Abstract
Ixodes scapularis transmits the agent of human granulocytic anaplasmosis, among other pathogens. The mechanisms used by the tick to control Anaplasma phagocytophilum are not known. We demonstrate that the I. scapularis Janus kinase (JAK)–signaling transducer activator of transcription (STAT) pathway plays a critical role in A. phagocytophilum infection of ticks. The A. phagocytophilum burden increases in salivary glands and hemolymph when the JAK-STAT pathway is suppressed by RNA interference. The JAK-STAT pathway exerts its anti-Anaplasma activity presumably through STAT-regulated effectors. A salivary gland gene family encoding 5.3-kDa antimicrobial peptides is highly induced upon A. phagocytophilum infection of tick salivary glands. Gene expression and electrophoretic mobility shift assays showed that the 5.3-kDa antimicrobial peptide–encoding genes are regulated by tick STAT. Silencing of these genes increased A. phagocytophilum infection of tick salivary glands and transmission to mammalian host. These data suggest that the JAK-STAT signaling pathway plays a key role in controlling A. phagocytophilum infection in ticks by regulating the expression of antimicrobial peptides.
The black-legged tick, Ixodes scapularis, is an important vector of several microbes [1–3], including Anaplasma phagocytophilum, the agent of human granulocytic anaplasmosis [4]. The tick innate immunity pathways that sense and respond to these pathogens are not well understood [5–7].
Arthropod innate immune responses are largely orchestrated by the Toll, immune deficiency (Imd), and Janus kinase (JAK)–signaling transducer activator of transcription (STAT) pathways [8–10]. The Toll pathway is mainly activated by gram-positive bacteria, fungi [11], and viruses [12] and largely controls the expression of antimicrobial peptides (AMPs). The Imd pathway is primarily responsive to gram-negative organisms and also controls the synthesis of several AMPs [13, 14]. The JAK-STAT pathway was originally identified as a cytokine-signaling pathway in mammals [15, 16] and has been shown to be a key player in antiviral defenses in humans [17], Drosophila [9], and Aedes aegypti [18]. The JAK-STAT signaling pathway has also been implicated in the control of bacteria in Drosophila [19, 20] and Plasmodium in mosquitoes [21]. In this study, we characterize how I. scapularis controls the obligate intracellular pathogen A. phagocytophilum.
MATERIALS AND METHODS
Cell Lines and A. phagocytophilum Infection
The promyelocytic cell line (HL-60) was acquired from the American Type Culture Collection (Manassas, VA) and maintained according to the supplier's specifications. A. phagocytophilum–infected cells were sustained as described previously [22]. A host cell–free preparation of A. phagocytophilum was obtained as described previously and used at a multiplicity of infection of 10 [23].
Ethics Statement
Female C3H/HeJ and C3H/SCID mice aged 4–6 weeks were purchased from The Jackson Laboratory. Animals were housed and handled under the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal experimental protocol was approved by Yale University's Institutional Animal Care & Use Committee (protocol permit number 2008-07941). All animal infection experiments were performed in a Biosafety Level 2 animal facility, in accordance with regulations of Yale University.
Infection of C3H/HeJ Mice With A. phagocytophilum
The A. phagocytophilum isolate NCH-1 [4] used in these studies was maintained through serial passage of infected blood in C3H/SCID mice [24]. Quantitative reverse-transcription polymerase chain reaction (qRT-PCR) was performed on an aliquot of blood collected from the SCID mice to quantify and standardize the infectious dose of A. phagocytophilum.
Ixodes Scapularis Ticks
To generate A. phagocytophilum–infected nymphs, the larvae fed to repletion on A. phagocytophilum–infected C3H/HeJ mice and molted into nymphs. To produce uninfected nymphs, larvae engorged on uninfected mice were then allowed to molt. Tick rearing was conducted in an incubator at 23°C with 85% relative humidity and a 14/10-hour light/dark photo-period regimen. Ten percent of the molted nymphs from each infection group were individually tested by qRT-PCR to confirm infection and determine infection prevalence.
RNA Interference (RNAi)
STAT, JAK, and 5.3-kD peptide gene-15 were amplified from complementary DNA (cDNA) from tick salivary glands, using the primers containing the T7 polymerase promoter sequence, as listed in Supplementary Table 1. The amplified DNA fragment was purified by a PCR product purification kit (Qiagen, CA), and double-stranded RNA (dsRNA) was synthesized using the Megascript RNAi kit (Ambion, TX). The dsRNA inoculation was performed as previously described [25]. A total of 5 nL (5 × 1012 molecules/μL) of dsRNA was injected into uninfected or A. phagocytophilum–infected nymphal ticks. The control ticks (ie, the mock-infected group) received 5 nL of injection buffer (10 mM Tris-HCl [pH 7.5] and 1 mM ethylenediaminetetraacetic acid [EDTA]). Ticks were allowed to feed for specific periods and were then collected for dissection. cDNA was made from salivary glands, midgut, and hemolymph pooled from 3 ticks.
Acquisition
For the acquisition experiments, control (ie, buffer-injected) ticks and ticks injected with dsRNA were allowed to feed on A. phagocytophilum–infected C3H/HeJ mice for 72 hours. Five mice were used in each group (experimental and control/mock), and 10 ticks were placed on each mouse. cDNA was made from salivary glands and hemolymph (pooled from 3 ticks), as described above.
