Abstract
Growing evidence suggests that type 2 diabetes mellitus (DM) is associated with age-dependent Alzheimer’s disease (AD), the latter of which has even been considered as type 3 diabetes. Several physiopathological features including hyperglycemia, oxidative stress, and dysfunctional insulin signaling relate DM to AD. In this study, high glucose-, oxidative stress-induced neuronal injury and intracerebroventricular-streptozotocin (ICV-STZ) animals as the possible models for diabetes-related AD were employed to investigate the effects of exendin-4 (Ex-4), a long-acting glucagon-like peptide-1 (GLP-1) receptor agonist, on diabetes-associated Alzheimer-like changes as well as the molecular mechanisms involved. Our study demonstrated that GLP-1/Ex-4 could exert a protective effect against reduced viability of PC12 cells caused by high glucose and that this protective effect was mediated via the PI3-kinase pathway. In addition, GLP-1/Ex-4 ameliorated oxidative stress-induced injury in PC12 cells. In rat models, bilateral ICV-STZ administration was used to produce impaired insulin signaling in the brain. Fourteen days following ICV-STZ injection, rats treated with twice-daily Ex-4 had better learning and memory performance in the Morris water maze test compared with rats treated with saline. Additionally, histopathological evaluation confirmed the protective effects of Ex-4 treatment on hippocampal neurons against degeneration. Furthermore, we demonstrated that Ex-4 reversed ICV-STZ-induced tau hyperphosphorylation through downregulation of GSK-3β activity, a key kinase in both DM and AD. Our findings suggests that Ex-4 can protect neurons from diabetes-associated glucose metabolic dysregulation insults in vitro and from ICV-STZ insult in vivo, and that Ex-4 may prove of therapeutic value in the treatment of AD especially DM-related AD.
Keywords: Alzheimer’s disease, Diabetes mellitus, Exendin-4, Streptozotocin, Tau-protein, Glycogen synthase kinase 3β
Introduction
Diabetes mellitus (DM) is defined as a group of metabolic diseases characterized by hyperglycemia resulting from defective insulin secretion or resistance to insulin signaling action, or both. In recent years, a growing body of epidemiological, clinical, and experimental evidence suggests a link between type 2 diabetes and Alzheimer’s disease (AD); Alzheimer’s disease has been attributed to, in part, neuro-endocrine disorder, even referred to by some as type 3 diabetes (Kroner 2009).
In an initial Rotterdam study (Ott et al. 1999), it was shown that having type 2 diabetes doubled the risk of developing dementia, and the risk increases fourfold in patients who need insulin treatment. Furthermore, the latest Rotterdam study (Schrijvers et al. 2010) suggests that insulin resistance increases the risk of developing AD within 3 years. The multiple factors contributing to the pathogenesis of diabetes-related Alzheimer’s disease are not yet fully elucidated but clearly share pathogenesis with brain ageing and diabetic neuropathy, involving not only metabolic changes related to chronic hyperglycemia, but probably also defects in insulin signaling action in the brain (Gispen and Biessels 2000). Current research suggests that insulin signaling may be involved in the process of Alzheimer’s disease; inhibition of neuronal insulin signal transduction may lead to the intracellular accumulation of Aβ and hyperphosphorylation of tau-protein, which are both important pathological features of Alzheimer’s disease (Hoyer 2004). Abnormal aggregates of tau molecules, as well as Aβ oligomers, are cytotoxic and can impair cognition (Querfurth and LaFerla 2010). Therefore, insulin treatment might not only ameliorate hyperglycemia but also exert a direct effect on the brain (Gispen and Biessels 2000).
The high prevalence of both DM and AD in the elderly population, coupled with the strong link between diabetes, insulin signaling, and AD prompted us to search for a desirable concomitant pharmacotherapy for DM-related AD based on current Food and Drug Administration (FDA)-approved drugs for the treatment of diabetes. Clinical findings indicate that insulin has beneficial effects on cognition in patients with dementia. A study by Plastino et al. (2010) evaluated the effect of anti-diabetic therapy on cognitive impairment in patients with both type 2 DM and AD. Their data demonstrated a significant slowing in cognitive decline both at 6 and 12 months of follow-up in patients treated with a combination of insulin and oral antidiabetic medications compared with patients treated with oral diabetes medication only. Moreover, clinical trials evaluating the peroxisome proliferator activated receptor-γ agonists rosiglitazone and pioglitazone, two of the FDA-approved thiazolidinediones for treating type 2 diabetes, showed that the use of rosiglitazone was associated with improved cognition and memory in mild to moderate AD patients who were not APOEε4 carriers (Risner et al. 2006), and also pioglitazone treatment exhibited cognitive and functional improvements in patients with mild AD accompanied with type 2 DM (Sato et al. 2009). In addition, Chen et al. (2009) found that metformin, another FDA-approved insulin-sensitizing drug, upregulated Aβ generation when administered alone, however, when in combined use, metformin enhanced insulin’s effect on reducing Aβ levels.
Glucagon-like peptide-1 (7–36)-amide (GLP-1), an attractive treatment for type 2 diabetes, is an endogenous insulinotropic peptide secreted from enteroendocrine L cells located in the intestine in response to food. GLP-1 has potent effects on not only glucose-dependent insulin secretion, but also proinsulin gene expression and pancreatic islet cell formation (Gao et al. 2010). Byetta, a synthetic version of Ex-4, is a long-acting GLP-1 receptor (GLP-1R) agonist and was the first of this new class of drugs to be approved by the FDA in 2005 to treat type 2 DM. Meanwhile, Hamilton et al. (2009) reported GLP-1Rs are expressed within the nervous system of rodents and humans along with pancreatic cells. GLP-1R stimulation improved functional outcome in well-characterized cellular and animal models of both stroke and Parkinson’s disease (Li et al. 2009). Also, it was confirmed that Ex-4 treatment could improve survival in a mouse model of Huntington’s disease (Martin et al. 2009). However, the relationship between GLP-1 receptor and DM-related AD is less clear. In the present experiments, we studied the effects as well as the molecular mechanisms of GLP-1 and especially its long-acting analogue Ex-4 on neuronal cell viability under high glucose or oxidative stress conditions in vitro and the effects of Ex-4 on memory deficit, histopathological changes, tau hyperphosphorylation, as well as the major tau kinase-glycogen synthase kinase 3β (GSK-3β) activity in rat models induced by impairing brain insulin signaling in vivo.
