCx occlusion at to identify the AAR. Lutetium (resting heart rate) and Europium (pacing to 160 beats/minute) labeled microspheres were injected at the final procedure while simultaneously withdrawing arterial blood from the femoral artery catheter. Harvested LV samples were completely dried in a 60° C oven, then exposed to neutron beams and microsphere densities measured (BioPAL). Myocardial blood flow in the AAR was determined using the following equation:
Blood flow = (withdrawal rate/tissue weight) × (tissue microsphere count/blood microsphere count)
Microvessel Studies
Coronary arterioles (80–180µm diameter) from the AAR were isolated and placed in a microvessel chamber. Vessels were maximally preconstricted with thromboxane-A2 analog U46619 (0.1–1.0 µM), then treated with endothelium-dependent vasodilator adenosine-5’-diphosphate (ADP, 10−9 to 10−4 mol/L) and endothelium-independent vasodilator sodium nitroprusside (SNP, 10−9 to 10−4 mol/L). Responses were defined as percent relaxation of the preconstricted diameter. All reagents were obtained from Sigma-Aldrich (St Louis, MO).
Quantification of Fibrosis
12-µm thick sections from the AAR were fixed and trichrome stained. Digital images of the stained slices were captured using Aperio slide scanning software (Aperio Technologies, Vista, CA). The amount of blue-stained collagen was quantified in a blinded fashion for three randomly selected 10X fields per section using Image J software (NIH, Bethesda, MD) and expressed as a percentage of the total section area. Measurements from the three fields were averaged to obtain representative % fibrosis for each section.
Immunoblotting
Sixty micrograms of total protein from AAR homogenates were fractionated by SDS-PAGE (Invitrogen, San Diego, CA) and transferred to PVDF membranes (Millipore, Bedford, MA). Membranes were incubated with antibodies against endothelial nitric oxide synthase (eNOS), phospho-eNOS, mammalian target of rapamycin (mTOR), phospho-mTOR, FOXO1, phospho-FOXO1 (Cell Signaling Technology, Danvers, MA), Sirt-1 (Santa Cruz Biotechnology, Santa Cruz CA), and vascular endothelial growth factor (VEGF) (Calbiochem, San Diego, CA) at dilutions recommended by the manufacturer, followed by the appropriate HRP-linked secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Immune complexes were detected with chemiluminescence (Amersham, Piscataway, NJ) and photographed using GeneSnap software (Syngene, Cambridge, England). Densitometry was performed using Image J software. α-tubulin (Cell Signaling Technology) was used as a loading control. Expression of phosphorylated proteins was expressed as a ratio of phosphorylated:total protein.
Protein Oxidative Stress
Dinitrophenylhydrazine-derivatized tissue homogenates containing 30 µg of total protein from the AAR were separated as above. Membranes were incubated with primary antibody to dinitrophenylhydrazine, followed by HRP-linked secondary antibody per manufacterer’s recommendations (Millipore, Billerica, MA). Immune complexes were visualized with chemiluminescence. Densitometric analysis of entire lanes was performed using Image J software.
Immunostaining for Capillary and Arteriolar Density
12 µm-thick frozen sections of myocardium from the AAR were formalin fixed, then incubated with antibodies against porcine endothelial marker CD-31 (R&D Systems, Minneapolis, MN) and smooth muscle actin (SMA, Sigma Aldrich), followed by the appropriate alexa-fluor conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA). Photomicrographs of three random 20X fields per section were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc, Thornwood, NY). Capillaries, defined as CD-31 positive structures between 5–25 µm2 in cross-sectional area, and arterioles, defined as structures co-staining for both SMA and CD-31, were counted using Image J software. Results were averaged for three slices per section and are presented as vessels/mm2.
Platelet activity
Whole blood samples drawn 1 hour postprandially were collected directly into tubes containing 3.2% trisodium citrate. Platelet function studies were performed using a Platelet Lumi-Aggregometer (Chronolog Corporation, Havertown, PA). Aggregation response to the agonists ADP (10 µM) and arachidonic acid (0.5 mM) was measured by impedance aggregation and ATP secretion using the firefly luciferin-luciferase system as previously described15.
Statistical Analysis
All results are presented as mean ± SEM. Microvessel responses were analyzed using two-way, repeated-measures ANOVA using the Bonferroni method to compare all pairwise contrasts between treatment-group means (K=3). Student’s t test was used to compared blood alcohol content between the HCW and HCV groups. All other comparisons were carried out using one-way ANOVA with a Neuman-Keuls post-hoc test to compare between groups using GraphPad Prism 5.0 Software (GraphPad Software Inc., San Diego, CA). Differences with a p value < 0.05 were considered statistically significant.
