Abstract
Cullin ring ligases (CRLs) constitute the largest group of RING finger ubiquitin ligases. Two recent studies in Molecular Cell describe glomulin as a CRL1 inhibitor that blocks interactions with its ubiquitin-conjugating enzyme (E2) (Duda et al., 2012; Tron et al., 2012). These findings and their significance are discussed.
Ubiquitination is an exquisitely regulated process that affects essentially all pathways in eukaryotes through targeting of proteins for proteasomal and nonproteasomal degradation as well as through nondegradative functions. Ubiquitination occurs through the combinatorial interaction of ubiquitin ligases (E3s) with ubiquitin-conjugating enzymes (E2s), which bind the C terminus of activated ubiquitin through their active site cysteine (E2~Ub). The majority of E3s are RING finger proteins, which simultaneously bind substrate and E2~Ub and mediate the direct transfer of ubiquitin from E2 to substrate or to growing substrate-bound ubiquitin chains. Two recent papers provide an in-depth look at the regulation of E2 interactions with the RING finger of cullin ring ligase (CRL) superfamily E3s (Duda et al., 2012; Tron et al., 2012).
Most of the focus on regulated ubiquitination is at the level of substrate-E3 interactions, where phosphorylation and other posttranslational modifications, association with regulatory proteins, and subcellular localization can affect recognition by the E3. Ubiquitin ligases are also regulated by ubiquitination and by transition between active and inactive states (Weissman et al., 2011; Kee and Huibregtse, 2007; Ryan et al., 2006; Duda et al., 2011). Such transitions can occur in response to posttranslational modifications that can either affect association with proteins that regulate function or change the conformational state of ligases, altering accessibility of substrates or E2s to their binding sites.
The CRL superfamily constitutes the largest group of substrate-specific RING finger E3s. There are multiple CRL subfamilies, each defined by a specific cullin protein. While there is variation in architecture, all CRLs are comprised of a cullin that serves as a scaffold, with most binding the small RING finger protein Rbx1 (also known as Roc1 or Hrt1). In general, CRL subfamilies associate with cullin-specific classes of interchangeable substrate receptors (Sarikas et al., 2011). Each receptor can potentially target multiple proteins for ubiquitination, allowing for recognition of myriad cellular substrates by CRLs (Figure 1A). The CRL1 complex (also known as the SCF) has been studied extensively and has numerous highly regulated substrates implicated in processes such as cell-cycle regulation and signaling. Their dysregulation contributes to oncogenesis and other pathologies. CRL1 is characterized by an adaptor protein known as Skp1 to which substrate receptor F box proteins bind; there are at least 69 human F box proteins (Cardozo and Pagano, 2004). CRL1, as well as other CRL subfamilies, are regulated by the dynamic covalent modification of the cullin with the ubiquitin-like protein Nedd8, which induces conformational changes in Cul1 and allows for activation by facilitating the binding of Skp1 F box modules. In the absence of Nedd8 modification, the CRL inhibitor CAND1 binds Cul1-Rbx1 leading to an inactive complex lacking a Skp1 F box module. When Cul1 is neddylated, CAND1 dissociates and Skp1 associates together with one of many F box proteins, reactivating CRL1 (Duda et al., 2011) (Figure 1B).