Materials and methods
Cell culture
Cells were cultured and treated as described by Qian et al. (2008). Briefly, PC12 cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco Life Technologies, NY, USA) supplemented with heat-inactivated 10% fetal bovine serum (Gibco Life Technologies), 100 U/ml penicillin, 100 μg/ml streptomycin in a water-saturated 5% CO2 atmosphere at 37°C.
Cell viability assay
Cell viability was determined using a modified 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium (MTT) (Sigma, St Louis, MO) assay. Briefly, cells were seeded onto flat-bottomed 96-well culture plates. To produce hyperglycemia or oxidative stress, PC12 cells were treated with high glucose or hydrogen peroxide (H2O2) for the indicated period in the absence and presence of Ex-4 or GLP-1 (TASH Biotechnology Co., Ltd, Shanghai, China). To study the mechanism involved in GLP-1/Ex-4 effects on high glucose-induced neurotoxicity, we pre-treated PC12 cells with a specific inhibitor of PI3 kinase (LY294002; 50 μM; Beyotime Institute of Biotechnology, China) or MAP-Kinase (PD98059; 100 μM; Beyotime Institute of Biotechnology) for 30 min. After removing the medium, MTT solution (5 mg/ml in phosphate buffer saline (PBS)) was added for 4 h resulting in the production of formazan dye, which was then solubilized in 150 μl dimethyl sulfoxide. The absorption was then quantified by measuring optical density at 570 nm (630 nm as a reference).
Animals and drug administration
Thirty male Wistar rats (210–260 g) were used in this study. All rats were group housed on a 12-h light/12-h dark cycle and had ad libitum access to food and water. After intracerebroventricular (ICV) injection of streptozotocin, either Ex-4 (10 μg/kg body weight; TASH Biotechnology Co., Ltd, Shanghai, China) or saline was administered by a twice-daily subcutaneous injection for 14 days. The experiments in vivo in this study were performed in compliance with the rules in the Guide for the Care and Use of Laboratory Animals as described by the US National Institutes of Health.
Glucose measurements
Glucose levels were measured in blood collected from the tail vein using a Bayer One-Touch Ultra Blood Glucose Meter.
ICV injection of streptozotocin
Adult rats were anesthetized with 60 mg/kg body weight napental intraperitoneally, and then were fixed on a stereotaxic frame (Second Military Medical University, Shanghai, China). The scalp was incised, the skull exposed, and then burr holes were drilled on both sides over the lateral cerebral ventricles using the following stereotaxic coordinates—1.0 mm posterior to bregma; 1.5 mm lateral to sagittal suture, and 3.5 mm beneath the surface of the brain. Through the prepared drill holes, bilateral microinjection was employed.
Streptozotocin (STZ) (Sigma, St Louis, MO) was dissolved in saline, and this solution was prepared immediately prior to injection. The rats received ICV injection of STZ (3 mg/kg body weight, 6 μl each side for a rat, n = 20) or saline (3 mg/kg body weight, 6 μl each side for a rat, n = 10). After surgery, the rats were kept in individual cages and were fed normally.
Morris water maze
To evaluate the spatial learning and memory of rats 14 days after administrating of STZ, the Morris water maze (Morris 1984) (a circular pool 150 cm in diameter and 60 cm high) was adopted. Forty-centimeter high of water at 25 ± 2°C was added into the circular water tank. The pool was divided into four equal quadrants, labeled north, south, east, and west. A transparent escape platform (10 cm in diameter) was hidden 2 cm below the surface of the water at a fixed location in one of the quadrants to ensure being invisible to the rats but high enough for the rats to stand on it. On the first five successive days considered as training period, rats were gently put into the water one by one from each quadrant once a day. They were allowed 90 s to freely search for the platform. The rats who found the platform in allotted time were allowed to stay on the platform for another 30 s, while those who failed to detect the platform in 90 s were guided to the destination and also allowed to stay for 30 s. The time required for reaching the platform (escape latency), distance swam to the platform, and swimming speed as well as the time and distance spent in each quadrant were recorded and measured by a video tracking system (EthoVision Image Analysis 3.1, Noldus Information Technology, Wageningen, The Netherlands).
To assess the degree of memory consolidation after learning, on the sixth day, a probe test was conducted by removing the platform. The rats were put down from one of the quadrants randomly and were allowed to swim freely in the pool for 90 s. The time spent in the target quadrant, the one that had previously contained the hidden platform and the frequency of crossing through the place of the platform were recorded.
Histopathological examination
Briefly, on the 22nd day after STZ or saline ICV injection, rats were anesthetized with 60 mg/kg body weight napental intraperitoneally and then subjected to cardiac perfusion with saline followed by 4% paraformaldehyde in 0.1 M PBS. Brains were removed quickly and then transferred to 30% sucrose in PBS until they sank. The tissues were then taken out of the sucrose solution and were kept at −80°C until frozen sectioning. Serial sections of 5-μm thickness were cut in the coronal plane and stained with hematoxylin (H) and eosin (E). Histological specimens were examined by light microscopy (Olympus, Tokyo, Japan).
As for the histological analysis of the neurons, the degree of hippocampal damage was determined by the number of normal pyramidal cells in the CA1 region of hippocampus (Dhar et al. 2006). An observer blind to group assignment counted the viable pyramidal neurons with blue staining, intact round-shaped nuclei and without any nuclear fragmentation or karyopyknosis in the hippocampal CA1 subfield at a magnification of ×400. Five different microscopical vision fields for each hemisphere of each section were randomly chosen for calculating the mean number of neurons (per millimeter) in the hippocampal CA1 region.