RESULTS
Serum cholesterol and alcohol
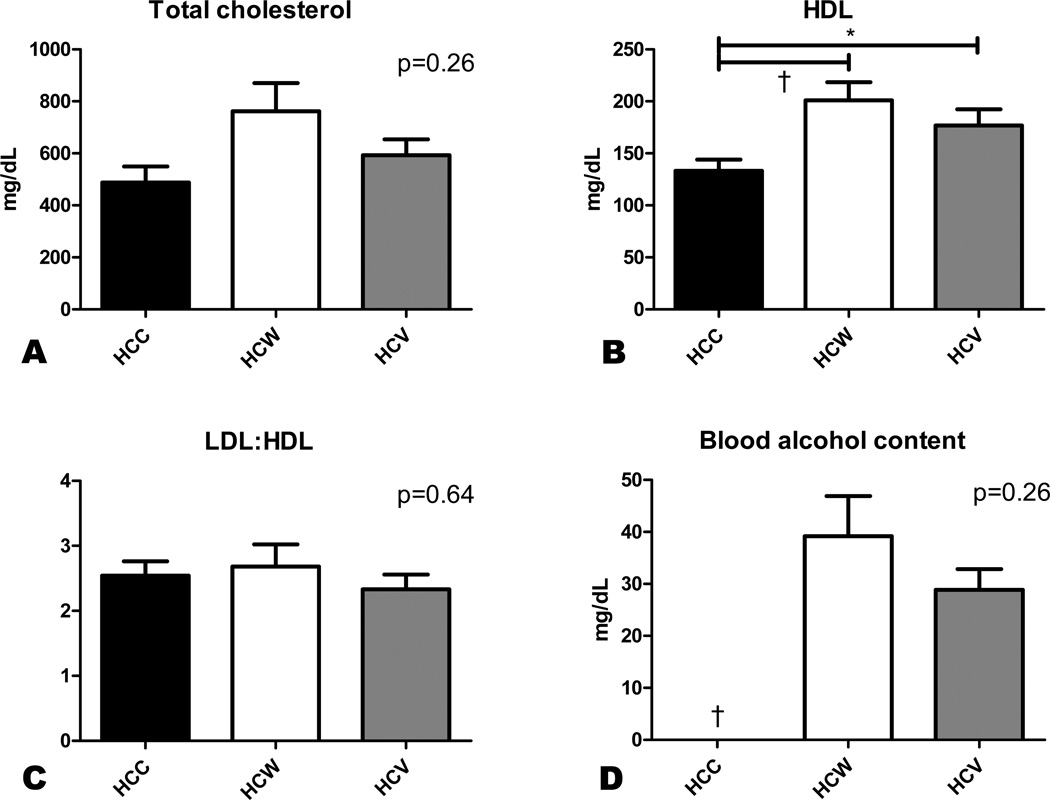
All groups had similarly elevated levels of total serum cholesterol at the end of the experiment (Figure 1A). HDL cholesterol, however, was significantly increased in the two alcohol treated groups compared to controls (Figure 1B). There was no difference in the calculated LDL:HDL ratio between groups (Figure 1C).
Figure 1. Serum chemistries.
Whole blood drawn at the terminal procedure was analyzed for total and HDL cholesterol, and the LDL:HDL ratio was calculated. Blood was also drawn one hour postprandially for quantification of blood alcohol levels. There was no difference in total cholesterol or LDL:HDL ratio between groups (A,C), but HDL cholesterol was significantly increased in HCW and HCV compared to HCC (B). Blood alcohol levels were similarly elevated in both HCW and HCV swine (D). *p<0.05, †p<0.01.
Alcohol was detected in the blood of HCW and HCV animals one hour after eating but not of HCC animals. There was no significant difference in blood alcohol content between the HCW and HCV groups (Figure 1D).
Coronary angiography
All animals demonstrated complete occlusion of the LCx at the terminal procedure. There was no significant difference in angiographic collateralization as assessed by the Rentrop score between groups. Radiographic perfusion as assessed by blush scoring tended to be higher in the alcohol treated groups compared to the control group, but this difference did not reach statistical significance (0.11±0.11 in HCC, 0.50±0.19 in HCW, and 0.44±0.24 in HCV, p=0.31).
