Abstract
Objective/Hypothesis
Needle electrode-based electromechanical reshaping (EMR) is a novel, ultra-low cost nascent surgical technology to reshape cartilage with low morbidity. EMR uses direct current (DC) to induce mechanical relaxation in cartilage that is first deformed into a required geometry, which in turn leads to permanent shape change. The objective of this study was to determine the effect of EMR voltage and time on the shape change of costal cartilage grafts.
Study Design
Electromechanical reshaping of ex vivo porcine costal cartilage.
Method
Graft specimens obtained from the central core of porcine costal cartilage were bent at a 90° angle with a custom jig and then reshaped via EMR. The effect of voltage (3–7 V) and application time (1–5 min) on the amount of shape change were systematically examined. Bend angles were analyzed using ANOVA (analysis of variance) and paired t-tests to determine significant reshaping times at each voltage setting.
Results
There is a threshold for voltage and time above which the retention of bend angle is statistically significant in treated specimens compared to the control (P<0.05). Above the threshold of 3V, shape retention initially increased with application time for all voltages tested and was then observed to reach a plateau. Shape retention was noted to be greatest at 6V without a rise in temperature.
Conclusions
EMR provides a novel method to bend and shape costal cartilage grafts for use in facial plastic surgery. A low voltage can reshape cartilage grafts within several minutes and without the heat generation. This study demonstrates the feasibility of EMR and brings this minimally invasive procedure closer to clinical implementation.
Keywords: Costal Cartilage, Reconstructive Surgery, Tissue Reshaping, Otolaryngology, Rhinoplasty, Shape Change
INTRODUCTION
Costal margin rib is a major reservoir of cartilage tissue used to reconstruct or create absent cartilaginous structures of the head and neck particularly when septal or auricular cartilage has been depleted.1–4 Traditional surgical approaches to shape costal cartilage require skill and experience with carving and suturing techniques to create simple curves. Complications can arise when performing these surgical maneuvers that leads to an increase in utilized resources, scaring at the donor site, and rigidity of the hyaline cartilage; much of this due to unintended warping or graft distortion.5, 6 As an alternative to these surgical maneuvers, lasers were investigated as a form of treatment to reshape cartilage, but because of cost issues and the esoteric nature of the technology, have not garnered mainstream attention.
Minimally invasive surgery utilizing laser sources have provided surgeons with an alternative way to alter cartilage structures of the face. Lasers have been used to reshape the septum of the nose and the antihelix of the ear with good results7 although some techniques require an incision to expose the cartilage.8 Using lasers offers such advantages as: being readily available, easily shaped, ability to avoid donor site morbidity, low risk of infection and excellent tissue tolerance.9 While lasers have shown good outcomes, a major disadvantage of using lasers is cost and the possible cutaneous injury.10, 11 Using lasers to reshape costal cartilage grafts have not been documented to date, but lasers have been shown to accelerate and stabilize the warping of rib grafts.12
As an alternative to reshaping with lasers, we have developed a technique to reshape cartilage that we termed electro-mechanical reshaping (EMR). The process of EMR involves deforming the cartilage into the desired shape using a jig and then applying low voltage (1–6 V) direct current to the composite tissue via strategically placed needles. The tissue between the anode and cathode is the media for complex electrochemical reactions that result in localized stress relaxation; resistive heating does not occur at the voltages required to achieve shape change. The structural changes in tissue macromolecules have not been identified as of yet, though clearly this is a redox driven process. The reshaping process can be accomplished using percutaneous platinum needles.7 The use of needles is amenable to a minimally invasive procedure that offers similar advantages to using lasers, but without the high cost and risk of heat generation.7, 13, 14
EMR has been examined in rabbit cartilage tissue obtained from both septum and pinna and the dependence of shape change on voltage and time has been established.7, 13 However, in clinical implementation for major reconstructive surgery, costal cartilage would be a potential target tissue for this technology. To date, reshaping precise sections of costal cartilage specimens using EMR has not been investigated. This study focused on analyzing the shape change in costal cartilage following EMR for various electrical dosimetry parameters (voltage and time) in order to determine the feasibility of reshaping thicker and more clinically relevant cartilage tissue.
