Abstract
Background. Although opportunistic infections due to Mycobacterium avium complex (MAC) have been less common since the introduction of highly active antiretroviral therapy, globally, human immunodeficiency virus-1 (HIV-1)–positive patients remain predisposed to these infections. Absence of a properly functioning acquired immune response allows MAC persistence within macrophages localized in lymph nodes coinfected with HIV and MAC. Although a deficiency in interferon γ appears to play a part in the ability of MAC to deflect the macrophage-associated antimicrobial attack, questions about this process remain. Our study examines the ability of MAC to regulate interleukin 17 (IL-17), a proinflammatory cytokine involved in host cell recruitment.
Methods. Coinfected lymph nodes were examined for IL-17 by immunohistochemical analysis. In vitro, macrophages exposed to mycobacteria were evaluated for transcription activities, proteins, and signaling pathways responsible for IL-17 expression. Infected macrophages were also analyzed for expression of interleukin 21 (IL-21) and negative regulators of immune responses.
Results. Infection of macrophages triggered synthesis of IL-17, correlating with IL-17 expression by macrophages in coinfected lymph nodes. Infected macrophages exposed to exogenous IL-17 expressed CXCL10, which favors recruitment of new macrophages as targets for infection. Blockade of nuclear factor κ-light-chain-enhancer of activated B cells and mitogen-activated protein kinase pathways suppressed mycobacteria-induced IL-17 expression. MAC triggered expression of IL-21, IRF4, and STAT3 genes related to IL-17 regulation, as well as expression of the negative immunoregulators CD274(PD-L1) and suppressors of cytokine signaling.
Conclusions. MAC-infected macrophages can provide an alternative source for IL-17 that favors accumulation of new targets for perpetuating bacterial and viral infection while suppressing host antimicrobial immune responses.
The immunocompromised individual remains at risk for opportunistic infections, including Mycobacterium avium complex (MAC), most evident in individuals infected with human immunodeficiency virus-1 (HIV-1) [1–4]. Although infection with opportunistic pathogens represented an early diagnostic feature of AIDS, the nature of such opportunistic infections has changed over the past 2 decades with the use of highly active antiretroviral therapy (HAART) [5]. However, viral resistance and noncompliance with HAART can contribute to the prevalence of opportunistic infections that are associated with morbidity and mortality in patients with advanced AIDS [3]. In these patients. MAC has a predilection for the gastrointestinal tract and for lymphoid tissues and may disseminate via the bloodstream [1, 3, 4]. Of interest, immune reconstitution inflammatory syndrome, a transient focal manifestation of variable duration that begins after the initiation of HAART and reactivates preexisting infections, such as those due to MAC, has been increasingly reported in HIV-infected individuals [4, 6].
The introduction of tumor necrosis factor α (TNF-α) blockers in the treatment of autoimmune diseases has also led to an increased risk of infection and reactivation of infection due to various mycobacterial species, with MAC responsible for most pulmonary nontuberculous mycobacterial and disseminated infections [7]. In some patients receiving anti–TNF-α therapy, pulmonary nontuberculous mycobacterial disease developed even when therapy was administered with antimycobacterial drugs. Individuals with genetic defects in interferon γ (IFN-γ) and interleukin 12 (IL-12) signaling pathways, as well as elderly individuals, are also susceptible to MAC [8, 9]. Two severe cases of MAC infection, one of which was fatal, have been reported in a new immunodeficiency syndrome associated with CXCR4 dysfunction [10]. More recently, and for reasons that are still being studied, an increase in the number of nontuberculous MAC infections in non–HIV-infected individuals has become more evident [11].
Macrophages are essential in controlling MAC infection but can become infected with substantial numbers of MAC organisms when the level of IFN-γ–producing CD4+ T cells decreases, which is typical in patients with AIDS [1]. Moreover, macrophages infected with mycobacteria can become refractory to IFN-γ in vitro, and evidence suggests that therapeutic administration of exogenous IFN-γ may not always resolve MAC coinfections, even in the presence of HAART [12]. We recently showed that macrophage IFN-γ unresponsiveness is due, at least in part, to the ability of MAC to induce suppressors of cytokine signaling (SOCS) and that coinfected lymph nodes express high levels of SOCS1 and SOCS3 proteins [13].
To delineate factors that may influence recruitment of macrophage hosts to the site of mycobacterial replication, we examined the potential role of interleukin 17A (IL-17A), which is recognized as pivotal, particularly in the early response to infection [14]. IL-17 has been mostly linked to the CD4+ helper T-cell 17 (Th17) lineage and is also produced by γδ T cells, natural killer cells, neutrophils, and Paneth cells [14]. IL-17 is not only involved in initiating and sustaining the inflammatory response, it also plays critical roles in chronic inflammation and autoimmunity [15]. The IL-17 family of cytokines consists of 6 members, IL-17A–IL-17F, but their individual roles in infectious diseases are poorly defined [14].
Here, we provide evidence that IL-17 is involved in the host immune response to MAC but that increased IL-17 originates in macrophages localized in coinfected lymph nodes of patients with AIDS and is detected in macrophages infected in vitro. MAC-induced IL-17, in turn, may recruit new bacterial hosts, even in the relative absence of IFN-producing T cells, by inducing chemokines, such as CXCL10, associated with disease progression in MAC-infected patients [16]. Our data demonstrate involvement of the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways in the regulation of MAC-induced IL-17 transcription. Exposure of macrophages to mycobacteria resulted in modulation of additional factors involved in regulation of IL-17 expression. On the other hand, enhanced expression of SOCS and CD274/PD-L1 may support an immunosuppressive environment favoring bacterial survival. MAC-induced IL-17 apparently triggers and sustains infiltration during the early and chronic immune response to mycobacteria, ensuring abundant target cells for both viral and mycobacterial replication, while dampening protective host-pathogen responses. Collectively, our data implicate MAC as modulating the immune response for its own benefit, thereby contributing to persistence of this opportunistic pathogen in the immunocompromised host.
MATERIALS AND METHODS
Ethics Statement
Paraffin-embedded lymphoid tissues from uninfected individuals, patients with HIV infection, and patients with HIV/MAC coinfection were obtained through the AIDS/Cancer Specimen Resource (ACSR; available at: http://acsr.ucsf.edu). The ACSR is a National Cancer Institute–funded tissue-banking program that obtains tissues from patients after appropriate consent and a deidentification procedure before sending tissues to ACSR-approved investigators. The ACSR is recognized by the Office of Biorepositories and Biospecimen Research at the National Institutes of Health (NIH) as being HIPAA (Health Insurance Portability and Accountability Act of 1996) compliant in accordance with ethical standards of the Declaration of Helsinki. All material was obtained under approval from the UCSF Committee on Human Research.