Western immunoblot analysis
Rats were decapitated, and hippocampal tissue samples of the rat brains were rapidly dissected. Whole hippocampal sample lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electro-transferred to nitrocellulose membranes (Amersham International, Buckinghamshire, UK). The membranes were preincubated for 1 h at room temperature in Tris-buffered saline containing 0.05% Tween 20 and 3% bovine serum albumin at pH 7.6. The nitrocellulose membranes were incubated with total GSK-3β-, ser9-phosphorylated GSK-3β-specific antibodies (Cell Signaling Technology, Beverly, MA, USA), tyr216-phosphorylated GSK-3β- (Signalway Antibody, Pearland, USA), ser473-phosphorylated Akt (Cell Signaling Technology), total Akt (Santa Cruz Biotechnology, USA), thr205-phosphorylated tau (Invitrogen, USA), tau-, ser396-phosphorylated tau-, and thr181-phosphorylated tau- and β-actin-specific antibodies (Santa Cruz Biotechnology). Immunoreactive bands were then detected by incubation with conjugates of secondary antibody with horseradish peroxidase and enhanced chemiluminescence reagents (Amersham).
Bax and Bcl-2 (antibodies bought from Santa Cruz Biotechnology) protein levels from PC12 cells treated with high glucose and/or GLP-1/Ex-4 were also assessed by Western immunoblot analysis.
Statistical analysis
The mean±standard error of the mean was determined for each treatment group in a given experiment. Comparison among groups was performed by the analysis of variance followed by Tukey’s test. P values less than 0.05 were considered significant.
Results
Effects of glucose on PC12 cell viability
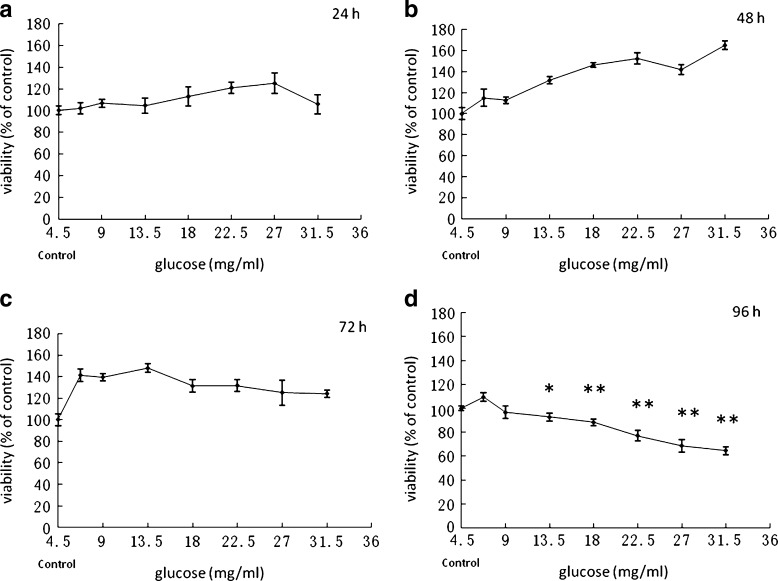
Since the optimal glucose concentration for PC12 cells is 4.5 mg/ml, we simulated in vitro hyperglycemia by increasing the medium glucose concentration up to 6.75, 9, 13.5, 18, 22.5, 27, and 31.5 mg/ml (1.5-, 2-, 3-, 4-, 5-, 6-, and 7-fold of the optimal glucose concentration). PC12 cells were exposed to increasing levels of glucose for 24 h (Fig. 1a), 48 h (Fig. 1b), 72 h (Fig. 1c), and 96 h, and a toxicity effect was obtained after 96 h (*P < 0.05, **P < 0.01) (Fig. 1d). This time point was selected for further study as a representation of hyperglycemic condition.
Fig. 1.
Effects of glucose on PC12 cell viability. Cells were treated with different concentrations of glucose for 24 (a), 48 (b), 72 (c), or 96 h (d), and then cell viability was estimated by MTT method. *P < 0.05, **P < 0.01 vs the control group in optimal glucose concentration. n = 6
Effect of Ex-4 on high glucose induced cellular toxicity and its downstream signaling pathways
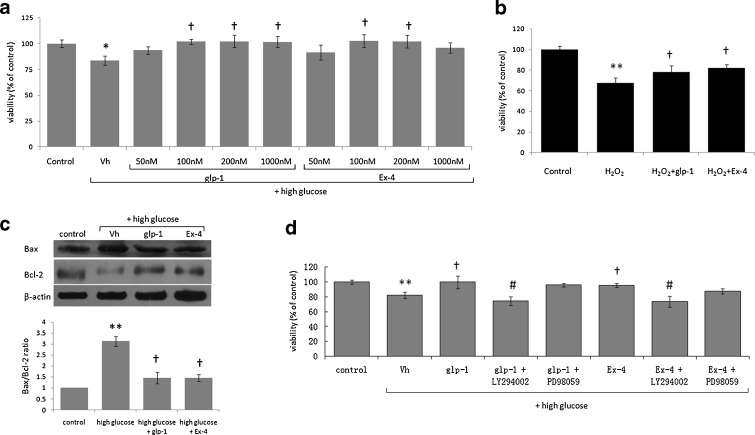
To elucidate the neuroprotective mechanism of Ex-4 in vitro, we studied the effects of GLP-1 and Ex-4 on cultured PC12 cells under hyperglycemic conditions. The cells treated with 50, 100, 200, and 1,000 nM of GLP-1 or Ex-4 in the presence of 27 mg/ml glucose (sixfold of the optimal glucose concentration for PC12 cells) for 96 h exhibited higher viability than the controls (†P < 0.05; Fig. 2a). In our study, the action of Ex-4 seemed to be saturable at 100 nM, as higher concentrations did not result in significantly elevation of cell viability. Exposure to high glucose (27 mg/ml) significantly reduced cell viability and produced an elevation of Bax and a decline in Bcl-2 protein resulting in an elevated Bax/Bcl-2 ratio, as assessed by Western blot analysis. Concurrent treatment with 100 nM GLP-1 or Ex-4 resulted in a decline in value of Bax/Bcl-2 ratio (Fig. 2c).
Fig. 2.