Myocardial function
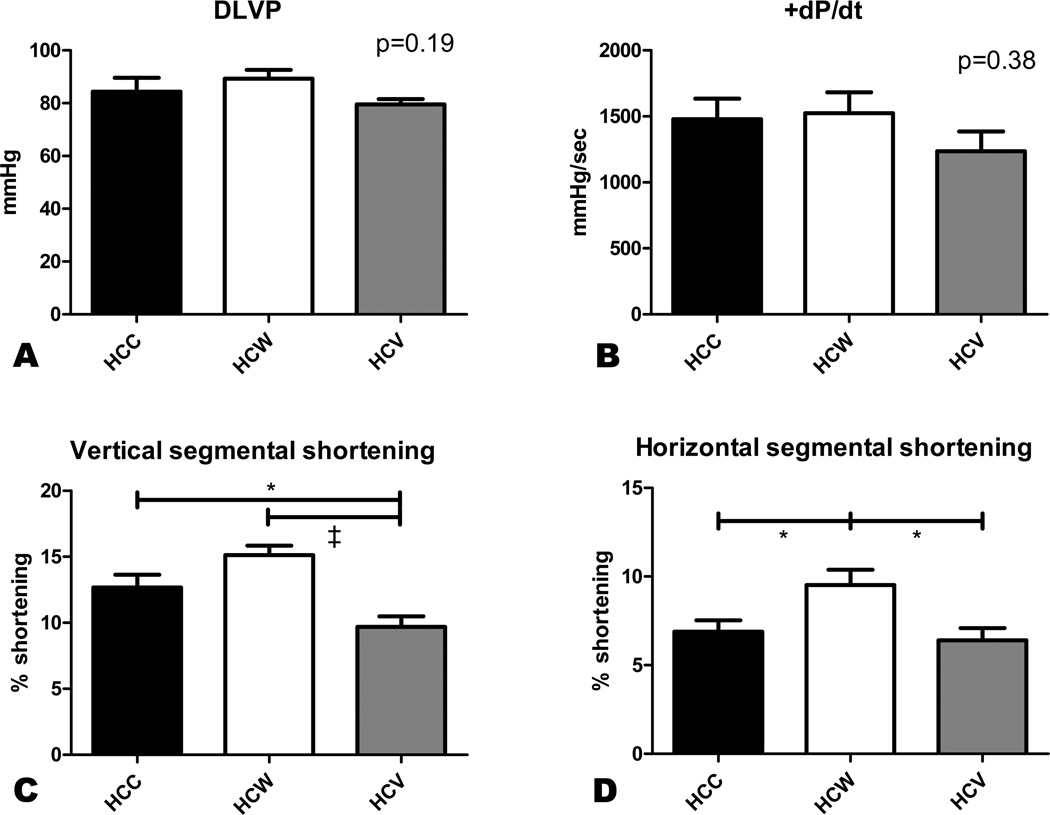
Heart rate at the time of harvest was significantly lower in the HCW group compared to both the HCC and HCV groups (102.7±8.3 bpm in HCC, 77.9±1.9 bpm in HCW, and 95.2±3.8 bpm in HCV, p=0.008). There was no significant difference in mean aortic arterial pressure between groups (69.3±5.4 mmHg in HCC, 69.2±2.2 mmHg in HCW, and 58.0±2.0 mmHg in HCV, p=0.08). DLVP and LV contractility as measured by +dP/dt were similar between groups (Figure 2A–B). Regional contractility in the AAR was measured as myocardial shortening in two axes. In the vertical axis, contractility was decreased in the HCV group compared to both other groups, while contractility in the HCW group was marginally improved compared to the HCC group (Figure 2C). On the horizontal axis, regional contractility was significantly improved in the HCW group compared to both HCC and HCV, while the contractility in the HCV group was similar to the HCC group (Figure 2D).
Figure 2. Myocardial function.
Developed left ventricular pressure (DLVP) and LV contractility (+dP/dt) were measured using a pressure catheter inserted directly into the LV. There was no significant difference in DLVP or +dP/dt between groups (A,B). Vertical segmental shortening was decreased in the HCV group compared to both other groups (C), while horizontal segmental shortening was improved in the HCW group compared to both other groups. *p<0.05, ‡p<0.001.
