Abstract
Background. The degree of cross-immunity between human papillomavirus (HPV) types is fundamental both to the epidemiological dynamics of HPV and to the impact of HPV vaccination. Epidemiological data on HPV infections has been repeatedly interpreted as inconsistent with cross-immunity.
Methods. We reevaluate the epidemiological data using a model to determine the odds ratios of multiple to single infections expected in the presence or absence of cross-immunity. We simulate a virtual longitudinal survey to determine the effect cross-immunity has on the prevalence of multiple infections. We calibrate our model to epidemiological data and estimate the extent of type replacement following vaccination against specific HPV types.
Results. We find that cross-immunity can produce odds ratios of infection comparable with epidemiological observations. We show that the sample sizes underlying existing surveys have been insufficient to identify even intense cross-immunity. We also find that the removal of HPV type 16, type 18, and types 6 and 11 would increase the prevalence of nontargeted types by 50%, 29%, and 183%, respectively.
Conclusions. Cross-immunity between HPV types is consistent with epidemiological data, contrary to previous interpretations. Cross-immunity may cause significant type replacement following vaccination, and therefore should be considered in future vaccine studies and epidemiological models.
Human papillomavirus (HPV) types 16 and 18 are responsible for 70% of cervical cancers, whereas the remaining 30% of cervical cancers are associated with more than a dozen other HPV types [1]. The degree of cross-immunity among types is key both to the epidemiology of HPV and to the public health impact of HPV vaccines. Current HPV vaccines targeting HPV types 16 and 18 hold tremendous promise for the reduction of cervical cancer [2]. However, these vaccines may modulate the prevalence of HPV infections by nontargeted types through type replacement, which is the increase in prevalence of nontargeted types following vaccination [3].
Concerns about type replacement mediated by HPV vaccine have motivated natural history surveys that found odds ratios (ORs) and hazard ratios of multiple to single infections of >1 [4–9]. Consequently, an individual infected with one HPV type has an elevated risk of multiple infections, both concurrent and sequential, even after adjusting for multiple HPV risk factors (Table 1). These observations have been interpreted as evidence that there is no significant cross-immunity among HPV types [4–8, 10, 11]. Furthermore, these interpretations are widely cited to justify disregarding cross-immunity in evaluations of HPV vaccines [10–12]. However, the premise that cross-immunity does not lead to elevated risks of concurrent or sequential HPV infections has not been assessed. We test this hypothesis here by using a mathematical model for HPV transmission to determine the effect that cross-immunity would have on the risk of multiple infections.
Table 1.
Odds Ratios of Epidemiological Surveys of Concurrent and Sequential Human Papillomavirus Infection
| Survey | Concurrent ORs, Adjusted |
Sequential ORs, Adjusted |
Cohort | Follow-up Timing |
|||
|---|---|---|---|---|---|---|---|
| Lowest | Highest | Lowest | Highest | Length | Interval | ||
| Thomas et al 2000 [7] | 3.3a | 12.2 (4.2–35.8)b | 0.5a | 9.4 (1.2–74.5)b,c | Women aged 18–20 y | 34 mo | 4 mo |
| Liaw et al 2001 [5] | NM | NM | 1.9a | 7.9 (3.6–17.2)b | Women; median age, 23 y | 25 mo | 12 mo |
| Rousseau et al 2001 [4] | NM | NM | 2.1 (1.5–3.1)b,c | 2.6 (1.7–4.2)b,c | Women aged 18–60 y | 5 y | 4–6 mo |
| Mendez et al 2005 [6] | 3.3a | 25d | 2.6a | 12.5d | Women aged 15–85 y | 4 y | 7 mo |
| Kjaer et al 2005 [9] | NM | NM | NM | 4.1 (1.4–12.3)b | Men aged 18–29 y | 6–8 mo | 6–8 mo |
| Chaturvedi et al 2011 [8] | NM | 2.25 (2.12–2.38)b | NM | NM | Women aged 18–25 y | NA | NA |
Surveys were adjusted for factors such as subject age, number of lifetime sexual partners, and recent sexual partners.
Abbreviations: NA, not applicable; NM, not measured; OR, odds ratio.
a Not statistically significant.
b 95% confidence interval.
c Hazard ratio.
d P value declared significant, but confidence interval not reported.
We classify interactions between HPV types in terms of the timescales over which the interactions occur. Immediate interactions occur when current infection with one HPV type affects the risk of simultaneous co-infection with another. Physiologically, immediate interactions may arise from changes in cytokine profiles associated with local inflammation and from competition to colonize cervical cells, as occurs with other sexually transmitted infections [4, 13]. Delayed interactions occur when a past infection with one HPV type that has been cleared affects the subsequent risk of infection with another type. Delayed interactions may be due to acquired immunity that develops upon clearance of an HPV infection [14].