METHODS
Tissue preparation
Fresh porcine costal rib cartilage was obtained from a local abattoir and the surrounding muscle, fat, and connective tissues were excised. A custom fabricated cutting device was used to cut a 0.7mm thick slice from the central core of each rib.15 The central portion of costal cartilage was used in this study to minimize the effect of warping.5, 6 Each specimen was then cut to a standard size of 24mm × 8mm.
Reshaping Protocol
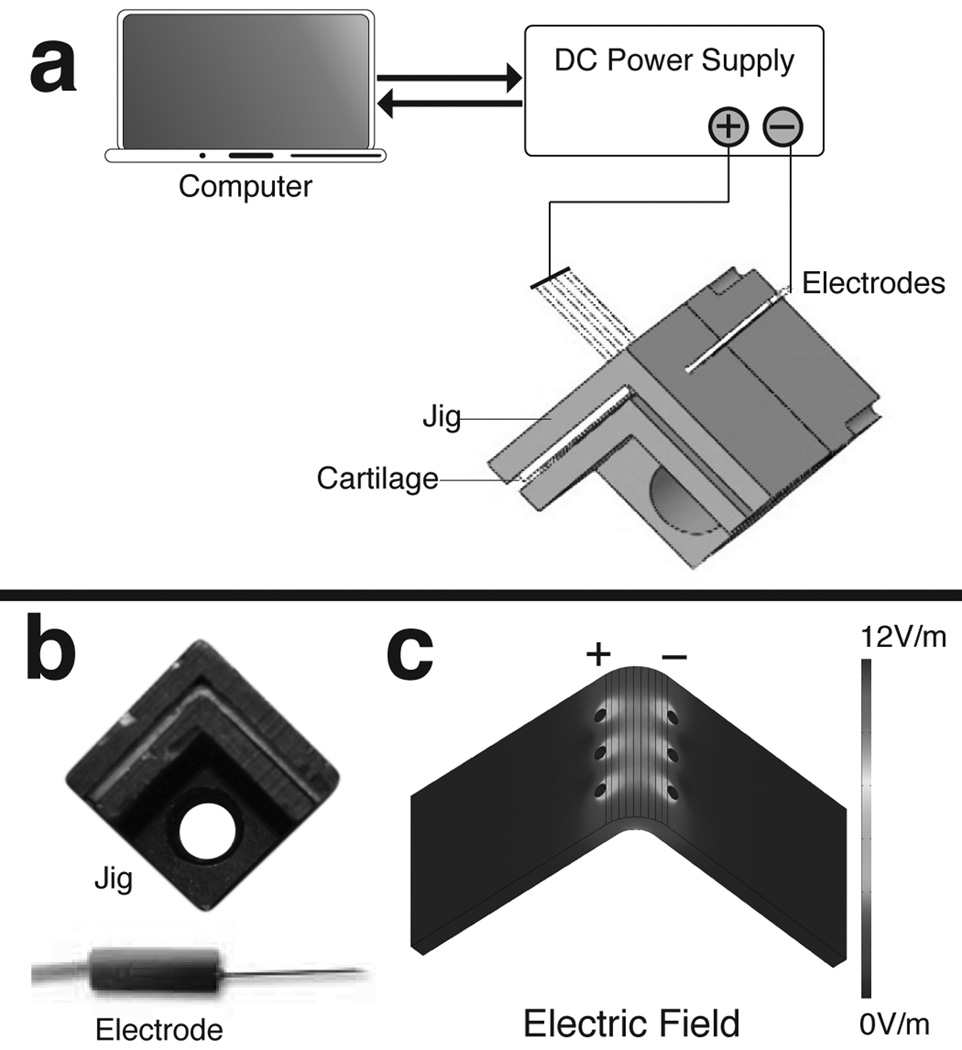
The EMR process began with mechanically deforming the specimen to a sharp 90° bend using a plastic jig (Fig. 1). Platinum needle electrodes (F-E2M-48, Grass Technologies, West Warwick, RI) were inserted into the cartilage through pre-drilled holes in the jig. The leads of the electrodes were connected to a DC power supply (E36446A, Agilent Technologies, Inc., Palo Alto, CA) with polarities arranged so that anodes and cathodes were on opposite sides of the bend (Fig. 1c). The voltage and application time of the power supply was set using a custom program (LabVIEW, National Instruments, Austin, TX). A low profile thermocouple (HH509R, Omega, Stamford, CT), to detect any potential resistive heat generation, was placed at the apex of the bend between the jig and specimen. A total of 145 specimens underwent reshaping using EMR. A range of voltages (3V, 4V, 5V, 6V, and 7V) and application times (1min, 2min, 3min, 4min and 5min) were systematically studied. The controls were bent in the jig and punctured with needles, but did not receive any application of voltage. All other specimens received EMR treatment with a single voltage and time combination. After EMR, the electrodes were removed and the jig containing the specimen was placed in a phosphate buffer solution at a pH of 7.4 and allowed to rehydrate for 15 minutes. After rehydration, the specimen was released from the jig and allowed to sit in open air for an additional minute before a digital photo was taken (Canon EOS 1000D Lake Success, NY). The bend angle was measured for each photograph using IMAGEJ software (National Institute of Health, Rockville, MD).