Myocardial perfusion
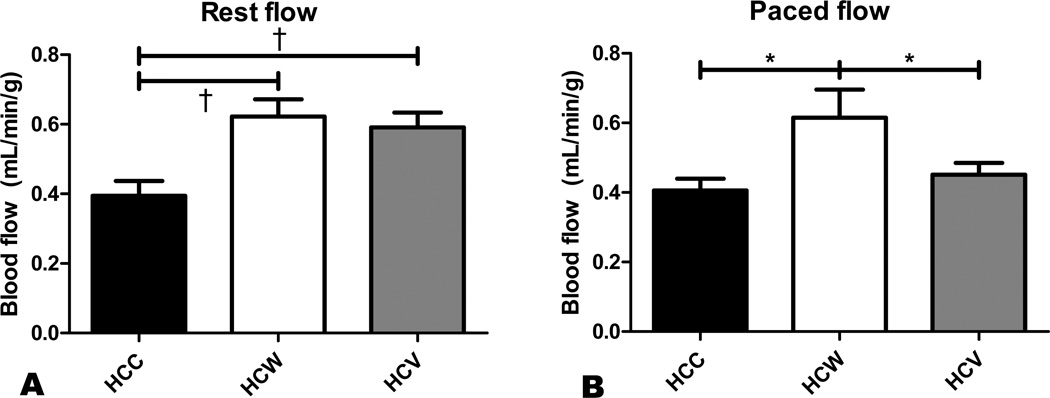
Under resting conditions, perfusion to the AAR was significantly increased approximately 1.5-fold in both the HCW and HCV groups compared to the HCC group (Figure 3A). There was no significant difference between the HCW and HCV groups at rest. However, under ventricular pacing, perfusion in the HCW group was significantly greater than in both HCC and HCV, and there was no longer a significant difference between the HCC and HCV groups (Figure 3B).
Figure 3. Myocardial perfusion.
Perfusion in the AAR was measured by microsphere injection at rest and under ventricular pacing to 160 bpm. At rest, both wine and vodka administration improved perfusion to the ischemic territory (A). However, under ventricular pacing, myocardial perfusion was improved only in the HCW group (B). *p<0.05, †p<0.01.
Microvessel Function
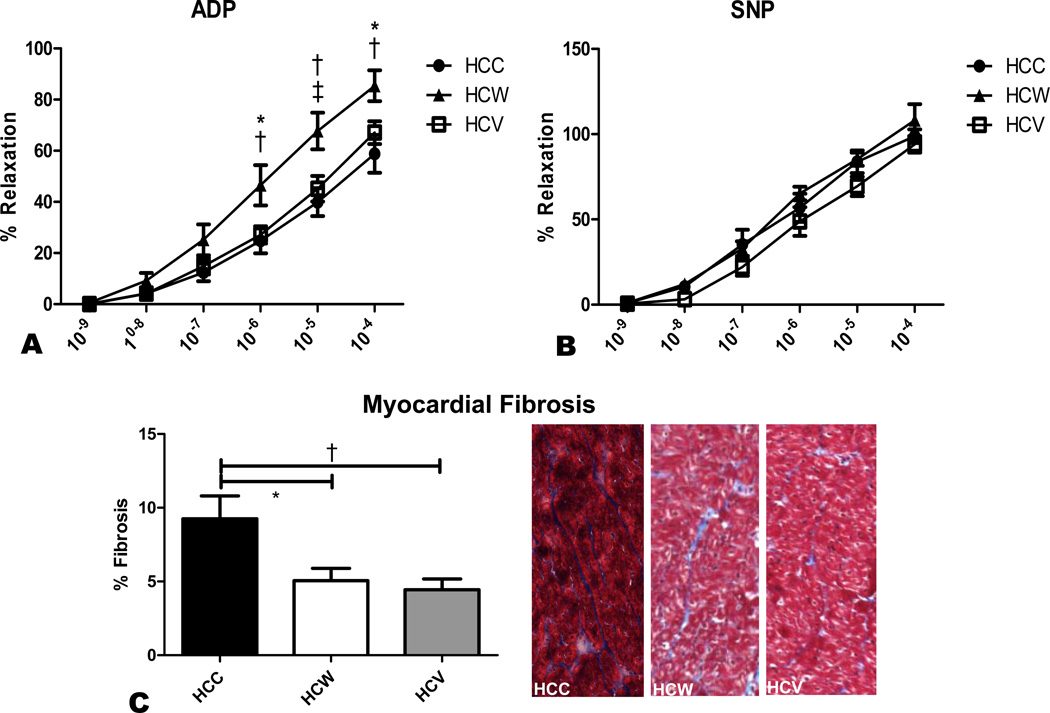
Microvessel relaxation response to ADP, an endothelium-dependent vasodilator, was significantly improved in the HCW group compared to both HCC and HCV (Figure 4A). There was no significant difference in the relaxation response to endothelium-independent SNP between groups (Figure 4B).
Figure 4. Microvessel function and myocardial fibrosis.
Microvessel relaxation responses to endothelium-dependent ADP and endothelium-independent SNP were measured in coronary arterioles from the AAR. A significant improvement in endothelium-dependent vasodilation was seen in the HCW group compared to both HCC and HCV (A). There was no difference between groups in endothelium-independent vasodilation (B). Myocardial fibrosis as measured by trichrome staining was significantly higher in the HCC group than in both other groups. Shown are representative trichrome stained myocardial sections from the three groups, with myocardium staining red and intervening connective tissue staining blue (C). *p<0.05, †p<0.01, ‡p<0.001.