We classify the interactions between HPV types as synergistic, independent, or cross-immune. Synergy increases the probability of infection with a second HPV type. Perhaps counterintuitively, synergy actually enhances the benefit of a vaccine, because the removal of the types that are targeted by vaccination reduces the transmission of other types [3]. Independence between 2 HPV types indicates that neither infection with nor resistance to one type has an effect on risk of infection with the second type. Cross-immunity decreases the probability of infection with another type. Under a scenario of cross-immunity, a reduction in vaccine-targeted types brought about by vaccination could cause a concomitant increase in prevalence of nontargeted HPV types through type replacement [3–6, 15]. Throughout this article, we assume that type interaction is partial rather than perfect, because perfect interactions would unrealistically preclude the possibility of multiple infections.
Type replacement following vaccination has been raised as a concern with other vaccines, notably vaccines against Haemophilus influenzae and Streptococcus pneumoniae. Lipsitch [15] predicted that such vaccines could result in type replacement. These predictions were borne out after pneumococcal conjugate vaccine (PCV7) and H. influenza type b conjugate vaccine (Hib) caused type replacement [16, 17].
In this article, we use an epidemiological model calibrated to HPV prevalence data to reevaluate clinical data on interactions among multiple HPV types and to assess whether cross-immunity can be ruled out on the basis of current epidemiological surveys. We find that the ORs of multiple infections observed in the epidemiological data are consistent with those expected under a model scenario of cross-immunity among HPV types. Taking into account this cross-immunity, we use our model to predict the degree of type replacement that could result from vaccine-mediated removal of targeted HPV types.
METHODS
Epidemiological Model
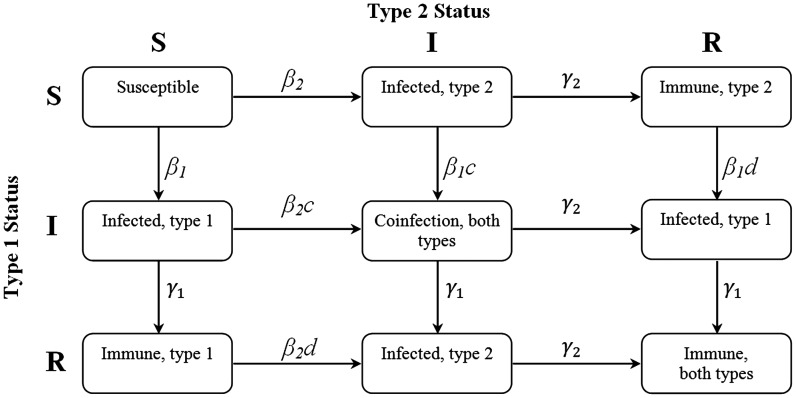
To evaluate the potential existence of cross-immunity among HPV types, we built upon our previous susceptible-infected-recovered model that incorporates interactions between 2 HPV types [3]. In our refined model (Supplementary Appendix 1), an individual can be in 1 of 9 infection states depending on whether they are susceptible to, infected with, or recovered from one or both HPV types (Figure 1). Our mathematical model provided the expected risks of multiple infections under 9 different scenarios that occur when immediate and delayed interactions each generate synergy, are independent, or elicit cross-immunity.
Figure 1.
Schematic of the possible infection and immunological status of individuals: susceptible (S), infected (I), or recovered (R). Immediate interactions between types are governed by the parameter c. Delayed interactions between types are governed by the parameter d.
First, to determine the expected effect of potential cross-immunity on epidemiological surveys, we used steady state prevalences from the HPV model to stochastically generate life histories of individuals, which were then “surveyed” at a random point in their lives, resulting in a virtual epidemiological survey mirroring real-world surveys [4–7]. Second, we calibrated our model to empirical observations of HPV prevalence to estimate the nature, duration, and intensity of interactions between HPV types. Third, we calculated the sample size necessary to identify type replacement from epidemiological surveys. Finally, to assess the repercussions of type replacement, we modeled the change in prevalence of nontargeted types following vaccination.
