Abstract
Many environmental conditions show rhythmic changes across the 24-h day; these include changes in light intensity, ambient temperature, food availability, and presence or absence of predators. Consequently, many organisms have developed corresponding adaptations, which ensure that specific physiological and behavioral events occur at an appropriate time of the day. In mammals, the underlying mechanism responsible for synchronizing internal biochemical processes with circadian environmental cues has been well studied and is thought to comprise three major components: (1) photoreception by the retina and transmission of neural signals along the retinohypothalamic tract, (2) integration of photoperiodic information with an internal reference circadian pacemaker located in the suprachiasmatic nucleus, and (3) dissemination of circadian information to target organs, via the autonomic nervous system and through humoral pathways. Given the importance that neuroendocrine rhythms play in coordinating normal circadian physiology and behavior, it is plausible that their perturbation during aging contributes to the etiology of age-related pathologies. This mini-review highlights some of the most dramatic rhythmic neuroendocrine changes that occur in primates during aging, focusing primarily on data from the male rhesus macaques (Macaca mulatta). In addition to the age-associated attenuation of hormone levels and reduction of humoral circadian signaling, there are also significant age-related changes in intracrine processing enzymes and hormone receptors which may further affect the functional efficacy of these hormones. Rhesus macaques, like humans, are large diurnal primates and show many of the same physiological and behavioral circadian changes during aging. Consequently, they represent an ideal translational animal model in which to study the causes and consequences of age-associated internal circadian disruption and in which to evaluate novel therapies.
Keywords: Adrenal gland, Circadian rhythms, Intracrinology, Neurosteroidogenesis
Introduction
Humans show a variety of biological rhythms that normally are synchronized with the daily light–dark cycle, including sleep, body temperature, and blood pressure (Hastings et al. 2003, 2007). These rhythms have a period of approximately 24 h and are regulated by a highly coordinated circadian neuroendocrine mechanism that comprises three major components: (1) photoreception by the retina and transmission of neural signals along the retinohypothalamic tract, (2) integration of photoperiodic information with an internal reference circadian pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus, and (3) dissemination of synchronized circadian information to target organs, via the autonomic nervous system and through humoral pathways, and integration with local oscillator circuits (Okamura 2004; Karatsoreos and Silver 2007; Maywood et al. 2007; Mendoza and Challet 2009; Bonnefont 2010; Urbanski 2011b). When functioning correctly, this mechanism ensures that various internal biochemical processes are compartmentalized not just spatially but also temporally and that physiological functions and behaviors occur at an appropriate time of day (Lemos et al. 2006; Kalsbeek et al. 2006). There are conditions, however, when this temporal synchronization of internal rhythms becomes disrupted, as when shift workers abruptly change their work schedule or when airline travelers fly across several meridians, resulting in a malaise commonly known as “jet lag.” Furthermore, perturbation of the circadian system is thought to contribute to the etiology of a winter depression known as “Seasonal Affective Disorder” or SAD (Urbanski 1999; Lewy et al. 1998, 2006, 2007; Skene and Arendt 2006; Lewy 2007, 2009; Arendt et al. 2008). Because much of the body’s circadian organization is mediated through the rhythmic release of various hormones, especially cortisol and melatonin, it is plausible that as the secretion patterns of these hormones change during aging so does the temporal organization of physiological and behavioral functions.
This mini-review highlights recent data from rhesus macaques showing how the 24-h release pattern of various hormones changes during aging and how this may contribute to circadian desynchronization. It also emphasizes that age-associated changes in intracrine processing enzymes and hormone receptors may further affect the functional efficacy of these hormones. Our hope is that a deeper understanding and appreciation of rhythmic neuroendocrine mechanisms could shed new light on the etiology of age-related pathologies and help with the development of more effective circadian-based therapies for the elderly (Yamakazi et al. 2002; Phillips 2009; Monteleone et al. 2011).
Age-related endocrine changes
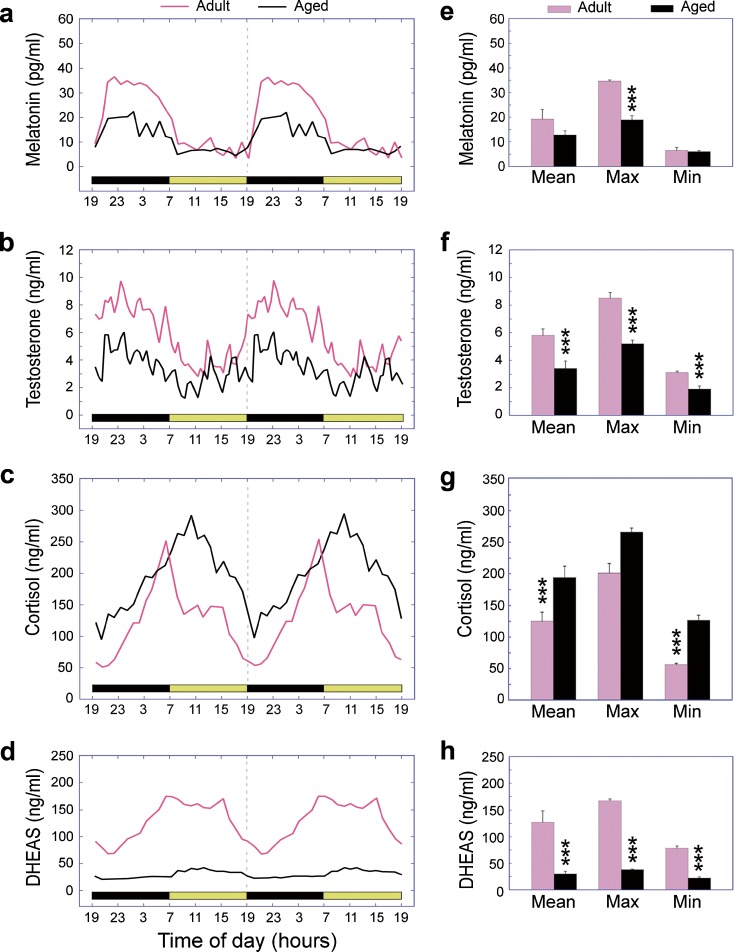
