Abstract
Inflammation may contribute to cognitive decline and dementia. This study examined the cross-sectional relationships between markers of systemic inflammation (C-reactive protein, interleukins-1β, -6, -8, -10, -12, plasminogen activator inhibitor, serum amyloid A, tumour necrosis factor-α and vascular adhesion molecule-1) and cognitive function in 873 non-demented community-dwelling elderly participants aged 70–90 years. Regression analyses were performed to determine the relationships between cognitive domains and inflammatory markers, controlling for age, sex, education, cardiovascular risk factors, obesity and other metabolic factors, smoking, alcohol consumption, depression and presence of the apolipoprotein ε4 genotype. Regression analyses were repeated using four factors derived from a factor analysis of the cognitive tests. After Bonferroni correction for multiple testing, associations remained between raised levels of interleukin-12 and reduced performance in processing speed. Marked sex differences were noted in the abovementioned findings, with only females being significantly affected. Using the four factors derived from the factor analyses of cognitive test as dependent variables, interleukins-12 and -6 were both associated with the processing speed/executive function factor, even after controlling for relevant confounding factors. Thus, markers of systemic inflammation are related to cognitive deficits in a non-clinical community-dwelling elderly population, independent of depression, cardiovascular or metabolic risk factors, or presence of apolipoprotein ε4 genotype. Additional research is required to elucidate the pathophysiology and longitudinal development of these relationships.
Keywords: Inflammation, Ageing, Cytokines, Inflammaging, Cognition, Dementia
Cognitive impairment and dementia are disabling conditions that are increasingly common with advancing age. With a rapidly ageing population, it is imperative to identify modifiable risk and/or protective factors associated with cognitive decline. One such factor may be low-grade systemic inflammation. A link between systemic inflammation and dementia was first hypothesised after discovery of up-regulated inflammatory processes localised to Alzheimer's disease (AD) pathology in post-mortem brain specimens (Ishii et al. 1975; Rogers et al. 1988). Subsequent research has identified an association between dementia and markers of systemic inflammation. In cross-sectional analysis of clinical populations, a reasonably consistent finding has been an association between dementia and higher levels of interleukin (IL)-1β, IL-6, C-reactive protein (CRP) and tumour necrosis factor-α (TNF-α) (Alvarez et al. 2007; Bermejo et al. 2008; Bruunsgaard et al. 1999; Dimopoulos et al. 2006; Engelhart et al. 2004; Licastro et al. 2000; Zuliani et al. 2007). Investigations have recently extended work on systemic inflammation to pre-dementia syndromes such as mild cognitive impairment (MCI) suggesting that increases in measures of low-grade systemic inflammation are linked to cognitive impairment. For example, studies have shown that increased levels of TNF-α are found in MCI patients compared to normal controls (Alvarez et al. 2007; Bermejo et al. 2008).
Similarly, systemic inflammation has been observed to increase with age in humans, of potential relevance for cognitive ageing. Termed ‘inflammaging’ (Franceschi et al. 2007), this age-related phenomenon includes a low-grade chronic inflammatory response, decline in adaptive immune mechanisms and concurrent up-regulation of the innate immune system (Giunta et al. 2008). This concept has been partially supported by initial studies of cognitively intact community-dwelling populations, which evaluate the impact of systemic inflammation on cognitive function. High levels of CRP and IL-6 have been commonly associated with poor cognitive performance in older cohorts (Alley et al. 2008; Komulainen et al. 2007; Marsland et al. 2006; Ravaglia et al. 2005; Schram et al. 2007; Weaver et al. 2002). However, several studies have not replicated these findings (Baune et al. 2008; Fischer et al. 2006; Schram et al. 2007). Instead, the MEMO study found that high levels of IL-8 were associated with low cognitive performance (Baune et al. 2008). Longitudinal data have not lessened the controversies of dominance of any particular inflammatory marker. For example, a 5-year longitudinal study found high IL-6 levels predicted cognitive decline (Schram et al. 2007); in contrast, a 3-year longitudinal study failed to show any predictive influence of IL-6 levels (Dik et al. 2005). Other studies have suggested that it is the cognitive ability that predicts the level of inflammation, with low cognitive ability in childhood or early adulthood predicting high levels of systemic inflammation in middle or old age (Luciano et al. 2009; Phillips et al. 2011).
The conflicting findings evident in the ‘inflammaging’ literature could be attributed to considerable methodological disparities between the abovementioned studies, which significantly reduce study comparability. Key discrepancies include measurement of circulating inflammatory markers, i.e. their type, method and assay sensitivity; and importantly, neuropsychological assessments of cognition. For example, some studies have relied only on the mini-mental state examination (MMSE), which is only a screening questionnaire. Another limitation of prior studies is residual confounding, i.e. not adjusting for, or else inaccurately measuring, confounding variables. Few comprehensive studies have been conducted with a large array of markers in a carefully characterised population with an extensive battery of neuropsychological tests.
Another key methodological concern arises from the interrelated biological variables that affect both systemic inflammation and cognition. Whilst some studies have controlled for only basic demographics (Dik et al. 2005; Schram et al. 2007), several utilised multiple adjustments including medical, psychological, genetic and lifestyle factors (Alley et al. 2008; Baune et al. 2008; Fischer et al. 2006; Ravaglia et al. 2005; Weaver et al. 2002). A consistent evidence-based approach is lacking in this regard.
This paper seeks to advance the ‘inflammaging’ literature by exploring the relationship between a broad array of systemic inflammatory markers and a comprehensive assessment of cognitive function in a large non-demented community-dwelling elderly cohort. Based on past findings, we expected to find an association between increased systemic inflammation and impaired cognitive performance, especially for biomarkers such as IL-6, IL-8 and CRP. We included vascular adhesion molecule-1 (VCAM-1) as it has been found to be elevated in patients with dementia but has not been previously measured in a community sample (Dimopoulos et al. 2006; Zuliani et al. 2008). TNF-α, IL-1β, IL-10 and IL-12 were included on the basis of a consistent association with MCI (Alvarez et al. 2007; Bermejo et al. 2008) and dementia (Bagnoli et al. 2007; Deniz-Naranjo et al. 2008; Motta et al. 2007). Plasminogen activator inhibitor-1 (PAI-1) and serum amyloid A (SAA) were included as they are activated by some of the previously mentioned inflammatory markers (Uhlar and Whitehead 1999; Soeda et al. 2008). Our approach differs from prior studies, not only in our comprehensive assessment of serum inflammatory markers and neuropsychological function, but by controlling for a range of well-measured possible confounders including depression, cardiovascular risk factors, obesity, metabolic factors, smoking, alcohol consumption and apolipoprotein E (APOE) genotype. In addition, this study explores sex differences on the impact of inflammation on cognition, as sex differences in dementia risk factors were recently outlined in a review (Azad et al. 2007).