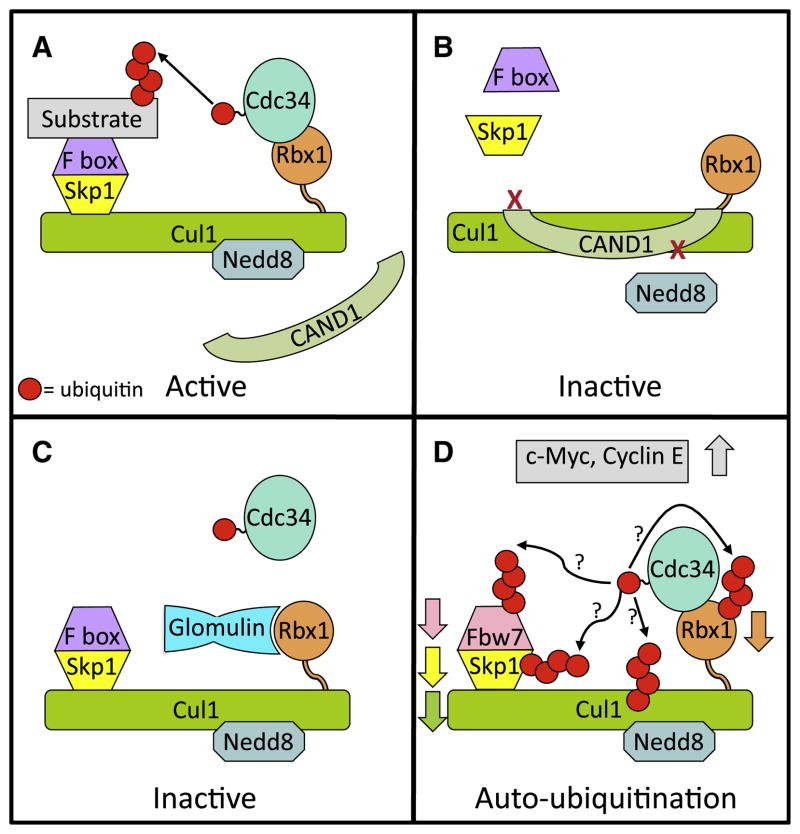
Figure 1. Regulation of CRL1.
(A) Functional CRL1. Cullin RING ligase 1 (CRL1) family members are comprised of Cul1, the RING finger Rbx1, which binds the active E2 (Cdc34~Ub), the adaptor protein Skp1, and one of many substrate-binding F box proteins. Activation of CRL1 requires modification of the cullin core with the ubiquitin-like protein Nedd8, which initiates assembly of the Skp1 and F box proteins on the CRL complex and prevents association of the CRL inhibitor CAND1.
(B) CRL1 inhibition through complex disassociation. Binding of the CRL inhibitor CAND1 to Cul1-Rbx1 inhibits neddylation and prevents assembly of the Skp1 F box complex.
(C) Inhibition of CRL1 activity by glomulin. A recently discovered mechanism for CRL inhibition is by association of the protein glomulin with Rbx1, preventing the RING finger of Rbx1 from binding to Cdc34~Ub.
(D) Speculative model of the effects of loss of glomulin. Absence of glomulin results in decreased levels of the F box protein Fbw7, Rbx1, and Cul1 through ubiquitination and proteasomal degradation leading to accumulation of the CRL1Fbw7 substrates c-Myc and Cyclin E. This is likely to occur through enhanced autoubiquitination of CRL1 components.
Two recent studies elucidate a new mode of CRL1 regulation where the CRL inhibitor glomulin (encoded by the GLMN gene) binds to Rbx1 and blocks its association with the CRL1 E2, Cdc34 (Duda et al., 2012; Tron et al., 2012). Mutations in GLMN, coupled with loss of the wild-type allele, result in the vascular disorder glomuvenous malformation (GVM), which is characterized by cutaneous venous lesions. Glomulin was initially observed to be associated with Cul7 (Arai et al., 2003). Now Tron et al. (2012) have discovered that Glomulin inhibits CRL1 by binding specifically to Rbx1. In vitro, this binding inhibits the functions of Rbx1 in ubiquitination and also in neddylation of Cul1. In cells and Glmn−/− mice, constitutively active CRL1, due to loss of glomulin, is manifest as a decrease in the F box protein Fbw7. This proteasome-mediated loss correlates with the ability of Fbw7 to bind ubiquitin (Pashkova et al., 2010). The net effect of loss of this CRL1 inhibitor in Glmn−/− mice is increased levels of the CRL1Fbw7 substrates c-Myc and Cyclin E (Tron et al., 2012). Notably, similar increases were seen in pathological samples from GVM patients. Moreover, the vascular abnormalities and embryonic death in Glmn−/− mice resembles those seen in Fbw7−/− mice. Interestingly, this work also implicates loss of glomulin in downregulation of Rbx1 and cullins to which Rbx1 binds including Cul1, Cul2, Cul3, and Cul4A. This loss presumably occurs through autoubiquitination (Figure 1D). Decreased Rbx1 is also observed in GVM patient samples. In contrast, the levels of several other well-studied and biologically important CRL1 F box proteins including β-TrCP (Fbw1) and Skp2 (Fbl1), both of which, like Fbw7, bind ubiquitin (Pashkova et al., 2010), were not affected by loss of glomulin expression.