Like humans, rhesus macaques (Macaca mulatta) are large, long-lived diurnal primates with similar organization of their brains and similar consolidated sleep–wake patterns. Importantly, from a research perspective, these nonhuman primates can be maintained under carefully controlled environmental conditions and can be subjected to serial blood sampling with no sedation and minimal restraint (Urbanski 2011a). We have made extensive use of a blood sampling setup that involves remote serial collection of blood via a surgically implanted subclavian vein catheter and a swivel/tether assembly. The advantage of this system is that it enables blood samples to be collected from an adjacent room at any time of the day without disturbing the subjects. Figure 1 depicts various plasma hormone profiles obtained from the same group of male rhesus macaques using this remote blood sampling system: melatonin (an indolamine produced by the pineal gland), cortisol and dehydroepiandrosterone sulfate (DHEAS) (two steroids produced by the adrenal cortex), and testosterone (an androgenic steroid produced by the testicular Leydig cells). All four hormones show clear 24-h rhythms, but their phase relationships are not identical. For example, in both humans and rhesus macaques plasma melatonin levels are high at night and very low in the daytime (Fig. 1a). This makes biological sense because in diurnal species melatonin is associated with sleep maintenance and restive physiological processes (Urbanski 1999; Lewy 2007; Arendt et al. 2008). Another hormone that shows a 24-h rhythm with a nocturnal peak, in both humans and rhesus monkeys, is the adipocytic hormone leptin (data not shown) (Sinha et al. 1996; Langendonk et al. 1998; Simon et al. 1998; Downs and Urbanski 2006a). Because leptin is generally associated with suppression of appetite, it also makes biological sense for its peak to occur at night, when diurnal species usually sleep. Similarly, plasma testosterone shows a nocturnal peak in both men and male rhesus macaques (Bremner et al. 1983; Tenover et al. 1988; Cooke et al. 1993; Downs et al. 2004; Garyfallou et al. 2005; Schlatt et al. 2008; Sitzmann et al. 2010). Note that because of the underlying pulsatile pattern of its release (Tenover et al. 1988; Plant and Witchel 2006), circulating testosterone levels display ultradian fluctuations as well as a 24-h pattern (Fig. 1b). The physiological significance of a nocturnal testosterone peak is unclear, but given its role in anabolic processes there may be physiological consequences if this nocturnal rhythm is perturbed. On the other hand, it is unlikely that the normal age-associated decrease in male sexual performance is triggered by these testosterone changes because in monkeys a decline in sexual behavior occurs well before the age-associated decrease in circulating testosterone levels (Chambers and Phoenix 1981). Even less is known about the physiological significance of the underlying ultradian testosterone pulses, but since there is much variability in the frequency of these pulses between individuals (unpublished observations), they may simply be an inconsequential side effect of the underlying episodic release of gonadotropin-releasing hormone and luteinizing hormone.
Fig. 1.
Age-related changes in the 24-h plasma concentrations of a melatonin, b testosterone, c cortisol, and d DHEAS in male rhesus macaques. The left panels depict mean hormone profiles from ten adult (~10 years) and ten aged (~26 years) animals, and the horizontal light and dark bars correspond to the 12L:12D lighting schedule; note that the profiles have been double plotted to facilitate observation of day–night differences. Each hormone showed a distinct 24-h rhythm, with a peak occurring either during the night (melatonin and testosterone) or early in the morning (cortisol and DHEAS). Statistical analysis of the hormone profiles is shown in the right panels. The mean, maximum (Max; average of five adjacent peak values), and minimum (Min; average of five adjacent nadir values) were calculated from each profile, and between-age comparisons were analyzed using Student’s t test. The bars represent the group means and the vertical lines represent SEMs. Note that in female rhesus macaques, plasma melatonin, cortisol, and DHEAS levels also show 24-h rhythms, but age-related changes are less clear. ***P < 0.001. Data adapted from Downs et al. (2004, 2008) and Garyfallou et al. (2005)
In marked contrast to melatonin, leptin, and testosterone, both cortisol and DHEAS show plasma peaks early in the morning when the animals wake up and a nadir late in the evening when they go to sleep (Fig. 1c, d). Cortisol and DHEAS are two of the most abundant steroids in the circulation of adult humans and rhesus macaques, and they are thought to play an important role in regulating responses to stress (Nguyen and Conley 2008; Schwartz and Pashko 2008). Additionally, elevated cortisol levels suppress the immune system, break down tissues, and have a general catabolic effect, whereas DHEA and DHEAS counterbalance the effects of cortisol by activating the immune system and building up tissues (Messaoudi et al. 2011).