To determine how interactions between HPV types change the risk of multiple infections over time, we explicitly modeled the formation and dissolution of sexual partnerships while tracking the HPV states of both partners (Supplementary Appendix 1). Individuals can be either single or partnered (even if for only one sexual act), and a partner's infection status can be any of the 9 states. To approximate the influence of highly sexually active individuals on the overall transmission dynamics, we used the effective partner change rate, which incorporates both the mean and the variance of partnership frequency in the population [18–20]. We decomposed the effective partner change rate of 4 per year into a partnership formation rate, r, and a partnership dissolution rate, s, taking into account an estimated average time outside of partnerships [12, 21, 22]. We assumed a population sampled across a 40-year time span of sexual activity, and a mean HPV infection duration of 18 months [23–25].
The transmission probability for a single HPV type over the course of a sexual partnership was previously found to be 60% [21]. From this empirical estimate, we calculated the rate of infection, β, taking into account empirical estimates of partnership durations (Supplementary Appendix 1). We defined 2 parameters, c and d, to represent the relative effects of immediate (c) and delayed (d) interaction between types. This produced effective rates of infection, cβ and dβ, representing the likelihood of infection with an HPV type given current (c) and previous (d) infection with a different type (Figure 1). We examined 9 hypothetical scenarios of c and d, choosing values representative of cross-immunity (c, d = 0.1), independence (c, d = 1), and synergy (c, d ≈ 1/β ≈ 1.665) (Table 2). The cross-immunity value of 0.1 was chosen as a value that was deemed sufficiently small; the synergy value of 1.665 was chosen to be slightly less than 1/β (β = 0.6), as the effective rates of infection must by definition be ≤1. Our transmission model predicted the type-specific HPV prevalence to vary between 1.5% and 2.8%, depending on the combinations of parameters c and d and in a range that is consistent with epidemiological data (Table 2) [26].
Table 2.
Equilibrium Type-specific Human Papillomavirus Prevalences for the 9 Model Scenarios
| Parameter | Delayed Cross-Immunity (d = 0.1) | Delayed Independence (d = 1) | Delayed Synergy (d = 1.665) |
|---|---|---|---|
| Immediate cross-immunity (c = 0.1) | 0.0152 | 0.0253 | 0.0272 |
| Immediate independence (c = 1) | 0.0162 | 0.0258 | 0.0274 |
| Immediate synergy (c = 1.665) | 0.0177 | 0.0262 | 0.0277 |
Virtual Epidemiological Survey
To evaluate the effect that cross-immunity would have on epidemiological surveys of multiple infections, we simulated a virtual longitudinal survey on HPV infection status of 50 000 individuals for each of our 9 parameter sets of HPV type interactions. We used HPV prevalences generated from our transmission model to parameterize a stochastic model of an individual's infection life history. Susceptible individuals entered the stochastic model at sexual debut, and their sexual life histories were subsequently tracked. Each individual was “enrolled” in our virtual survey at a random time in their life, and “followed-up” 1 year later (Supplementary Appendix 2).
We used logistic regression to obtain ORs for infection with multiple types under the different type-interaction scenarios (Table 2), where the independent variable was an infection status of one HPV type and the dependent variable was an infection status of another type. In our regressions we adjusted for age, number of lifetime sexual partners, and number of sexual partners in the intervening year. We calculated ORs for concurrent infection using data from the follow-up phase of our simulated surveys and ORs for sequential infection using data from both the entry and follow-up of our simulated surveys.
Empirical Calibrations
To evaluate the behavior of the type interaction parameters c and d beyond the 9 transmission scenarios (Table 2) and to evaluate how well these parameters correspond to field observations of HPV prevalence, we ran our model invoking both parameters assigned to each value between 0 and 1.66 in steps of 0.01. For every (c, d) pair, we calculated the χ2 statistic between the observed prevalence of infection for each of the 3 field surveys [6, 7, 26] and the prevalence of 0, 1, and 2 concurrent infections predicted by our model. For each of the 3 surveys we obtained the (c, d) pair that minimizes the χ2 statistic. To estimate 95% confidence intervals (CIs), we assumed Gaussian errors in the χ2 statistic and noted the parameter range within 2 log units of the maximum likelihood value.
Vaccine Trial Sample Size
We determined whether the vaccine trials that have been conducted [27, 28] had sufficient sample sizes to detect cross-immunity. Using a standard sample size formula, we calculated the sample size necessary to give an 80% statistical power of detecting a statistically significant difference in the prevalence that would arise from cross-immunity of HPV infections between the vaccine trial group and the placebo group for each of the 3 actual epidemiological surveys (Supplementary Appendix 3).