Purification of Human Monocytes
Human peripheral blood mononuclear cells obtained by leukapheresis from normal volunteers in the Department of Transfusion Medicine at the NIH (Bethesda, MD) were diluted in endotoxin-free phosphate-buffered saline without Ca2+ and Mg2+ (BioWhittaker) for density sedimentation. Monocytes in the mononuclear cell layer and T lymphocytes were purified by counterflow centrifugal elutriation within 4 hours after leukapheresis [13, 17]. Freshly elutriated monocytes were resuspended in DMEM (2 mM/L glutamine, 50 μg/mL gentamicin; BioWhittaker), plated in 6-well plates at 6 × 106 cells/well, and allowed to adhere for 2–4 hours, after which 10% fetal bovine serum was added. Cells were allowed to differentiate into monocyte-derived macrophages (MDMs) by culturing for 6–7 days at 37°C in 5% CO2. T cells were exposed to anti-CD3/CD28 antibodies (eBioscience) or phytohemagglutinin (Sigma).
Infection of MDMs
Macrophages were infected with M. avium strain 2-151, which is virulent and has a smooth, transparent morphotype, or with HIV-1 [18]. MAC was added at a ratio of 5:1 or 10:1 to macrophages or monocytes from 1 to 18 hours. Some cultures were exposed to the NF-κB inhibitor Bay-11-7082, the MAPK p38 inhibitor SB203580, or the Erk1/2 inhibitor U0126 (Calbiochem) for 60 minutes before mycobacteria were added.
Real-Time Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Total cellular RNA was extracted from adherent control or infected macrophages by use of the RNeasy mini kit and were exposed to DNase (Qiagen). For real-time RT-PCR, 1 μg of total RNA was used for reverse transcription by oligodeoxythymilic acid primer, and resulting complementary DNA was amplified by PCR, using the ABI 7500 sequence detector (Applied Biosystems). Amplification was performed using Taqman expression gene assays for IL-17A (Hs_00174383_m1), IL-17F (Hs_00369400_m1), CXCL10 (Hs_00171042_m1), IL-21 (Hs_00222337_m1), CXCR3 (Hs_00171041_m1), SOCS1 (Hs_00705164_s1), SOCS3 (Hs_00171041_m1), IRF4 (Hs_01056533_m1), STAT3 (Hs_01047580_m1), CD274 (Hs_01125301-m1), and GAPDH (Hs_99999905_m1) as normalization control (Applied Biosystems). Data were examined using the 2−ΔΔCT method [19], and results are expressed as fold increases. Conventional PCR was performed on RNA samples for IL-17 (forward: 5′-GTGAAGGCAGGAATCACAATC-3′; reverse: 5′-ACCAGGATCTCTTGCTGGAT-3′).
Immunohistochemical Analysis
Biopsy specimens from lymph node tissues were obtained from 3 patients with AIDS-defining opportunistic infection or from HIV-1–seropositive subjects without evidence of opportunistic infection and were fixed in 10% neutral-buffered formalin, paraffin embedded, and sectioned. Tissue sections were dewaxed with xylene, rehydrated through graded alcohol solutions, and processed for antigen retrieval in a decloaking chamber (Biocare Medical) in unmasking solution (Vector Laboratories), followed by cooling at room temperature. Endogenous peroxidase activity was blocked with 3% H2O2 in 50% methanol (for 15 minutes). Prior to adding primary antibody, tissue sections were incubated with blocking serum for 30 minutes, followed by incubation with anti–IL-17A (5 μg/mL; Santa Cruz Biotechnology), anti–IL-21 (0.5 μg/mL; eBioscience), anti-CD3 (25 μg/mL; Abcam), or anti-CD68 antibody (Invitrogen) overnight at 4°C. Sections were incubated for 30 minutes with biotinylated secondary antibody. IL-17A–immunoreactive staining was performed using ABC reagent from Vectastain Elite Kit (Vector Laboratories) for 30 minutes. Bound antibodies were visualized using 3,3-diaminobenzidine-tetrahydrochloride substrate chromogen (Zymed). Slides were counterstained with Meyer hematoxylin, dehydrated, and mounted with Permount (Fisher Scientific). Immunohistochemical staining was also performed using isotype-matched control primary antibody (Jackson ImmunoResearch Laboratories). Alexa-Fluor-488 and Alexa-Fluor-546 secondary antibodies and Hoechst stain (Invitrogen) were used for immunofluorescence analysis. Staining of mycobacteria was performed as described elsewhere [1, 17, 18].
Western Blot
Macrophages were exposed to MAC, and protein lysates were harvested at indicated time points. Lysates were prepared using a protein-extraction reagent, and cell debris was removed [13]. Protein concentration was determined using the Bio-Rad DC Protein Assay (Bio-Rad). Samples were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, followed by Western blot [13], for p38, P-p38, Erk1/2, P-Erk1/2, Iκbα, and P-Iκbα (Cell-Signaling Technology); IL-21 (eBioscience); and tubulin (Sigma). Signal was developed using the SuperSignal West Pico Chemiluminescent Substrate (Pierce).
IL-17 and Viral p24 Antigen Enzyme-Linked Immunosorbent Assay (ELISA)
Macrophage culture supernatants were collected after exposure to mycobacteria and evaluated for IL-17A by ELISA (R&D Systems). Culture supernatants were also examined for p24 viral antigen (PerkinElmer).
Flow Cytometry
Cells were washed with phosphate-buffered saline containing 0.1% bovine serum albumin and 0.01% sodium azide and were stained with CD14-FITC (clone:M5E2), PD1-PE (clone:MH4), and PDL1-PEcy7 (clone:MIH1) (BD Biosciences) with predetermined concentrations, according to the manufacturer's instructions. Data acquisition was performed using LSR-II, and data were analyzed using FlowJo Software. Data are expressed as the percentage of positive cells.
Statistical Analysis
Data are presented as means ± standard error of the mean and were analyzed using the Student t test, with P values ≤.05 considered statistically significant.