Importantly, all of the abovementioned hormones show marked age-related changes in their 24-h plasma profiles. Aged male rhesus macaques, like aged men, show a significant decline in the amplitude of their 24-h melatonin (Figs. 1a, e; Iguchi et al. 1982; Waldhauser et al. 1988; Sharma et al. 1989; Thomas and Miles 1989; Haimov et al. 1994; Zeitzer et al. 1999; Roth et al. 2001; Downs et al. 2004) and testosterone rhythms (Figs. 1b, f; Plymate et al., 1989; Feldman et al. 2002; Luboshitzky et al. 2003; Garyfallou et al. 2005; Kaufman and Vermeulen 2005; Page et al. 2007; Schlatt et al. 2008; Bremner 2010), and an even more pronounced attenuation of their 24-h DHEAS rhythm (Figs. 1d, h; Orentreich et al. 1992; Labrie et al. 1997; Wise 1999; Urbanski et al. 2004; Downs et al. 2008). An age-related decline has also been observed for leptin in old male rhesus macaques (data not shown; Downs and Urbanski 2006a). In marked contrast, circulating cortisol levels do not typically decline with age, but generally stay the same or show an age-associated increase, both in humans and rhesus macaques. Moreover, a well-defined 24-h plasma cortisol rhythm is clearly evident well into old age (Figs. 1c, g; Wise 1999; Purnell et al. 2004; Urbanski et al. 2004; Downs et al. 2008). Although the functional significance of all these age-related hormonal changes is unclear, a significant elevation of the cortisol baseline means that the brain and peripheral organs, such as the liver, will receive little or no daily respite from the influence of cortisol, which may predispose the elderly to insomnia as well as to metabolic disorders (Scheer et al. 2009). Similarly, there is evidence that the age-associated disruption of the circadian leptin rhythm may contribute to the development of metabolic disorders and obesity (Laughlin and Yen 1997; Matkovic et al. 1997; Balligand et al. 1998; Støving et al. 1998), and may have a negative impact on the maintenance of bone mass (Ducy et al. 2000; Elefteriou et al. 2005). Activity rhythms of primates appear to be closely linked to the circadian cortisol rhythm, and they show parallel phase shifts when the photoperiod is changed (Lemos et al. 2009). Consequently, an age-associated alteration in the cortisol and melatonin rhythms is likely to have a profound effect on activity and sleep cycles, which in turn could negatively impact other physiological functions such as immune response and cognition (Haley et al. 2009, 2011; Messaoudi et al. 2011). DHEAS and the non-sulfated form of the steroid (DHEA) are capable of attenuating the deleterious effects of high circulating cortisol levels. Additionally, lower levels of DHEA and DHEAS have been associated with cognitive disorders with a higher prevalence in the elderly, such as Alzheimer’s disease (Weill-Engerer et al. 2002) and depression (Michael et al. 2000), while in healthy old men, elevated endogenous DHEAS levels have been linked to better cognitive performance (van Niekerk et al. 2001). Consequently, an age-associated decline in circulating DHEA and DHEAS levels, as well as a decrease in the DHEA/cortisol ratio, may contribute to age-associated cognitive decline (van Niekerk et al. 2001; Karishma and Herbert 2002).
The age-associated decline in circulating testosterone levels observed in males during normal aging is much less abrupt and extreme than the decline in circulating estradiol and progesterone levels that occurs in females after menopause (Handelsman and Liu 2005; Downs and Urbanski 2006b). For example, during normal aging, circulating testosterone levels generally continue to be maintained above the prepubertal level, and so the physiological consequences of the normal age-related decline could be minimal. On the other hand, the circadian pattern of testosterone becomes less well defined during aging, and so this dampening of the rhythm may further contribute to the general impairment of circadian physiology in the elderly, including perturbed sleep–wake cycles.
Hormonal supplementation
Because circulating melatonin, DHEA/DHEAS, and testosterone levels decline significantly during aging, it is tempting to speculate that hormonal supplements could alleviate or counteract some of the negative symptoms of aging (Wolf and Kirschbaum 1999). Interestingly, in the USA melatonin and DHEA are both classified as food additives and so are not regulated by the Food and Drug Administration. This means that these hormones are readily available to the public without prescription and are widely used for self-medication by the elderly. Various testosterone preparations are also available to elderly men, under prescription, to help promote healthy aging. However, as emphasized by the plasma profiles depicted in Fig. 1, these hormones normally have clear 24-h circulating patterns in the circulation, with specific phase relationships. From a clinical perspective, this means that accurate assessment of endocrine function is dependent upon collecting blood samples at an appropriate time of day relative to phase of the underlying hormonal rhythm. For some hormones, such as melatonin, it might be more appropriate to collect the blood samples during the night in order to detect peak levels, while for other hormones, such as cortisol, it might be more appropriate to collect the samples at the time of the rhythm’s nadir in the evening (i.e., if the purpose is to test for elevated baseline levels). The situation is further complicated when dealing with testosterone; not only does the circadian peak of testosterone occur at night but its underlying ultradian rhythm means that single blood samples can yield misleading results (Kaler et al. 1986). To more accurately assess testicular endocrine function, multiple blood samples should be collected and ideally as close to the nocturnal peak as practical. With some hormones, it may also be necessary to collect serial blood samples during a specific phase of the menstrual cycle or at a specific time of the year.