Transmission
A. phagocytophilum–infected and STAT- or 5.3-kD peptide gene-15–deficient or control nymphs were allowed to feed on 10 naive C3H/HeJ mice (3–4 ticks per mouse). Once the ticks fed to repletion, the mice were bled on days 3, 5, and 7. Total genomic DNA was isolated from the peripheral blood using the DNeasy tissue kit (Qiagen), and the level of A. phagocytophilum 16S ribosomal DNA was measured by qRT-PCR.
Protein Expression and Preparation of Polyclonal Antibody
The full length of STAT was amplified using primers rSTATF and rSTATR (Supplementary Table 1) and was cloned into pGEX6P-2 vector (GE Healthcare, NJ) for bacterial expression. The recombinant protein fused with a glutathione S-transferase (GST) tag was purified by a glutathione–Sepharose 4B column (GE Healthcare) according to the manufacturer's protocol. The GST fusion tag was cleaved with PreScission Protease (GE Healthcare). Polyclonal antibodies against bacterially expressed STAT (henceforth referred to as “rSTAT”), was generated in rabbits using standard protocols. Full-length stat was also cloned into pMT/V5/His vector (Invitrogen, CA) for Drosophila S2 expression. STAT was expressed in a Drosophila S2 cells (Invitrogen) and purified using Talon affinity chromatography (Clontech Laboratories, CA) and is henceforth referred to as “rSTAT-DES.” rSTAT-DES was used for functional studies.
Immunoblots
Cell lysate or salivary gland extracts were electrophoresed on a sodium dodecyl sulfate 4%–15% polyacrylamide gel and processed for immunoblotting. rSTAT antisera was used to probe the tick STAT. Anti-FLAG immunoglobulin G (IgG; Sigma, USA) was use to probe the 5.3-kD peptide gene-15 and control gene, which encoded bacterial alkaline phosphatase (BAP), overexpressed in HL-60 cells. The bound antibodies were detected by using horseradish peroxidase–conjugated rabbit anti-mouse or goat anti-rabbit secondary antibodies (Sigma), and the blots were developed as previously described [26].
Electrophoretic Mobility Shift Assay (EMSA) and DNA Pulldown
EMSA was performed to examine the binding activity of tick STAT to the 5.3-kD peptide gene-15 promoter region according to the manufacturer's protocol (Pierce, USA). Biotinylated probes are listed in Supplementary Table 1. The interaction between tick STAT and the 5.3-kD peptide gene-15 promoter was performed by streptavidin-agarose pulldown analysis, as previously described [27]. Biotinylated probe and scrambled oligonucleotides were coupled to streptavidin-coated agarose beads (Pierce). We washed 400-μL beads twice with phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) and 1 M NaCl and then with TE buffer (10 mM Tris-Cl and 1 mM EDTA [pH 8.0]). Next, 250 pmol of oligonucleotides diluted in 0.5 mL of TE buffer were added to the washed beads and incubated with rotation for 30 minutes at room temperature. Unbound oligonucleotides were removed by washing with TE buffer. rSTAT-DES or tick salivary gland nuclear extracts were incubated with beads for 1 hour at room temperature with rotation. After incubation, unbound proteins were removed by washing the beads with PBS (1% BSA). Beads were collected and proteins were recovered by boiling in Laemmli sample buffer. Protein samples were analyzed by immunoblotting, as described above.
5.3-kD Peptide gene-15 Overexpression in HL-60 Cells
cDNA of 5.3-kD peptide gene-15 was amplified using primers g15FLAGF and g15FLAGR (Supplementary Table 1) and cloned into p3xFlag-CMV expression vector (Sigma). The recombinant plasmid was transfected into HL-60 cells, using the Nucleofector Kit V (Lonza, Germany) according to the manufacturer's instruction. The cells were infected with A. phagocytophilum 2 days after transfection.
Confocal Microscopy
The levels of A. phagocytophilum within the HL-60 cells were analyzed by confocal microscopy, as described previously [26]. In brief, the acetone-fixed cells were incubated with anti-P44 antisera followed by incubation with Alexa555-conjugated secondary antibody. The samples were counterstained with nuclear stain TO-PRO-3 (Invitrogen) and viewed under a LSM510 scanning laser confocal microscope (Carl Zeiss MicroImaging, Germany).
Statistical Analysis
The statistical significance of differences observed in experimental and control groups was analyzed using GraphPad Prism, version 4.00 (GraphPad Software, CA). A 2-tailed Student t test and Mann-Whitney U test was used to compare the median values, and a P value of < .05 was considered statistically significant.
Bioinformatic Analysis
In silico analysis of the primary structure of STAT was performed using the Basic Local Alignment Search Tool (available at: http://www.ncbi.nlm.nih.gov/blast) to search for homology with proteins in the public database. The alignment and phylogenetic tree with related proteins were constructed using MEGA 5.0 software.