RESULTS
Expression of IL-17 in HIV/MAC-Coinfected Lymph Nodes
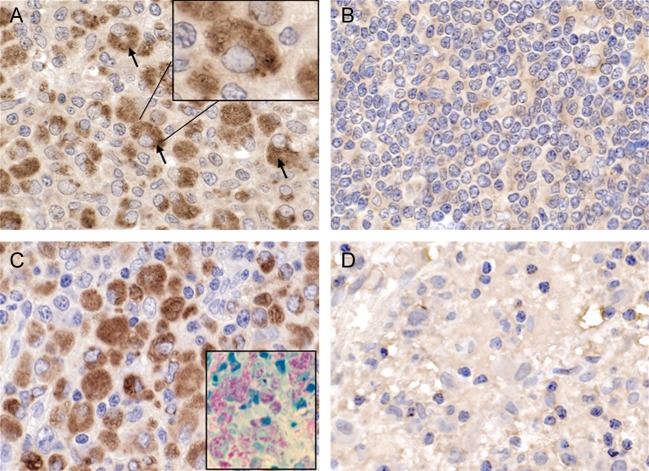
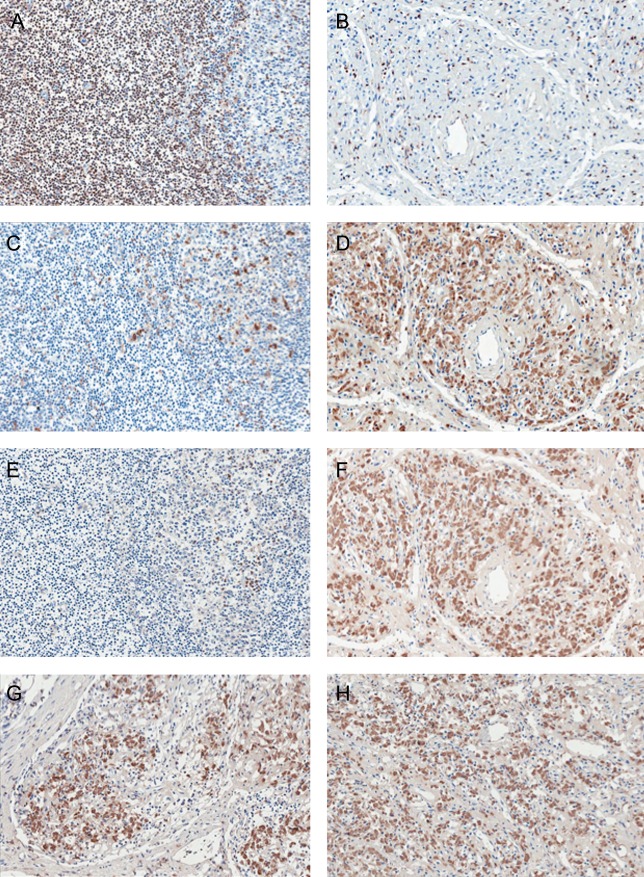
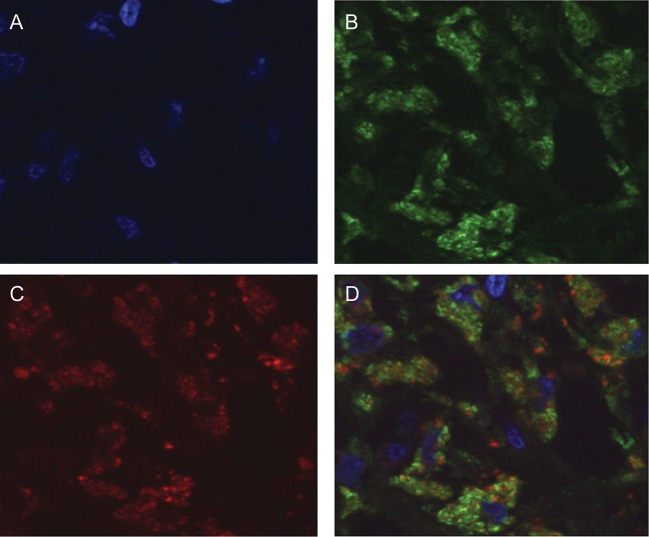
Coinfection with MAC and HIV provides an environment propitious for replication of both pathogens [13, 18]. In previous studies, we demonstrated that massive infiltration of host macrophages is evident in coinfected lymph nodes of patients with AIDS [1, 13]. To investigate mechanisms contributing to an influx of host cells to the site of infection despite a paucity of T cells [1], we examined lymph node tissues for IL-17, a proinflammatory cytokine. Immunohistochemical analysis of tissue from coinfected lymph nodes revealed enhanced IL-17 expression, compared with tissues infected with HIV alone, in which IL-17 was minimally expressed (Figure 1A and 1B). The majority of the cells positive for IL-17 were determined to be macrophages (Figure 1A) on the basis of size, morphology, macrophage-specific CD68 immunohistochemical staining (Figure 1C), and presence of intracellular mycobacteria (Figure 1C). By comparison, infiltration of monocytes into the lymph nodes occurs typically after an unsuccessful attempt to control mycobacterial infection [20]. Macrophages appeared to be minimally represented in lymph nodes lacking MAC (Figure 1B), as did detection of IL-17, further implicating macrophages as the originating cell population for this cytokine in the context of coinfection. Staining was not observed in isotype-matched antibody-stained coinfected tissues (Figure 1D). To further document macrophages as the IL-17–producing cell population, we used serial tissue sections to stain for IL-17, CD68, and CD3+ T cells (Figure 2). First, the distribution of T cells and macrophages in uninfected and HIV/MAC-coinfected lymph nodes was strikingly different. CD3+ T cells are the predominant population in uninfected lymph nodes (Figure 2A), whereas in coinfected tissues this population is depleted (Figure 2B). By comparison, relatively modest numbers of CD68+ cells were detected in uninfected lymph nodes but accumulated in large numbers in infected tissues (Figure 2C and 2D). Staining of serial sections of uninfected and infected lymph nodes revealed minimal IL-17 in uninfected, CD3+ T-cell–enriched tissues but dramatically positive staining in infected lymph nodes infiltrated by CD68+ macrophages (Figure 2E and 2F). Similar IL-17–staining patterns were observed in 2 additional coinfected specimens obtained from patients with AIDS, further suggesting that this was a shared response to MAC in HIV-positive populations (Figure 2G and 2H). Finally, we performed colocalization studies using immunofluorescence with antibodies to CD68 and IL-17 and documented that CD68+ macrophages were indeed also positive for IL-17 (Figure 3).
Figure 1.
Interleukin 17 (IL-17) expression in macrophages from patients with AIDS who were coinfected with human immunodeficiency virus-1 (HIV-1) and Mycobacterium avium complex (MAC). A, Representative lymph node tissue sections from 3 individuals coinfected with MAC and HIV-1 show IL-17 protein detected by immunohistochemical analysis. B, Lymph node tissue sections for 2 individuals positive only for HIV show minimal IL-17 protein. C, Lymph node tissue sections from 3 individuals coinfected with MAC and HIV-1 show massive numbers of CD68+ macrophages, reflecting similar pattern as IL-17 staining. The inset shows detection of MAC (red) in coinfected lymph nodes, as described in Materials and Methods. D, No staining was observed with an isotype-matched control antibody (coinfected tissue shown). Original magnification in all panels is 63×.
Figure 2.
CD68-positive macrophages are also positive for interleukin 17 (IL-17). Serial lymph node tissue sections from a representative uninfected individual (A, C, and E) and a patient with AIDS coinfected with human immunodeficiency virus-1 (HIV-1) and Mycobacterium avium complex (MAC; B, D, and F–H) were stained for CD3 (A and B), CD68 (C and D), and IL-17 (E–F) by immunohistochemical assay. Overlapping CD68 and IL-17 staining was detected using serial sections of tissue from patients with AIDS who were coinfected with HIV-1 and MAC (D and F) with minimal CD3+ cells (B). G and H, Additional tissues from coinfected patients that are positive for IL-17 staining, demonstrating that this is a typical presentation in patients with AIDS who are infected with MAC. Original magnification is 20×.