Moreover, clinical treatment of low testosterone levels through androgen supplementation should ideally follow the underlying physiological 24-h profile. For practical reasons, however, this is rarely the case. Common androgen supplementation paradigms typically involve long-term continuous release capsules, which have the advantage of low maintenance but which completely obliterate the 24-h rhythm. Other paradigms involve cyclic transdermal delivery of testosterone, via the daily application of gels. However, the instructions typically specify application of the gel in the morning rather than at night (to avoid accidental transfer of the steroid to a sleeping partner); unfortunately, this means that the daily plasma testosterone peak generated by the gel can be several hours out of phase with the endogenous testosterone rhythm. The long-term impact of these nonphysiological androgen supplementation paradigms is unclear, and alternative approaches may prove to be more beneficial to overall physiology. An interesting novel approach has recently been demonstrated in men (Amory and Bremner 2005; Amory et al. 2006), which involves oral administration of micronized testosterone in sesame oil. Normally, orally administered testosterone has little physiological potency because after being taken up by the gut it is immediately transported to the liver via the hepatic portal vein and then passed back into the gut rather than into the general circulation. It appears that much of this enterohepatic circulation of testosterone can be bypassed if the steroid is mixed with sesame oil. Although the exact mechanism is unclear, it may involve preferential absorption by the lymphatic system, bypassing the liver and reaching the circulation via the thoracic duct. Studies have shown that this administration paradigm can more closely mimic the natural 24-h plasma testosterone rhythm (Amory and Bremner 2005; Amory et al. 2006).
Age-related intracrine changes
Clinically, it is convenient and relatively noninvasive to measure hormone levels in the blood or body secretions, such as urine or saliva. However, these measurements do not necessarily reflect the activity of the hormones at their target site. This is because some hormones can be further processed within target tissues through intracrine enzymatic conversion, and also because the receptors for the hormones may also show dynamic changes during aging. While the vast majority of research on steroid actions in the brain has focused on hormones of peripheral or exogenous origin (e.g., adrenal glands and gonads), another oft-overlooked source of neuroactive steroids is the brain itself. These steroids may be derived de novo from cholesterol (auto- or paracrine) or may be the result of conversion of precursor steroids, such as DHEA, to a target hormone such as testosterone and estradiol (intracrine). The likely involvement of these neurosteroids in cognitive function is supported by findings from rodent studies, showing that locally produced estradiol derived from DHEA can increase hippocampal spine density and synapse frequency, and in turn can improve learning and memory (Hajszan et al. 2004; Hirshman et al. 2004; Rune and Frotscher 2005; Mukai et al. 2010). Depending on negative and positive feedback mechanisms, these auto-, para-, and intracrine hormones ultimately determine how much steroid reaches the brain from the periphery. This additional source of neuroactive hormones provides another point of age modulation of cognition as changes in not only peripheral but central steroid production may contribute to age-related cognitive decline (Sorwell and Urbanski 2010).
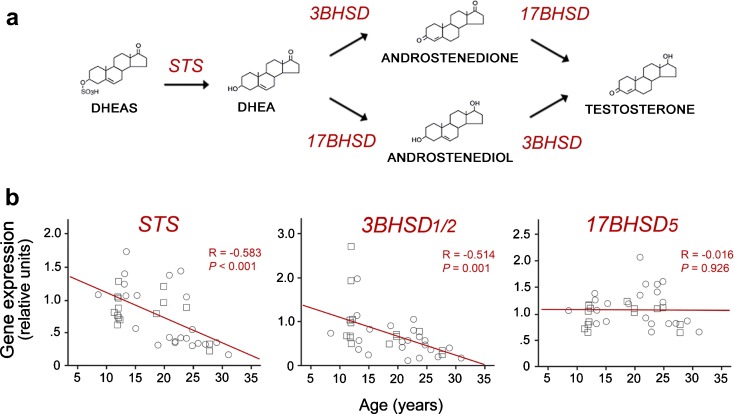
As illustrated in Fig. 2a, adrenal DHEAS can be converted into testosterone within target tissues if the appropriate enzymes are present. This enzymatic conversion thus creates a surge of central sex steroids concurrent with the morning peak of circulating DHEAS. This first key enzymatic step involves removal of the sulfate group by the enzyme steroid sulfatase (STS). 3BHSD and AKR1C3 (also referred to as 17BHSD5) then convert DHEA to testosterone, with androstenedione or androstenediol as intermediaries. If aromatase is also expressed, this testosterone may be further converted to estradiol. Importantly, there is evidence that hippocampal expression of STS and 3BHSD2 declines significantly with age in rhesus macaques (Fig. 2b; Sorwell et al. 2011), suggesting a reduction in the ability to convert circulating DHEAS to testosterone. These decreases in enzyme expression amplify the age-related decline in DHEA/S input from the adrenal glands. Thus, not only is the brain exposed to lower levels of peripherally derived testosterone and estradiol in old age but it may also be less able to synthesize its own sex steroids in situ.
Fig. 2.
Biosynthesis of neurosteroids. a A schematic diagram showing key enzymes involved in the conversion of DHEAS to testosterone. b Age-related changes in the expression of STS, 3BHSD2, but not 17BHSD5, in the rhesus macaque hippocampus, as determined by quantitative real-time PCR. Note that testosterone can be further converted to estradiol by aromatization. Together, these findings suggest neurosteroidogenesis is likely to be impacted by aging. Data adapted from Sorwell et al. (2011)
Elucidation of the pathways involved in auto-, para-, and intracrine sex steroid synthesis is crucial for understanding the impact of steroids on cognition during aging because they offer three additional mechanisms by which testosterone and estradiol concentrations may be modulated within the brain: (a) a decline in adrenal DHEAS reaching the brain, (b) reduced expression of the enzymes necessary to metabolize DHEAS to testosterone and estradiol, and (c) possible reduction of de novo synthesis of DHEA within the brain itself.