Figure 1.
(a) Experiment set-up. (b) Actual jig and electrode. (c) Finite-element model of the tangential electric field using 5V.
Analysis of Bend Angles
Electrical current traces measured during EMR were analyzed to ensure electrical contact and to quantify the amount of energy used to create the new shape. The total charge transfer was calculated from the electrical current data and the corresponding bend angle was compared to all other reshaped specimens.
The bend angles of reshaped specimens can range from 0° to 90° with 90° being the absolute maximum shape change achievable with this jig. A greater EMR effect corresponds to a larger bend angle, likewise a shallow bend angle correlates to a less effective EMR setting. A statistical analysis was performed on measured bend angles using one-way general linear model analysis of variance (ANOVA) to determine if the means are different within each voltage group. When significant effects were detected by ANOVA, a paired t-test was performed to determine which parameter sets exhibited statistically significant results (P < 0.05).
RESULTS
Reshaping of Costal Cartilage
The evolution of gas at the anode and cathode was observed during EMR, similar to a previous study on rabbit septal cartilage.7, 13 Following EMR, the tissue surrounding anode electrodes was observed to be transparent, while that surrounding the cathode was opaque white; this observation was seen within a 1–3 mm diameter region surrounding the needle electrodes. The diameter of these anode and cathode regions increased as expected with application time and voltage. The transparency of the anode region and the white color of the cathode region returned to its native opacity after rehydration in PBS.
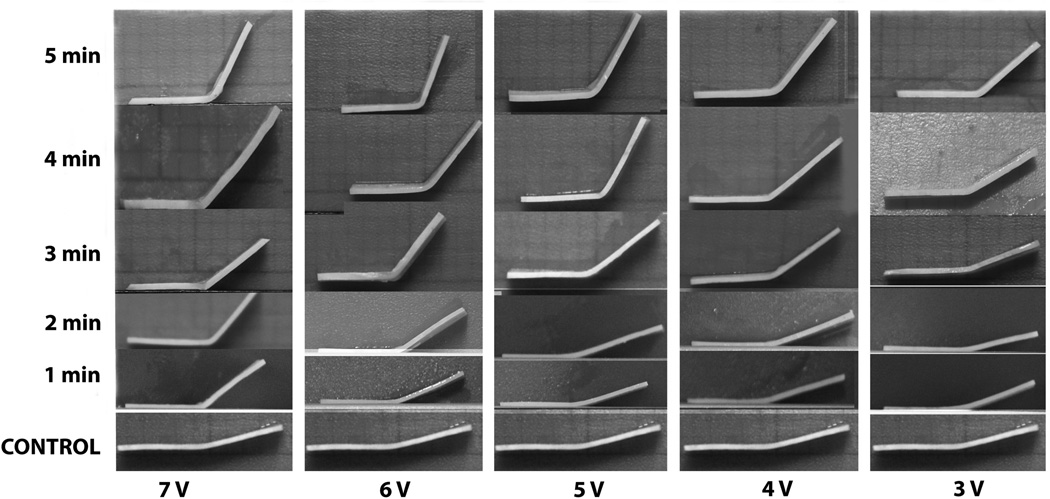
A total of 145 rib grafts were modified with EMR and an additional 8 specimens were used as controls that did not undergo EMR treatment. The average thickness of all specimens was 0.71±0.08mm. The resultant shape change of rib grafts increased with increasing voltage and application time (Fig. 2). Control specimens remained fairly flat and had slight residual shape memory produced by the sharp 90° bend of the jig and the perforations created by platinum needles.