In this issue, through extensive biophysical analysis, Duda and colleagues provide structural data demonstrating the intricacies of how the Cul1-Rbx1 subcomplex of CRL1 is regulated by glomulin, which interestingly has structurally similar N- and C-terminal domains (Duda et al., 2012). Identification of specific residues at the interface between the C-terminal half of glomulin and Rbx1 reveal that glomulin masks regions on the Rbx1 RING finger essential for E2 binding, thereby inhibiting CRL1 (Figure 1C). It was further demonstrated that glomulin regulates CRL1 activity through competitive inhibition of E2 binding by interacting with the Rbx1 RING finger independently of neddylation and of Rbx1 association with Cul1. Duda et al. (2012) also demonstrate that glomulin exhibits a markedly higher affinity for Rbx1 than does Cdc34. Importantly, to our knowledge, this work provides the first clear example of a protein acting in trans to directly block an E2-E3 interaction.
The focus of most work on CRLs has been on degradation of their highly regulated substrates and not on degradation of CRL components themselves. With depletion or loss of glomulin, the primary observation is enhanced degradation of CRL components, particularly Fbw7. However, the basic RING finger and E2 dependent mechanism of both autoubiquitination of CRL1 components and substrate degradation are presumably the same. One might envision, given the relative affinities of Cdc34 and glomulin for Rbx1, that small changes in glomulin levels could profoundly affect CRL activity. Additionally, whether the N-terminal half of glomulin plays a role in regulating CRL function remains to be determined. It now becomes of great interest to assess how levels and cellular distribution of glomulin might affect the balance between targeting of substrates and CRL components and potentially fine tune CRL superfamily function.
In the ubiquitin system, the identification of a new means of intracellular regulation often portends discovery of other related mechanisms. Regulating E2~Ub binding through intramolecular interactions is a well-known mechanism of regulation in the ubiquitin system. The findings in these two studies will likely be followed by other discoveries of proteins not intrinsic to E3s regulating the all-important interface between ubiquitin ligases and their cognate E2s.
References
- Arai T, Kasper JS, Skaar JR, Ali SH, Takahashi C, DeCaprio JA. Proc Natl Acad Sci USA. 2003;100:9855–9860. doi: 10.1073/pnas.1733908100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo T, Pagano M. Nat Rev Mol Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- Duda DM, Olszewski JL, Tron AE, Hammel M, Lambert LJ, Waddell MB, Mittag T, Decaprio JA, Schulman BA. Mol Cell. 2012;47:371–382. doi: 10.1016/j.molcel.2012.05.044. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DM, Scott DC, Calabrese MF, Zimmerman ES, Zheng N, Schulman BA. Curr Opin Struct Biol. 2011;21:257–264. doi: 10.1016/j.sbi.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y, Huibregtse JM. Biochem Biophys Res Commun. 2007;354:329–333. doi: 10.1016/j.bbrc.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashkova N, Gakhar L, Winistorfer SC, Yu L, Ramaswamy S, Piper RC. Mol Cell. 2010;40:433–443. doi: 10.1016/j.molcel.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PE, Davies GC, Nau MM, Lipkowitz S. Trends Biochem Sci. 2006;31:79–88. doi: 10.1016/j.tibs.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Sarikas A, Hartmann T, Pan ZQ. Genome Biol. 2011;12:220. doi: 10.1186/gb-2011-12-4-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tron AE, Arai T, Duda DM, Kuwabara H, Olszewski JL, Fujiwara Y, Bahamon BN, Signoretti S, Schulman BA, DeCaprio JA. Mol Cell. 2012;46:67–78. doi: 10.1016/j.molcel.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AM, Shabek N, Ciechanover A. Nat Rev Mol Cell Biol. 2011;12:605–620. doi: 10.1038/nrm3173. [DOI] [PMC free article] [PubMed] [Google Scholar]