RESULTS
The JAK-STAT Pathway Controls A. phagocytophilum Infection in I. scapularis
The immune responses of I. scapularis ticks to microbes are poorly understood. The Toll, Imd, and JAK-STAT pathways represent the major innate signaling pathways in arthropods [10, 28]. The completion of the I. scapularis genome sequence [29] and comparative analyses with the genome sequences of Drosophila melanogaster and mosquitoes have revealed orthologs for Toll, the core JAK-STAT, and some of the Imd pathway components in ticks. According to the I. scapularis genome sequence, 13 genes were annotated as putative Toll. One of the genes, which we name Toll-1 (ISCW018193), has most identity (30%) with D. melanogaster Toll (FBgn0262473). We therefore selected Toll-1 as representative gene for studying the tick Toll pathway. We first assessed the potential roles of these 3 pathways by RNAi-mediated silencing of I. scapularis STAT (ISCW005692), Toll-1 (ISCW018193), or transforming growth factor β (TGF-β)–activated kinase 1 (TAK1; ISCW023496) and TAK1 adaptor protein 1 (TAB1) (ISCW009346) in Anaplasma-infected ticks. Toll-1, TAK1, or TAB1 knockdown ticks did not show any significant effect on the A. phagocytophilum burden in salivary glands, compared with buffer-injected ticks (the mock-infected group) (Supplementary Figure 1A and B). However, STAT knockdown ticks showed a significantly increased A. phagocytophilum burden (Figure 1A and B). The knockdown efficiency was significant in the STAT dsRNA injected group, compared with the mock-infected group (Figure 1C and D). We therefore examined in further detail the role of the JAK-STAT signaling pathway in A. phagocytophilum infection.
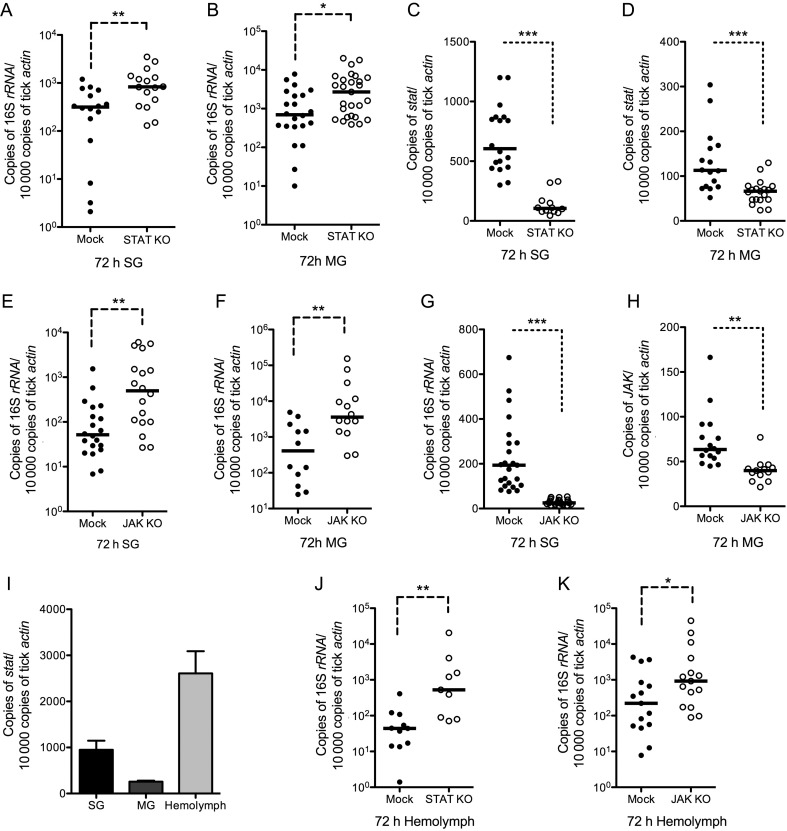
Figure 1.
The Janus kinase (JAK)–signaling transducer activator of transcription (STAT) pathway modulates Ixodes ticks susceptibility to Anaplasma phagocytophilum infection. A. phagocytophilum burden in the salivary glands, midguts, and hemolymph of STAT double-stranded RNA (dsRNA; A, B, and J) and JAK dsRNA (E, F, and K)–injected ticks and the knockdown efficiency of STAT (C and D) and JAK (G and H) in tick salivary glands and midguts were determined by quantitative reverse-transcription polymerase chain reaction 3 days after ticks fed on A. phagocytophilum–infected mice. Buffer-injected ticks were used as control (Mock). I, The expression profile of tick stat in the salivary glands (SG), midgut (MG), and hemolymph. Three independent experiments yielded similar results. The horizontal line represents the medians. Error bars show means ± standard error of the mean. *P < .05, **P < .01, and ***P < .001. Three independent experiments yielded similar results.
RNAi-mediated silencing of STAT in nymphal ticks that fed on A. phagocytophilum–infected mice showed a significantly increased A. phagocytophilum burden in the salivary glands and midguts of STAT-deficient ticks, compared with buffer-injected ticks (Figure 1A and B). To confirm the function of the STAT pathway in controlling A. phagocytophilum infection, another key component of STAT pathway, JAK (ISCW016158), was knocked down by RNAi (Figure 1G and H). The result indicated that the A. phagocytophilum burden also increased in the salivary glands and midguts of JAK-deficient ticks as compared to the buffer-injected ticks, which is consistent with the STAT knockdown phenotype (Figure 1E and F).
Gene expression analysis indicated that STAT could be detected in tick salivary glands, midguts, and hemolymph, tissues that are important for A. phagocytophilum infection of ticks (Figure 1I). Hemolymph is an immunocompetent tissue [13] and mounts critical innate immune responses to invading microbes. A. phagocytophilum has been shown to infect Ixodes tick hemocytes, which is essential for successful infection of salivary glands [26]. We therefore examined whether the STAT pathway could also control the pathogen in hemolymph. The results showed that the A. phagocytophilum burden in tick hemolymph significantly increased in STAT- or JAK-deficient ticks, compared with control groups (Figure 1J and K). These data suggested that the JAK-STAT pathway potentially functions to control the A. phagocytophilum burden in Ixodes ticks.