Hormone receptors
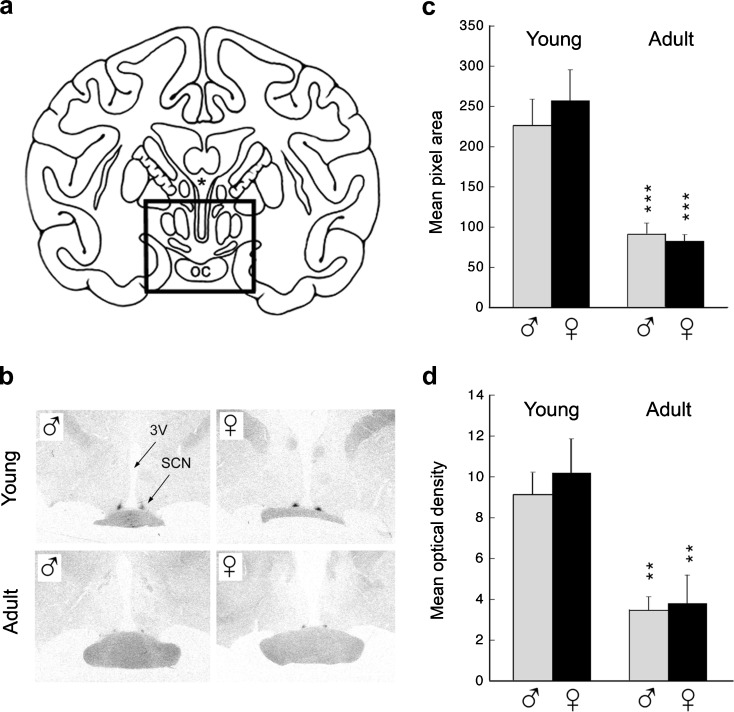
Modulation of hormone receptors can have a major effect on the efficacy of endogenous hormones or hormonal supplements, and so a better understanding of how these receptors change during aging would help with the development of more effective therapies. For example, in the context of hormonal supplementation, there is evidence that melatonin can act as a Zeitgeber or synchronizer of the neuronal activity in the SCN and can entrain activity rhythms (Arendt et al. 1997; Lewy et al. 1998; Masuda and Zhdanova 2010), and not surprisingly the mammalian SCN has been shown to contain high-affinity melatonin receptors, of which MT1 (Mel1a) and MT2 (Mel1b) are the principal subtypes (Reppert et al. 1988, 1994; von Gall et al. 2002). Furthermore, the functional relevance of these receptors has been demonstrated in mice, through targeted deletion of the MT1 receptor which completely blocked the acute inhibitory effects of melatonin on SCN multiunit activity. On the other hand, the phase-shifting responses to melatonin appeared normal in these animals, suggesting that a functional MT2 receptor is partially able to compensate for the absence of the MT1 receptor. From the perspective of human aging, it is pertinent that MT1 gene expression in the SCN is markedly lower in adults than in children (Weaver and Reppert 1996), and similar age-related differences have been observed in the rhesus macaque SCN (Fig. 3). Together, these findings emphasize that hormone receptor gene expression is dynamic and that marked age-associated changes may occur well before the onset of extreme old age. Such changes in receptor expression could undermine the efficacy of hormonal supplementation in the elderly and so may need to be taken into consideration when developing hormone-based therapies.
Fig. 3.
Age-related changes in melatonin receptor gene expression within the SCN of rhesus macaques. a A schematic coronal section through the rhesus macaque brain showing location of the SCN. OC optic chiasm, * third ventricle. b Representative autoradiographs showing in situ hybridization of a 35S-labeled antisense MT1 (Mel1a) riboprobe in the hypothalamus of young (<1 year) and adult (10–15 years) males and females. SCN suprachiasmatic nucleus, 3V third ventricle. Image analysis of the autoradiographs involved measuring mean pixel area (c) and mean optical density (d). The bars represent mean values from three males or three females, and the vertical lines represent SEMs. **P < 0.01, ***P < 0.001 (between-age comparison). Data adapted from Urbanski et al. (2000)
Conclusions
Neuroendocrine rhythms play an important role in coordinating a wide range of physiological and behavioral functions. It is highly likely, therefore, that perturbation of their release patterns during aging contributes to the etiology of various pathologies in the elderly. What is often overlooked or underappreciated, however, is that many hormones have well-defined 24-h profiles, with particular phase relationships, which help to temporally synchronize internal biochemical functions with that of the changing external environment. Consequently, disruption of these 24-h hormone patterns, due to normal aging or as a side effect of some pharmaceutical interventions, could lead to internal physiological disharmony. The situation is further complicated because many enzymes involved in the biosynthesis of hormones may also show age-related changes, and the corresponding hormone receptors may themselves be downregulated. Taken together, the data suggest that in cognitive brain areas such as the hippocampus, a decrease in exposure, production, response, and synchrony of neuroactive steroids may collectively contribute to impaired cognitive function during old age. Furthermore, the data suggest that the efficacy of hormone replacement therapies or pharmaceutical interventions in the elderly could be enhanced, and have fewer side effects, if appropriate circadian phase is taken into consideration.
Acknowledgments
This work was supported by National Institutes of Health Grants AG-029612, AG-036670, and RR-000163.