Figure 3.
CD68 and interleukin 17 (IL-17) colocalization in macrophages in lymph node tissues from patients coinfected with human immunodeficiency virus-1 (HIV-1) and Mycobacterium avium complex (MAC). Lymph node tissue sections were stained by indirect immunofluorescence, using both mouse anti-CD68 and rabbit anti–IL-17 antibodies, followed by corresponding secondary antibodies conjugated to either Alexa-488 or Alexa-546. Sections were also stained with Hoechst. A, Nuclear detection as determined by Hoechst staining (blue). B, Macrophages in coinfected lymph nodes show positive immunofluorescence staining for CD68 (green). C, The same section shown in A and B is also positive for IL-17 (red). D, Overlay images (A, B, and C) demonstrate colocalization of CD68 and IL-17 in macrophages of coinfected lymph nodes. Original magnification is 100×.
IL-17 Gene Expression in MAC-Infected Macrophages In Vitro
Although macrophages infected with MAC in lymph nodes stained positively for IL-17, they could have been the source of the cytokine and/or could have acquired IL-17 from another source. To determine whether MAC triggered IL-17 synthesis in this population, we examined the effect of MAC on IL-17 production in vitro, using human MDMs [17, 18, 21]. Induction of IL-17 messenger RNA (mRNA) was evident early, with a 15-fold increase in production detected 1–2 hours after MAC exposure (Figure 4A), as demonstrated by conventional PCR (Figure 4A) or real-time RT-PCR, and a 30–50-fold increase detected by 3–4 hours (P ≤ .05; Figure 4A). Incubation of macrophages with MAC triggered transcription of the closely related cytokine IL-17F at levels relatively similar to those of IL-17 (Figure 4B). IL-17 isoforms such as IL-17C/B/D that were examined in parallel were minimally induced (approximately 2-fold for IL-17C) or not induced (data not shown). In contrast, MAC did not directly trigger IL-17 transcription in T lymphocytes, compared with CD3/CD28 or phytohemagglutinin (Figure 4C). Culture supernatants from MAC-exposed macrophages revealed increased IL-17 expression, as demonstrated by ELISA (Figure 4D). Macrophages coinfected in vitro with HIV/MAC revealed higher IL-17 expression, compared with cultures exposed to MAC alone (P ≤ .05), and no transcriptional induction occurred as a result of HIV infection (Figure 4E). Although IL-17 may itself trigger myeloid cell recruitment, it is also known to increase CXCL10 levels [22]. Infected macrophages express elevated mRNA levels of CXCL10, a chemokine known to participate in recruitment of T lymphocytes and monocytes/macrophages [23, 24] and to be associated with poor response to treatment in patients with nontuberculous mycobacteria [16]. Moreover, concomitant exposure to MAC and IL-17 resulted in higher levels of CXCL10, compared with cells exposed to either MAC or cytokines alone (P < .05) (Figure 4F). Of interest, exposure of blood-derived monocytes to MAC resulted in an increase in CXCR3 mRNA, the CXCL10 receptor (Figure 4F).
Figure 4.
Induction of interleukin 17 (IL-17) messenger RNA (mRNA) and protein by Mycobacterium avium complex (MAC). A, IL-17 mRNA expression in unexposed and MAC-exposed (ratios, 10:1 or 5:1) adherent macrophage cultures after 1–4 hours, as determined by conventional (inset) or real-time reverse-transcription polymerase chain reaction (RT-PCR) analysis of total mRNA from cultures (*P ≤ .05; n = 4). B, Transcriptional analysis of levels of IL-17F mRNA in unexposed and MAC-exposed macrophages, as determined by real-time RT-PCR (*P ≤ .05; n = 3). C, Levels of IL-17 transcription after exposure of T lymphocytes to medium alone, MAC (ratio, 10:1), anti-CD3/CD28 antibodies, or phytohemagglutinin for 4 hours (*P ≤ .01; n = 3). D, Increased IL-17 protein in culture supernatants of macrophages that or were not exposed to MAC (ratio, 10:1) for 4–8 hours in vitro (*P < .01; n = 3). E, IL-17 transcription and viral p24 antigen levels (inset) in macrophages exposed to MAC for 4 hours at periods of 2 hours or 7 days after HIV infection (*P ≤ .05, **P < .01; n = 3). F, CXCL10 gene expression after macrophages were incubated with MAC and/or IL-17 for 4 hours, showing modulation of CXCL0 by MAC and IL-17, as determined by real-time RT-PCR (*P < .01 for no exposure vs MAC exposure, **P < .05 for MAC- or IL-17-alone exposure vs both IL-17 and MAC exposure; n = 3). The inset shows levels of CXCR3 mRNA in monocytes exposed to MAC for 4 hours (*P < .05; n = 3). Representative donor data are shown.
MAC-Induced Signal Transduction Leading to IL-17
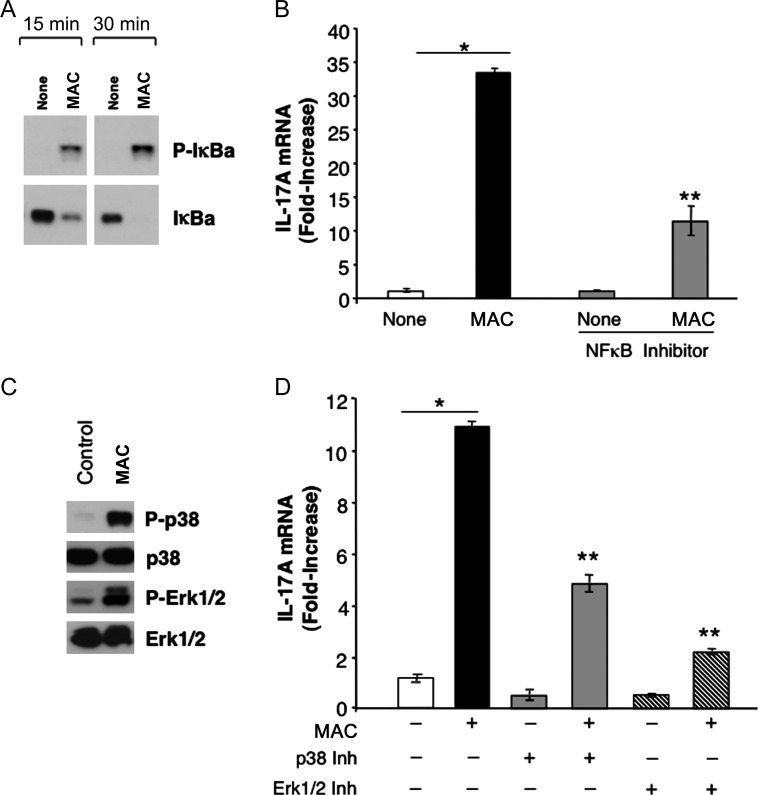
Interaction of mycobacteria with Toll-like receptor 2 (TLR2) on macrophages engages several signaling pathways, including the NF-κB signaling pathway [17, 25] (Figure 5A). Activation of this pathway by MAC occurred within 15–30 minutes, as detected by phosphorylation of IkB (Figure 5A). Evaluation of NF-κB after using the NF-κB inhibitor Bay-11-7082 (administered 1 hour prior to MAC exposure) to inhibit bacteria-induced phosphorylation of IκBα (Figure 5A) showed significant reduction in IL-17 transcription (Figure 5B). Since interaction of MAC with macrophages also triggers activation of MAPK pathway (Figure 5C), preexposure of cultures to a p38 or Erk1/2 MAPK inhibitor resulted in diminished induction of IL-17, compared with cells exposed to MAC alone (P ≤ .01; Figure 5D). Our findings indicate that NF-κB and MAPK signaling pathways contribute to MAC-induced IL-17 transcription in macrophages and that disruption of these pathways blunts IL-17 expression.