Neuroprotective effect of GLP-1/Ex-4 against high glucose-induced toxicity, the downstream signaling pathways involved and the effect of GLP-1/Ex-4 on protecting PC12 cells from oxidative stress-induced injuries. a Exposure to 27 mg/ml glucose and GLP-1/Ex-4 (50, 100, 200, and 1,000 nM) for 96 h, cell viability was increased compared with GLP-1/Ex-4 untreated group. n = 6. b Pretreatment with GLP-1/Ex-4 protected PC12 cells from 0.025 mM H2O2-induced loss of cell viability. n = 6. c In PC12 cells, the ratio of Bax/Bcl-2 (marker of apoptosis) was elevated by high glucose (27 mg/ml) and was lowered by GLP-1/Ex-4-treatment. n = 3. d The effect of GLP-1/Ex-4 on protecting PC12 cells from high glucose (27 mg/ml) induced injury was lost in cells treated with an inhibitor of PI3K (LY294002; 50 μM) but not with an inhibitor of MAP-kinase (PD98059; 100 μM). n = 5. *P < 0.05, **P < 0.01 vs the control group; †P < 0.05 vs the high glucose or H2O2 group; #P < 0.05 vs the high glucose + GLP-1 or the high glucose + Ex-4 group
To study the mechanism involved in GLP-1/Ex-4 effects on high glucose-induced neurotoxicity, we pre-treated PC12 cells with a specific inhibitor of PI3 kinase (LY294002; 50 μM) or MAP-Kinase (PD98059; 100 μM) for 30 min. As illustrated in Fig. 2d, concurrent treatment with LY294002 resulted in a loss of GLP-1/Ex-4-mediated protective effects in PC12 cells, whereas PD98059 did not significantly influence cell viability.
Ex-4 protects PC12 cells from oxidative stress-induced injury
The results in Fig. 2b show that application of hydrogen peroxide induced a loss of viability (P < 0.01) of PC12 cells. Pretreatment of PC12 cells with 100 nM of GLP-1 (P < 0.05) or Ex-4 (P < 0.05) reduced the cytotoxicity triggered by 0.025 mM H2O2 in 24 h. (Fig. 2b)
Effect of Ex-4 and ICV STZ on body weight and plasma glucose level in rats
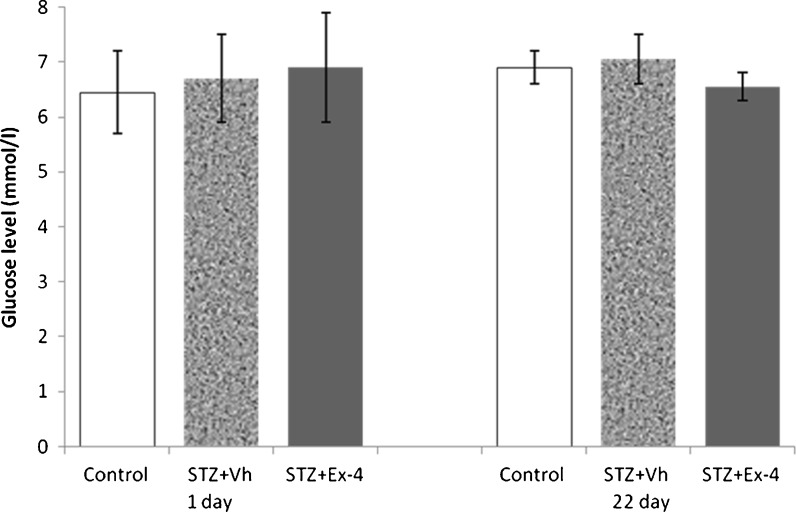
At study initiation, the mean weight of rats was approximately 230 g; weights were similar among groups, and the blood glucose levels of all experimental rats were in the normal range. Correspondingly, there was no significant difference in mean random blood glucose concentration (Fig. 3) and mean body weight (data not shown) among groups before sacrifice.
Fig. 3.
Effects of Ex-4 and ICV-STZ on blood glucose levels in rats. Blood glucose concentration (millimolars per liter) was measured at initiation (day 1) and prior to sacrifice (day 22) using the One-Touch Ultra Blood Glucose Meter. n = 6
Morris water maze test
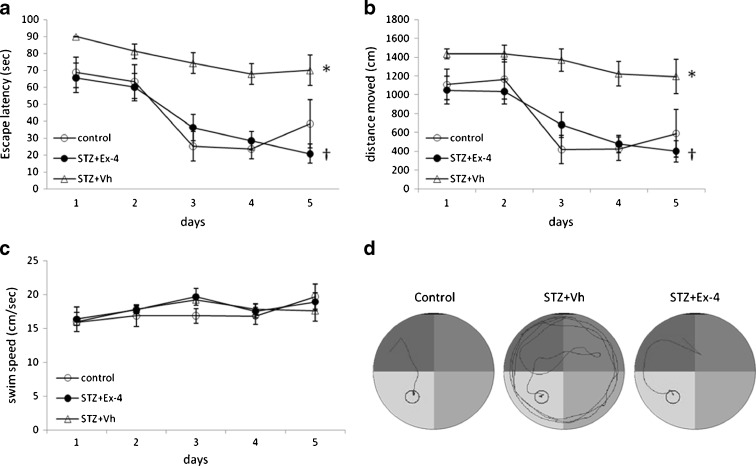
The general trends of the escape latencies (Fig. 4a) and distances moved (Fig. 4b) to reach the hidden platform decreased in all groups during the 5 days of training in the Morris water maze (MWM) test, and no statistically significant differences were noted among groups for swim speed (Fig. 4c). The mean escape latency and the mean distance moved were significantly (P < 0.05) prolonged in the STZ-treated group compared with the control group, showing a poorer learning performance due to ICV-STZ infusion. The latency and the distance in the STZ-treated rats were significantly (P < 0.05) decreased by concurrent administration of Ex-4. Typical swimming patterns found on day 5 of the training period suggested that rats in the control and STZ + Ex-4 groups found the platform mainly by tendency and linear patterns; the swimming patterns of STZ-treated animals always remained marginal and random (Fig. 4d).
Fig. 4.