References
- Amory JK, Bremner WJ. Oral testosterone in oil plus dutasteride in men: a pharmacokinetic study. J Clin Endocrinol Metab. 2005;90:2610–2617. doi: 10.1210/jc.2004-1221. [DOI] [PubMed] [Google Scholar]
- Amory JK, Page ST, Bremner WJ. Oral testosterone in oil: pharmacokinetic effects of 5alpha reduction by finasteride or dutasteride and food intake in men. J Androl. 2006;27:72–78. doi: 10.2164/jandrol.05058. [DOI] [PubMed] [Google Scholar]
- Arendt J, Skene DJ, Middleton B, Lockley SW, Deacon S. Efficacy of melatonin treatment in jet lag, shift work, and blindness. J Biol Rhythms. 1997;12:604–617. doi: 10.1177/074873049701200616. [DOI] [PubMed] [Google Scholar]
- Arendt J, Someren EJ, Appleton R, Skene DJ, Akerstedt T. Clinical update: melatonin and sleep disorders. Clin Med. 2008;8:381–383. doi: 10.7861/clinmedicine.8-4-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand JL, Brichard SM, Brichard V. Hypoleptinemia in patients with anorexia nervosa: loss of circadian rhythm and unresponsiveness to short-term refeeding. Eur J Endocrinol. 1998;138:415–420. doi: 10.1530/eje.0.1380415. [DOI] [PubMed] [Google Scholar]
- Bonnefont X. Circadian timekeeping and multiple timescale neuroendocrine rhythms. J Neuroendocrinol. 2010;22:209–216. doi: 10.1111/j.1365-2826.2010.01955.x. [DOI] [PubMed] [Google Scholar]
- Bremner WJ. Testosterone deficiency and replacement in older men. N Engl J Med. 2010;363:189–191. doi: 10.1056/NEJMe1006197. [DOI] [PubMed] [Google Scholar]
- Bremner WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56:1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Phoenix CH. Diurnal patterns of testosterone, dihydrotestosterone, estradiol, and cortisol in serum of rhesus males: relationship to sexual behavior in aging males. Hormone Behav. 1981;15:416–426. doi: 10.1016/0018-506X(81)90006-4. [DOI] [PubMed] [Google Scholar]
- Cooke RR, McIntosh JE, McIntosh RP. Circadian variation in serum free and non-SHBG-bound testosterone in normal men: measurements, and simulation using a mass action model. Clin Endocrinol (Oxf) 1993;39:163–171. doi: 10.1111/j.1365-2265.1993.tb01769.x. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Aging-related sex-dependent loss of the circulating leptin 24-h rhythm in the rhesus monkey. J Endocrinol. 2006;190:117–127. doi: 10.1677/joe.1.06745. [DOI] [PubMed] [Google Scholar]
- Downs JL, Urbanski HF. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta) Biol Reprod. 2006;75:539–546. doi: 10.1095/biolreprod.106.051839. [DOI] [PubMed] [Google Scholar]
- Downs JL, Garyfallou VT, Aghazadeh-Sanai M. Effect of aging and caloric restriction on circadian hormone release in male rhesus macaques. Soc Neurosci Abstr. 2004;30:545.1. [Google Scholar]
- Downs JL, Mattison JA, Ingram DK, Urbanski HF. Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol Aging. 2008;29:1412–1422. doi: 10.1016/j.neurobiolaging.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/S0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Feldman HA, Longcope C, Derby CA. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jc.87.2.589. [DOI] [PubMed] [Google Scholar]
- Garyfallou VT, Brown DI, Downs JL, James LJ, Urbanski HF (2005) Effect of aging on circulating testosterone levels and on the expression of genes associated with testosterone biosynthesis. Endocr Soc Abstr 87:OR4.6
- Haimov I, Laudon M, Zisapel N, Souroujon M, Nof D, Shlitner A, Herer P, Tzischinsky O, Lavie P. Sleep disorders and melatonin rhythms in elderly people. BMJ. 1994;309:167. doi: 10.1136/bmj.309.6948.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, Maclusky NG, Leranth C. Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized mice. Endocrinology. 2004;145:1042–1045. doi: 10.1210/en.2003-1252. [DOI] [PubMed] [Google Scholar]
- Haley G, Landauer N, Renner L, Hooper K, Urbanski HF, Kohama SG, Neuringer M, Raber J. Circadian activity associated with spatial learning and memory in aging rhesus monkeys. Exp Neurol. 2009;217:55–62. doi: 10.1016/j.expneurol.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley GE, Urbanski HF, Kohama SG, Messaoudi I, Raber J. Spatial memory performance associated with measures of immune function in elderly female rhesus macaques. Eur Geriatr Med. 2011;2:117–121. doi: 10.1016/j.eurger.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelsman DJ, Liu PY. Andropause: invention, prevention, rejuvenation. Trends Endocrinol Metab. 2005;16:39–45. doi: 10.1016/j.tem.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Hastings M, O'Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195:187–198. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- Hirshman E, Merritt P, Wang CC, Wierman M, Budescu DV, Kohrt W, Templin JL, Bhasin S. Evidence that androgenic and estrogenic metabolites contribute to the effects of dehydroepiandrosterone on cognition in postmenopausal women. Horm Bheav. 2004;45:144–155. doi: 10.1016/j.yhbeh.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Iguchi H, Kato K-I, Ibayashi H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. J Clin Endcorinol Metab. 1982;55:27–29. doi: 10.1210/jcem-55-1-27. [DOI] [PubMed] [Google Scholar]