Figure 5.
Mycobacterium avium complex (MAC)–induced interleukin 17 (IL-17) is mediated by NF-κB and mitogen-activated protein kinase (MAPK) pathways. A, Human macrophages were incubated with MAC, and whole protein cell lysates were generated after 15 and 30 minutes and examined for P-IκB activation and IκB by Western blot. B, Preexposure of macrophages to the NF-κB inhibitor Bay-11-7082 (for 1 hour) suppressed MAC-induced IL-17A, as shown by real-time polymerase chain reaction (*P < .01 for no exposure vs MAC exposure, **P < .05 for both MAC and inhibitor exposure vs MAC-only exposure; n = 3). C, Whole protein cell lysates (30 minutes) from unexposed and MAC-exposed macrophages were analyzed for phosphorylation of p38 and Erk1/2 MAPK and total MAPK (n = 3). D, IL-17 mRNA expression in macrophage cultures preexposed to a p38 or Erk1/Erk2 MAPK inhibitor for 1 hour prior to exposure to MAC for 4 hours (*P < .01 for no exposure vs MAC-only exposure, **P ≤ .01 for both MAC and inhibitor exposure vs MAC-only exposure; n = 3). Representative donor data are shown.
MAC Regulation of IL-17 Transcription
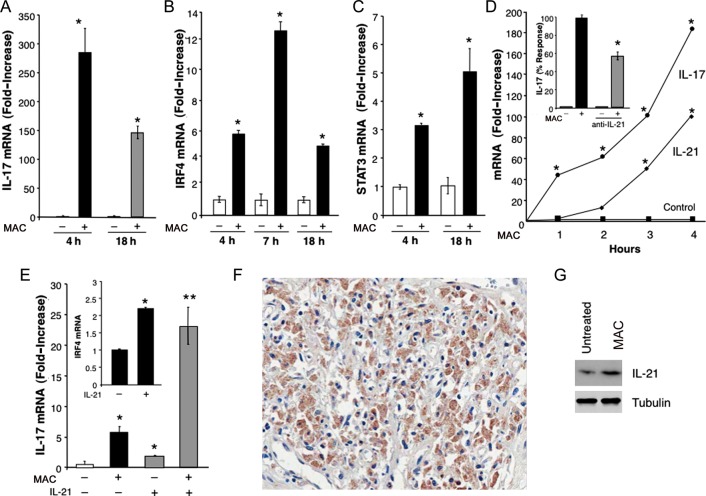
Further analyses of MAC-infected macrophages revealed that these cells express sustained elevated levels of IL-17 mRNA, with levels remaining high 18 hours after infection (Figure 6A), suggesting that additional elements may participate in supporting IL-17. We analyzed gene expression of RORc, which has been described as playing a role in IL-17 expression in lymphoid cells [26], but we did not detect modulation of this transcription factor (data not shown). Of interest, IRF4, which has been reported to regulate IL-17 transcription [27], was significantly increased in MAC-infected macrophages, with optimal gene expression evident at 4–7 hours and declining but still significantly elevated expression 18 hours after infection (Figure 6B). The level of STAT3, another transcription factor that regulates IL-17 transcription, was enhanced in macrophages exposed to MAC (Figure 6C). Despite a 10–20-fold increase in IL-17A/F expression 1 hour after exposure to MAC, IRF4 and STAT3 levels were not significantly elevated within 3–4 hours, potentially sustaining IL-17 expression in infected cells.
Figure 6.
Mycobacterium avium complex (MAC) induces interleukin 17 (IL-17)–related transcription factors IRF4, STAT3, and interleukin 21 (IL-21). A, IL-17 messenger RNA (mRNA) levels in total mRNA from macrophages exposed to MAC (ratio, 10:1) for 4–18 hours (*P < .01; n = 3). B, Findings of kinetic transcriptional analysis of IRF4 mRNA in macrophage cultures incubated with MAC for various intervals (*P < .05; n = 3). C, STAT3 mRNA levels in macrophages that were or were not exposed to MAC (*P < .05; n = 3). D, Findings of kinetic transcriptional analysis of IL-21 and IL-17 by real-time reverse-transcription polymerase chain reaction in macrophage cultures incubated with MAC for 1–4 hours (*P < .01; n = 3). The inset shows findings for macrophages that were incubated with MAC overnight in the presence or absence of neutralizing IL-21 antibodies and evaluated for IL-17 transcription. Results are expressed as percentage response, compared with cultures that did not receive antibodies (*P < .05). E, IL-17 mRNA levels in macrophages exposed to MAC and/or IL-21 (10 ng/mL) for 4 hours (*P ≤ .05, **P < .01; n = 3). The inset shows IRF4 mRNA levels in macrophages that were exposed to IL-21 for 4 hours (*P < .05; n = 3). Data are for a representative donor. F, Immunohistochemical staining revealed IL-21–positive cells in lymph node tissue from an individual with AIDS who was coinfected with MAC and HIV-1. G, Whole cell protein extracts of macrophages exposed to MAC showed enhanced IL-21 protein expression, compared with unexposed cultures, as determined by Western blot (n = 3).
Another potential regulatory molecule is IL-21, known to participate in IL-17 regulation in Th17 cells by mechanisms involving IRF4 and STAT3 [27, 28]. MAC induced transcriptional IL-21 activation in macrophages, but, of interest, this occurred in a somewhat delayed fashion. Rather than preceding detection of IL-17, IL-21 was detected after 3–4 hours of bacterial exposure (Figure 6D), and the level was declining by 8 hours (data not shown), which suggests maintenance rather than induction of IL-17. Blockade of IL-21 in infected cultures resulted in a 40%–50% reduction in MAC-induced levels of IL-17 (Figure 6D). In this regard, exogenous IL-21, modestly (by 2–3-fold) increased IL-17 levels in control macrophages (Figure 6E), whereas higher IL-17 transcription was evident when IL-21 was added concomitantly with MAC (Figure 6E). These results may be explained, at least in part, by the ability of IL-21 to enhance transcription of IRF4 (by approximately 2-fold) (Figure 6E), which may support not only IL-17 transcription, but also its own expression [27]. Moreover, IL-21 was detected in coinfected lymph nodes (Figure 6F) in populations corresponding with CD68 staining and also in infected macrophages in vitro (Figure 6G).