Materials and methods
Participants
Participants were drawn from the Sydney Memory and Aging Study (MAS), which has been described in detail elsewhere (Sachdev et al. 2010). In brief, MAS is a prospective population-derived cohort comprised of 1,037 non-demented community-dwelling adults aged 70–90 years at enrolment. The sample is not more economically deprived than the general population. Exclusion criteria included insufficient English, sensory deficits precluding neuropsychological assessment, major neurological or psychiatric disorder (multiple sclerosis, motor neuron disease, central nervous system inflammation, developmental disability, psychotic symptoms, schizophrenia or bipolar disorder) or progressive malignancy. Individuals were also excluded if they had a prior diagnosis of dementia (DSM-IV-TR criteria; American Psychiatric Association 2000) or a baseline MMSE (Folstein et al. 1975) score of less than 24 after adjustment for age, years of education and non-English speaking background (NESB)(Anderson et al. 2007).
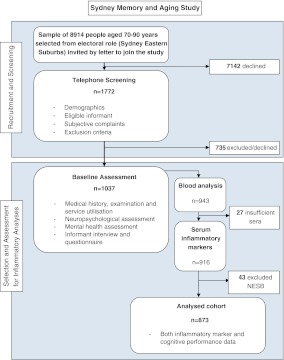
MAS participants underwent an extensive assessment including a detailed medical and lifestyle history, physical and mental status examination, comprehensive neuropsychological assessment and fasting early morning blood tests (including routine biochemistry, glucose and systemic inflammatory markers). Because of uncertainty of the validity of their neuropsychological test scores, NESB participants were excluded from the present study. Thus, a total of 873 participants with both neuropsychological and biomarker data was available for the analysis (see Fig. 1). Ethics approval for this study was granted by the University of New South Wales and the South-Eastern Illawarra Area Health Service—Eastern sector (HREC 05037) and consent was obtained for all participants.
Fig. 1.
Cohort selection and assessment
Neuropsychological tests
A comprehensive neuropsychological test battery was administered by trained psychology graduates. Twelve tests were conducted representing the diverse array of cognitive functions impaired with ageing, producing a total of six cognitive domains (see Table 1). A high z-score represented good performance.
Table 1.
Cognitive domains and component tests
| Cognitive domain | Neuropsychological test scores included |
|---|---|
| Processing speed | Digit Symbol, Trail Making Test A |
| Fine motor | Grooved Pegboard Test |
| Memory | Logical Memory Story A (immediate and delayed), RAVLT (total learning; trials 1–5, short-term recall; trial 6 and long-term ecall; trial 7), BVRT recognition |
| Language | Animal naming, 30-item Boston Naming Test |
| Spatial | Block Design |
| Executive | Trail Making Test B, FAS, Stroop Interference |
NB Domain scores are composites of component test Z-scores. Fine motor and spatial domains were represented by only one test each
RAVLT Rey Auditory Verbal Learning Test; BVRT Benton Visual Retention Test; FAS phonemic fluency
To assess memory domain, several tests were conducted, including Logical Memory Story A (immediate and delayed recall; Wechsler 1997), Rey Auditory Verbal Learning Test (total learning, short-term and long-term delayed recall, RAVLT; Rey 1964) and Benton Visual Retention Test recognition (BVRT; Benton Sivan and Spreen 1996). Trail Making Test B (TMTB; Reitan and Wolfson 1993), phonemic fluency (FAS; Benton 1967) and a computerised 40-item version of the Stroop Test (colour-word interference score; Stroop 1935) were part of the executive function domain. Both the 30-item Boston Naming Test (BNT, Fastenau et al. 1998; Kaplan et al. 2001) and semantic fluency (Animal Naming Task; Spreen and Benton 1969) were utilised to examine language domain. Trail Making Test A (TMTA; Reitan and Wolfson 1993) and Digit–Symbol Coding task (Wechsler 1997) were used to assess processing speed domain. The Block Design task (Wechsler 1981) and the Grooved Pegboard Test (Klove 1963) assessed visuo-spatial domain and fine motor domain, respectively.
Inflammatory markers
Blood was collected after an overnight fast, clotted, aliquoted and frozen at −80°C. An array of inflammatory biomarkers were analysed, including interleukins (IL-) -1β, -6, - 8, -10, -12p70, serum vascular cell adhesion molecule-1 (sVCAM-1), PAI-1, SAA, TNF-α and CRP.
sVCAM-1, PAI-1 and SAA levels were measured using commercially available sandwich enzyme-linked immunosorbant assay (ELISA) kits. The sVCAM-1 and PAI-1 ELISA kits were obtained from Bender Medsystems GmbH (Austria, Europe). The detectable range was 3.1–100 ng/ml for sVCAM-1, and 78–5,000 pg/ml for PAI-1. SSA ELISA kit was obtained from United States Biological (USA) and had a detectable range of 9.4–600 ng/ml. High sensitivity CRP was measured via Near Infrared Particle Immunoassay rate methodology using Beckman Coulter Synchron LXi (Beckman Coulter, USA).
The cytokines IL-1β, IL-6, IL-8, IL-10, IL-12 and TNF-α concentrations were measured using cytometric bead array (CBA, BD Biosciences, San Diego, CA, USA). Six bead populations with distinct fluorescence intensities were coated with capture antibodies specific for the corresponding proteins. These bead populations were mixed together to form the BD CBA, which resolved in the FL3 channel of a flow cytometer (BD FACSCalibur). The capture beads, PE-conjugated detection antibodies and recombinant standards were incubated together to form sandwich complexes. Following acquisition of sample data using the flow cytometer, the results were generated in graphical and tabular format using the BD CBA Analysis Software. The intra-assay coefficients of variation were 4–7% for IL-1 β, 5–8% for IL-6, 2–5% for IL-8, 5–6% for IL-10, 3–6% for IL-12 and 6–10% for TNF-α. The inter-assay coefficients of variation were 8–13% for IL-1β, 8–10% for IL-6, 4–7% for IL-8, 8–11% for IL-10, 6–9% for IL-12 and 8–15% for TNF-α.