The training period in the Morris water maze (MWM) test. Comparison of the acquisition performance according to escape latency (a), distances moved (b), swim speed (c), and representative swim paths (d) during the training period on the Morris water maze task among the three groups of rats (the control group, the STZ + Vehicle (Vh) group, and the STZ + Ex-4 group). The training task was performed with four trials per day for five successive days. n = 6–8. *P < 0.05 for STZ + Vh vs control; †P < 0.05 for STZ + Ex-4 vs STZ + vh
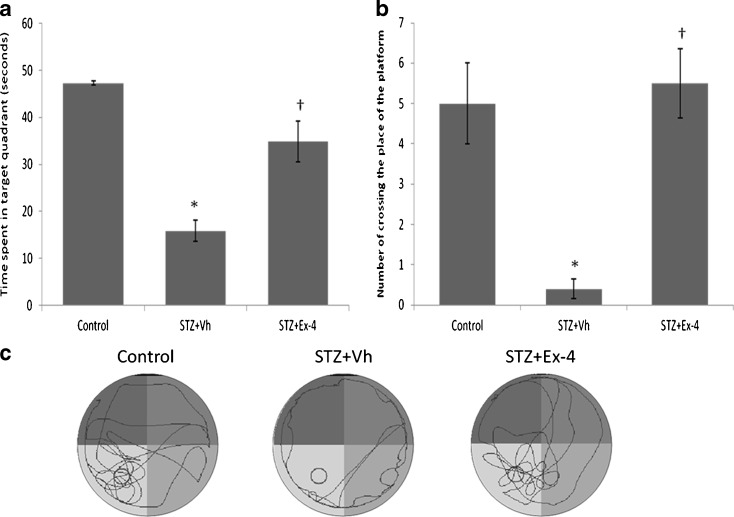
In the probe test, after the platform was removed, the STZ group failed to remember the precise location of the platform and spent significantly (P < 0.05) less time in the target quadrant than the control group (Fig. 5a). Mean percent time spent in the target quadrant (Fig. 5a) and the number of crossing (Fig. 5b) the original location of the platform increased significantly (P < 0.05) by supplementation with Ex-4 in the STZ + Ex-4 group compared with the STZ group. The typical swimming patterns (Fig. 5c) in the probe test showed that the rats in control and STZ + Ex-4 groups looked for the platform by means of shuttling back and forth. In contrast to the control, STZ group rats swam along the pool border to search for the platform still using marginal pattern.
Fig. 5.
The probe test in the MWM task. The percentages of time spent in the target area (a), number of crossing the place of the platform (b), and representative swim paths (c) during the probe test are shown. This test was performed on the sixth day after the first five training days. n = 6–8. *P < 0.05 for STZ + Vh vs control; †P < 0.05 for STZ + Ex-4 vs STZ + vh
Histopathological analysis
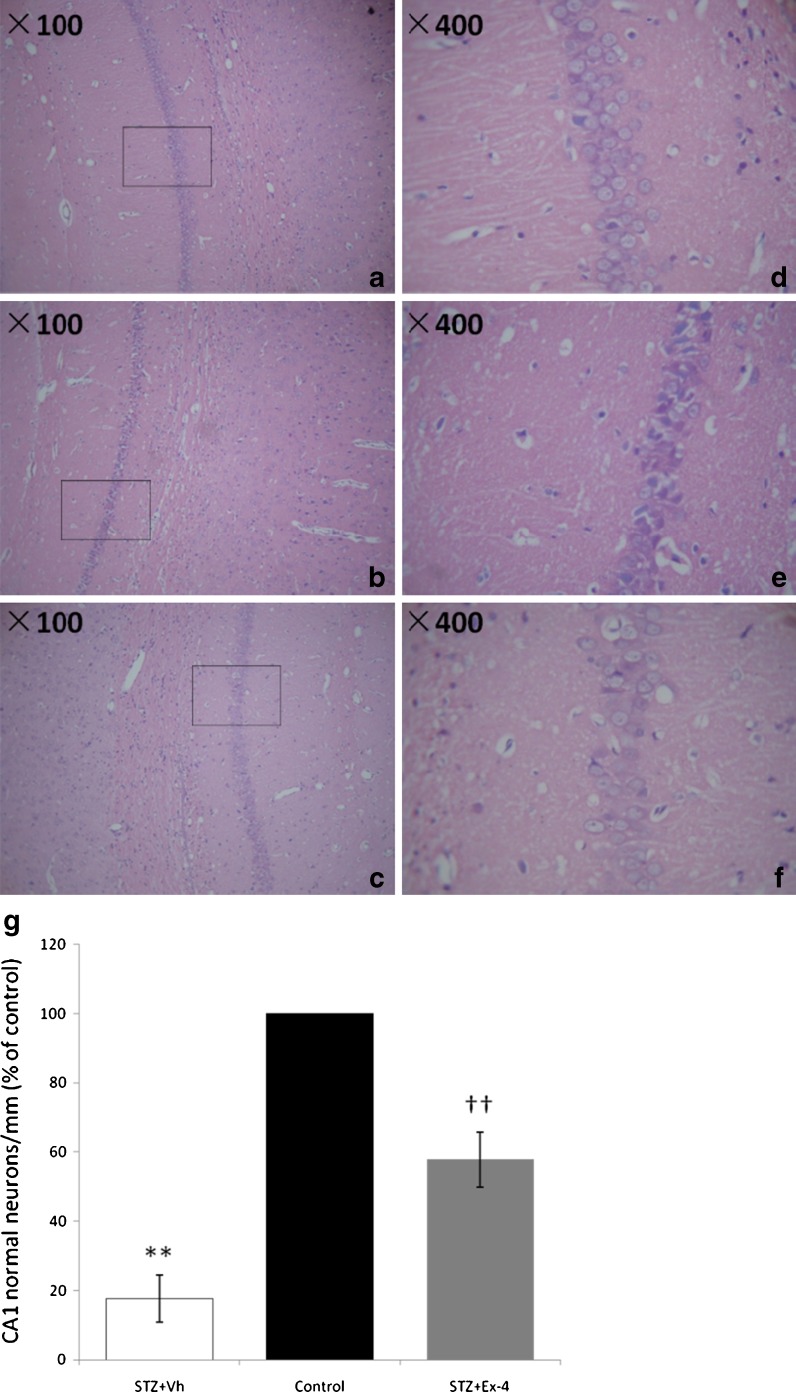
Histopathological examination revealed that the pyramidal neurons decreased in number and were distributed irregularly in the CA1 areas of the hippocampus of the rats in ICV-STZ-infused group (Fig. 6b, e), and structural damage in the CA1 area was more prominent compared with the control group (Fig. 6a, d). The neurons in the hippocampal CA1 areas in STZ + Ex-4 group (Fig. 6c, f) were increased and distributed more regularly compared with the model group, and the structural damage was also alleviated. The result of cell counting (Fig. 6g) was expressed as percentage of normal cells per millimeter compared with the control group.
