Abstract
HIV-1 capsid protein (CA) encloses the viral RNA genome and forms a conical-shaped particle in the mature HIV-1 virion, with orderly capsid assembly and disassembly critically important for viral infectivity. The 231 residue CA is composed of two helical domains, connected by a short linker sequence. In solution, CA exhibits concentration dependent dimerization which is mediated by the C-terminal domain (CTD). Here, we present nearly complete 1H, 15N and 13C assignments for the 20 kDa homodimeric CA–CTD, a prerequisite for structural characterization of the CA–CTD dimer.
Keywords: HIV-1, Capsid protein, CA, C-terminal domain, Dimerization
Biological context
Concomitant with release of HIV from the infected cell, the viral protease cleaves the Gag polyprotein into its individual components, which rearrange to form a mature, conical shaped viral protein shell, the capsid. HIV-1 capsid is composed of about 1,500 capsid proteins (CA). Elegant cryo-electron microscopy structural analyses provided a CA hexameric molecular model for mature HIV-1 capsid (Ganser et al. 1999; Li et al. 2000) in which the interactions between the N-terminal domains (NTD) of CA create the hexameric assembly while the C-terminal domains (CTD) of CA connect neighboring hexamers.
In solution, HIV-1 full-length CA dimerizes via its CTD with a Kd of 18 μM, and Trp184 and Met185 were identified as crucial residues for dimerization (Gamble et al. 1997). Although many crystal structures of dimeric CTD are available (Gamble et al. 1997; Ternois et al. 2005), these CTD structures display considerable variability with regard to the relative orientations of the two monomers, while the structures of the individual monomeric units are very similar. No solution structure or NMR assignment of a CTD dimer has been reported to date, and previous structural characterizations of monomeric CTD by NMR made use of W184A and W184A/M185A mutants, that are deficient for dimerization (Alcaraz et al. 2007; Wong et al. 2008). Furthermore, none of the dimer crystal structures exhibited a good fit to the recently reported EM density map of 2D sheets comprising hexameric in vitro assembled capsids (Ganser-Pornillos et al. 2007). Here we report 1H, 15N and 13C assignments for the dimeric CTD of HIV-1 CA, prerequisites for the determination of the solution structure of the CTD dimer and for NMR screening studies of potential capsid assembly inhibitors targeting the CTD dimer interface.
Methods and experiments
The cDNA encoding the gag polyprotein, pr55gag, was obtained from the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. The region encoding the CA–CTD (Met144-Leu231) was amplified and subcloned into pET21 (EMD chemicals, Inc. San Diego, CA) using NdeI and XhoI sites. The construct encodes CA–CTD with only native sequences. 13C/15N-labeled CA–CTD protein was expressed in E. coli, Rosetta 2 (DE3), cultured in modified minimal medium using 15NH4Cl and 13C6-glucose as the sole nitrogen and carbon sources, respectively, and induced with 0.4 mM IPTG at 23°C for 16 h. The protein was purified over a 5 ml Hi-Trap QP column (GE Healthcare, Piscataway, NJ) in 25 mM sodium phosphate, pH 7.0, 1 mM DTT, and 0.02% sodium azide, followed by further purification over 5 ml Hi-Trap SP columns (GE Healthcare) using a 0–1 M NaCl gradient in 25 mM sodium phosphate, pH 5.8, 1 mM DTT, and 0.02% sodium azide. The final purification was performed by gel-filtration over Hi-Load Superdex 200 16/60 columns (GE Healthcare) in 25 mM sodium phosphate, pH 6.5, 100 mM NaCl, 1 mM DTT, and 0.02% sodium azide. Molecular mass of the purified CA–CTD was confirmed by LC-TOF mass spectrometry (Bruker Daltonics, Billerica, MA).
The NMR sample contained 2 mM 13C/15N-labeled CA–CTD in 25 mM sodium phosphate buffer, pH 6.5, with 0.02% azide and 2 mM DTT. CA–CTD exists predominantly as a dimer (~95%) under the condition. All NMR spectra were recorded at 25°C using Bruker AVANCE900, 800 and 700 spectrometers, equipped with 5 mm triple-resonance and z-axis gradient cryoprobes. Backbone and side chain resonance assignments were carried out using 2D 1H-15N HSQC and 1H-13C HSQC and 3D HNCACB, HN(CO)CACB, HNCA, HN(CO)CA, HBHA(CO)NH and HCCH-TOCSY experiments (Bax and Grzesiek 1993; Clore and Gronenborn 1998). 3D simultaneous 13C- and 15N-edited NOESY (Sattler et al. 1995), 13C-edited NOESY, and 2D NOESY data were used to obtain complete side chain assignments especially for aromatic residues.