Covariates
All analyses of the relationships between inflammatory markers and cognition were performed with participants' age, sex and years of education as control variables. Further covariates were selected based on their impact on cognition and inflammation in our sample. Factors demonstrated in the literature, to have an effect on inflammation and/or cognitive function in the elderly, were also taken into account. The final covariates were depression (van den Biggelaar et al. 2007), cardiovascular and metabolic factors (Danesh et al. 2000; Kuo et al. 2005; Lezak et al. 2004; Strachan et al. 2008; Warnberg et al. 2009; Wilson et al. 2002) and APOE genotype (Henderson et al. 1995). Cardiovascular factors were diagnosed history of angina, acute myocardial infarction (AMI), cerebrovascular accident (CVA), transient ischaemic attack, hypertension, alcohol consumption and regular current or past smoking. Alcohol consumption was defined as abstainers, one drink per day, or more than one drink per day in the last year. Each participant identified whether they engaged in regular smoking, either currently or in the past. Metabolic factors were recorded: body mass index (BMI, weight/height2), diagnosis of diabetes mellitus (DM) and fasting blood glucose. DM was defined as either having a previous diagnosis made or current fasting glucose >7 mmol/L. Normal and IFG states were defined using the American Diabetes Association criteria: normal ≤5.5 mmol/L, IFG defined by glucose between 5.6 and 6.9 mmol/L. Depressive symptoms were assessed by the 15-item Geriatric Depression Scale (GDS; Sheikh and Yesavage 1986), which has been shown to be valid and have excellent test re-test reliability (Sheikh et al. 1991). The continuous GDS was included as a covariate.
Of 873 participants included in analysis 861 (99%) had data relating to APOE genotype. Genomic DNA was extracted from peripheral blood leukocytes using standard procedures and stored at Genetics Repositories Australia. APOE genotyping was undertaken by genotyping the two single nucleotide polymorphisms (SNPs, rs7412 and rs429358) that distinguish between the three APOE alleles ε2, ε3 and ε4. Genotyping was performed using Taqman assays (Applied Biosystems Inc. [ABI], Foster City, CA, USA). The validity of the APOE genotyping was confirmed in a subsample using an alternate genotyping method (Hixson and Vernier 1990). APOE genotyping results were available for more than 99% of the DNA samples and the allele frequencies in Caucasians for each of the two SNPs were in Hardy-Weinberg equilibrium (p > 0.05). In analysis, participants were coded as either carriers or non-carriers of the ε4 allele.
Statistical analyses
Data analyses were performed using software programme PASW Statistics 18.0 (SPSS Statistics 2008). Biomarker distributions were highly skewed, and attempts to transform to approximately normal distributions were not successful. Therefore, regression analyses were carried out using the four quartiles of each inflammatory marker as an independent variable and cognitive domains as the dependent variables. Cognitive domain composite scores were formed by averaging the z-scores of the component neuropsychological tests (see Table 1) and transforming to normal scores, using Blom's (1958) procedure.
Preliminary analyses were carried out to ensure no violation of relevant assumptions including, normality, linearity, homoscedasticity, homogeneity of variances and homogeneity of regression slopes. Age and sex were included as covariates in an initial set of analyses and in all subsequent analyses. Education was included subsequently. Data were analysed again to explore the impact of the following additional covariates, namely depression, cardiovascular factors, metabolic factors, alcohol consumption and APOE ε4 genotype. Bonferroni correction to the type 1 error rate was implemented to account for multiple testing, with the adjusted value being set at 0.008 (0.05 divided by six tests). To examine any sex differences, data were also categorised by sex and the regression analyses were conducted again, but only for those inflammatory markers that had a significant association with cognition after Bonferroni correction for multiple testing.
Two additional analyses were carried out. The inflammatory markers and the cognitive tests both underwent a factor analysis procedure and three and four factors were extracted, respectively. The three factors of inflammation were then replaced instead of the ten inflammatory markers within a series of regression analyses, similar to the ones described above, keeping the cognitive domains and including all available covariates. In addition, the four cognitive factors replaced the six cognitive domains and were included in a series of regression analyses.
Results
Descriptive statistics
Table 2 presents general characteristics of the MAS cohort for which inflammatory analysis was undertaken. The mean age was 78.7 ± 4.8 years, 52% of the sample was female and mean duration of education was 11.6 ± 3.5 years. These characteristics were not significantly different to those of the total sample. Twelve percent had suffered an MI, whilst 4% had a prior diagnosis of CVA. Mean BMI was 27.1 ± 4.5 and 13.4% of participants had pre-existing diagnosis of diabetes mellitus. Although 54% of the sample had a past history of regular tobacco smoking only 7% of these had smoked in the past month. Consistent with epidemiological findings for a Caucasian population, carriers of an APOE ε4 allele (Christensen et al. 2008) were 20% of the cohort. There were a number of differences between males and females, including education, BMI, percentage of people who had/have angina, CVA, AMI, DM, fasting blood glucose, and the levels of HDL cholesterol, smoking and alcohol consumption.
Table 2.
General characteristics for participants in the Sydney MAS cohort in which inflammatory analyses were performed
| Males, n = 448; mean (SD) or percentage with the condition | Females, n = 425; mean (SD) or percentage with the condition | Total sample, n = 873; mean (SD) or percentage with the condition | |
|---|---|---|---|
| Age | 78.76 (4.70) | 78.89 (4.92) | 78.84 (4.82) |
| Education | 12.30 (3.82)* | 11.04 (3.05)* | 11.59 (3.47) |
| Body Mass Index (kg/m2) | 27.61 (4.17)* | 26.64 (4.70)* | 27.08 (4.50) |
| Depression | 9.9% | 10.7% | 10.3% |
| Anginaa | 18.1%* | 8.9%* | 13% |
| Cerebrovascular accident (CVA)a | 5.9%* | 2.5%* | 4% |
| Acute myocardial infarction (AMI)a | 18.3%* | 6.2%* | 11.6% |
| Transient ischaemic attack (TIA)a | 6.3% | 7.2% | 6.8% |
| Past or current regular smoking | 67.9%* | 42.7%* | 54% |
| Alcohol consumption | * | * | |
| Abstainer | 8.4% | 15.9% | 12.5% |
| ≤1 drink per day | 44.5% | 61.7% | 54.1% |
| >1 drink per day | 47.5% | 22.2% | 33.4% |
| HDL-cholesterol | 1.29 (.39)* | 1.57 (.43)* | 1.44 (.44) |
| Hypertension | 58.6% | 62.8% | 61% |
| Triglycerides | 1.06 (.56) | 1.06 (.52) | 1.06 (.54) |
| Diabetes mellitus | 18.2%* | 9.4%* | 13.4% |
| Fasting blood glucose | * | * | |
| >10 mmol | 1.6% | 0.8% | 1.2% |
| 7.0–10 mmol | 12.4% | 6% | 9.2% |
| 5.6–6.9 mmol | 50.3% | 41.8% | 56.3% |
| <5.6 mmol | 35.7% | 51.4% | 42.7% |
| Apolipoprotein ε4 allele | 21% | 18.9% | 19.9% |
aIndicates variables determined by prior doctor diagnosis or confirmation
*p < 0.05 indicates a statistically significant difference between males and females
Correlations between inflammatory markers
Table 3 shows the Pearson's correlation coefficients for the inflammatory biomarkers. Whilst PAI-1 correlated significantly only with VCAM-1, TNF-α and CRP, most of the other inflammatory markers correlated with each other. In fact, out of the 45 correlations, 29 were statistically significant. Table 4 shows the mean and standard deviation for the raw data of each quartile for each inflammatory marker.