Myocardial Fibrosis
Fibrosis was significantly higher in the HCC group compared to HCW and HCV. There was no significant difference in fibrosis between HCW and HCV animals. (Figure 4C)
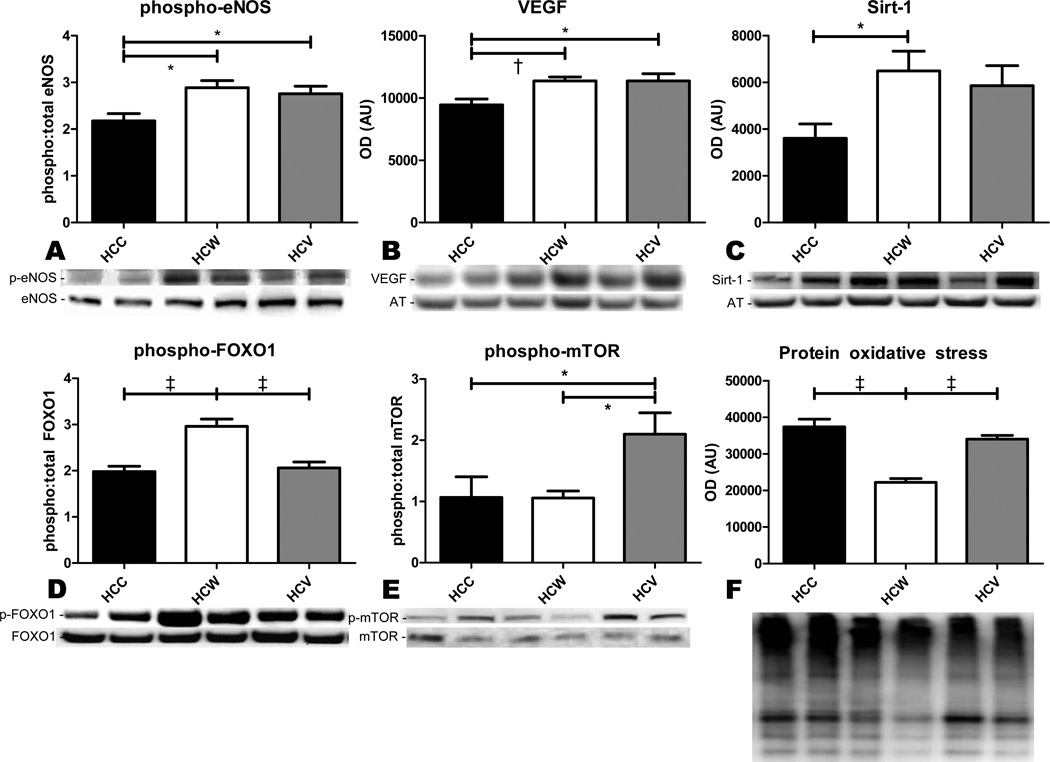
Protein expression
Expression of pro-angiogenic proteins phospho-eNOS and VEGF was significantly increased in both HCW and HCV compared to HCC (Figure 5A–B). Expression of Sirt-1 was significantly increased only in the HCW group, though it tended to be higher than control in the HCV group as well (Figure 5C). However, expression of antioxidant transcription factor phospho-FOXO1 was higher in the HCW group than both other groups (Figure 5D), while expression of pro-angiogenic phospho-mTOR was highest in the HCV group (Figure 5E).
Figure 5. Immunoblotting.
Expression of proangiogenic proteins phospho-eNOS, VEGF, and phospho-mTOR, antioxidant proteins Sirt-1 and phospho-FOXO1, and total protein oxidation in the AAR were measured by immunoblotting. Both alcoholic beverages increased the expression of phospho-eNOS and VEGF (A,B), while only red wine significantly increased the expression of Sirt-1 and phospho-FOXO1 (C,D), though Sirt-1 tended to be elevated in the HCV group as well. Phospho-mTOR was only upregulated in the HCV group (E). Protein oxidative stress was significantly reduced in the HCW group compared to both other groups (F). *p<0.05, †p<0.01, ‡p<0.001.
Protein oxidative stress
Total oxidative stress in the AAR as measured by the OxyBlot assay was significantly lower in the HCW group than both other groups (Figure 5F).