A Salivary Gland Gene Family Encoding 5.3-kD Proteins Is Involved in Anti-A. phagocytophilum Defense
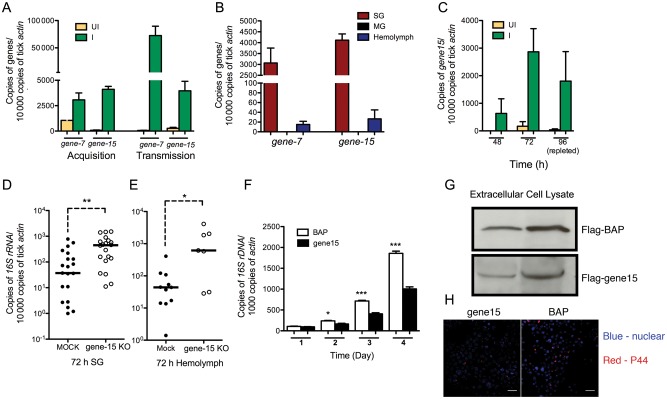
In our previous work, several I. scapularis salivary gland proteins that were upregulated in A. phagocytophilum–infected tick salivary glands were identified by 2-dimensional fluorescence difference gel electrophoresis [30]. Among them, the messenger RNA (mRNA) levels of gene-7 and gene-15, which belong to a 5.3-kD salivary gland peptide gene family, were dramatically increased in tick salivary glands during A. phagocytophilum acquisition and transmission (Figure 2A). These genes were highly expressed in tick salivary glands and could also be detected in hemolymph but were undetectable in tick midguts (Figure 2B). We selected gene-15 as a representative member of the family to study the role of 5.3-kD peptide encoding genes in A. phagocytophilum infection. qRT-PCR results showed that gene-15 was not expressed in clean ticks during feeding but was highly induced in salivary glands of clean ticks that fed on A. phagocytophilum–infected mice (Figure 2C). RNAi results showed that the A. phagocytophilum burden in both salivary glands and hemolymph was significantly increased in gene-15–deficient ticks, compared with the mock-infected group (Figure 2D and E).
Figure 2.
A 5.3-kD salivary gland peptide gene family involved in anti-Anaplasma phagocytophilum defense. A, Expression profiles of 5.3-kD peptide gene-7 and gene-15 in tick salivary glands during A. phagocytophilum acquisition and transmission. B, Tissue-specific expression of 5.3-kD peptide genes in ticks salivary glands (SGs), midguts (MGs), and hemolymph. C, 5.3-kD peptide gene-15 expression profiles at 48 hours, 72 hours, and 96 hours in tick salivary glands during A. phagocytophilum acquisition. D and E, A. phagocytophilum burden in the salivary glands and hemolymph of 5.3-kD peptide gene-15–deficient ticks. F, Overexpression of 5.3-kD peptide gene-15 in HL-60 cells and extracellular medium could be detected by immunoblot; BAP was used as control. G, A. phagocytophilum burden in BAP and 5.3-kD peptide gene-15–overexpressed HL-60 cells. H, Confocal microscopy of A. phagocytophilum in control (bacterial alkaline phosphatase) and 5.3-kD peptide gene-15–overexpressed HL-60 cells. Error bars show means ± standard error of the mean. *P < .05 and ***P < .001. Three independent experiments yielded similar results. Scale bar represents 20 μm.
Pichu et al identified a 5.3-kD novel secreted salivary AMP from I. scapularis [31]. Bioinformatics analysis showed that this protein and the 5.3-kD peptides we identified belong to the same gene family (Supplementary Figure 2), suggesting that these 5.3-kD peptide genes might belong to a novel AMP gene family.
We then determined whether the 5.3-kD peptide could inhibit A. phagocytophilum infection in vitro, using a HL-60 cell line [26]. As a representative, gene-15 was cloned into a mammalian-secreted protein expression vector, p3xFlag-CMV, and transfected into HL-60 cells. The expression of gene-15 in HL-60 cells was confirmed by immunoblotting from protein extracts of HL-60 cells and the extracellular medium 2 days after transfection with anti-FLAG IgG (Figure 2G). The expression vector with BAP was used as control. Cell-free A. phagocytophilum was added into the HL-60 cells 2 days after transfection. qRT-PCR assessment of the A. phagocytophilum burden showed a significant decrease in the HL-60 cells that overexpressed 5.3-kD peptide Gene-15, compared with the control group (Figure 2F). Assessment of bacterial burden by confocal microscopy also showed decreased numbers of bacteria in HL-60 cells expressing the 5.3-kD peptide Gene-15 (Figure 2H).