MAC Induces Immunosuppressive Molecules
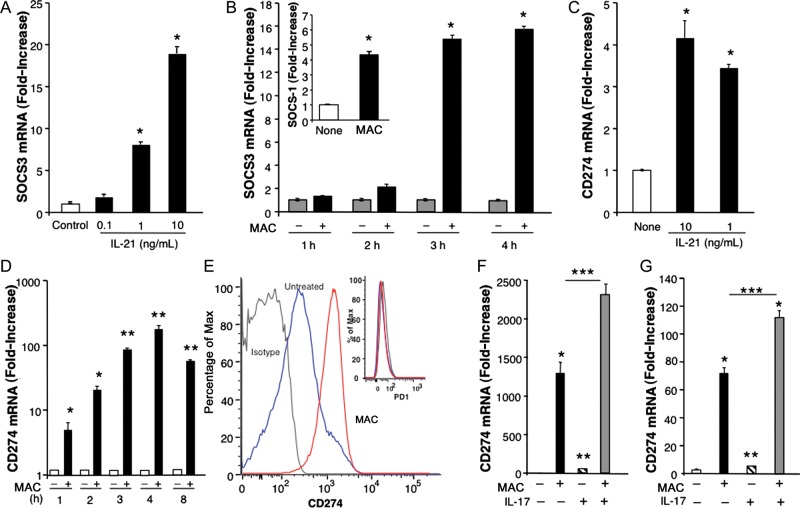
Recent evidence suggests that IL-21 may also be of significance in regulating dendritic cell expression of SOCS [29]. Here, we show that addition of exogenous IL-21 to human macrophages in vitro significantly augmented SOCS1 (data not shown) and SOCS3 transcription (Figure 7A). As we previously showed, SOCS is also induced by MAC, with maximal levels detectable within 3–4 hours (Figure 7B) [13], and collectively, these pathways leading to SOCS could dampen host responses to the protective cytokine IFN-γ. Reportedly, IL-17 and IL-21 can influence programmed death 1 (PD1) and its ligands (PD-L1/PD-L2) to further exert suppressive roles on myeloid cells restricting T-lymphocyte function [30, 31]. Of importance, PD1/PD-L1 play key roles in chronic viral infections, including those due to HIV [32]. In our studies, exposure of macrophages to IL-21 resulted in an increase in CD274/PD-L1 mRNA (Figure 7C), while exposure of macrophages to MAC led to striking levels of CD274/(PD-L1) mRNA and cell surface expression (60% increase), compared with values in unexposed macrophages. Moreover, a 2–4-fold increase in mean fluorescence intensity was noted in MAC-exposed cultures (Figure 7D and 7E) but not for PD1 (Figure 7E). An increased CD274/PD-L1 level was evident 1 hour after infection, and significantly elevated transcription was still seen 8 hours after infection. Even higher levels of CD274/PD-L1 mRNA were detected following concomitant exposure of monocytes or macrophages to IL-17 and MAC (Figure 7F and 7G). Augmentation of CD274/(PD-L1) appeared to be relatively specific in that gene expression for PD-L2 and PD-1 was variable or unaltered in multiple donors. These data shed light on novel MAC-induced immunosuppressive mechanisms that may permit this opportunistic pathogen to prevail in immunocompromised hosts.
Figure 7.
Expression of suppressors of cytokine signaling (SOCS) and CD274 by Mycobacterium avium complex (MAC) and MAC-induced cytokines. A, SOCS3 gene expression with and without exposure to exogenous interleukin 21 (IL-21) (*P ≤ .01; n = 3). B, SOCS messenger RNA (mRNA) levels in macrophages after no exposure or 1–4 hours of exposure to MAC, as determined by analysis of total mRNA levels (*P ≤ .01; n = 3). C, CD274 mRNA levels in macrophages with or without exposure to IL-21, as determined by real-time reverse-transcription polymerase chain reaction (RT-PCR) detection of CD274/PD-L1 (*P < .05; n = 3). D, CD274 mRNA levels in macrophage cultures with or without exposure to MAC alone for 1–8 hours, as determined by real-time RT-PCR analysis of total mRNA for detection of CD274/(PD-L1) (*P ≤ .05, **P ≤ .01 for no exposure vs MAC exposure; n = 3). E, Flow cytometry analyses of unexposed (blue line) and MAC-exposed macrophages (red line) for CD274 or PD1 (inset). The gray line denotes isotype. F, CD274 mRNA levels in monocytes with or without exposure to MAC and interleukin 17 (IL-17) for 4 hours, as determined by real-time RT-PCR (*P < .005 for no exposure vs MAC-only exposure, **P < .05 for no exposure vs IL-17–only exposure, ***P = .03 for both MAC and IL-17 exposure vs MAC-only exposure; n = 3) G, CD274 mRNA levels in macrophage cultures exposed to MAC and/or IL-17, as determined by real-time RT-PCR (*P = .002 for no exposure vs MAC exposure, *P = .002 for no exposure vs both MAC and IL-17 exposure, **P < .05 for no exposure vs IL-17 exposure, ***P < .05 for MAC-only exposure vs both MAC and IL-17 exposure; n = 3). Representative donor data are shown (n = 3).
DISCUSSION
Our studies reveal the involvement of IL-17 in the host innate immune response to the opportunistic bacterium MAC. However, unanticipated was the finding that macrophages rather than T cells were the cellular source of IL-17. We provide data supporting the notion that IL-17 plays a role during early host immune responses against mycobacteria, evident shortly after in vitro exposure of macrophages to MAC. Moreover, detection of IL-17 in macrophages from coinfected lymph nodes of patients with AIDS suggests that IL-17 contributes to the immune response and persistence of MAC infection in the immunocompromised host, where IL-17 can lead to recruitment of new hosts and support IFN nonresponsiveness. IL-17F, one of the members of the IL-17 family with the most homology to IL-17A induced in activated monocytes [33], was also elevated in MAC-infected macrophages.
IL-17A/F are potent inducers of inflammatory mediators, including chemokines such as CXCL10 [22]. CXCL10 participates in recruitment of T lymphocytes, monocytes, and macrophages [23, 24], and elevated levels of CXCL10 correlate with poor response to treatment in patients with MAC infections [16]. IL-17 may further sustain inflammation by enhancing the stability of chemokine mRNA transcripts [34]. Therefore, enhanced CXCL10 induction by MAC and IL-17 suggests that this mechanism may be operational and contribute to continuous recruitment of new targets for bacterial and viral infections.