Table 3.
Pearson's correlation coefficient of all normalised inflammatory markers
| VCAM-1 | PAI-1 | SAA | TNF | CRP | IL-1β | IL-6 | IL-8 | IL-10 | IL-12 | |
|---|---|---|---|---|---|---|---|---|---|---|
| VCAM-1 | – | −0.076* | 0.056 | 0.046 | 0.142* | 0.067* | 0.159** | 0.097** | 0.179* | 0.073* |
| PAI-1 | – | 0.023 | 0.072* | 0.178** | 0.043 | 0.012 | 0.053 | −0.054 | −0.007 | |
| SAA | – | −0.047 | 0.423** | −0.048 | 0.205** | 0.054 | 0.076* | 0.053 | ||
| TNF-α | – | 0.044 | 0.507** | 0.187** | 0.039 | 0.421** | 0.493** | |||
| CRP | – | 0.035 | 0.306** | 0.076* | 0.082* | 0.025 | ||||
| IL-1β | – | 0.273** | 0.180** | 0.389** | 0.486** | |||||
| IL-6 | – | 0.313** | 0.349** | 0.351** | ||||||
| IL-8 | – | 0.206** | 0.202** | |||||||
| IL-10 | – | 0.543** | ||||||||
| IL-12 | – |
PAI-1 plasminogen activator inhibitor-1, VCAM-1 vascular adhesion molecule-1, TNF-α tumour necrosis factor alpha, CRP C-reactive protein, SAA serum amyloid A, IL-1β interleukin-1β, IL-6 interleukin-6, IL-8 interleukin-8, IL-10 interleukin-10, IL-12 interleukin-12p70
*significant at .05
**significant at .01
Table 4.
Mean (SD) raw scores of each quartile for each inflammatory marker
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| sVCAM-1 (n = 872) | 647.3 (103.7) (n = 226) | 859.2 (46.2) (n = 226) | 1067.3 (74.5) (n = 226) | 1517.9 (284.6) (n = 194) |
| PAI-1 (n = 873) | 48.7 (9.8) (n = 226) | 70.1 (4.4) (n = 225) | 86.1 (5.3) (n = 226) | 112.5 (11.9) (n = 196) |
| SAA (n = 873) | 7.15 (2.93) n = 226 | 17.7 (3.2) (n = 225) | 34.9 (7.5) (n = 226) | 90.3 (36.9) (n = 196) |
| IL-12 (n = 873) | 0.85 (0.89) (n = 221) | 2.56 (0.25) (n = 222) | 3.47 (0.26) (n = 220) | 5.05 (0.98) (n = 210) |
| TNF-α (n = 873) | 0.00 (0.0) (n = 253) | 1.86 (0.32) (n = 190) | 2.84 (0.32) (n = 220) | 4.65 (1.1) (n = 210) |
| IL-10 (n = 872) | 0.92 (0.78) (n = 226) | 2.21 (0.19) (n = 226) | 2.89 (0.20) (n = 224) | 4.05 (0.74) (n = 196) |
| IL-6 (n = 872) | 2.61 (1.16) (n = 226) | 4.45 (0.39) (n = 226) | 6.05 (0.64) (n = 226) | 10.11 (2.67) (n = 194) |
| IL-1β (n = 872) | 0.76 (0.76) (n = 226) | 2.16 (0.24) (n = 224) | 3.12 (0.34) (n = 226) | 5.31 (1.24) (n = 196) |
| IL-8 (n = 873) | 10.36 (2.14) (n = 225) | 15.33 (1.22) (n = 223) | 19.99 (1.79) (n = 226) | 29.22 (5.05) (n = 199) |
| CRP (n = 873) | 0.20 (0.00) (n = 269) | 1.00 (0.009) (n = 184) | 2.40 (0.49) (n = 226) | 6.02 (2.6) (n = 194) |
PAI-1 plasminogen activator inhibitor-1, VCAM-1 vascular adhesion molecule-1, TNF-α tumour necrosis factor alpha, CRP C-reactive protein, SAA serum amyloid A, IL-10 interleukin-10, IL-6 interleukin-6, IL-8 interleukin-8, IL-1β interleukin-1β, IL-12 interleukin-12p70
Relationships between inflammatory markers and cognition
Table 5 shows the results for the regression analyses when using each domain as a dependent variable and all of the inflammatory markers (represented as quartiles) as the independent variables. Significant relationships were found suggesting that as inflammation increases cognitive function decreases. Model 1 was adjusted for age and sex, model 2 was adjusted for age, sex and years of education, while Model 3 included all the covariates described above. This was done to show the importance of the inclusion of the covariates when understanding the relationship between systemic inflammation and cognition. Education was included separately because education could be used as a proxy marker for cognitive ability in earlier life, and if the correlation between inflammation and cognition without adjustment for education is stronger it could suggest that cognition affects inflammation.
Table 5.