Fig. 6.
Histopathological analysis of the brains from the three groups of rats. Histopathological examination showed the normal CA1 areas of hippocampus (a H&E × 100, d H&E × 400) in the control group, neuronal loss in CA1 area in the STZ + Vh group (b H&E × 100, e H&E × 400) and less neuronal loss in the STZ + Ex-4 group (c H&E × 100, f. H&E × 400) compared with the STZ + Vh group. The result of cell counting (g) was expressed as percentage of normal cells per millimeter compared with the control group. n = 4. **P < 0.01 for STZ + Vh vs Control; ††P < 0.01 for STZ + Ex-4 vs STZ + vh
Reversal of ICV-STZ-induced tau hyperphosphorylation in response to Ex-4 treatment
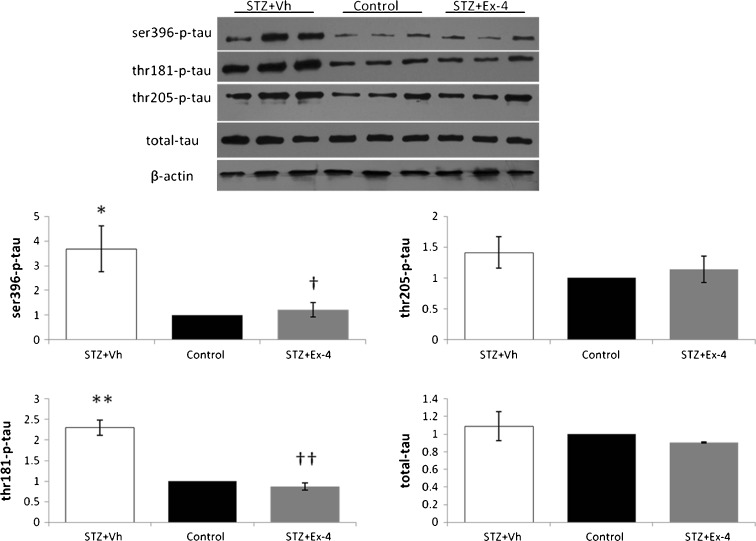
To further investigate the molecular mechanism of Ex-4 mediated amelioration of neurodegeneration in ICV-STZ-infused rats, tau hyperphosphorylation was evaluated via Western blot (Fig. 7). After Ex-4 administration, the STZ-induced hyperphosphorylation of tau at Ser396 (P < 0.05 vs. control), Thr181 (P < 0.01 vs. control), and Thr205 in the hippocampus was decreased. There was no significant difference in total tau-protein expression between the groups in our study.
Fig. 7.
Effects of Ex-4 on ICV-STZ-induced phosphorylated tau. Hippocampal tissue extracts were prepared and subjected to Western immunoblotting using antibodies specific for ser396-phosphorylated tau, thr181-phosphorylated tau, thr205-phosphorylated tau, total tau, and β-actin. n = 6. *P < 0.05, **P < 0.01 for the STZ + Vh group vs the control group; †P < 0.05, ††P < 0.01 for the STZ + Ex-4 group vs the STZ + vh group
Downregulatory effect of Ex-4 on ICV-STZ-induced activation of GSK-3β and GSK-3β protein expression
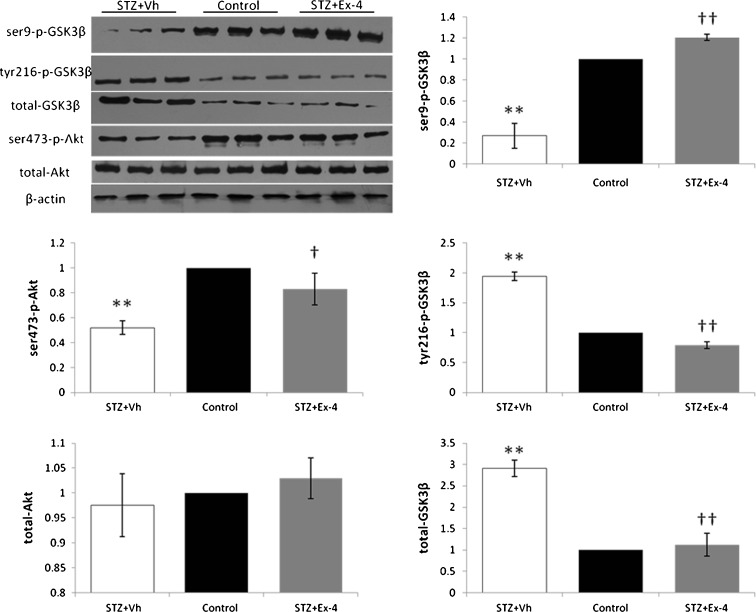
Because GSK-3β is an important kinase responsible for tau protein hyperphosphorylation, we assessed the effect of Ex-4 on GSK-3β kinase activation (Fig. 8). GSK-3β is inactivated by phosphorylation at Ser9, which can be regulated by Akt, and activation of GSK-3β kinase requires phosphorylation at tyrosine residues. Immunoblot analysis with phospho- GSK-3β-specific (Ser9, Tyr216) antibodies was performed in hippocampal lysates. After Ex-4 administration, ICV-STZ-induced phosphorylation (P < 0.01 vs. control) of GSK-3β at Tyr216 was decreased (P < 0.01), and a corresponding decrease in GSK-3β protein expression (P < 0.01) was also noted in this group. Ex-4 treatment reversed the ICV-STZ-induced decline (P < 0.01 vs. control) in the levels of phosphorylation of GSK-3β at Ser9 and Akt at Ser473.
Fig. 8.