Figure 2.
A montage reshaped rib grafts compared to the control.
We identified an onset and saturation threshold of shape change of the specimens for each voltage group. The bend angle mean and standard deviation of each EMR dosimetry is plotted in Fig. 3. For a specific voltage, the bend angle increased with time except for 3V and 7V group. At 4Vand 5V, the onset of significant reshaping as compared to the control group began at 2 minutes of application time. The next significant increase in bend angle occurred at 4 minutes of application time, which was also the point where saturation effects were observed. At 6V, the onset of significant shape change began at 1 minute and saturated at 4 minutes. The greatest resultant shape change using 7V was identified at 2 minutes compared to all other application times in the same voltage group. At 7V, a slight temperature increase (< 2°C) was noted starting at 2 minutes. No heat evolution was detected for all other voltages. The tabulated range of bend angles and sample size on specific EMR voltage and time parameters used in this study are shown in Table 1.
Figure 3.
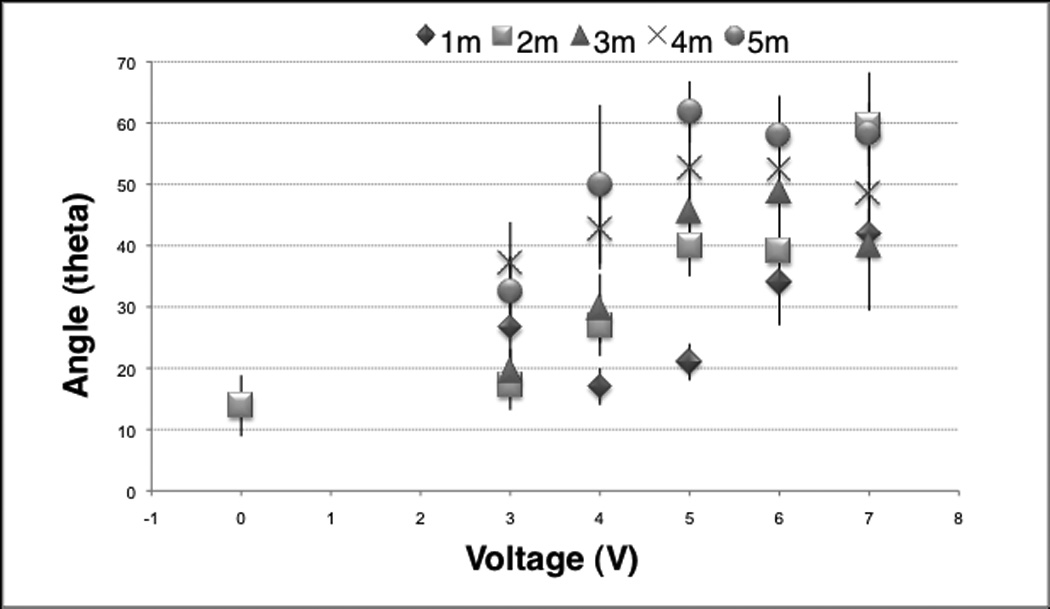
The mean and standard deviation of resultant bend angles after EMR compared to control the group.
Table I.
Sample size and bend angles ranges of treated grafts.