Our study shows that MAC induces IL-17 production through a mechanism involving the MAPK and NF-κB pathways. Recent reports have shown that chitin, a ubiquitous polysaccharide in fungi, insects, and parasites, regulates IL-17 and acute tissue inflammation in macrophages [35] and that p38MAPK influences IL-17 [36]. In addition, TLR2 stimulation in combination with T-cell receptor activation can promote Th17 differentiation [37].
Prolonged IL-17 expression in mycobacteria-exposed macrophages correlated with high IRF4 and STAT3 levels, which are known to support IL-17 [14, 26]. These findings correlated with enhanced expression of IL-21, a regulator of IL-17 that is important for Th17 polarization and differentiation, representing an alternative pathway for generation of the Th17 subset [38], which we considered to be a potential regulator of macrophage IL-17. The synergistic effect of MAC and IL-21 on IL-17 transcription can be explained, at least in part, by the fact that macrophages cultured with IL-21 or MAC show enhanced gene expression of IRF4, which is known to participate in the transcriptional regulation of IL-17 and IL-21 [27, 28]. IL-21, in turn, activates the MAPK pathway [39] and influences phosphorylation of IRF4 [27]. While we cannot rule out the participation of additional factors in the regulation of IL-17 in macrophages [14], RORγt gene expression linked to regulation of IL-17 downstream of IRF4 [14] was not significantly increased in macrophages by MAC (data not shown).
Despite the IL-17–propagated inflammatory response, macrophages in the immunocompromised host do not clear MAC, in part because of enhanced SOCS1/3 interference with IFN-γ signaling [13]. Here, we show that abrogation of IFN-γ–protective innate immune responses may be further exacerbated by IL-21–driven SOCS, consistent with its effects in dendritic cells [29]. Higher SOCS expression is found in patients with tuberculosis and recurrent tuberculosis, and reduction of SOCS expression has been considered a plausible approach to improve host protective responses [40]. SOCS may also interfere with protective antiviral activity of type I and type II interferons [13, 41]. Further complicating this scenario is the fact that infection of macrophages with MAC in the presence or absence of IL-21 enhanced levels of immunosuppressive CD274/PD-L1, as did coexposure to IL-17 and mycobacteria, likely via MAPK [30, 42]. This can be further aggravated by the ability of HIV-1 to enhance CD274/PD-L1 on macrophages, mediated via TLR [43]. CD274/PD-L1, considered a marker for disease progression, and its receptor, PD1, are augmented on monocytes/macrophages and T cells during HIV-1 infection, suggesting dampening of the HIV-specific effector T-cell function [32, 43, 44]. Although IL-21–producing CD4+ T cells have been associated with retroviral control by affecting CD8+ T lymphocytes in patients with a low viral load [45], in immunocompromised hosts, IL-21 may deflect innate and adaptive protective immune responses by inducing SOCS and CD274 expression. In addition, the PD-1/PD-L1/PD-L2 pathway has been connected to suppression of effector T-cell function against Mycobacterium tuberculosis [46].
Although appropriate levels of IL-17 are thought to have a protective role in response to infection, especially during the early stages of infection with HIV-1 and M. tuberculosis, persistent IL-17 is also associated with tissue-damaging inflammation and negative outcomes [22, 47]. For instance, IL-17 has been reported to have immunopathological roles during infection with multidrug-resistant M. tuberculosis and persistently elevated levels of antigen [48]. IL-17 contributes to the pathogenesis of autoimmune/inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosis, multiple sclerosis, Sjögren syndrome, asthma, and Crohn disease [15, 22]. IL-17 in CD68+ monocytes/macrophages found in inflamed mucosa of patients with inflammatory bowel disease has been linked to induction and persistence of mucosal inflammation [49]. Recently, tissue macrophages expressing IL-17 have been described in breast tumors, where they promote invasiveness [50].
MAC regulates multiple host molecules in macrophages in vitro, corresponding to evidence of their dysregulation in vivo [13, 18]. Here, we exposed a new strategy used by this opportunistic pathogen to promote persistence within the macrophage. It is possible that, initially, IL-17 production by MAC-infected macrophages may aid in recruiting cells and thereby mediate resistance/protection activities that are important during the early innate immune response. In immunocompromised individuals and during advanced disease, dysregulated production of IL-17 in the absence of Th1 lymphocytes, IFN-γ, or appropriate counterregulatory mechanisms to disengage IL-17 responses could drive pathogenesis.
Notes
Acknowledgments. We are grateful to Dr Ke-jian Lei, Wenwen Jin, and Vichit Lorn, for technical assistance; to Calley Grace, for editorial assistance; and Drs Alfredo Molinolo and Ramiro Iglesias-Bartolome, for help with immunoflourescence staining of tissue.
Financial support. This work was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, NIH.
Potential conflicts of interests. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–61. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 2.Wahl SM, Greenwell-Wild T, Hale-Donze H, Moutsopoulos N, Orenstein JM. Permissive factors for HIV-1 infection of macrophages. J Leukoc Biol. 2000;68:303–10. [PubMed] [Google Scholar]
- 3.Karakousis PC, Moore RD, Chaisson RE. Mycobacterium avium complex in patients with HIV infection in the era of highly active antiretroviral therapy. Lancet Infect Dis. 2004;4:557–65. doi: 10.1016/S1473-3099(04)01130-2. [DOI] [PubMed] [Google Scholar]
- 4.Tuon FF, Mulatti GC, Pinto WP, de Siqueira Franca FO, Gryschek RC. Immune reconstitution inflammatory syndrome associated with disseminated mycobacterial infection in patients with AIDS. AIDS Patient Care STDS. 2007;21:527–32. doi: 10.1089/apc.2006.0121. [DOI] [PubMed] [Google Scholar]
- 5.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 6.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–73. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 7.Winthrop KL, Chang E, Yamashita S, Iademarco MF, LoBue PA. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg Infect Dis. 2009;15:1556–61. doi: 10.3201/eid1510.090310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest. 1998;101:2364–9. doi: 10.1172/JCI2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haerynck F, Holland SM, Rosenzweig SD, Casanova JL, Schelstraete P, De Baets F. Disseminated Mycobacterium avium infection in a patient with a novel mutation in the interleukin-12 receptor-beta1 chain. J Pediatr. 2008;153:721–2. doi: 10.1016/j.jpeds.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 10.Doncker AV, Balabanian K, Bellanne-Chantelot C, et al. Two cases of disseminated Mycobacterium avium infection associated with a new immunodeficiency syndrome related to CXCR4 dysfunctions. Clin Microbiol Infect. 2011;17:135–9. doi: 10.1111/j.1469-0691.2010.03187.x. [DOI] [PubMed] [Google Scholar]
- 11.Parrish SC, Myers J, Lazarus A. Nontuberculous mycobacterial pulmonary infections in Non-HIV patients. Postgrad Med. 2008;120:78–86. doi: 10.3810/pgm.2008.11.1942. [DOI] [PubMed] [Google Scholar]