A. phagocytophilum Transmission Was Enhanced in STAT-Deficient Ticks
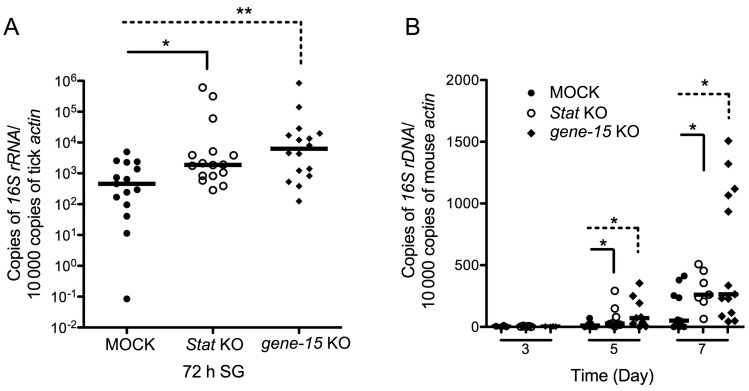
We also examined whether the STAT pathway and 5.3-kD peptide were involved in controlling A. phagocytophilum infection during Anaplasma transmission from infected ticks to a mammalian host. The expression of stat or 5.3-kD peptide encoding gene-15 was silenced by RNAi, and the pathogen burden was examined by qRT-PCR. The results showed that the burden of bacteria increased in tick salivary glands in stat- or gene-15–deficient ticks, compared with the mock-infected group (Figure 3A). Consequently, the A. phagocytophilum burden in mouse blood was also significantly increased in the mice on which stat- or gene-15–deficient ticks fed, compared with the mice on which buffer-injected ticks fed (Figure 3B).
Figure 3.
Anaplasma phagocytophilum transmission was enhanced in signaling transducer activator of transcription (STAT)–deficient ticks. A, A. phagocytophilum burden in the salivary glands of stat and 5.3-kD gene-15–deficient ticks. B, A. phagocytophilum burden in the blood of mice on which STAT or gene-15–deficient ticks fed, 3, 5, and 7 days after feeding. Horizontal lines represent median values. *P < .05. Three independent experiments yielded similar results. Abbreviation: KO, knockout.
5.3-kD Peptides Represent One of the Effectors Regulated by Tick JAK-STAT Pathway
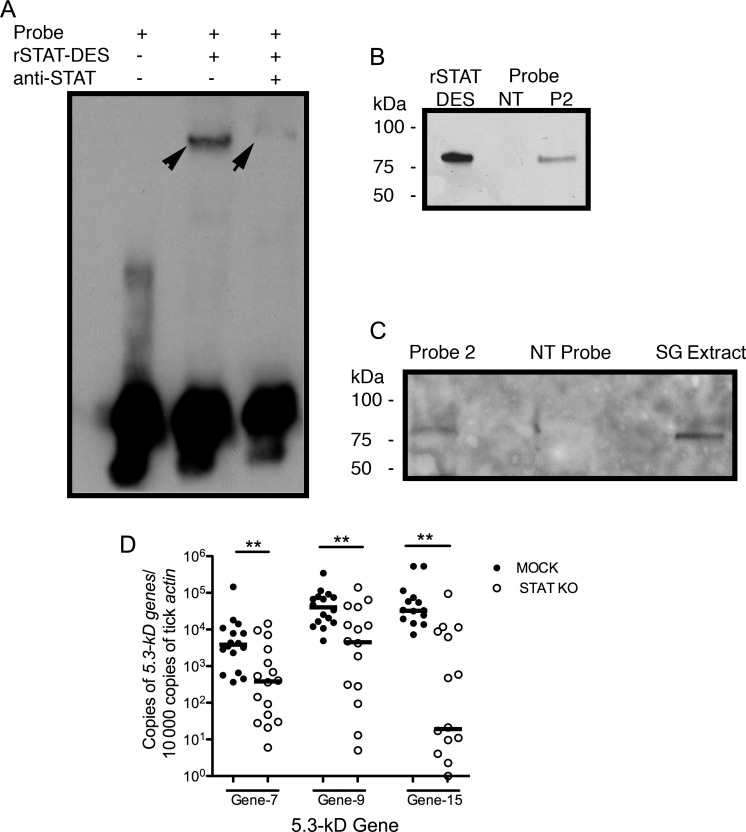
The upstream DNA sequence of the 5.3-kD peptide gene was analyzed, and 5 potential STAT-binding sites [32] were identified in the promoter region (Supplementary Figure 2). Biotin-labeled probes were designed, and EMSA was performed to assess the binding of tick STAT to the promoter of 5.3-kD peptide gene-15. The result suggested that STAT bound to the gene-15 promoter, as seen by the shift in mobility when STAT interacted with probe 2 (Figure 4A). DNA pulldown using purified recombinant tick STAT protein or tick salivary gland nuclear extract also confirmed the binding (Figure 4B and C). These results indicated that tick STAT might bind to the promoter region of 5.3-kD peptide gene and enhance the transcription of 5.3-kD peptide genes that encode AMPs detrimental to A. phagocytophilum survival in ticks.
Figure 4.
The 5.3-kD peptide genes are regulated by tick Janus kinase (JAK)–signaling transducer activator of transcription (STAT) pathway. A, Findings of an electrophoretic mobility shift assay. The shift could be detected when the rSTAT-DES protein was added (middle line). The anti-STAT antibody was used for the supershift assay (right line). B and C, DNA pulldown assay revealed that probe 2 could bind to rSTAT-DES protein (B) or the tick STAT from salivary gland extract (C). Nontarget (NT) probe was used as a control. D, The gene expression profiles of 5.3-kD peptide genes in the salivary glands of STAT-deficient ticks. Horizontal lines represent median values. **P < .01. Three independent experiments yielded similar results.