Regression analyses using each cognitive domain as the dependent variable and all the inflammatory markers as the independent variables
| PAI-1 t(p) | VCAM-1 t(p) | TNF-α t(p) | CRP t(p) | SAA t(p) | IL-1β t(p) | IL-6 t(p) | IL-8 t(p) | IL-10 t(p) | IL-12 t(p) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 (N = 763) | ||||||||||
| Processing speed | 0.655 (0.512) | −0.398 (0.691) | 0.264 (0.792) | −1.62 (0.106) | −2.02 (0.043) | −1.52 (0.129) | 1.20 (0.229) | −0.537 (0.592) | 1.36 (0.174) | −1.88 (0.060) |
| Fine motor | −0.781 (0.435) | −1.39 (0.164) | 0.159 (0.874) | −1.72 (0.085) | −1.48 (0.139) | −0.538 (0.591) | −0.066 (0.948) | −0.604 (0.546) | −1.42 (0.155) | −0.158 (0.875) |
| Memory | 0.343 (0.732) | −0.710 (0.478) | −1.27 (0.206) | 0.342 (0.733) | −1.24 (0.215) | −0.481 (0.631) | 0.873 (0.403) | −0.686 (0.493) | −0.590 (0.556) | −0.410 (0.682) |
| Language | 0.677 (0.499) | 1.95 (0.051) | −0.959 (0.338) | −0.760 (0.448) | −0.615 (0.539) | −1.41 (0.158) | 0.346 (0.730) | −0.882 (0.378) | 0.242 (0.809) | −0.253 (0.800) |
| Spatial | −0.855 (0.393) | −0.271 (0.787) | 0.504 (0.614) | −0.563 (0.574) | −1.36 (0.175) | −1.54 (0.123) | 0.490 (0.624) | −1.82 (0.069) | 0.173 (0.863) | −0.579 (0.562) |
| Executive | −1.17 (0.242) | 0.523 (0.601) | −0.140 (0.889) | −1.96 (0.050) | 0.508 (0.612) | −1.25 (0.212) | 0.698 (0.485) | −0.928 (0.354) | 0.011 (0.991) | −0.996 (0.320) |
| Model 2 (N = 753) | ||||||||||
| Processing speed | 0.900 (0.369) | −0.497 (0.619) | 0.782 (0.434) | −1.18 (0.239) | −2.17 (0.030) | −1.18 (0.238) | 1.43 (0.152) | −0.197 (0.844) | −0.901 (0.368) | −2.10 (0.036) |
| Fine motor | −0.687 (0.492) | −1.45 (0.146) | 0.383 (0.702) | −1.53 (0.127) | −1.53 (0.127) | −0.377 (0.706) | 0.029 (0.977) | −0.463 (0.644) | −1.63 (0.103) | −0.237 (0.813) |
| Memory | 0.626 (0.531) | −0.893 (0.372) | −0.738 (0.461) | 0.978 (0.328) | −1.44 (0.149) | −0.009 (0.993) | 0.245 (0.798) | −0.251 (0.802) | −1.13 (0.260) | −0.655 (0.513) |
| Language | 0.961 (0.337) | 1.87 (0.062) | −0.369 (0.712) | −0.333 (0.739) | −0.755 (0.451) | −1.02 (0.307) | 0.644 (0.520) | 0.151 (0.880) | −0.320 (0.749) | −0.494 (0.621) |
| Spatial | −0.595 (0.552) | −0.382 (0.702) | 1.08 (0.279) | −0.058 (0.953) | −1.50 (0.135) | −0.931 (0.352) | 0.737 (0.461) | −1.17 (0.239) | −0.362 (0.718) | −0.796 (0.426) |
| Executive | −0.901 (0.368) | 0.418 (0.676) | 0.666 (0.506) | −1.49 (0.135) | 0.399 (.690) | −0.798 (0.425) | 1.04 (0.298) | −0.414 (0.679) | −0.594 (0.552) | −1.38 (0.167) |
| Model 3 (N = 659) | ||||||||||
| Processing speed | 0.761 (0.447) | 0.116 (0.908) | 0.219 (0.827) | −1.50 (0.134) | −2.35 (0.019) | −1.34 (0.180) | 2.29 (0.022) | 0.218 (0.827) | 1.29 (0.197) | −2.90 (0.004)* |
| Fine motor | −0.035 (0.354) | −1.72 (0.087) | −0.159 (0.873) | −1.33 (0.185) | −2.30 (0.022) | −0.225 (0.822) | 1.05 (0.293) | −0.712 (0.477) | −1.60 (0.110) | −0.235 (0.814) |
| Memory | 0.487 (0.626) | −0.154 (0.877) | −0.582 (0.561) | 0.181 (0.856) | −1.60 (0.111) | −0.013 (0.990) | 1.59 (0.113) | −0.667 (0.505) | −1.11 (0.267) | −1.10 (0.273) |
| Language | 0.760 (0.447) | 1.97 (0.049) | −1.06 (0.129) | −1.01 (0.314) | −1.24 (0.214) | −0.445 (0.656) | 1.12 (0.263) | −0.382 (0.702) | 0.450 (0.653) | −1.03 (0.306) |
| Spatial | −0.169 (0.866) | −0.159 (0.874) | 0.417 (0.677) | −0.399 (0.690) | −1.72 (0.086) | −0.764 (0.445) | 2.13 (0.033) | −1.66 (0.098) | −0.376 (0.707) | −0.994 (0.321) |
| Executive | −0.273 (0.785) | 0.688 (0.492) | −0.221 (0.825) | −1.10 (0.275) | −0.718 (0.473) | −1.23 (0.218) | 1.94 (0.053) | −0.670 (0.503) | −0.355 (0.723) | −1.52 (0.129) |
The direction of all significant findings was always negative, with raised systemic inflammation associated with low cognitive performance
Model 1: covariates included are sex and age; model 2: covariates included are sex, age and education; model 3: covariates included are sex, age, education, metabolic and cardiovascular factors, depression and APOE
PAI-1 plasminogen activator inhibitor-1, VCAM-1 vascular adhesion molecule-1, TNF-α tumour necrosis factor alpha, CRP C-reactive protein, SAA serum amyloid A, IL-1β interleukin-1β, IL-6 interleukin-6, IL-8 interleukin-8, IL-10 interleukin-10, IL-12 interleukin-12
*p ≤ 0.008 level of significance after Bonferroni correction of 6X test
Model 1 and model 2 suggest that some relationships between cognition and inflammation were stronger when not correcting for education (e.g. model 1 showed stronger significant associations compared to model 2). Although suggestive, this could indicate that cognitive ability predicts inflammation, and that in turn inflammation affects cognitive ability.
Model 3 outlines the relationship between inflammation and cognition when controlling for all the covariates. After Bonferroni correction for multiple testing, only one relationship remained significant. High levels of IL-12 were associated with poor performance on attention/processing speed domain (F(1,659) = −2.90, p = 0.004; partial eta squared, 0.032). It is important to note that removing those individuals with DM from the sample did not diminish any significant result; on the other hand, it strengthened many associations (data not shown). Results were repeated after data was categorised by sex for the significant findings. It was found that the results of IL-12 were significant in females (F(1,374) = −2.85, p = 0.005) but not in males (F(1,317) = −1.64, p = 0.102).
Principal component analysis of inflammatory markers
It was logical to examine, with multiple parameters of inflammation, whether we could derive general inflammatory factors using factor analysis (oblique). The ten inflammatory markers loaded on three different factors: first factor was comprised of TNF, IL-1, IL-6, IL-10 and IL-12, we named it cellular inflammation; second factor was comprised of CRP and amyloid A and we named it vascular inflammation; and third factor PAI was by itself, which was to be expected since it is suspected to be neuroprotective. IL-8 did not load more than 0.39 in any of the factors and thus it was excluded. We conducted six regression analyses (for each cognitive domain), including all covariates and the three inflammatory markers as independent variables. We found that high cellular inflammation was associated with poor performance in memory (p = .033). No other significant results were found.
Principal component analysis of cognitive tests
Because cognitive tests are multifactorial and usually measure more than one domain, factor analysis was done on all the cognitive tests to construct four factors (described in Table 6). Regression analyses were again conducted and results can be found in Table 7. Results showed that after Bonferroni correction, IL-6 and IL-12 were both associated with executive function/processing speed domain.
Table 6.