Type Replacement
HPV types have been classified into phylogenetic clades (http://hpv-web.lanl.gov/) within which cross-immunity may occur [5]. Type 16 is classified in clade A9, type 18 in clade A7, and types 6 and 11 in clade A10. The proportion of HPV infections within each clade due to vaccine-targeted types varies: type 16 causes 38% of infections in clade A9, type 18 causes 27% in clade A7, and types 6 and 11 cause 67% in clade A10 [29]. To assess the impact of type interaction on the effectiveness of HPV vaccines, we modeled the change in prevalence of nontargeted types within each clade following vaccination and compared this change with the reduction in prevalence of the targeted type caused by vaccination (Supplementary Appendix 4).
RESULTS
Virtual Epidemiological Survey
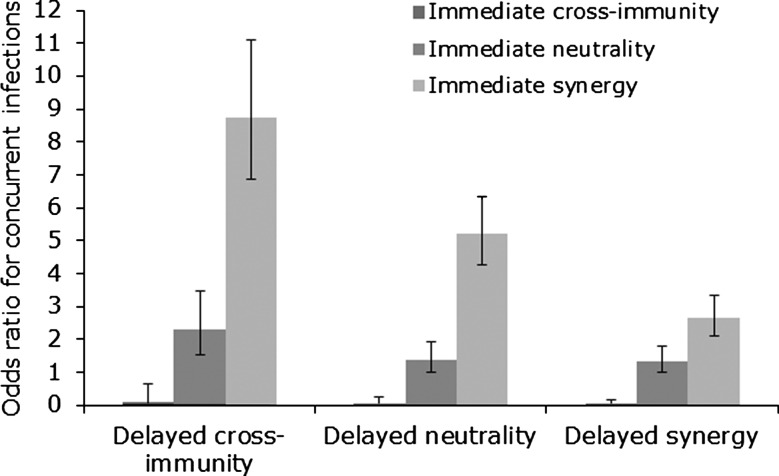
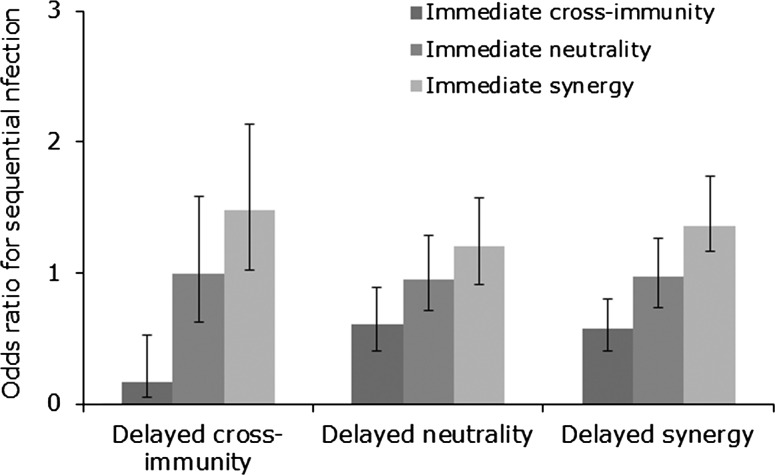
Using ORs to measure the risk of concurrent and sequential infection with different HPV types, we found that both delayed and immediate cross-immunity increased ORs for concurrent and sequential infection (Figures 2 and 3), but that delayed cross-immunity with immediate synergy produced the greatest increase in ORs, increasing the odds of multiple infection by 8.8 times for concurrent infection (Figures 2 and 3). These results argue that the elevated risk of concurrent infections previously found (Table 1) does not preclude the existence of delayed cross-immunity.
Figure 2.
Odds ratios for concurrent infection in 9 simulated longitudinal studies. Odds ratios were adjusted for age and lifetime number of sexual partners. Error bars represent 95% confidence intervals.
Figure 3.
Odds ratios for sequential infection in 9 simulated longitudinal studies. Odds ratios were adjusted for age, lifetime number of sexual partners, and number of new sexual partners since enrollment. Error bars represent 95% confidence intervals.