Further, the expression levels of 5.3-kD peptide genes in STAT-deficient ticks were examined by qRT-PCR. The results showed that the mRNA levels of 5.3-kD peptide genes were decreased in the salivary glands of STAT knockdown ticks, compared with the mock group (Figure 4D). This result, combined with results of EMSA and the DNA pulldown assay, indicated that the 5.3-kD peptide genes are likely regulated by tick STAT.
DISCUSSION
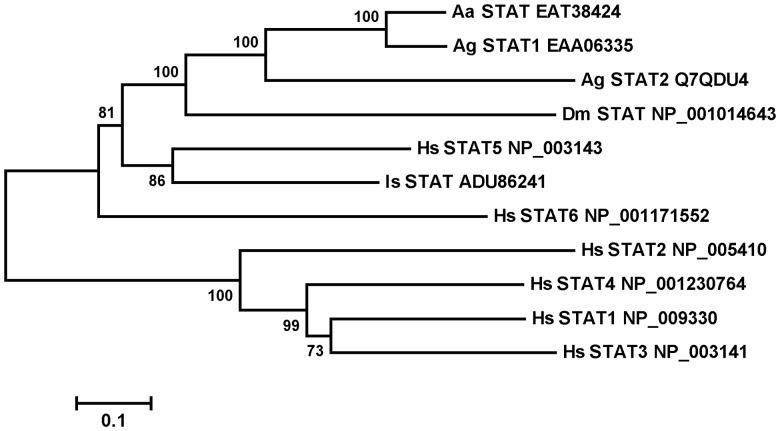
The evolutionarily conserved JAK-STAT signaling pathway mediates mammalian cytokine signaling and is involved in innate immune defense in mammals, flies, and mosquitoes [9, 17, 18]. Here, we show that the I. scapularis JAK-STAT pathway plays a predominant role in limiting the A. phagocytophilum burden in tick tissues. In Drosophila, the activation of the JAK-STAT pathway is initiated by the extracellular binding of the unpaired ligand Upd to the transmembrane receptor Domeless (DOME) [33, 34], and activated STAT translocates to the nucleus, where it initiates the transcription of target genes. While in silico analysis did not reveal an ortholog of Upd in the I. scapularis genome, orthologs of Domeless, JAK, and STAT were represented (available at: http://www.vectorbase.org). There is only one STAT ortholog represented in the assembled I. scapularis genome. However, the annotated tick stat gene sequence is not full length and lacks the 3′ exons, compared with full-length Drosophila STAT genes (FBgn0016917). Rapid amplification of 3′ cDNA ends was performed to amplify the 3′ sequence of tick STAT from I. scapularis salivary gland and midgut cDNA templates, and the full-length sequence of I. scapularis STAT cDNA was submitted to GenBank (accession number HQ710832). The alignment of the amino acid sequences of members of the STAT family from different species was used to build a phylogenetic tree (Figure 5). I. scapularis STAT showed highest homology to vertebrate STAT5 (Figure 5) and clustered with STAT orthologs from other insects (mosquito and Drosophila).
Figure 5.
Phylogenetic and gene expression analysis of Ixodes scapularis signaling transducer activator of transcription (STAT). A, Bootstrap neighbor-joining phylogenetic tree of tick STAT protein. The phylogenetic tree was constructed by using the neighbor-joining algorithm (MEGA 5.0) from full sequence alignments computed in ClustalX. Accession numbers and assigned names are used for Homo sapiens (Hs), Drosophila melanogaster (Dm), Aedes aegypti (Aa), Anopheles gambiae (Ag), and I. scapularis (Is).
The susceptibility of Ixodes ticks to A. phagocytophilum infection in salivary glands, hemolymph, and midguts increased when the JAK-STAT pathway was suppressed by RNAi-mediated depletion of JAK and STAT (Figure 1). We then examined whether the 5.3-kD AMP family identified in earlier studies [30, 31] might be one of the effectors activated by STAT. Two representative genes from this family, gene-7 and gene-15, were significantly increased upon A. phagocytophilum infection of ticks (Figure 2A). RNAi-mediated knockdown of gene-15 showed a significant increase in the A. phagocytophilum burden in tick tissues during pathogen acquisition from the murine host and also enhanced transmission to the murine host (Figure 2). Further, the 5.3-kDa peptide–deficient ticks phenocopied STAT knockdown ticks (Figure 2), and STAT knockdown ticks showed decreased expression of 5.3-kDa peptide–encoding genes (Figure 4). Finally, EMSA and DNA pulldown assays suggested that STAT might indeed bind to the promoter regions of the 5.3-kD peptide–encoding gene family (Figure 4A and B). Taken together, these observations implicate a major role for the I. scapularis JAK-STAT pathway in controlling the A. phagocytophilum burden in ticks. We also provide evidence that the 5.3-kDa antimicrobial peptide–encoding gene family might serve as one of the effectors of the STAT activation, providing an effective humoral response to restrict A. phagocytophilum survival in hemolymph and salivary glands.