Cognitive factors after undergoing factor analysis and component tests
| Factors | Neuropsychological test scores included |
|---|---|
| Learning | RAVLT (total learning; trials 1–5, short-term recall; trial 6 and long-term recall) |
| Language | Animal naming, 30-item Boston Naming Test, FAS |
| Memory | Logical Memory Story A (immediate and delayed) |
| Executive/processing speed | Trail Making Test B, Trail Making Test A, Stroop Interference, Block Design Grooved Pegboard Test, BVRT recognition, Grooved Pegboard Test, Digit Symbol, |
Table 7.
Regression analyses using each cognitive factor as the dependent variable and all the inflammatory markers as the independent variables
| PAI-1 t(p) | VCAM-1 t(p) | TNF-α t(p) | CRP t(p) | SAA t(p) | IL-1β t(p) | IL-6 t(p) | IL-8 t(p) | IL-10 t(p) | IL-12 t(p) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 (N = 712) | ||||||||||
| Language | 0.655 (0.512) | −0.398 (0.691) | 0.264 (0.792) | −1.62 (0.106) | −2.02 (0.043) | −1.52 (0.129) | 1.20 (0.229) | −0.537 (0.592) | 1.36 (0.174) | −1.88 (0.060) |
| Executive/processing speed | 0.545 (0.586) | −0.124 (0.901) | 0.773 (0.440) | −2.10 (0.036) | −2.07 (0.039) | −1.06 (0.292) | 3.00 (0.003)* | −0.926 (0.355) | −0.500 (0.617) | −3.36 (0.001)* |
| Memory | 0.343 (0.732) | −0.710 (0.478) | −1.27 (0.206) | 0.342 (0.733) | −1.24 (0.215) | −0.481 (0.631) | 0.873 (0.403) | −0.686 (0.493) | −0.590 (0.556) | −0.410 (0.682) |
| Learning | 0.677 (0.499) | 1.95 (0.051) | −0.959 (0.338) | −0.760 (0.448) | −0.615 (0.539) | −1.41 (0.158) | 0.346 (0.730) | −0.882 (0.378) | 0.242 (0.809) | −0.253 (0.800) |
The direction of all significant findings was always negative, with raised systemic inflammation associated with low cognitive performance
All covariates included: sex, age, education, metabolic and cardiovascular factors, depression and APOE
PAI-1 plasminogen activator inhibitor-1, VCAM-1 vascular adhesion molecule-1, TNF-α tumour necrosis factor alpha, CRP C-reactive protein, SAA serum amyloid A, IL-1β interleukin-1β, IL-6 interleukin-6, IL-8 interleukin-8, IL-10 interleukin-10, IL-12 interleukin-12
*p ≤ 0.0125 level of significance after Bonferroni correction of 4× test
Discussion
This study analysed the relationship between a comprehensive array of systemic inflammatory markers and cognitive performance in a large non-demented community-dwelling elderly cohort, whilst taking a step-wise approach to covariate inclusion. Sex differences, which have been shown to be important in understanding the risk factors for dementia, were also examined in this study. As hypothesised, significant associations between inflammatory status and cognitive function were observed which indicated that higher levels of low-grade systemic inflammation were associated with poorer cognitive function. When a comprehensive array of covariates was included in the analysis, the majority of these associations were strengthened.
After Bonferroni correction, a raised level of IL-12 was associated with poorer performance in processing speed domain. There were marked sex differences in the relationship of IL-12 with cognition, with elevated IL-12 significantly associated with low scores on processing speed only in females. Our finding suggests that there could be a biological sex difference that increases inflammation and impairs cognition. Together with our finding of higher glucose and HDL levels in females, this finding highlights the need for further research on sex differences in risk factors for cognitive decline. The only other study to assess IL-12 in a non-clinical elderly sample did not find such relationships (Baune et al. 2008), however their sample was younger. Elevated levels of IL-12 have however been found in mild and moderate AD (Motta et al. 2007). Longitudinal examination of the association with MCI and its subtypes in our sample may illuminate whether our association is specific for a pattern of cognitive performance heralding AD.
Because cognitive tests are usually multi-factorial and assess a range of domains, some more strongly than others, we also conducted factor analysis of all cognitive tests to yield four factors. The association of IL-2 and cognition was again evident. However, IL-6 reached significance after Bonferroni correction. Both were associated with executive function/processing speed domain. Our results were similar to previous studies that found a negative association between IL-6 levels and cognition (Marsland et al. 2006; Wright et al. 2006).
The association of inflammation with processing speed/executive domain suggests a potential impact of systemic inflammation on frontal–subcortical structures thought to subserve this domain. Furthermore, as processing speed and executive function are key cognitive domains known to be affected by the ageing process (Buckner 2004; Lezak et al. 2004; Salthouse 1996), the results support a phenomenon of ‘inflammaging’ which directly affects cognitive function. The way in which the impact of low-grade systemic inflammation on cognition is mediated is at present unclear. The known association between cognitive ageing and white matter pathology (Gunning-Dixon et al. 2009) raises the possibility that a proinflammatory state could result in acceleration of white matter damage or progressive loss of white matter integrity on the basis of microvascular damage. This idea is supported by the Rotterdam Study of 1,033 dementia-free elders (aged 60–90 years) which reported a significant association between elevated CRP and the presence and 3-year progression of white matter lesions on magnetic resonance imaging (van Dijk et al. 2005). The relationship between other inflammatory markers and white matter pathology awaits further study in the present cohort.
Factor analysis on the inflammatory markers revealed three separate constructs, which we named cellular inflammation, vascular inflammation and PAI-1. Elevated cellular inflammation, comprised of TNF, IL-1, IL-6, IL-10 and IL-12, was associated with poor memory performance. The predictive value of this construct on cognitive decline requires further study in the present cohort.
Systemic inflammatory markers, such as CRP, which have been previously documented to be elevated when measures of cognitive performance are poor (Gimeno et al. 2008), showed no associations with cognition in this sample. Contrary to a longitudinal study that did not find an association between SAA and cognitive decline (Jordanova et al. 2008), our study demonstrated an association between high SAA levels and lower performance on processing speed and fine motor domains, but not after Bonferroni correction.
Although TNF-α has been repeatedly implicated in dementia (Bruunsgaard et al. 1999; Paganelli et al. 2002; Zuliani et al. 2007) and predementia populations (Alvarez et al. 2007; Bermejo et al. 2008), in the present study, raised TNF-α was not associated with low performance in any cognitive domain. Nor did we find an association between the plasminogen activator inhibitor-1 (PAI-1) and any cognitive domain, even though it was expected that PAI-1 would be protective for cognition. It could be that PAI-1 is only protective when there is already some cognitive impairment. This is supported by a study that also used the MAS sample, which showed that levels of PAI-1 were lower in those amnestic multiple domain mild cognitive impairment compared to those with normal cognition (Trollor et al. 2010).