- Kaler LW, Gliessman P, Hess DL, Hill J. The androgen status of aging male rhesus macaques. Endocrinology. 1986;119:566–571. doi: 10.1210/endo-119-2-566. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Palm IF, Fleur SE. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Silver R. The neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology. 2007;148:5640–5647. doi: 10.1210/en.2007-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons, and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jc.82.8.2396. [DOI] [PubMed] [Google Scholar]
- Langendonk JG, Pijl H, Toornvliet AC, Burggraaf J, Frölich M, Schoemaker RC, Doornbos J, Cohen AF, Meinders AE. Circadian rhythm of plasma leptin levels in upper and lower body obese women: influence of body fat distribution and weight loss. J Clin Endocrinol Metab. 1998;83:1706–1712. doi: 10.1210/jc.83.5.1706. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Yen SS. Hypoleptinemia in women athletes: absence of a diurnal rhythm with amenorrhea. J Clin Endocrinol Metab. 1997;82:318–321. doi: 10.1210/jc.82.1.318. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Downs JL, Urbanski HF. Twenty-four hour rhythmic gene expression in the rhesus macaque adrenal gland. Molec Endocrinol. 2006;20:1164–1176. doi: 10.1210/me.2005-0361. [DOI] [PubMed] [Google Scholar]
- Lemos DR, Downs JL, Raitiere MN, Urbanski HF. Photoperiodic modulation of adrenal gland function in the rhesus macaque: effect on 24-h plasma cortisol and dehydroepiandrosterone sulfate rhythms and adrenal gland gene expression. J Endocrinol. 2009;201:275–285. doi: 10.1677/JOE-08-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ. Melatonin and human chronobiology. Cold Spring Harb Symp Quant Biol. 2007;72:623–636. doi: 10.1101/sqb.2007.72.055. [DOI] [PubMed] [Google Scholar]
- Lewy AJ. Circadian misalignment in mood disturbances. Curr Psychiatry Rep. 2009;11:459–465. doi: 10.1007/s11920-009-0070-5. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CM, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiol Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proc Natl Acad Sci USA. 2006;103:7414–7419. doi: 10.1073/pnas.0602425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Rough JN, Songer JB, Mishra N, Yuhas K, Emens JS. The phase shift hypothesis for the circadian component of winter depression. Dialogues Clin Neurosci. 2007;9:291–300. doi: 10.31887/DCNS.2007.9.3/alewy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luboshitzky R, Shen-Orr Z, Herer P. Middle-aged men secrete less testosterone at night than young healthy men. J Clin Endocrinol Metab. 2003;88:3160–3166. doi: 10.1210/jc.2002-021920. [DOI] [PubMed] [Google Scholar]
- Masuda K, Zhdanova IV. Intrinsic activity rhythms in Macaca mulatta: their entrainment to light and melatonin. J Biol Rhythms. 2010;25:361–371. doi: 10.1177/0748730410379382. [DOI] [PubMed] [Google Scholar]
- Matkovic V, Ilich JZ, Badenhop NE, Skugor M, Clairmont A, Klisovic D, Landoll JD. Gain in body fat is inversely related to the nocturnal rise in serum leptin level in young females. J Clin Endocrinol Metab. 1997;82:1368–1372. doi: 10.1210/jc.82.5.1368. [DOI] [PubMed] [Google Scholar]
- Maywood ES, O’Neill JS, Chesham JE, Hastings MH. The circadian clockwork of the suprachiasmatic nuclei—analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology. 2007;148:5624–5634. doi: 10.1210/en.2007-0660. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Challet E. Brain clocks: from the suprachiasmatic nuclei to a cerebral network. The Neurosientist. 2009;15:477–488. doi: 10.1177/1073858408327808. [DOI] [PubMed] [Google Scholar]
- Messaoudi I, Urbanski HF, Kohama SG. Integrative models of aging in the nonhuman primate. In: Williams RM, editor. Monkeys: biology, behavior and disorders. New York: Nova Science Publishers; 2011. pp. 1–54. [Google Scholar]
- Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000;48:989–995. doi: 10.1016/S0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Martiadis V, Maj M. Circadian rhythms and treatment implications in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1569–1574. doi: 10.1016/j.pnpbp.2010.07.028. [DOI] [PubMed] [Google Scholar]
- Mukai H, Kimoto T, Hojo Y, Kawato S, Murakami G, Higo S, Hatanaka Y, Ogiue-Ikeda M. Modulation of synaptic plasticity by brain estrogen in the hippocampus. Biochim Biophys Acta. 2010;1800:1030–1044. doi: 10.1016/j.bbagen.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Conley AJ (2008) Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. 13:33–54 [DOI] [PubMed]
- Okamura H. Clock genes in cell clocks: roles, actions, and mysteries. J Biol Rhythms. 2004;19:388–399. doi: 10.1177/0748730404269169. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75:1002–1004. doi: 10.1210/jc.75.4.1002. [DOI] [PubMed] [Google Scholar]
- Page ST, Matsumoto AM, Bremner WJ. DHEA and testosterone in the elderly. N Engl J Med. 2007;356:635–637. doi: 10.1056/NEJMc063190. [DOI] [PubMed] [Google Scholar]
- Phillips ML. Circadian rhythms: of owls, larks and alarm clocks. Could out-of-sync body clocks be contributing to human disease? Nature. 2009;458:142–144. doi: 10.1038/458142a. [DOI] [PubMed] [Google Scholar]
- Plant TM, Witchel SF. Puberty in nonhuman primates and humans. In: Neill JD, editor. Knobil and Neill’s physiology of reproduction, 3rd edn. St. Loius: Elsevier; 2006. pp. 2177–2230. [Google Scholar]