Empirical Calibrations
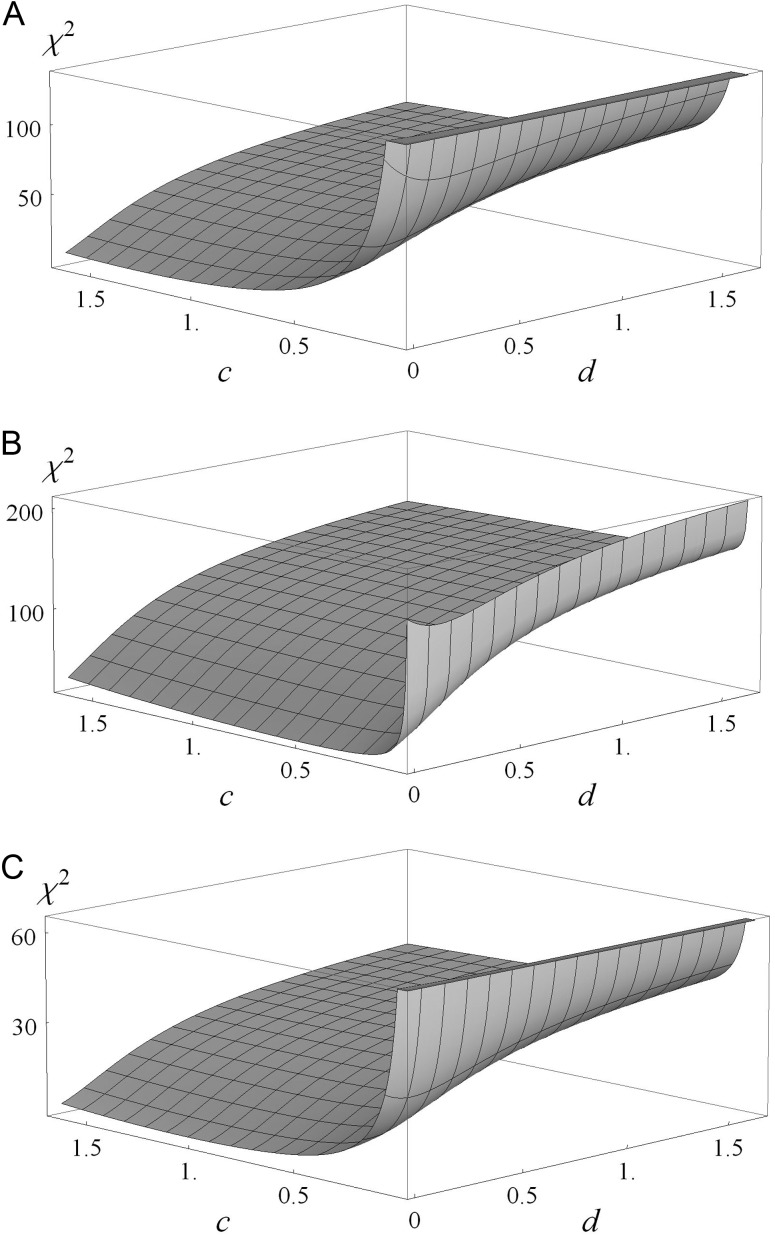
To estimate values for the immediate and delayed cross-immunity parameters, c and d respectively, we calibrated our model to epidemiological surveys by determining the χ2 statistic for the 3 epidemiological surveys [6, 7, 26] as c and d are varied. We also calculated the values of c and d that minimize the χ2 statistic for each survey (Figure 4). These results indicate that empirical observations are best approximated by a small value for d, indicating strong delayed cross-immunity.
Figure 4.
Fit of χ2 model of 3 data sets to model predictions of 0, 1, and 2 concurrent human papillomavirus infections based on immediate (c) and delayed (d) type interactions. Lower values of χ2 indicate better fit. 95% confidence intervals are based on normal error models underlying χ2 and are indicated in parentheses; an asterisk indicates that the 95% confidence interval exceeds the realistic parameter space. For Thomas et al [7], χ2 is minimized by c = 1.3 (0.94, 1.665*) and d = 0 (0*, 0.028) (A). For Mendez et al [6], χ2 is minimized by c = 0.5 (0.24, 0.97) and d = 0 (0*, 0.014) (B). For Dunne et al [26], χ2 is minimized by c = 1.1(0.56, 1.665*) and d = 0 (0*, 0.065) (C).
Vaccine Trial Sample Size
Our calculations of the sample size necessary to observe type interactions within a clinical trial indicate that >140 000 person-years of observation would be needed to detect even perfect cross-immunity based on the event rates of HPV infection in Harper et al [27], compared with 2500 person-years (1113 subjects for an enrollment of up to 27 months) that were used in that survey. More than 36 million person-years would be required using the event rates in Mao et al [28], compared with the 9500 person-years used (2391 subjects over 4 years). Thus, cross-immunity cannot be ruled out on the basis of these existing surveys.