Our study has major strengths including sample size, the use of a comprehensive battery of inflammatory markers and neuropsychological tests. Our incorporation of multiple covariates in a hierarchical approach clearly demonstrates robust relationships between inflammatory markers and cognition, which could not be attributed to the confounding influence of depression, measured cardiovascular and metabolic factors, or APOE genotype. A weakness in this analysis is the single-point measurement of inflammatory markers. The study is currently limited by its cross-sectional design and hence we cannot definitely attribute causality in the relationships we have detected. However, future longitudinal observations in this well-defined population can help delineate the nature of the relationships.
In conclusion, this study supports the growing evidence of an increase of low-grade systemic inflammation associated with decreased cognitive function, especially in processing speed, in the elderly. This relationship remains robust despite adjusting for potential confounders including current depression, cardiovascular and metabolic factors and APOE genotype. However, this study underscores the importance of examining sex differences in inflammation. Longitudinal follow-up is required to assess the validity of these inflammatory markers in predicting cognitive decline and dementia progression in this cohort. In addition, future studies examining the relationship between systemic inflammation and structural brain changes in the elderly are required to further explore the effect of inflammation on brain integrity.
Acknowledgements
This study was supported by a Dementia Research Grant through the Australian National Health and Medical Research Council (Grant ID 510124).
The Sydney MAS is supported by the Australian National Health and Medical Research Council Program Grant (Grant ID 350833). The authors wish to acknowledge the contributions of Brain and Ageing Research Program Staff especially Kristan Kang, Simone Reppermund and Melissa Slavin as well all MAS participants.
DNA was extracted by Genetic Repositories Australia, which is supported by an Australian National Health and Medical Research Council Grant (grant ID 401184). Arezoo Assareh and Karen Mather undertook the APOE genotyping in the laboratory of Peter Schofield and John Kwok at Neuroscience Research Australia.
We would also like to acknowledge and thank the accredited laboratory SEALS for their contribution.
References
- Alley DE, Crimmins EM, Karlamangla A, Hu P, Seeman TE. Inflammation and rate of cognitive change in high-functioning older adults. J Gerontol. 2008;63A(1):50–55. doi: 10.1093/gerona/63.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez A, Cacabelos R, Sanpedro C, Garcia-Fantini M, Aleixandre M. Serum TNF-α levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging. 2007;28:533–536. doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Diagnostic and statistical manual of mental disorders. 4. Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- Anderson TM, Trollor JN, Sachdev PS, Brodaty H. Effects of sociodemographic and health variables on Mini-Mental State Exam scores in older Australians. Am J Geriatr Psychiatry. 2007;15:467–476. doi: 10.1097/JGP.0b013e3180547053. [DOI] [PubMed] [Google Scholar]
- Azad NA, Al Bugami M, Loy-English I. Gender differences in dementia risk factors. Gend Med. 2007;4:120–129. doi: 10.1016/S1550-8579(07)80026-X. [DOI] [PubMed] [Google Scholar]
- Bagnoli S, Celline E, Tede A, Nacmias B, Piacentini S, Bessi V, Bracco L, Sorbi S. Association of IL-10 promoter polymorphism in Italian Alzeimer's disease. Neurosci Lett. 2007;418:262–265. doi: 10.1016/j.neulet.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Baune BT, Ponath G, Golledge J, Varga G, Arolt V, Rothermundt M, Berger K. Association between IL-8 cytokine and cognitive performance in an elderly general population – The MEMO-Study. Neurobiol Aging. 2008;29:937–944. doi: 10.1016/j.neurobiolaging.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Benton AL. Problems of test construction in the field of aphasia. Cortex. 1967;3:32–58. [Google Scholar]
- Benton Sivan AB, Spreen O. Der Benton Test. 7. Bern: Huber; 1996. [Google Scholar]
- Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Ribera JM, et al. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer's disease. Immunol Lett. 2008;117:198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Blom G. Statistical estimates and transformed beta-variables. New York: John Wiley and Sons; 1958. [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-α is associated with dementia in centenarians. J Gerontol. 1999;54A(7):357–364. doi: 10.1093/gerona/54.7.m357. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Christensen H, Batterham PJ, Mackinnon AJ, Jorm AF, Mack HA, et al. The association of APOE genotype and cognitive decline in interaction with risk factors in a 65–69 year old community sample. BMC Geriatr. 2008;8:14. doi: 10.1186/1471-2318-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz-Naranjo MC, Munoz-Fernandez C, Alemany-Rodriguez MJ, Perez-Vieitez MC, Aladro-Benito Y, Irurita-Latasa J, Sanchez-Garcia F. Cytokine IL-1 beta but not IL-1 alpha promoter polymorphism is associated with Alzheimer disease in a population from the Canary Islands, Spain. Eur J Neurol. 2008;15:1080–1084. doi: 10.1111/j.1468-1331.2008.02252.x. [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64:1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- Dimopoulos N, Piperi C, Salonicioti A, Mitropoulos P, Kallai E, Liappas I, et al. Indicies of low-grade chronic inflammation correlate with early cognitive deterioration in an elderly Greek population. Neurosci Lett. 2006;398:118–123. doi: 10.1016/j.neulet.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Engelhart M, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, Swieten JC, Stijnen T, Hofman A, Witteman JCM, Breteler MMB. Inflammatory proteins in plasma and the risk of dementia. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- Fastenau PS, Denburg NL, Mauer BA. Parallel short forms for the Boston Naming Test: psychometric properties and norms for older adults. J Clin Exp Neuropsychol. 1998;20(6):828–834. doi: 10.1076/jcen.20.6.828.1105. [DOI] [PubMed] [Google Scholar]
- Fischer P, Zehetmayer S, Bauer K, Huber K, Jungwirth S, Tragl KH. Relation between vascular risk factors and cognition at age 75. Acta Neurol Scand. 2006;114:84–90. doi: 10.1111/j.1600-0404.2006.00597.x. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Marmot MG, Singh MA. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008;33:1322–1334. doi: 10.1016/j.psyneuen.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta B, Fernandez F, Nikolic WV, Obregon D, Rrapo E, Town T, Tan J. Inflammaging as a prodrome to Alzheimer's disease. J Neuroinflammation. 2008;5:51–66. doi: 10.1186/1742-2094-5-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Ageing of cerebral white matter: a review of MRI findings. Int J Geriatric Psychiatry. 2009;24:109–117. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson AS, Easteal S, Jorm AF, Mackinnon AJ, Korten AE, et al. Apolipoprotein E allele ε4, dementia, and cognitive decline in a population sample. Lancet. 1995;346(8987):1387–90. doi: 10.1016/S0140-6736(95)92405-1. [DOI] [PubMed] [Google Scholar]
- Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hha1. J Lipid Res. 1990;31(3):545–8. [PubMed] [Google Scholar]
- Ishii T, Haga S, Shimizu F. Identification of components of immunoglobins in senile plaques by means of fluorescent antibody technique. Acta Neuropath. 1975;32:157–162. doi: 10.1007/BF00689569. [DOI] [PubMed] [Google Scholar]
- Jordanova V, Stewart R, Davies E, Sherwood R, Prince M. Markers of inflammation and cognitive decline in an African-Caribbean population. Int J Geriatr Psychiatry. 2008;22:966–973. doi: 10.1002/gps.1772. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- Klove H. Clinical neuropsychology. In: Forster FM, editor. The Medical Clinics of North America. New York: Saunders; 1963. [PubMed] [Google Scholar]
- Komulainen P, Lakka TA, Kivipelto M, Hassinen M, Penttila IM, Helkala E, et al. Serum high sensitivity C-reactive protein and cognitive function in elderly women. Age Aging. 2007;36:443–448. doi: 10.1093/ageing/afm051. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systemic review and meta-analysis. Lancet Neurol. 2005;4:371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. 4. New York: Oxford University Press; 2004. [Google Scholar]
- Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, et al. Increased plasma levels of interleukin-1, interleukin-6 and α-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J Neuroimmunology. 2000;103:97–102. doi: 10.1016/S0165-5728(99)00226-X. [DOI] [PubMed] [Google Scholar]
- Luciano M, Marioni RE, Gow AJ, Starr JM, Deary IJ. Reverse causation in the association between C-reactive protein and fibrinogen levels and cognitive abilities in an aging sample. Psychosom Med. 2009;71:404–9. doi: 10.1097/PSY.0b013e3181a24fb9. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Petersen KL, Sathanoori R, Muldoon MF, Neumann SA, Ryan C, Flory JD, Manuck SB. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom Med. 2006;68:895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- Motta M, Imbesi R, Rosa M, Stivala F, Malaguarnera L. Altered plasma cytokine levels in Alzheimer's disease: correlation with the disease progression. Immunol Lett. 2007;114:46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Paganelli R, Iorio A, Patricelli L, Ripani F, Sparvieri E, Faricelli R, Iarlori C, Porreca E, Gioacchino M, Abate G. Proinflamatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer's disease patients. Exp Gerontol. 2002;37:257–263. doi: 10.1016/S0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Batty GD, Zanten JJ, Mortensen LH, Deary IJ, Calvin CM, Carroll D. Cognitive ability in early adulthood is associated with systemic inflammation in middle age: the Vietnam experience study. Brain Behav Immun. 2011;25:298–301. doi: 10.1016/j.bbi.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Mailoi F, Brunetti N, Martelli M, Servadei L, et al. Serum C-reactive protein and cognitive function in healthy elderly Italian community dwellers. J Gerontol: Med Sci. 2005;60A(8):1017–1021. doi: 10.1093/gerona/60.8.1017. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead–Reitan neuropsycholgical test battery: theory and clinical interpretation. Tucson: Neuropsychological Press; 1993. [Google Scholar]
- Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's Disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/S0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Brodaty H, Reppermund S, Kochan NA, Trollor JN, Draper B, Slavin M, Crawford J, Kang K, Broe A, Mather K, Lux O, Memory and Ageing Study Team The Sydney Memory and Ageing Study (MAS): methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non-demented cohort of Australians aged 70–90 years. Int Psychogeriatr. 2010;19:1–17. doi: 10.1017/S1041610210001067. [DOI] [PubMed] [Google Scholar]
- Schram MT, Euser SM, Craen AJM, Witteman JC, Frolich M, Hofman A, Jolles J, Breteler MMB, Westendorp RGJ. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55:708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: a guide to assessment and intervention. NY: The Hawthorn Press; 1986. pp. 165–173. [Google Scholar]
- Soeda S, Koyanagi S, Kuramoto Y, Kimura M, Oda M, Kozako T, Hayashida S, Shimeno H. Anti-apoptotic roles of the plasminogen activator inhibitor-1 as a neurotropic factor in the central nervous system. Thromb Hemost. 2008;100:1014–1020. doi: 10.1160/th08-04-0259. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory center comprehensive examination for Aphasia: manual of instructions (NCCEA) Victoria: University of Victoria; 1969. [Google Scholar]
- Rel. 17.0.0. Chicago: SPSS Inc; 2008. [Google Scholar]
- Strachan MWJ, Reynolds RM, Frier BM, Mitchell RJ, Price JF. The relationship between type 2 diabetes and dementia. Br Med Bull. 2008;88:131–146. doi: 10.1093/bmb/ldn042. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- Sheikh JI, Yesavage JA, Brooks JO, 3rd, Friedman L, Gratzinger P, Hill RD, Zadeik A, Crook T. Proposed factor structure of the Geriatric Depression Scale. Int Psychogeriatr. 1991;3(1):23–8. doi: 10.1017/S1041610291000480. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295X.103.3.403. [DOI] [PubMed] [Google Scholar]
- Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, Samaras K, Crawford J, Brodaty H, Sachdev P. Systemic inflammation is associated with MCI and its subtypes - the Sydney Memory and Aging Study. Dement Geriatr Cogn Disord. 2010;30(6):569–578. doi: 10.1159/000322092. [DOI] [PubMed] [Google Scholar]
- Uhlar CM, Whitehead AS. Serum amyloid A, the major vertabrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- Biggelaar AHJ, Gussekloo J, Craen AJM, Frolich M, Stek ML, Mast RC, Westendorp RGJ. Inflammation and interleukin-1 signalling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42:693–701. doi: 10.1016/j.exger.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Wright CB, Sacco RL, Rundek TR, Delman JB, Rabbani LE, Elkind MSV. Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. J Stroke Cerebrovasc Dis. 2006;15:34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MMB. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation. 2005;112:900–905. doi: 10.1161/CIRCULATIONAHA.104.506337. [DOI] [PubMed] [Google Scholar]
- Warnberg J, Gomez-Martinez S, Romeo J, Diaz LE, Marcos A. Nutrition, inflammation and cognitive function. Neuroimmunomodulation: annuals New York Academy Sci. 2009;1153:164–175. doi: 10.1111/j.1749-6632.2008.03985.x. [DOI] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur Studies of Successful Aging. Neurology. 2002;59:371–378. doi: 10.1212/WNL.59.3.371. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D (1981) Wechsler adult intelligence scale — Revised: Manual. Psychological Corporation: New York
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition: the case for a head-to-toe paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Cavalieri M, Galvani M, Passaro A, Munani MR, Bosi C, et al. Markers of endothelial dysfunction in older subjects with late onset Alzheimer's disease or vascular dementia. J Neurological Sci. 2008;272:164–170. doi: 10.1016/j.jns.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti AR, Ble A, Fellin R. Plasma cytokines profile in older subjects with late onset Alzheimer's disease or vascular dementia. J Psychiatr Res. 2007;41:686–693. doi: 10.1016/j.jpsychires.2006.02.008. [DOI] [PubMed] [Google Scholar]