Effects of Ex-4 on ICV-STZ-induced GSK-3β activation and phosphorylated Akt. Hippocampal tissue extracts were prepared and subjected to Western immunoblotting using antibodies specific for ser9-phosphorylated GSK-3β, tyr216-phosphorylated GSK-3β, total GSK-3β, ser473-phosphorylated Akt, total Akt, and β-actin. n = 6. **P < 0.01 for the STZ + Vh group vs the control group; †P < 0.05, ††P < 0.01 for the STZ + Ex-4 group vs the STZ + vh group
Discussion
Type 2 diabetes and glucose intolerance are considered to be the risk factors for AD (Takeda et al. 2010; Profenno et al. 2010). In line with this, deterioration of insulin signaling in brain has been found in some studies of Alzheimer’s disease (Frisardi et al. 2010). Aging and life span are also influenced by insulin/insulin receptor action. Inhibition of neuronal insulin signaling may render neurons energy-deficient, and disturbances of oxidative energy metabolism or other metabolic insults and impaired synaptic plasticity can occur. Moreover, hyperglycemia also directly damages hippocampal structures, hyperphosphorylates tau-protein, upregulates glycogen synthase kinase 3β (GSK-3β) activity, and reduces levels of insulin-degrading enzyme in the brain in patients with dementia (Querfurth and LaFerla 2010).
It has been well documented that some pathological neuronal loss in AD occurs through apoptosis. Glucose is essential for neuronal function and metabolism. Interestingly, both serum/glucose deprivation (Mousavi et al. 2010a, b) and hyperglycemia (Mousavi et al. 2010a, b) have been shown to be toxic to neuronal cells. Incubating with elevated glucose levels can also induce cell death in PC12 cells, providing a possible cellular model for diabetic encephalopathy. Protection of brain cells from degeneration is a useful preventive strategy for AD. In our previous study, we have made considerable efforts to search for natural substances with neuro-protective potential, and particular attention has been paid to anti-oxidative effects (Wang et al. 2007, 2008; Qian et al. 2008; Cai et al. 2008). The neurotoxicity of hyperglycemia arises from high glucose levels and also from an increased production of reactive oxygen and nitrogen species, and subsequent oxidative stress, similar to that induced by Aβ (Frisardi et al. 2010). In our experiments, incubation of PC12 cells with either GLP-1 or Ex-4 proved to be protective against high glucose as well as oxidative stress (hydrogen peroxide, H2O2)-induced cell injury, representing two pathways involved in AD especially DM-related AD (Frisardi et al. 2010). Similar protective results have been reported previously in PC12 cells, showing the effects of GLP-1/Ex-4 on β-amyloid, glutamate, and growth factor challenge (Perry et al. 2002, 2003); however, in this study, we were interested in studying the effects as well as the mechanisms of Ex-4 on diabetes-related AD. Several physiopathological features including hyperglycemia and oxidative stress relate type 2 diabetes to AD (Mousavi et al. 2010a, b; Frisardi et al. 2010), and our results emphasized that both GLP-1 and Ex-4 can inhibit high glucose-induced apoptosis in neurons. Furthermore, detailed study of the molecular mechanism has been carried out to evaluate the inhibitory effects of GLP-1/Ex-4 on high glucose-induced toxicity in neuronal cells as a suitable in vitro model for DM-related AD. In the present study, LY294002 (inhibitor of PI3 kinase) but not PD98059 (inhibitor of MAP-Kinase) appeared to fully inhibit GLP-1/Ex-4-mediated protective effects in PC12 cells.
Type 2 diabetes is a major risk factor for AD, illustrating an involvement of dysfunctional insulin signaling in the emergence of AD pathology in DM (Frisardi et al. 2010). In general, STZ, a glucosamine derivative of nitrosourea, is a drug used as a tool for creating experimental diabetes because of its selective toxicity for glucose transporter 2 (GLUT2)-expressing neuroendocrine cells (Wang and Gleichmann 1998). After entering the cells via GLUT2, STZ exerts its effects by causing insulin resistance and finally leads to diabetes mellitus (Schnedl et al. 1994). Furthermore, the STZ-transporter GLUT2 has also been found to be distributed inside the mammalian brain (Brant et al. 1993). Therefore, direct injection of STZ into the brain has been attempted to selectively impair central insulin signaling. The data available so far demonstrate that STZ can cause prolonged impairment of brain glucose and energy metabolism accompanied with impaired learning memory and cognition when injected intracerebroventricularly in rats (Isik et al. 2009), and in the ICV-STZ-treated brains, impaired insulin signaling was associated with increased levels of phosphor-tau and activated GSK-3β (Lester-Coll et al. 2006). Thus, to undertake our study in vivo, ICV-STZ-injected rats were utilized as they exhibit characteristics of AD-type neurodegeneration by selectively impairing brain insulin signaling which is thought to be a core contributor to so-called type-3 diabetes. GLP-1 and Ex-4 have been shown to protect cultured hippocampal neurons against death induced by Aβ in vitro (Perry et al. 2003), and previous studies also showed that Ex-4 can cross through the blood–brain barrier (Kastin and Akerstrom 2003). However, GLP-1 receptor stimulation and brain insulin signaling have not been investigated together in rats treated with ICV-STZ, which was suggested as an experimental model (Isik et al. 2009) of type 3 diabetes related to sporadic Alzheimer’s disease. Consequently, this ICV-STZ method was utilized in our present study to test Ex-4 in ICV-STZ-induced cognitive deficits and neurodegeneration, as confirmed by Morris water maze tasks. In this study, results from the water maze test showed that Ex-4-treated ICV-STZ animals exhibited significantly shorter escape latencies, increasing numbers of crossing the place of the platform and percentages of time spent in the target quadrant. Histopathological examination of the brains from these rats also revealed a neuroprotective effect of Ex-4, and this neuroprotective effect is consistent with the previous literatures (Perry et al. 2003; Li et al. 2009; Martin et al. 2009).