Rib graft EMR dataset.
| 1min | 2min | 3min | 4min | 5min | |
|---|---|---|---|---|---|
| 3V | 18°–32° n = 5 |
13°–23° n = 5 |
14°–24° n = 5 |
29°–45° n = 5 |
23°–41° n = 8 |
| 4V | 14°–21° n = 5 |
21°–33° n = 5 |
23°–39° n = 6 |
36°–52° n = 5 |
36°–75° n = 7 |
| 5V | 18°–25° n = 5 |
31°–45° n = 6 |
36°–53° n = 6 |
41°–60° n = 5 |
54°–67° n = 5 |
| 6V | 24°–43° n = 5 |
35°–45° n = 5 |
29° – 57° n = 10 |
37° – 71° n = 5 |
50° – 67° n = 5 |
| 7V | 37°–47° n = 5 |
50°–69° n = 5 |
22° – 51° n = 8 |
38° – 58° n = 6 |
48° – 63° n = 6 |
Electrical current traces increased with voltage (Fig. 4) and showed a transient exponential decrease in current during the first seconds of the reshaping process for all voltage categories. The current then remained steady in amplitude for the 3V, 4V, and 5V tracings. At 6V and 7V, the current slightly increased and then decreased with time. An increase in the transferred electric charge correlated with greater reshaping (Fig. 5).
Figure 4.
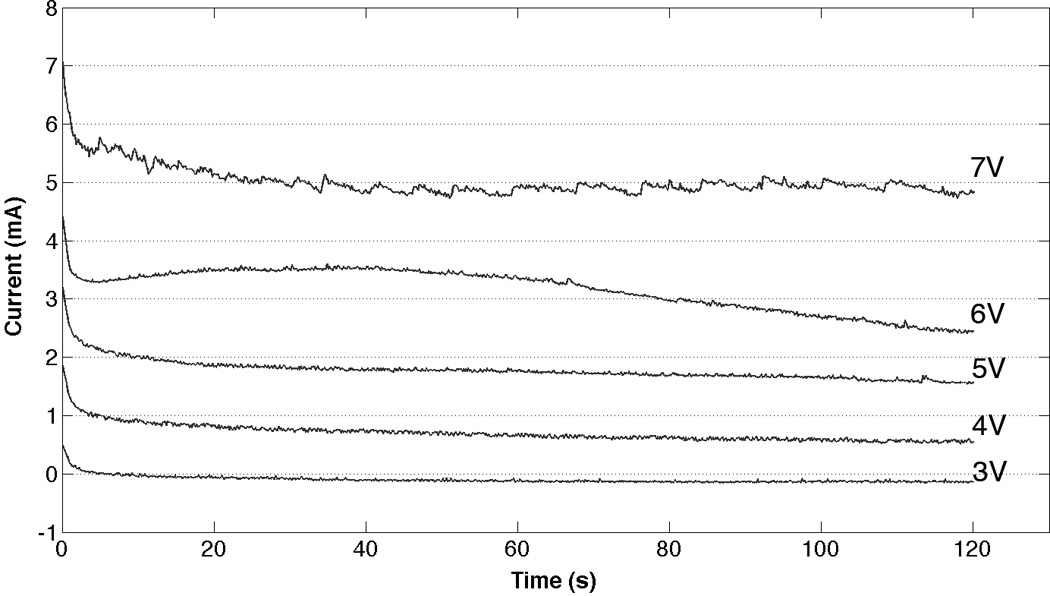
Typical electric current traces during the first two minutes of EMR.
Figure 5.
