Abstract
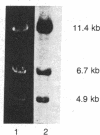
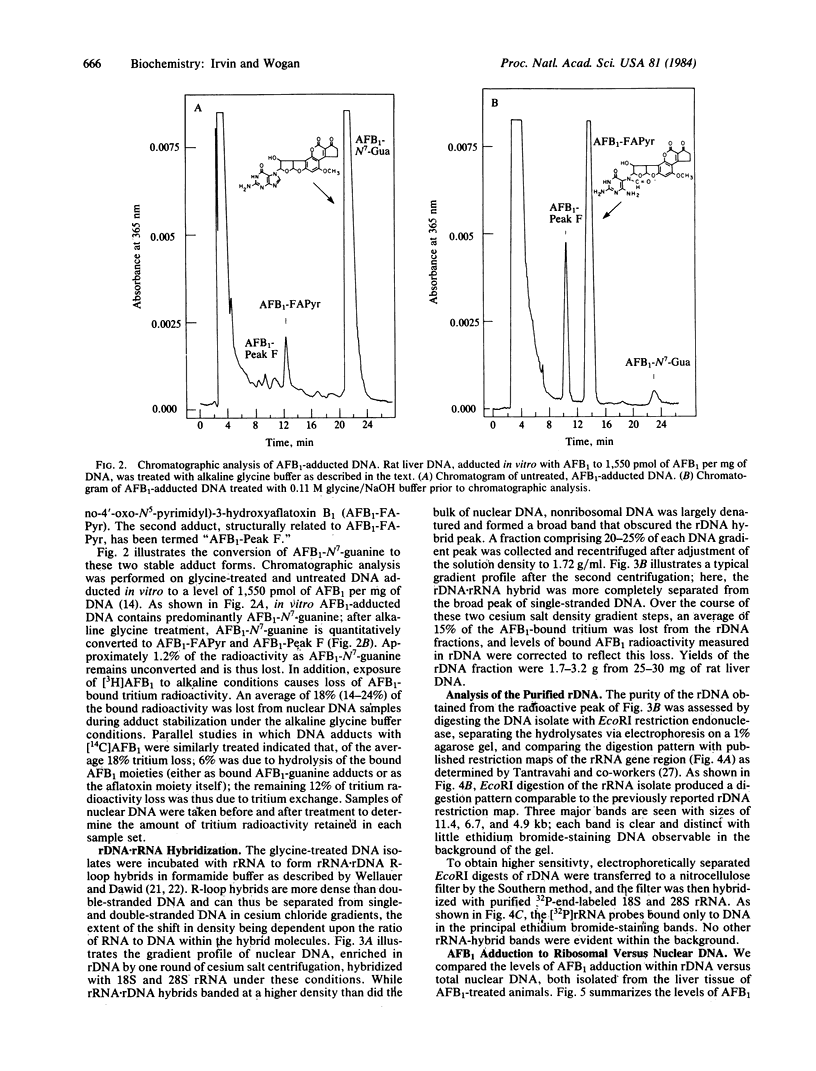
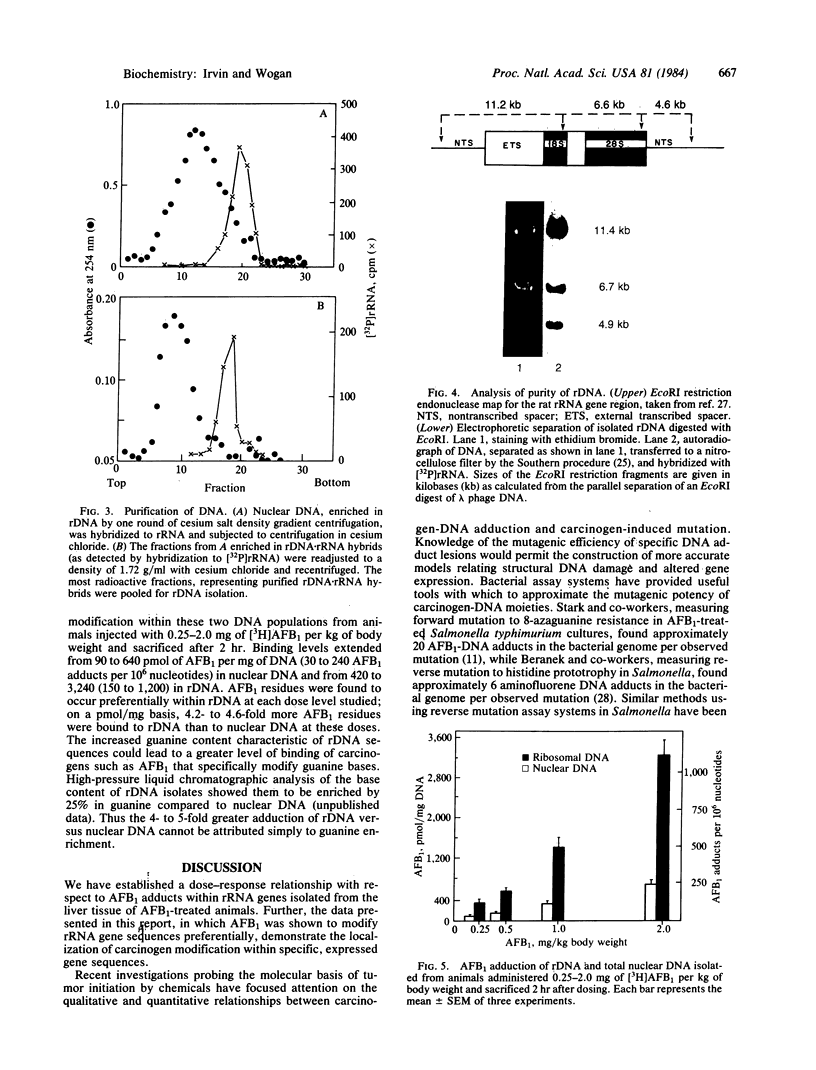
The in vivo formation of covalent aflatoxin B1 (AFB1)-DNA adducts within the rRNA gene sequences of nuclear DNA has been studied in AFB1-treated rats. Liver nuclear DNA, enriched in ribosomal DNA (rDNA) by one round of cesium salt density gradient centrifugation, was treated under buffered alkaline conditions to convert unstable AFB1-N7-guanine adducts to stable AFB1-formamidopyrimidine derivatives. The alkali-treated DNA was hybridized to 18S and 28S rRNA in 70% formamide buffer to form rRNA X rDNA hybrids. These hybrids were separated from the bulk of nuclear DNA by two rounds of centrifugation in CsCl, and the level of AFB1 adduction to rDNA versus total nuclear DNA was compared as a function of dose 2 hr after AFB1 administration. Over an 8-fold dose range (0.25-2.0 mg of AFB1 per kg of body weight), rDNA contained 4- to 5-fold more AFB1 residues than nuclear DNA, indicating that rDNA is preferentially accessible to carcinogen modification in vivo. While aflatoxin B1 forms adducts with DNA principally at guanine residues, the guanine enrichment of rDNA was insufficient to explain the magnitude of observed preferential AFB1 modification of rDNA. These results support the hypothesis that rDNA regions are preferentially accessible to carcinogen modification because of the diffuse conformation maintained within transcribed genes. This experimental approach permits the quantitative description of carcinogen modification within a defined gene sequence; further refinement of this approach may be useful in defining the precise relationships between covalent chemical-DNA interactions and the alterations in gene expression that result.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranek D. T., White G. L., Heflich R. H., Beland F. A. Aminofluorene-DNA adduct formation in Salmonella typhimurium exposed to the carcinogen N-hydroxy-2-acetylaminofluorene. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5175–5178. doi: 10.1073/pnas.79.17.