Type Replacement
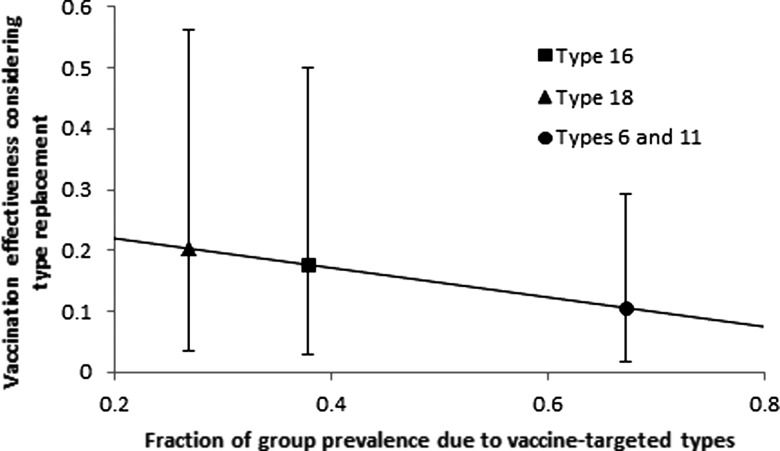
Modeling the effect of delayed cross-immunity on HPV prevalence following the elimination of the vaccine-targeted types and parameterizing the immediate and delayed interaction parameters based on the results of our empirical calibrations, we found that the vaccination effectiveness as a result of type replacement was 11% (95% CI, 1.8%–29%) for types 6 and 11 in the A10 clade, 18% (95% CI, 3.0%–50.%) for type 16 in the A9 clade, and 20% (95% CI, 3.5%–56%) for type 18 in the A7 clade. The prevalence of nontargeted types increased by 50% (95% CI, 31%–58%) following vaccination for type 16, by 29% (95% CI, 18%–33%) for type 18, and by 183% (95% CI, 145%–216%) for types 6 and 11. We observed a negative correlation between the prevalence of the vaccine-targeted type within its clade and the corresponding vaccination effectiveness, indicating that those HPV types that constitute a larger proportion of their clade leave open a wider niche to fill upon elimination, resulting in greater type replacement by competing types (Figure 5).
Figure 5.
Vaccine effectiveness under cross-immunity. Effectiveness is calculated as the ratio of total human papillomavirus infection prevalence between the scenarios of type replacement and no type replacement, following vaccination against the designated type. Under the scenario of cross-immunity, a greater fraction of a targeted type within its clade results in greater type replacement and lower benefit of removing that type.
DISCUSSION
The hypothesis that ORs would decrease with cross-immunity had not been quantitatively evaluated. The assumption has been that cross-immunity would decrease ORs for concurrent and sequential HPV infections, and therefore that ORs above unity implied the absence of cross-immunity [5–7, 10, 11]. However, our analysis shows that the elevated ORs identified in empirical surveys do not disprove the existence of cross-immunity between HPV types, contrary to previous interpretations [5–7, 11]. We also show that sample sizes of vaccine trials have been insufficient to detect even strong cross-immunity.
By using a mathematical model to determine the expected risks of multiple HPV infections under different scenarios of immediate and delayed interaction between HPV types, we have shown that delayed cross-immunity produces elevated ORs of multiple infections similar to those seen in empirical surveys [4–7]. This cross-immunity is predicted to reduce the efficacy of vaccination as a result of type replacement. However, while we calculate a significant increase in nontargeted HPV types due to vaccine-induced type replacement, these types are less carcinogenic than are HPV type 16 and 18 [30]. Infection with one of these nontargeted types presents a lower cancer risk, therefore partially ameliorating the negative public health impact of type replacement.
To focus our analysis on the interactions among HPV types and to minimize the considerable uncertainty involved in parameterizing human sexual behavior, we made several simplifying assumptions in our model. We treated the formation of sexual partnerships as homogeneous and well-mixing; in reality, human sexual networks have been shown to exhibit scale-free properties with a small number of individuals engaging in the most epidemiologically important behavior [31, 32]. Additionally, we excluded concurrent partnerships from our model, which, although rare within the population, have been shown to play an important role in sexual transmission of infection, and indicate a primary risk factor for infection [33, 34]. Finally, we assumed symmetry in transmission rates among partnerships regardless of whether the infected partner is male or female, although previous studies have suggested the HPV transmission rate from women to men is 3–4 times that from men to women [35].
The effective partner change rate partially compensates for these assumptions by implicitly including the variance of sexual behavior. Although the remaining factors are epidemiologically important, we do not think they would have a significant effect on our results due to the structure of our analysis. Our analysis first determines the equilibrium levels of infection and then stochastically simulates individual life histories. To affect our calculated ORs of multiple infection prevalence, these sexual network details would have to significantly shift the equilibrium levels of infection. This seems unlikely due to the small fraction of the population engaged in concurrent and high-risk sexual activity [34]. A thorough analysis of these factors would require an individually based computational or network simulation, adding formidable challenges of parameterization and exacerbating the difficulty of arriving at general conclusions about the interactions between HPV types.