The gene expression analysis showed that the 5.3-kD AMP–encoding genes are only expressed in tick salivary glands and hemolymph, not in midguts (Figure 2B). However, the A. phagocytophilum burden is increased in the guts of JAK or STAT knockdown ticks (Figure 1B and C), suggesting that the 5.3-kD AMPs are perhaps not the only effectors regulated by the STAT pathway. Additional JAK-STAT pathway–regulated A. phagocytophilum restriction factors in I. scapularis midguts might be revealed by global transcriptome analysis. Since the 5.3-kD AMP also decreased A. phagocytophilum burden in HL60 cells in vitro (Figure 2F and G), it is likely that the 5.3-kD salivary AMP secreted into the bite site might impair A. phagocytophilum survival at the bite site. This might, in part, account for the increased burden in the midguts of STAT or JAK knockdown ticks that fed on A. phagocytophilum–infected hosts (Figure 1) and increased transmission by STAT or 5.3-kD AMP gene-15 knockdown ticks to the murine host (Figure 3B).
In Drosophila, the Toll and Imd pathways represent the hallmark AMP-inducing pathways in response to fungi and gram-positive bacteria (for Toll) and to gram-negative bacteria (for Imd) [35]. While in silico analysis revealed components of the Toll and Imd pathways in the I. scapularis genome, RNAi-mediated knockdown of key components of the 2 pathways, namely Toll-1 [36] in the Toll pathway and TGF-β–activated kinase 1 (TAK1) and TAK1 adaptor protein 1 (TAB1), which are essential in the Drosophila Imd pathway [37, 38], did not demonstrate a significant impact on the A. phagocytophilum burden in ticks (Supplementary Figure 1). Gene expression analysis also indicated that the expression of 5.3-kD AMP genes was not altered in Toll-1– and TAB1-deficient ticks (Supplementary Figure 1F and G), suggesting that the 5.3-kD AMP genes are probably not under the control of Toll or Imd pathways in I. scapularis.
The 5.3-kD peptide genes differ from known defensins (Supplementary Figure 1H), presumed to be linear AMP [31]. The tissue expression profile showed that the 5.3-kD AMP genes are expressed in salivary glands and hemolymph but not in midguts (Figure 2B), in clear contrast to I. scapularis defensin, which is expressed mostly in midguts and fat bodies [39]. These results indicated that the 5.3-kD AMP genes belong to a novel AMP gene family. The members of the 5.3-kD AMP gene family were shown to be upregulated in ticks infected with Borrelia burgdorferi [40]. The role of these 5.3-kD AMPs in the context of B. burgdorferi infection of ticks remains to be determined.
Ticks are vectors for human and animal pathogens worldwide, and yet the innate immune responses of ticks to microbial pathogens are not well understood [7, 41]. We have demonstrated for the first time that the I. scapularis JAK-STAT signaling pathway is triggered in response to A. phagocytophilum and limits the pathogen burden. It would be interesting to determine whether the JAK-STAT pathway might play a role in the control of other related vector-borne Rickettsia pathogens. Understanding these mechanisms might offer new strategies to control ticks and prevent the transmission of pathogens by ticks.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Jinyi Pan, for providing technical support during mice and tick experiments, and Lindsay Rollend, for maintaining and providing uninfected and A. phagocytophilum–infected I. scapularis nymphal ticks.
L. L. and J. D. designed the research protocol; L. L., J. D., Y. O. Z., Y. Y., and L. Z. performed the research; L. L., J. D., Y. O. Z., S. N., Y. Y., and L. Z. analyzed data; and L. L. and E. F. wrote the paper.
Financial support. This work was supported by the National Institutes of Health (grant R01AI041440). E. F. is an investigator of the Howard Hughes Medical Institute. J. Dai was supported by a project funded by the Priority Academic Program, Development of Jiangsu Higher Education Institutions and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1075).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–9. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2.Mather TN, Telford SR, 3rd, Moore SI, Spielman A. Borrelia burgdorferi and Babesia microti: efficiency of transmission from reservoirs to vector ticks (Ixodes dammini) Exp Parasitol. 1990;70:55–61. doi: 10.1016/0014-4894(90)90085-q. [DOI] [PubMed] [Google Scholar]
- 3.Telford SR, 3rd, Armstrong PM, Katavolos P, et al. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis. 1997;3:165–70. doi: 10.3201/eid0302.970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Telford SR, 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci U S A. 1996;93:6209–14. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johns R, Ohnishi J, Broadwater A, Sonenshine DE, De Silva AM, Hynes WL. Contrasts in tick innate immune responses to Borrelia burgdorferi challenge: immunotolerance in Ixodes scapularis versus immunocompetence in Dermacentor variabilis (Acari: Ixodidae) J Med Entomol. 2001;38:99–107. doi: 10.1603/0022-2585-38.1.99. [DOI] [PubMed] [Google Scholar]
- 6.Demar T. Innate immunity in ticks: A review. J Acarological Soc Japan. 2006;15:109–27. [Google Scholar]
- 7.Kopacek P, Hajdusek O, Buresova V, Daffre S. Tick innate immunity. Adv Exp Med Biol. 2010;708:137–62. [PubMed] [Google Scholar]