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Croy R. G., Essigmann J. M., Reinhold V. N., Wogan G. N. Identification of the principal aflatoxin B1-DNA adduct formed in vivo in rat liver. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1745–1749. doi: 10.1073/pnas.75.4.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essigmann J. M., Croy R. G., Nadzan A. M., Busby W. F., Jr, Reinhold V. N., Büchi G., Wogan G. N. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci U S A. 1977 May;74(5):1870–1874. doi: 10.1073/pnas.74.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahl W. E., Scarpelli D. G., Gill K. Relationship between benzo(a)pyrene-induced DNA base modification and frequency of reverse mutations in mutant strains of Salmonella typhimurium. Cancer Res. 1981 Sep;41(9 Pt 1):3400–3406. [PubMed] [Google Scholar]
- Fahl W. E., Scarpelli D. G., Gill K. Relationship between benzo(a)pyrene-induced DNA base modification and frequency of reverse mutations in mutant strains of Salmonella typhimurium. Cancer Res. 1981 Sep;41(9 Pt 1):3400–3406. [PubMed] [Google Scholar]
- Groopman J. D., Croy R. G., Wogan G. N. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5445–5449. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groopman J. D., Croy R. G., Wogan G. N. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5445–5449. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. K., Kennan K. A., Miller E. C., Miller J. A. Reduced nicotinamide adenine dinucleotide phosphate-dependent formation of 2,3-dihydro-2,3-dihydroxyaflatoxin B1 from aflatoxin B1 by hepatic microsomes. Cancer Res. 1978 Aug;38(8):2424–2428. [PubMed] [Google Scholar]
- Lutz W. K. Constitutive and carcinogen-derived DNA binding as a basis for the assessment of potency of chemical carcinogens. Adv Exp Med Biol. 1981;136(Pt B):1349–1365. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E., Gurdon J. B. Purified DNAs are transcribed after microinjection into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1502–1506. doi: 10.1073/pnas.74.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R. P., Muench K. F., Humayun M. Z. Covalent and noncovalent interactions of aflatoxin with defined deoxyribonucleic acid sequences. Biochemistry. 1983 Jul 5;22(14):3351–3359. doi: 10.1021/bi00283a008. [DOI] [PubMed] [Google Scholar]