The degree to which a vaccine itself generates cross-immunity will affect the extent of type replacement. For example, currently available vaccines based on L1 viral proteins produce type-specific immunity without significant cross-immunity [2]. Clinical trials of L1 vaccines reveal that although the prevalence of infection with HPV types 16 and 18 declined, the overall prevalence of infection with nontargeted HPV types remains steady among vaccinated individuals, suggesting that other HPV types increased in prevalence [27]. Conversely, L2 vaccines under development, which comprise a cross-neutralization epitope against genital HPV infection, may elicit cross-immunity and reduce type replacement while the vaccine is used [2, 36]. Vaccine-mediated cross-immunity beneficially reduces nontargeted HPV types during a vaccination program. However, the effect of this cross-immunity will only last for as long as the vaccine is used. Once the vaccine is no longer administered, the full effect of type replacement as a result of the removal of other HPV types will occur.
Previous studies of HPV vaccination have observed elevated risks of multiple infections, and interpreted these findings as evidence against significant cross-immunity among types. These interpretations have affected both vaccination policy and modeling [10–12]. Many of the models of effectiveness and cost-effectiveness of HPV vaccination have not addressed the possibility of cross-immunity, with a few exceptions [37, 38]. Our results suggest that type replacement may reduce vaccine effectiveness. Therefore, including cross-immunity factors may alter cost-effectiveness analyses and the optimization of public health strategies. Consequently, future evaluations of strategies to control HPV should not exclude the possibility of cross-immunity among HPV types.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are grateful for discussion with and the assistance of Jan Medlock, Traci Craig Green, Martina Morris, Lara Kidoguchi, Emlyn Jones, Angelika Hofmann, and Dave Thomas in the preparation of this manuscript.
E. M. P. and A. P. G. conceptualized the idea for this paper. E. M. P., A. P. G., and J. P. T. framed the analysis. D. P. D., Y. I., and E. M. P. conducted the analysis. All authors interpreted the results, discussed the findings, and contributed to writing the manuscript. All authors revised the paper for important intellectual content. All authors approved the final version of this paper.
Financial support. This work was supported by the James McDonnell Foundation (grant 220020114); the National Science Foundation (grant SBE-0624117); the National Institute of Health (grant R01A1072706); and the Miriam Burnett Trust.
Potential conflicts of interest. A. P. G. has consulted for Merck and Sanofi. These organizations did not fund or play any role in the research described in this manuscript. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Muñoz N, Bosch FX, Castellsagué X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- 2.Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One. 2011;6:e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbasha EH, Galvani AP. Vaccination against multiple HPV types. Math Biosci. 2005;197:88–117. doi: 10.1016/j.mbs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Rousseau MC, Pereira JS, Prado JC, Villa LL, Rohan TE, Franco EL. Cervical coinfection with human papillomavirus (HPV) types as a predictor of acquisition and persistence of HPV infection. J Infect Dis. 2001;184:1508–17. doi: 10.1086/324579. [DOI] [PubMed] [Google Scholar]
- 5.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 6.Mendez F, Munoz N, Posso H, et al. Cervical coinfection with human papillomavirus (HPV) types and possible implications for the prevention of cervical cancer by HPV vaccines. J Infect Dis. 2005;192:1158–65. doi: 10.1086/444391. [DOI] [PubMed] [Google Scholar]
- 7.Thomas KK, Hughes JP, Kuypers JM, et al. Concurrent and sequential acquisition of different genital human papillomavirus types. J Infect Dis. 2000;182:1097–102. doi: 10.1086/315805. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Katki HA, Hildesheim A, et al. for the CVT Group. Human papillomavirus infection with multiple types: pattern of coinfection and risk of cervical disease. J Infect Dis. 2011;203:910–20. doi: 10.1093/infdis/jiq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjaer SK, Munk C, Winther JF, Jørgensen HO, Meijer CJLM, van den Brule AJC. Acquisition and persistence of human papillomavirus infection in younger men: a prospective follow-up study among Danish soldiers. Cancer Epidemiol Biomarkers Prev. 2005;14:1528–33. doi: 10.1158/1055-9965.EPI-04-0754. [DOI] [PubMed] [Google Scholar]