- Plymate SR, Tenover JS, Bremner WJ. Circadian variation in testosterone, sex hormone-binding globulin, and calculated non-sex hormone-binding globulin bound testosterone in healthy young and elderly men. J Androl. 1989;10:366–371. doi: 10.1002/j.1939-4640.1989.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89:281–287. doi: 10.1210/jc.2003-030440. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Science. 1988;242:78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lesnikov V, Lesnikov M, Ingram DK, Lane MA. Dietary caloric restriction prevents the age-related decline in plasma melatonin levels of rhesus monkeys. J Clin Endocrinol Metab. 2001;86:3292–3295. doi: 10.1210/jc.86.7.3292. [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neurosceince. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta) Biol Reprod. 2008;79:93–99. doi: 10.1095/biolreprod.107.066126. [DOI] [PubMed] [Google Scholar]
- Schwartz AG, Pashko LL. Dehydroepiandrosterone, cortisol, and aging. Open Longev Sci. 2008;2:1–6. doi: 10.2174/1876326X00802010001. [DOI] [Google Scholar]
- Sharma M, Palacios-Bois J, Schwartz G, Iskandar H, Thakur M, Quirion R, Nair NP. Circadian rhythms of melatonin and cortisol in aging. Biol Psychiatry. 1989;25:305–319. doi: 10.1016/0006-3223(89)90178-9. [DOI] [PubMed] [Google Scholar]
- Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;183:1893–1899. doi: 10.1210/jc.83.6.1893. [DOI] [PubMed] [Google Scholar]
- Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, Marco C, Caro JF. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97:1344–1347. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitzmann BD, Leone EH, Mattison JA, Ingram DK, Roth GS, Urbanski HF, Zelinski MB, Ottinger MA. Effects of moderate calorie restriction on testosterone production and semen characteristics in young rhesus macaques (Macaca mulatta) Biol Reprod. 2010;83:635–640. doi: 10.1095/biolreprod.110.084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene DJ, Arendt J. Human circadian rhythms: physiological and therapeutic relevance of light and melatonin. Ann Clin Biochem. 2006;43:344–353. doi: 10.1258/000456306778520142. [DOI] [PubMed] [Google Scholar]
- Sorwell KG, Urbanski HF. Dehydroepiandrosterone and age-related cognitive decline. Age (Dordr) 2010;32:61–67. doi: 10.1007/s11357-009-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorwell KG, Kohama SG, Urbanski HF (2011) Age-related steroidogenic enzyme expression in the rhesus macaque hippocampus. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2011.05.004
- Støving RK, Vinten J, Handberg A. Diurnal variation of the serum leptin concentration in patients with anorexia nervosa. Clin Endocrinol (Oxf) 1998;48:761–768. doi: 10.1046/j.1365-2265.1998.00434.x. [DOI] [PubMed] [Google Scholar]
- Tenover JS, Matsumoto AM, Clifton DK, Bremner WJ. Age-related alterations in the circadian rhythms of pulsatile luteinizing hormone and testosterone secretion in healthy men. J Gerontol. 1988;43:M163–M169. doi: 10.1093/geronj/43.6.m163. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Miles A. Melatonin secretion and age. Biol Psychiatry. 1989;25:365–367. doi: 10.1016/0006-3223(89)90187-X. [DOI] [PubMed] [Google Scholar]
- Urbanski HF. Influence of light and the pineal gland on biological rhythms. In: Conn PM, Freeman ME, editors. Neuroendocrinology in physiology and medicine. Totowa: Human Press Inc; 1999. pp. 405–420. [Google Scholar]
- Urbanski HF. Circadian variation in the physiology and behavior of humans and nonhuman primates. In: Raber J, editor. Animal models of behavioral analysis. New York: Springer; 2011. pp. 217–235. [Google Scholar]
- Urbanski Role of circadian neuroendocrine rhythms in the control of behavior and physiology. Neuroendocrinol. 2011;93:211–222. doi: 10.1159/000327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Garyfallou VT, Latimer VS, Kohama SG. Age-related changes in the expression of Mel1A melatonin receptor mRNA in the rhesus macaque suprachiasmatic nucleus. Soc Neurosci Abstr. 2000;26:1263. [Google Scholar]
- Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann N Y Acad Sci. 2004;1019:443–447. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- Niekerk JK, Huppert JA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26:591–612. doi: 10.1016/S0306-4530(01)00014-2. [DOI] [PubMed] [Google Scholar]
- Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tiss Res. 2002;309:151–162. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Weiszenbacher G, Tatzer E. Alterations in nocturnal serum melatonin levels in humans with growth and aging. J Clin Endocrinol Metab. 1988;66:648–652. doi: 10.1210/jcem-66-3-648. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Reppert SM. The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. Neuroreport. 1996;8:109–112. doi: 10.1097/00001756-199612200-00022. [DOI] [PubMed] [Google Scholar]
- Weill-Engerer S, David JP, Sazdovitch V. Neurosteroid quantification in human brain regions: comparison between Alzheimer’s and nondemented patients. J Clin Endocrinol Metab. 2002;87:5138–5143. doi: 10.1210/jc.2002-020878. [DOI] [PubMed] [Google Scholar]
- Wise PM. Neuroendocrine correlates of aging. In: Conn PM, Freeman ME, editors. Neuroendocrinology in physiology and medicine. Totowa: Human Press Inc; 1999. pp. 371–387. [Google Scholar]
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Brain Res Rev. 1999;30:264–288. doi: 10.1016/S0165-0173(99)00021-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Daniels JE, Duffy JF, Klerman EB, Shanahan TL, Dijk DJ, Czeisler CA. Do plasma melatonin concentrations decline with age? Am J Med. 1999;107:432–436. doi: 10.1016/S0002-9343(99)00266-1. [DOI] [PubMed] [Google Scholar]