- Muench K. F., Misra R. P., Humayun M. Z. Sequence specificity in aflatoxin B1--DNA interactions. Proc Natl Acad Sci U S A. 1983 Jan;80(1):6–10. doi: 10.1073/pnas.80.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchase I. F., Longstaff E., Ashby J., Styles J. A., Anderson D., Lefevre P. A., Westwood F. R. Evaluation of six short term tests for detecting organic chemical carcinogens and recommendations for their use. Nature. 1976 Dec 16;264(5587):624–627. doi: 10.1038/264624a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stark A. A., Essigmann J. M., Demain A. L., Skopek T. R., Wogan G. N. Aflatoxin B1 mutagenesis, DNA binding, and adduct formation in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1343–1347. doi: 10.1073/pnas.76.3.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz D. R., Poirier L. A., Irving C. C., Stich H. F., Weisburger J. H., Grice H. C. Evaluation of short-term tests for carcinogenicity. Toxicol Appl Pharmacol. 1974 Aug;29(2):157–180. doi: 10.1016/0041-008x(74)90054-4. [DOI] [PubMed] [Google Scholar]
- Tantravahi U., Guntaka R. V., Erlanger B. F., Miller O. J. Amplified ribosomal RNA genes in a rat hepatoma cell line are enriched in 5-methylcytosine. Proc Natl Acad Sci U S A. 1981 Jan;78(1):489–493. doi: 10.1073/pnas.78.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Isolation and sequence organization of human ribosomal DNA. J Mol Biol. 1979 Mar 5;128(3):289–303. doi: 10.1016/0022-2836(79)90089-5. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- Wong J. J., Hsieh D. P. Mutagenicity of aflatoxins related to their metabolism and carcinogenic potential. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2241–2244. doi: 10.1073/pnas.73.7.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. L. Mechanism of aflatoxin B1 inhibition of rat hepatic nuclear RNA synthesis. J Biol Chem. 1977 May 25;252(10):3245–3251. [PubMed] [Google Scholar]