- 10.Dillner J, Arbyn M, Dillner L. Translational mini-review series on vaccines: monitoring of human papillomavirus vaccination. Clin Exp Immunol. 2007;148:199–207. doi: 10.1111/j.1365-2249.2007.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roden R, Wu T-C. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006;6:753–63. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes JP, Garnett G, Koutsky L. The theoretical population-level impact of a prophylactic human papilloma virus vaccine. Epidemiology. 2002;13:631–9. doi: 10.1097/00001648-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Burchell AN, Winer RL, de Sanjosé S, Franco EL. Chapter 6: epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(Suppl 3):S3/52–61. doi: 10.1016/j.vaccine.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Malik ZA, Hailpern SM, Burk RD. Persistent antibodies to HPV virus-like particles following natural infection are protective against subsequent cervicovaginal infection with related and unrelated HPV. Viral Immunol. 2009;22:445–9. doi: 10.1089/vim.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lipsitch M. Bacterial vaccines and serotype replacement: lessons from Haemophilus influenzae and prospects for Streptococcus pneumoniae. Emerg Infect Dis. 1999;5:336–45. doi: 10.3201/eid0503.990304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196:1346–54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro GS, Reis JN, Cordeiro SM, et al. Prevention of Haemophilus influenzae type b (Hib) meningitis and emergence of serotype replacement with type a strains after introduction of Hib immunization in Brazil. J Infect Dis. 2003;187:109–16. doi: 10.1086/345863. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RM, Medley GF, May RM, Johnson AM. A preliminary study of the transmission dynamics of the human immunodeficiency virus (HIV), the causative agent of AIDS. IMA J Math Appl Med Biol. 1986;3:229–63. doi: 10.1093/imammb/3.4.229. [DOI] [PubMed] [Google Scholar]
- 19.May RM, Anderson RM. Transmission dynamics of HIV infection. Nature. 1987;326:137–42. doi: 10.1038/326137a0. [DOI] [PubMed] [Google Scholar]
- 20.Poolman EM, Elbasha EH, Galvani AP. Vaccination and the evolutionary ecology of human papillomavirus. Vaccine. 2008;26:C25–30. doi: 10.1016/j.vaccine.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Barnabas RV, Laukkanen P, Koskela P, Kontula O, Lehtinen M, Garnett GP. Epidemiology of HPV 16 and cervical cancer in Finland and the potential impact of vaccination: mathematical modelling analyses. PLoS Med. 2006;3:e138. doi: 10.1371/journal.pmed.0030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laumann EO, Gagnon JH, Michael RT, Michaels S. The social organization of sexuality: sexual practices in the United States. University of Chicago Press, Chicago; 1994. [Google Scholar]
- 23.Insinga RP, Dasbach EJ, Elbasha EH, Liaw K-L, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev. 2007;16:709–15. doi: 10.1158/1055-9965.EPI-06-0846. [DOI] [PubMed] [Google Scholar]
- 24.Kulmala S, Shabalova IP, Petrovitchev N, et al. Type-specific persistence of high-risk human papillomavirus infections in the New Independent States of the former Soviet Union Cohort Study. Cancer Epidemiol Biomarkers Prev. 2007;16:17–22. doi: 10.1158/1055-9965.EPI-06-0649. [DOI] [PubMed] [Google Scholar]
- 25.Richardson H, Kelsall G, Tellier P, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003;12:485. [PubMed] [Google Scholar]
- 26.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 27.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 28.Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107:18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 29.Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–8. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 30.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. The Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 31.Liljeros F, Edling CR, Amaral LA, Stanley HE, Åberg Y. The web of human sexual contacts. Nature. 2001;411:907–8. doi: 10.1038/35082140. [DOI] [PubMed] [Google Scholar]
- 32.Blythe SP, Castillo-Chavez C. Scaling of sexual activity. Nature. 1990;344:202. doi: 10.1038/344202a0. [DOI] [PubMed] [Google Scholar]
- 33.Ghani AC, Swinton J, Garnett GP. The role of sexual partnership networks in the epidemiology of gonorrhea. Sex Transm Dis. 1997;24:45–56. doi: 10.1097/00007435-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Adimora AA, Schoenbach VJ, Taylor EM, Khan MR, Schwartz RJ. Concurrent partnerships, nonmonogamous partners, and substance use among women in the United States. Am J Public Health. 2011;101:128–36. doi: 10.2105/AJPH.2009.174292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez BY, Wilkens LR, Zhu X, et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–94. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slupetzky K, Gambhira R, Culp TD, et al. A papillomavirus-like particle (VLP) vaccine displaying HPV16 L2 epitopes induces cross-neutralizing antibodies to HPV11. Vaccine. 2007;25:2001–10. doi: 10.1016/j.vaccine.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359:821–32. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorigatti I, Pugliese A. Analysis of a vaccine model with cross-immunity: when can two competing infectious strains coexist? Math Biosci. 2011;234:33–46. doi: 10.1016/j.mbs.2011.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.