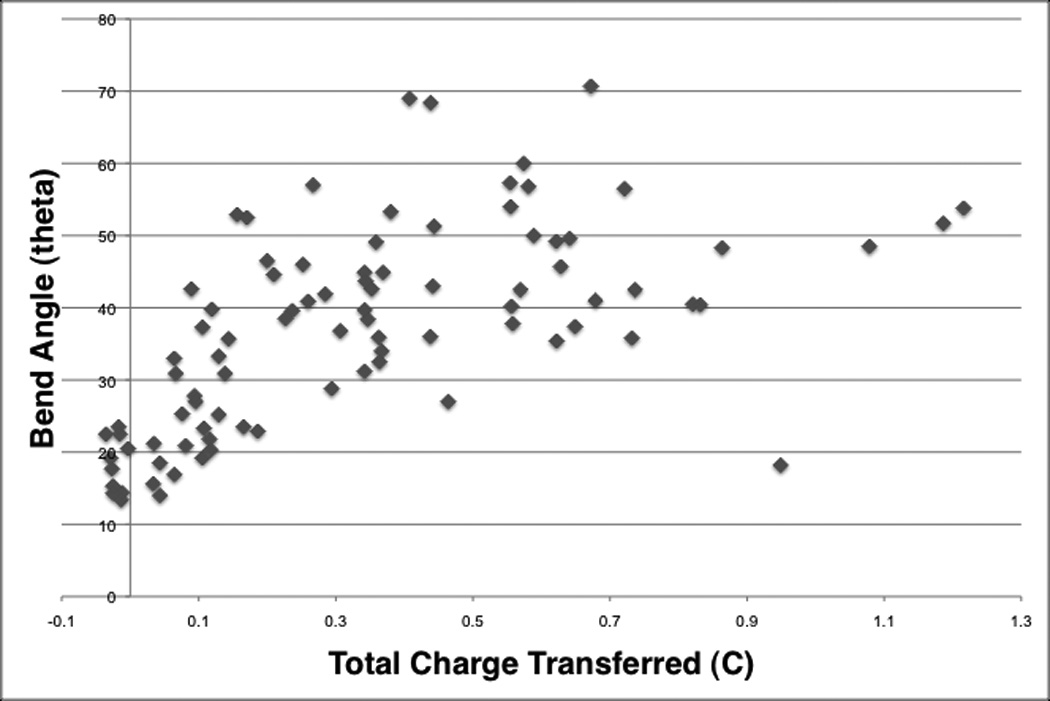
Scatter plot of bend angles and the corresponding electrical charge transfer.
DISCUSSION
We have shown that EMR reshapes costal cartilage grafts without the generation of significant heat. (Fig. 2) A low voltage is required for ideal reshaping and can be powered with disposable batteries. (Fig. 4) This minimally invasive and spatially selective treatment modality has the clinical potential to progressively reshape cartilage structures of the face in situ or as demonstrated herein with rib grafts.
The general trend in EMR is that increased voltage and application time result in more effective reshaping. Voltage level is clearly important on individual molecules, macromolecules, and moieties as they will have different standard oxidation-reduction potentials. At present, the molecular components of cartilage beyond water that participate in EMR are unknown, though this is an active area of investigation in our laboratory and those of others. At 7V, resistive heating becomes detectable and thus using lower voltages is ideal in order to minimize heat induced tissue damage. Nonetheless, the heat increase at even 7V is slight (< 2°C) and inadequate to cause protein denaturation.
The EMR voltage and time parameters investigated in this study showed an onset of reshaping at 3V and saturation beginning at 5V. Between 4V to 6V, reshaping increased with application time. A different trend however, was noted at 3V and 7V (Fig. 3) where bend angles did not increase with increasing application time. The fluctuation of bend angles noted at 3V could be due to the voltage threshold required for reshaping 0.7mm grafts. Furthermore, a likely reason for the inconsistent trends at both 3V and 7V can be due to the anisotropic property of costal cartilage and slight differences in tissue thickness. There is also a variance in the amount of electrical charge transferred at a specific voltage and time. This can be attributed to the small amount of play of the needles within the jig, which can result in inconsistent positioning of the needles during insertion into the cartilage. Lastly, the variance can be due to regional variability in the location from which the costal rib cartilage was anatomically obtained; for example ribs towards the superior region have a greater natural curve than those inferior.
Surgeons may be able to use EMR to reshape cartilage. For example, in operations requiring reconstruction of the lower lateral cartilages, flat pieces of rib grafts can be reshaped using EMR to create an acute angle and then possibly further stabilized with sutures. This treatment modality has the potential to also reshape cartilage in situ such as in a needle-based method to create anti-helical folds in patients with prominent ears. Whether with grafts or in situ, needle-based EMR can modestly reshape cartilage as a sole form of treatment, or in combination with time-honored cut and suture techniques.
This was an ex vivo study, and in vivo work must be performed to observe the long-term stability and shape retention of EMR treated specimens. This work is currently underway in our laboratory.
CONCLUSION
EMR provides a novel method to bend and shape costal cartilage grafts for use in facial plastic surgery. Costal cartilage can be reshaped using (EMR) with platinum needle electrodes to create bends up to 70°. Maximum reshaping is accomplished using voltages 4V, 5V or 6V and with an application time of 5 minutes. Animal studies are needed to examine the long term healing response of treated cartilage and are warranted prior to human trials.