Vessel density
Capillary density in the AAR was higher in the HCV group than in both HCC and HCW (608±30 vessels/mm2 in HCC, 600±39 vessels/mm2 in HCW, and 786±73 vessels/mm2 in HCV, p=0.02). There was no significant difference in arteriolar density between the groups (36.1±5.1 vessels/mm2 in HCC, 39.6±6.0 vessels/mm2 in HCW, and 37.5±5.4 vessels/mm2 in HCV, p=0.90).
Platelet function
When stimulated with ADP, there was no significant difference in platelet aggregation as measured by impedance (9.67±1.33 ohm in HCC, 10.33±0.56 in HCW, and 12.17±1.40 in HCV, p=0.52) or ATP secretion (0.85±0.18 Lum in HCC, 0.93±0.56 in HCW, and 0.67±0.10 in HCV, p=0.26). Similarly, when stimulated with arachidonic acid, there was no significant difference in aggregation as measured by impedance (6.67±3.38 ohm in HCC, 6.83±1.89 in HCW, and 11.83±1.19 in HCV, p=0.14) or ATP secretion (1.31±0.96 Lum in HCC, 1.39±0.46 in HCW, and 1.73±0.39 in HCV, p=0.47).
DISCUSSION
In this study, we demonstrated that both red wine and vodka improve perfusion to ischemic myocardium, but they do so by different mechanisms. Both beverages also decrease myocardial fibrosis in ischemic myocardium, but only red wine increases perfusion during ventricular pacing, improves segmental shortening and microvessel function, and reduces oxidative stress.
Serum alcohol levels in the swine were somewhat lower than we expected, considering the body weight of the animals at the time of sacrifice was only 30–40 kg. It may simply be that swine metabolize alcohol differently than humans, or that swine serum alcohol levels peak at a different time than that which we chose to draw their blood. Fortunately, the serum alcohol levels achieved in the swine in this experiment approximate that of an adult human after 1–2 alcoholic drinks, and were sufficient to produce significant effects.
We specifically chose to use a model of hypercholesterolemia and chronic myocardial ischemia in this study because these comorbidities are very likely to be present in the at-risk patient population that might benefit from the cardioprotective effects of alcohol. Like other studies16, we found that alcohol supplementation was associated with increased serum HDL cholesterol, while total cholesterol levels were unaffected. Though some groups, including our own, have shown that high doses of resveratrol decreases total cholesterol and the LDL:HDL ratio17, we did not see these effects with the low doses of resveratrol present in our red wine. HDL cholesterol transports LDL from the periphery to the liver where it is metabolized; thus the favorable lipid profile created by alcohol supplementation may play a role in cardioprotection and the prevention of atherosclerosis.
A key finding in this study is that both red wine and vodka significantly increased perfusion in the ischemic territory at rest. Two mechanisms can lead to increased blood flow in the setting of chronic ischemia: neogenesis of vessels, or dilation of resistance arterioles. Hypercholesterolemia has been shown to cause endothelial dysfunction, reducing the ability of coronary arterioles to dilate18. In a previous study, we found that high-dose purified resveratrol reversed this endothelial dysfunction in the ischemic territory of hypercholesterolemic swine, related to its antioxidant effects17. Similarly, despite a many-fold reduction in resveratrol content, red wine in this study significantly decreased oxidative stress and improved endothelium-dependent microvessel relaxation, phenomena which were not seen in vodka-treated swine. The finding that Sirt-1 and the antioxidant transcription factor phospho-FOXO1 were also upregulated only in the wine group suggests that these effects were in fact mediated by resveratrol.
On the other hand, the increased perfusion in the vodka-treated group appears to be related to increased capillary density, mediated by increases in phospho-eNOS, VEGF, and phospho-mTOR, all potent mediators of angiogenesis in ischemic myocardium19,20. Interestingly, alcohol has actually been shown to inhibit mTOR activity in cultured myocytes21, but it may act differently in vivo or in the setting of ischemia. Phospho-eNOS and VEGF were upregulated in the wine-treated group as well, but likely did not stimulate neogenesis of capillaries since perfusion was already improved by arteriolar relaxation.
The different mechanisms by which red wine and vodka improve perfusion may also explain why perfusion was only increased in the wine group under ventricular pacing. In the setting of greater oxygen demand, capillaries in the ischemic territory of vodka-treated swine would provide minimal benefit with regard to perfusion because of their small size and fixed number. On the other hand, the improved ability of resistance arterioles to relax in the wine-treated animals likely allows them to adjust for the increased oxygen demand of ventricular pacing and increase blood flow accordingly. Thus, the antioxidant properties of resveratrol-containing red wine provide additional benefit over alcohol alone with regard to myocardial perfusion.