- 12.Lauw FN, van Der Meer JT, de Metz J, Danner SA, van Der Poll T. No beneficial effect of interferon-gamma treatment in 2 human immunodeficiency virus-infected patients with Mycobacterium avium complex infection. Clin Infect Dis. 2001;32:e81–2. doi: 10.1086/318705. [DOI] [PubMed] [Google Scholar]
- 13.Vazquez N, Greenwell-Wild T, Rekka S, Orenstein JM, Wahl SM. Mycobacterium avium-induced SOCS contributes to resistance to IFN-gamma-mediated mycobactericidal activity in human macrophages. J Leukoc Biol. 2006;80:1136–44. doi: 10.1189/jlb.0306206. [DOI] [PubMed] [Google Scholar]
- 14.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–89. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 15.Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjogren's syndrome immunopathogenesis. Am J Pathol. 2009;175:1167–77. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim A, Allison C, Tan DB, Oliver B, Price P, Waterer G. Immunological markers of lung disease due to non-tuberculous mycobacteria. Dis Markers. 2010;29:103–9. doi: 10.3233/DMA-2010-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwell-Wild T, Vazquez N, Sim D, et al. Mycobacterium avium infection and modulation of human macrophage gene expression. J Immunol. 2002;169:6286–97. doi: 10.4049/jimmunol.169.11.6286. [DOI] [PubMed] [Google Scholar]
- 18.Wahl SM, Greenwell-Wild T, Peng G, et al. Mycobacterium avium complex augments macrophage HIV-1 production and increases CCR5 expression. Proc Natl Acad Sci U S A. 1998;95:12574–9. doi: 10.1073/pnas.95.21.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Hale-Donze H, Greenwell-Wild T, Mizel D, et al. Mycobacterium avium complex promotes recruitment of monocyte hosts for HIV-1 and bacteria. J Immunol. 2002;169:3854–62. doi: 10.4049/jimmunol.169.7.3854. [DOI] [PubMed] [Google Scholar]
- 21.Wahl SM, Greenwell-Wild T, Peng G, Hale-Donze H, Orenstein JM. Co-infection with opportunistic pathogens promotes human immunodeficiency virus type 1 infection in macrophages. J Infect Dis. 1999;179(Suppl 3):S457–60. doi: 10.1086/314814. [DOI] [PubMed] [Google Scholar]
- 22.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–7. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Tang PC, Qin L, et al. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J Exp Med. 2010;207:1951–66. doi: 10.1084/jem.20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taub DD, Lloyd AR, Conlon K, et al. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–14. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocco JM, Irani VR. Mycobacterium avium and modulation of the host macrophage immune mechanisms. Int J Tuberc Lung Dis. 2011;15:447–52. doi: 10.5588/ijtld.09.0695. [DOI] [PubMed] [Google Scholar]
- 26.Hirahara K, Ghoreschi K, Laurence A, Yang XP, Kanno Y, O'Shea JJ. Signal transduction pathways and transcriptional regulation in Th17 cell differentiation. Cytokine Growth Factor Rev. 2010;21:425–34. doi: 10.1016/j.cytogfr.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswas PS, Gupta S, Chang E, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120:3280–95. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huber M, Brustle A, Reinhard K, et al. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci U S A. 2008;105:20846–51. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strengell M, Lehtonen A, Matikainen S, Julkunen I. IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells. J Leukoc Biol. 2006;79:1279–85. doi: 10.1189/jlb.0905503. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Q, Xiao X, Wu Y, et al. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol. 2011;41:2314–22. doi: 10.1002/eji.201041282. [DOI] [PubMed] [Google Scholar]
- 31.Kinter AL, Godbout EJ, McNally JP, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 32.Kaufmann DE, Walker BD. Programmed death-1 as a factor in immune exhaustion and activation in HIV infection. Curr Opin HIV AIDS. 2008;3:362–7. doi: 10.1097/COH.0b013e3282f9ae8b. [DOI] [PubMed] [Google Scholar]
- 33.Starnes T, Robertson MJ, Sledge G, et al. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J Immunol. 2001;167:4137–40. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 34.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J Immunol. 2007;179:4135–41. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 35.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–86. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noubade R, Krementsov DN, Del Rio R, et al. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood. 2011;118:3290–300. doi: 10.1182/blood-2011-02-336552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyirenda MH, Sanvito L, Darlington PJ, et al. TLR2 stimulation drives human naive and effector regulatory T cells into a Th17-like phenotype with reduced suppressive function. J Immunol. 2011;187:2278–90. doi: 10.4049/jimmunol.1003715. [DOI] [PubMed] [Google Scholar]
- 38.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 39.Fuqua CF, Akomeah R, Price JO, Adunyah SE. Involvement of ERK-1/2 in IL-21-induced cytokine production in leukemia cells and human monocytes. Cytokine. 2008;44:101–7. doi: 10.1016/j.cyto.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mistry R, Cliff JM, Clayton CL, et al. Gene-expression patterns in whole blood identify subjects at risk for recurrent tuberculosis. J Infect Dis. 2007;195:357–65. doi: 10.1086/510397. [DOI] [PubMed] [Google Scholar]
- 41.Fenner JE, Starr R, Cornish AL, et al. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat Immunol. 2006;7:33–9. doi: 10.1038/ni1287. [DOI] [PubMed] [Google Scholar]
- 42.Wolfle SJ, Strebovsky J, Bartz H, et al. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–24. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez-Garcia M, Porichis F, de Jong OG, et al. Expression of PD-L1 and PD-L2 on human macrophages is up-regulated by HIV-1 and differentially modulated by IL-10. J Leukoc Biol. 2011;89:507–15. doi: 10.1189/jlb.0610327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boasso A, Hardy AW, Landay AL, et al. PDL-1 upregulation on monocytes and T cells by HIV via type I interferon: restricted expression of type I interferon receptor by CCR5-expressing leukocytes. Clin Immunol. 2008;129:132–44. doi: 10.1016/j.clim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yue FY, Lo C, Sakhdari A, et al. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J Immunol. 2010;185:498–506. doi: 10.4049/jimmunol.0903915. [DOI] [PubMed] [Google Scholar]
- 46.Jurado JO, Alvarez IB, Pasquinelli V, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol. 2008;181:116–25. doi: 10.4049/jimmunol.181.1.116. [DOI] [PubMed] [Google Scholar]
- 47.Torrado E, Cooper AM. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 2010;21:455–62. doi: 10.1016/j.cytogfr.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basile JI, Geffner LJ, Romero MM, et al. Outbreaks of Mycobacterium tuberculosis MDR strains induce high IL-17 T-cell response in patients with MDR tuberculosis that is closely associated with high antigen load. J Infect Dis. 2011;204:1054–64. doi: 10.1093/infdis/jir460. [DOI] [PubMed] [Google Scholar]
- 49.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu X, Mulcahy LA, Mohammed RA, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]