ACKNOWLEDGEMENTS
This work was supported by the Department of Defense Deployment Related Medical Research Program (DR090349), Air Force Office of Scientific Research (FA9550–04–1–0101) and the National Institutes of Health (DE019026, DC005572, DC 00170, RR 01192).
Footnotes
Conflict of interest: None
BIBLIOGRAPHY
- 1.Quatela VC, Jacono AA. Structural grafting in rhinoplasty. Facial Plast Surg. 2002;18:223–232. doi: 10.1055/s-2002-36490. [DOI] [PubMed] [Google Scholar]
- 2.Gentile P, Cervelli V. Nasal dorsum reconstruction with 11th rib cartilage and auricular cartilage grafts. Ann Plast Surg. 2009;62:63–66. doi: 10.1097/SAP.0b013e31817433dc. [DOI] [PubMed] [Google Scholar]
- 3.Brent B. Technical advances in ear reconstruction with autogenous rib cartilage grafts: personal experience with 1200 cases. Plast Reconstr Surg. 1999;104:319–334. doi: 10.1097/00006534-199908000-00001. discussion 335–318. [DOI] [PubMed] [Google Scholar]
- 4.Brent B. Ear reconstruction with an expansile framework of autogenous rib cartilage. Plast Reconstr Surg. 1974;53:619–628. doi: 10.1097/00006534-197406000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Kim DW, Shah AR, Toriumi DM. Concentric and eccentric carved costal cartilage: a comparison of warping. Arch Facial Plast Surg. 2006;8:42–46. doi: 10.1001/archfaci.8.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Harris S, Pan Y, Peterson R, et al. Cartilage warping: an experimental model. Plast Reconstr Surg. 1993;92:912–915. [PubMed] [Google Scholar]
- 7.Manuel CT, Foulad A, Protsenko DE, et al. Needle electrode-based electromechanical reshaping of cartilage. Ann Biomed Eng. 2010;38:3389–3397. doi: 10.1007/s10439-010-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ragab A. Carbon dioxide laser-assisted cartilage reshaping otoplasty: a new technique for prominent ears. Laryngoscope. 2010;120:1312–1318. doi: 10.1002/lary.20951. [DOI] [PubMed] [Google Scholar]
- 9.Kridel RWHAF, Liu ES, Hart CG. Long-term use and follow-up of irradiated homologous costal cartilage grafts in the nose. Arch Facial Plast Surg. 2009;11(6):378–394. doi: 10.1001/archfacial.2009.91. [DOI] [PubMed] [Google Scholar]
- 10.Olbricht SM, Stern RS, Tang SV, et al. Complications of cutaneous laser surgery. A survey. Arch Dermatol. 1987;123:345–349. [PubMed] [Google Scholar]
- 11.Alster TS, Lupton JR. Prevention and treatment of side effects and complications of cutaneous laser resurfacing. Plast Reconstr Surg. 2002;109:308–316. doi: 10.1097/00006534-200201000-00048. discussion 317–308. [DOI] [PubMed] [Google Scholar]
- 12.Foulad A, Ghasri P, Garg R, et al. Stabilization of costal cartilage graft warping using infrared laser irradiation in a porcine model. Arch Facial Plast Surg. 2010;12:405–411. doi: 10.1001/archfacial.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Protsenko DE, Ho K, Wong BJ. Stress relaxation in porcine septal cartilage during electromechanical reshaping: mechanical and electrical responses. Ann Biomed Eng. 2006;34:455–464. doi: 10.1007/s10439-005-9051-y. [DOI] [PubMed] [Google Scholar]
- 14.Protsenko DE, Ho K, Wong BJ. Survival of Chondrocytes in Rabbit Septal Cartilage After Electromechanical Reshaping. Ann Biomed Eng. 2010 doi: 10.1007/s10439-010-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foulad AMC, Wong B. Practical device for rapid and precise cutting of costal cartilage grafts to uniform thickness. Arch Facial Plast Surg. 2010 doi: 10.1001/archfacial.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]