Assignments and data deposition
The CA–CTD protein used for the NMR assignments contained CA residues Met144-Leu231 without any added amino acids from the cloning procedure. Using 1H–15N HSQC spectra of the CA–CTD samples at several different concentrations ranging from 12.4 μM to 2 mM, we determined the dimerization constant for CA–CTD as Kd = 9.8 ± 0.6 μM (I. -J. L. Byeon, J. Jung, J. Ahn, J. Concel, and A. M. Gronenborn, unpublished data). This value is similar to the one previously reported value (10 ± 3 μM) from equilibrium sedimentation studies on the CA–CTD construct containing residues Ser146-Leu231 (Gamble et al. 1997). At submillimolar concentrations, many resonances in the 1H–15N HSQC and 1H–13C HSQC spectra exhibited severe line broadening and were, therefore, of low intensity, caused by milli- and micro-second timescale exchange involving the association/dissociation of CA–CTD (I.-J. L. Byeon, J. Jung, J. Ahn, J. Concel, and A. M. Gronenborn, unpublished data). However, using highly concentrated CA–CTD samples (≥2 mM) that contain predominantly dimeric CA–CTD (≥95%), it was possible to record spectra of sufficient quality to permit total NMR assignments. Nevertheless, the large variability in intensity due to dimer-monomer exchange can clearly be observed in the 1H–15N HSQC (Fig. 1a) and 1H–13C HSQC (Fig. 1b) spectra. Resonances of residues that reside in the dimer interface (colored gold in the structure insert in Fig. 1a) exhibited severe line broadening at low concentrations and thus could only be observed at concentrations >2 mM in a predominately dimeric sample (Fig. 1a). Note, that for several resonances large chemical shift differences between the monomer and dimer are observed. For example, the Tyr 169 amide of the dimeric species resonates at 9.10 ppm (1H)/117.6 ppm (15N) while the reported frequencies for a monomer mutant are 8.66 ppm (1H)/116.5 ppm (15N) (Wong at al. 2008).
Fig. 1.
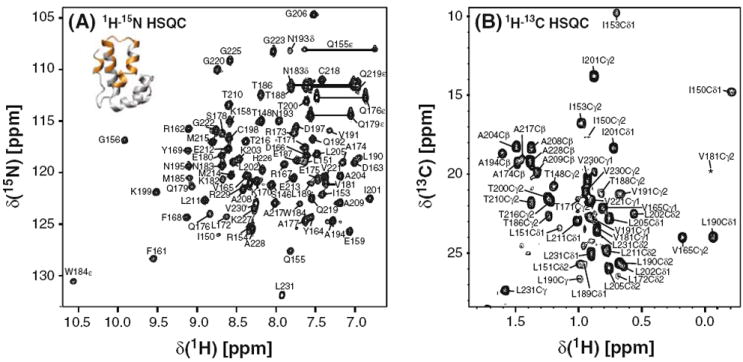
2D 1H–15N HSQC (a) and 1H–13C HSQC (b) spectra recorded using a 2 mM CA–CTD sample at 25°C. a The 1H–15N HSQC spectrum at 800 MHz. b A selected region of 1H–13C HSQC spectrum at 900 MHz showing mainly Ala, Thr, Leu, Val and Ile methyl resonances. The insert in a shows a ribbon diagram of the CA–CTD monomer in which backbone amides for which resonances are only observed in the dimer state are colored in gold
In summary, all backbone 1H–15N resonances, except for Met144, Tyr145 and Ser149, and more than 95% of all CA–CTD resonances were assigned. Assignments have been deposited in the BMRB database at Madison, WI, with accession number 16555.
Acknowledgments
We thank Dr. Teresa Brosenitsch for critical reading of the manuscript. This work was supported by the National Institutes of General Medical Sciences (NIH Grant P50GM082251) and is a contribution from the Pittsburgh Center for HIV Protein Interactions.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- Alcaraz LA, del Alamo M, Barrera FN, Mateu MG, Neira JL. Flexibility in HIV-1 assembly subunits: solution structure of the monomeric C-terminal domain of the capsid protein. Biophys J. 2007;93:1264–1276. doi: 10.1529/biophysj.106.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax A, Grzesiek S. Methodological advances in protein NMR. Acc Chem Res. 1993;26:131–138. [Google Scholar]
- Clore GM, Gronenborn AM. Determining the structures of large proteins and protein complexes by NMR. Trends Biotechnol. 1998;16:22–34. doi: 10.1016/S0167-7799(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Gamble TR, Yoo S, Vajdos FF, von Schwedler UK, Worthylake DK, Wang H, McCutcheon JP, Sundquist WI, Hill CP. Structure of the carboxyl-terminal dimerization domain of the HIV-1 capsid protein. Science. 1997;278:849–853. doi: 10.1126/science.278.5339.849. [DOI] [PubMed] [Google Scholar]
- Ganser BK, Li S, Klishko VY, Finch JT, Sundquist WI. Assembly and analysis of conical models for the HIV-1 core. Science. 1999;283:80–83. doi: 10.1126/science.283.5398.80. [DOI] [PubMed] [Google Scholar]
- Ganser-Pornillos BK, Cheng A, Yeager M. Structure of full-length HIV-1 CA: a model for the mature capsid lattice. Cell. 2007;131:70–79. doi: 10.1016/j.cell.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Li S, Hill CP, Sundquist WI, Finch JT. Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature. 2000;407:409–413. doi: 10.1038/35030177. [DOI] [PubMed] [Google Scholar]
- Sattler M, Maurer M, Schleucher J, Griesinger C. A Simultaneous 15N, 1H-HSQC and 13C, 1H-HSQC with sensitivity enhancement and a heteronuclear gradient-echo. J Biomol NMR. 1995;5:97–102. doi: 10.1007/BF00227475. [DOI] [PubMed] [Google Scholar]
- Ternois F, Sticht J, Duquerroy S, Krausslich HG, Rey FA. The HIV-1 capsid protein C-terminal domain in complex with a virus assembly inhibitor. Nat Struct Mol Biol. 2005;12:678–682. doi: 10.1038/nsmb967. [DOI] [PubMed] [Google Scholar]
- Wong HC, Shin R, Krishna NR. Solution structure of a double mutant of the carboxy-terminal dimerization domain of the HIV-1 capsid protein. Biochemistry. 2008;47:2289–2297. doi: 10.1021/bi7022128. [DOI] [PubMed] [Google Scholar]