Though wine and vodka supplementation had no effect on global LV function as measured by DLVP and +dP/dt, there were significant differences in regional function in the ischemic territory. Red wine supplementation improved contractility in the ischemic territory, likely related to the improvements in blood flow discussed above. Vodka supplementation, however, actually decreased regional contractility in the vertical axis, though myocardial fibrosis in the AAR was decreased compared to controls. Chronic ethanol ingestion has been associated with increased myocardial fibrosis and global contractile dysfunction22, but the amounts of alcohol given in this study were insufficient to cause cardiomyopathy. It may be that at lower doses, alcohol actually decreases fibrosis in the ischemic territory. Though we did not specifically examine the molecular basis for decreased fibrosis in these animals, this finding likely relates to the increased myocardial perfusion in the ischemic myocardium in these groups. Antioxidants have also been shown to decrease myocardial remodeling and fibrosis after ischemic injury, so this may play a role in the wine treated animals as well, though they did not demonstrate any less fibrosis than the vodka-treated animals. The decreased contractility in the vodka group could be related to some other mechanism that we did not examine, such as increased apoptosis.
A number of studies have shown that the cardioprotective effects of alcohol may actually be related to an inhibitory effect on platelet aggregation23,24. Our results showed no significant difference in platelet aggregation with either red wine or vodka supplementation, though the small numbers of animals assayed led to substantial margins of error. Nonetheless, both red wine and vodka clearly had beneficial effects on myocardial perfusion, fibrosis, and serum lipid profiles, with red wine leading to additional improvements in perfusion and regional function due to its antioxidant properties. These findings shed new light on the mechanisms by which moderate alcohol intake might reduce cardiovascular risk. Whether these beneficial effects are also seen in patients remains to be seen.
LIMITATIONS
Though the beneficial effects of red wine are frequently ascribed to resveratrol, red wine is a complex substance containing many other compounds, about some of which very little is known. Furthermore, the activation of Sirt-1 may not be specific to resveratrol as once thought. A recent study showed that white wine, which contains little resveratrol, and tyrosol, a phenolic compound found in white wines and olive oil, also increased expression of Sirt-1 and FOXO in rat hearts, though cardioprotection as measured by reduction in infarct size was greatest with resveratrol and red wine12. Thus the antioxidant effect we saw in this study may not be specific to red wine, and future studies investigating other beverages or compounds present in red wine would be useful. In addition, even among red wines there is a large variation in actual resveratrol content. Though Californian pinot noir is reported to have one of the highest resveratrol contents, the amount of resveratrol in the wine we chose for this study was lower than that reported for other red wines25. Whether or not the effects we saw are dose-dependent remains to be seen.
ACKNOWLEDGEMENTS
We would like to thank the veterinary and animal care staff at Rhode Island Hospital for their excellent care of the animals used in this study.
FUNDING SOURCES
Funding for this study was provided by NIH T32 grants HL094300 (Dr. Chu), HL076134 (Dr. Lassaletta), HL007734 (Dr. Robich), and NIH grants RO1HL46716, RO1HL69024, and RO1HL85647 (Dr. Sellke).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Saremi A, Arora R. The cardiovascular implications of alcohol and red wine. Am J Ther. 2008;15:265–277. doi: 10.1097/MJT.0b013e3180a5e61a. [DOI] [PubMed] [Google Scholar]
- 2.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2010;55:1339–1347. doi: 10.1016/j.jacc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Gaziano JM, Buring JE, Breslow JL, Goldhaber SZ, Rosner B, VanDenburgh M, Willett W, Hennekens CH. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–1834. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- 4.Jensen T, Retterstel LJ, Sandset PM, Godal HC, Skjonsberg OH. A daily glass of red wine induces a prolonged reduction in plasma viscosity: a randomized controlled trial. Blood Coagul Fibrinolysis. 2006;17:471–476. doi: 10.1097/01.mbc.0000240920.72930.63. [DOI] [PubMed] [Google Scholar]
- 5.Teragawa H, Fukuda Y, Matsuda K, Higashi Y, Yamagata T, Matsuura H, Chayama K. Effect of alcohol consumption on endothelial function in men with coronary artery disease. Atherosclerosis. 2002;165:145–152. doi: 10.1016/s0021-9150(02)00193-4. [DOI] [PubMed] [Google Scholar]