- 8.De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J. 2002;21:2568–79. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dostert C, Jouanguy E, Irving P, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol. 2005;6:946–53. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 10.Welchman DP, Aksoy S, Jiggins F, Lemaitre B. Insect immunity: from pattern recognition to symbiont-mediated host defense. Cell Host Microbe. 2009;6:107–14. doi: 10.1016/j.chom.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Tzou P, Reichhart JM, Lemaitre B. Constitutive expression of a single antimicrobial peptide can restore wild-type resistance to infection in immunodeficient Drosophila mutants. Proc Natl Acad Sci U S A. 2002;99:2152–7. doi: 10.1073/pnas.042411999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4:e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffmann JA, Reichhart JM. Drosophila innate immunity: an evolutionary perspective. Nat Immunol. 2002;3:121–6. doi: 10.1038/ni0202-121. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko T, Silverman N. Bacterial recognition and signalling by the Drosophila IMD pathway. Cell Microbiol. 2005;7:461–9. doi: 10.1111/j.1462-5822.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- 15.Shuai K, Ziemiecki A, Wilks AF, et al. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993;366:580–3. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- 16.Watling D, Guschin D, Muller M, et al. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993;366:166–70. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- 17.Dupuis S, Jouanguy E, Al-Hajjar S, et al. Impaired response to interferon-alpha/beta and lethal viral disease in human STAT1 deficiency. Nat Genet. 2003;33:388–91. doi: 10.1038/ng1097. [DOI] [PubMed] [Google Scholar]
- 18.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci U S A. 2009;106:17841–6. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boutros M, Agaisse H, Perrimon N. Sequential activation of signaling pathways during innate immune responses in Drosophila. Dev Cell. 2002;3:711–22. doi: 10.1016/s1534-5807(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 20.Buchon N, Broderick NA, Poidevin M, Pradervand S, Lemaitre B. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe. 2009;5:200–11. doi: 10.1016/j.chom.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Gupta L, Molina-Cruz A, Kumar S, et al. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe. 2009;5:498–507. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas V, Samanta S, Wu C, Berliner N, Fikrig E. Anaplasma phagocytophilum modulates gp91phox gene expression through altered interferon regulatory factor 1 and PU.1 levels and binding of CCAAT displacement protein. Infect Immun. 2005;73:208–18. doi: 10.1128/IAI.73.1.208-218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas V, Fikrig E. Anaplasma phagocytophilum specifically induces tyrosine phosphorylation of ROCK1 during infection. Cell Microbiol. 2007;9:1730–7. doi: 10.1111/j.1462-5822.2007.00908.x. [DOI] [PubMed] [Google Scholar]
- 24.Hodzic E, Ijdo JW, Feng S, et al. Granulocytic ehrlichiosis in the laboratory mouse. J Infect Dis. 1998;177:737–45. doi: 10.1086/514236. [DOI] [PubMed] [Google Scholar]
- 25.Narasimhan S, Montgomery RR, DePonte K, et al. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc Natl Acad Sci U S A. 2004;101:1141–6. doi: 10.1073/pnas.0307669100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Narasimhan S, Dai J, Zhang L, Cheng G, Fikrig E. Ixodes scapularis salivary gland protein P11 facilitates migration of Anaplasma phagocytophilum from the tick gut to salivary glands. EMBO Rep. 2011;12:1196–203. doi: 10.1038/embor.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy SV, Alcantara O, Boldt DH. Analysis of DNA binding proteins associated with hemin-induced transcriptional inhibition. The hemin response element binding protein is a heterogeneous complex that includes the Ku protein. Blood. 1998;91:1793–801. [PubMed] [Google Scholar]
- 28.Ferrandon D, Imler JL, Hetru C, Hoffmann JA. The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol. 2007;7:862–74. doi: 10.1038/nri2194. [DOI] [PubMed] [Google Scholar]
- 29.Lawson D, Arensburger P, Atkinson P, et al. VectorBase: a data resource for invertebrate vector genomics. Nucleic Acids Res. 2009;37:D583–7. doi: 10.1093/nar/gkn857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai J, Narasimhan S, Zhang L, Liu L, Wang P, Fikrig E. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the lyme disease agent. PLoS Pathog. 2010;6:e1001205. doi: 10.1371/journal.ppat.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichu S, Ribeiro JM, Mather TN. Purification and characterization of a novel salivary antimicrobial peptide from the tick, Ixodes scapularis. Biochem Biophys Res Commun. 2009;390:511–5. doi: 10.1016/j.bbrc.2009.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehret GB, Reichenbach P, Schindler U, et al. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem. 2001;276:6675–88. doi: 10.1074/jbc.M001748200. [DOI] [PubMed] [Google Scholar]
- 33.Brown S, Hu N, Hombria JC. Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol. 2001;11:1700–5. doi: 10.1016/s0960-9822(01)00524-3. [DOI] [PubMed] [Google Scholar]
- 34.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–8. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- 36.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 37.Vidal S, Khush RS, Leulier F, Tzou P, Nakamura M, Lemaitre B. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-kappaB-dependent innate immune responses. Genes Dev. 2001;15:1900–12. doi: 10.1101/gad.203301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman N, Zhou R, Erlich RL, et al. Immune activation of NF-kappaB and JNK requires Drosophila TAK1. J Biol Chem. 2003;278:48928–34. doi: 10.1074/jbc.M304802200. [DOI] [PubMed] [Google Scholar]
- 39.Hynes WL, Ceraul SM, Todd SM, Seguin KC, Sonenshine DE. A defensin-like gene expressed in the black-legged tick, Ixodes scapularis. Med Vet Entomol. 2005;19:339–44. doi: 10.1111/j.1365-2915.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, et al. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol. 2006;36:111–29. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Jaworski DC, Zou Z, Bowen CJ, et al. Pyrosequencing and characterization of immune response genes from the American dog tick, Dermacentor variabilis (L.) Insect Mol Biol. 2010;19:617–630. doi: 10.1111/j.1365-2583.2010.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.