- 6.Imhof A, Woodward M, Doering A, Helbecque N, Loewel H, Amouyel P, Lowe GD, Koenig W. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille) Eur Heart J. 2004;25:2092–2100. doi: 10.1016/j.ehj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, MJ S. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–468. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 8.Gronbaek M, Becker U, Johansen D, Gottschau A, Schnohr P, Hein HO, Jensen G, Sorensen TI. Type of alcohol consumed and mortality from all causes, coronary heart disease, and cancer. Ann Intern Med. 2000;133:411–419. doi: 10.7326/0003-4819-133-6-200009190-00008. [DOI] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Shaper AG. Type of alcoholic drink and risk of major coronary heart disease events and all-cause mortality. Am J Public Health. 1999;89:685–690. doi: 10.2105/ajph.89.5.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 11.Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E, Yu X, Tian B, Wagner T, Vatner SF, Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee S, Lekli I, Gurusamy N, Bertelli AA, Das DK. Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med. 2009;46:573–578. doi: 10.1016/j.freeradbiomed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Sorajja P, Gersh BJ, Mehran R, Lansky AJ, Krucoff MW, Webb J, Cox DA, Brodie BR, GW S. Impact of collateral flow on myocardial reperfusion and infarct size in patients undergoing primary angioplasty for acute myocardial infarction. Am Heart J. 2007;154:379–384. doi: 10.1016/j.ahj.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 14.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Sellke FW. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweeney JD, Labuzetta JW, Fitzpatrick JE. The effect of the platelet count on the aggregation response and adenosine triphosphate release in an impedance lumi-aggregometer. Am J Clin Pathol. 1988;89:655–659. doi: 10.1093/ajcp/89.5.655. [DOI] [PubMed] [Google Scholar]
- 16.Koppes LL, Twisk JW, Van Mechelen W, Snel J, Kemper HC. Cross-sectional and longitudinal relationships between alcohol consumption and lipids, blood pressure and body weight indices. J Stud Alcohol. 2005;66:713–721. doi: 10.15288/jsa.2005.66.713. [DOI] [PubMed] [Google Scholar]
- 17.Robich MP, Osipov RM, Nezafat R, Feng J, Clements RT, Bianchi C, Boodhwani M, Coady MA, Laham RJ, Sellke FW. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010;122:S142–S149. doi: 10.1161/CIRCULATIONAHA.109.920132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quillen JE, Sellke FW, Armstrong ML, Harrison DG. Long-term cholesterol feeding alters the reactivity of primate coronary microvessels to platelet products. Arterioscler Thromb. 1991;11:639–644. doi: 10.1161/01.atv.11.3.639. [DOI] [PubMed] [Google Scholar]
- 19.Penumathsa SA, Koneru S, Samuel SM, Maulik G, Bagchi D, Yet SF, Menon VP, Maulik N. Strategic targets to induce neovascularization by resveratrol in hypercholesterolemic rat myocardium: role of caveolin-1, endothelial nitric oxide synthase, hemeoxygenase-1, and vascular endothelial growth factor. Free Radic Biol Med. 2008;45:1027–1034. doi: 10.1016/j.freeradbiomed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang XD, Gu TX, Shi EY, Lu CM, Wang C. Effect and mechanism of panaxoside Rg1 on neovascularization in myocardial infarction rats. Chin J Integr Med. 2010;16:162–166. doi: 10.1007/s11655-010-0162-4. [DOI] [PubMed] [Google Scholar]
- 21.Hong-Brown LQ, Brown CR, Huber DS, CH L. Alcohol and indinavir adversely affect protein synthesis and phosphorylation of MAPK and mTOR signaling pathways in C2C12 myocytes. Alcohol Clin Exp Res. 2006;30:1297–1307. doi: 10.1111/j.1530-0277.2006.00157.x. [DOI] [PubMed] [Google Scholar]
- 22.Ren J, Wold LE. Mechanisms of alcoholic heart disease. Ther Adv Cardiovasc Dis. 2008;2:497–506. doi: 10.1177/1753944708095137. [DOI] [PubMed] [Google Scholar]
- 23.de Lange DW, Scholman WL, Kraaijenhagen RJ, Akkerman JW, van de Wiel A. Alcohol and polyphenolic grape extract inhibit platelet adhesion in flowing blood. Eur J Clin Invest. 2004;34:818–824. doi: 10.1111/j.1365-2362.2004.01432.x. [DOI] [PubMed] [Google Scholar]
- 24.Ruf JC. Alcohol, wine and platelet function. Biol Res. 2004;37:209–215. doi: 10.4067/s0716-97602004000200006. [DOI] [PubMed] [Google Scholar]
- 25.Paulo L, Domingues F, Queiroz JA, E G. Development and validation of an analytical method for the determination of trans- and cis-resveratrol in wine: analysis of its contents in 186 Portuguese red wines. J Agric Food Chem. 2011;59:2157–2168. doi: 10.1021/jf105004y. [DOI] [PubMed] [Google Scholar]