The potential mechanisms of the action by which Ex-4 provides neuroprotection against cognitive deficits and oxidative damage in ICV-STZ in rats remain uncertain. In Alzheimer’s disease, neurodegeneration is associated with intracellular accumulation of Aβ and neurofibrillary tangles (NFTs), which are the most prominent pathological markers of the severity of AD (Querfurth and LaFerla 2010). Previously, Perry et al. (2003) had already shown that Ex-4 treatment significantly lowered the level of Aβ in the brain in vivo. Neurofibrillary tangles, which are filamentous inclusions in the pyramidal cells of the hippocampus and in neurons of the cerebral cortex, occur in senile dementia of the Alzheimer type and other neurodegenerative disorders termed tauopathies, and the main components of NFTs are abnormally hyper-phosphorylated microtubule-associated tau proteins. Normally, tau phosphorylation is a key regulator of the ability of tau to bind and stabilize microtubules, but hyper-phosphorylated tau is insoluble, inactive in promoting microtubules assembly, and self-associates into paired helical filament structures (Querfurth and LaFerla 2010). Although the aberrant tau hyperphosphorylation alone is not sufficient to cause neurodegeneration, abnormally hyper-phosphorylated tau proteins may sensitize neurons to subsequent or concomitant insults related with Alzheimer’s disease (Clodfelder-Miller et al. 2006). Additionally, an increase of hyperphosphorylation in the microtubule-binding tau proteins was found in mouse brains after central nervous system (CNS) depletion of insulin and insulin receptor expressed in CNS cells by ICV-STZ treatment (Lester-Coll et al. 2006), raising the possibility that the reported link between diabetes and Alzheimer’s disease could in part be due to aberrant phosphorylation of tau caused by insulin signaling impairment. These data encouraged us to investigate the effect of Ex-4 treatment in tau hyperphosphorylation induced by ICV-STZ. Tau can be phosphorylated at multiple sites. It is worthy of attention that hyperphosphorylation of tau protein at ser396, thr181, and thr205 could exert neurotoxic effects, as well as contribute to neurite degeneration (Clodfelder-Miller et al. 2006). Here, we found that Ex-4 could reduce hippocampal tau phosphorylation at ser396, thr181, and thr205 in ICV-STZ-induced model rats. GSK-3β, a key kinase in fundamental processes during both DM (Mussmann et al. 2007) and AD (Hur and Zhou 2010), is a major tau kinase among the various kinases involved in tau protein hyperphosphorylation (Yoon et al. 2010). At present, in order to determine the role of GSK-3β in our study, we further investigated the phosphorylation pattern of GSK-3β in model rats after ICV-STZ insult. Consistent with the downregulatory effect of Ex-4 on tau hyperphosphorylation, phosphorylation pattern suggesting decreased GSK-3β activity was also observed, suggesting that the effect of Ex-4 on GSK-3β may play a critical role in reversal of ICV-STZ-induced tau hyperphosphorylation.
In summary, our study demonstrated that both GLP-1 and its long-acting analogue Ex-4 could increase the viability of neuronal cells under either high glucose or oxidative stress conditions in vitro, and in vivo Ex-4 treatment led to improved memory and cognitive performance in ICV-STZ rats. Based on our findings, Ex-4 has potential effects in the treatment of neurodegenerative diseases such as AD especially DM-related AD, and the most likely mechanism that can account for this effect in vivo involves a decrease of tau hyperphosphorylation levels and GSK-3β activity. However, further studies demonstrating the efficacy of Ex-4 therapy and elucidating the intracellular processes of the brain insulin signaling pathway interactions should be undertaken. It is possible that studying the interaction between diabetes medication and AD may also clarify the relationship between DM and AD.
Acknowledgements
This work was co-financed by the National Natural Science Foundation (No. 30973667), the Jiangsu Provincial Research Innovation Program for College Graduates (CX10B-377Z), Qing Lan Project and the 111 Project (111-2-07).
Footnotes
Song Chen and Ai-ran Liu contributed equally to this work.
Contributor Information
Wen-bing Yao, Phone: +86-25-83271218, FAX: +86-25-83302827, Email: wbyao@cpu.edu.cn.
Xiang-dong Gao, Phone: +86-25-83271298, FAX: +86-25-83271249, Email: xiangdong_gao@yahoo.com.cn.
References
- Brant AM, Jess TJ, Milligan G, Brown CM, Gould GW. Immunological analysis of glucose transporters expressed in different regions of the rat brain and central nervous system. Biochem Biophys Res Commun. 1993;192(3):1297–1302. doi: 10.1006/bbrc.1993.1557. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang H, Li Q, Qian Y, Yao W. Salidroside inhibits H2O2-induced apoptosis in PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Acta Biochim Biophys Sin. 2008;40(9):796–802. [PubMed] [Google Scholar]
- Chen Y, Zhou K, Wang R, Liu Y, Kwak YD, Ma T, Thompson RC, Zhao Y, Smith L, Gasparini L, Luo Z, Xu H, Liao FF. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci U S A. 2009;106(10):3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clodfelder-Miller BJ, Zmijewska AA, Johnson GV, Jope RS. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes. 2006;55(12):3320–3325. doi: 10.2337/db06-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar A, Kaundal RK, Sharma SS. Neuroprotective effects of FeTMPyP: a peroxynitrite decomposition catalyst in global cerebral ischemia model in gerbils. Pharmacol Res. 2006;54(4):311–316. doi: 10.1016/j.phrs.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A, Sancarlo D, Vendemiale G, Pilotto A, Panza F. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res Rev. 2010;9(4):399–417. doi: 10.1016/j.arr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Gao M, Tian H, Ma C, Gao X, Guo W, Yao W. Expression, purification, and C-terminal site-specific PEGylation of cysteine-mutated glucagon-like peptide-1. Appl Biochem Biotechnol. 2010;162(1):155–165. doi: 10.1007/s12010-009-8725-6. [DOI] [PubMed] [Google Scholar]
- Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23(11):542–549. doi: 10.1016/S0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Hölscher C. Receptors for the incretin glucagon-like peptide-1 are expressed on neurons in the central nervous system. Neuroreport. 2009;20(13):1161–1166. doi: 10.1097/WNR.0b013e32832fbf14. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11(8):539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik AT, Celik T, Ulusoy G, Ongoru O, Elibol B, Doruk H, Bozoglu E, Kayir H, Mas MR, Akman S. Curcumin ameliorates impaired insulin/IGF signalling and memory deficit in a streptozotocin-treated rat model. Age. 2009;31(1):39–49. doi: 10.1007/s11357-008-9078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27(3):313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- Kroner Z. The relationship between Alzheimer’s disease and diabetes: type 3 diabetes? Altern Med Rev. 2009;14(4):373–379. [PubMed] [Google Scholar]
- Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9(1):13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106(4):1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Golden E, Carlson OD, Pistell P, Zhou J, Kim W, Frank BP, Thomas S, Chadwick WA, Greig NH, Bates GP, Sathasivam K, Bernier M, Maudsley S, Mattson MP, Egan JM. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington’s disease. Diabetes. 2009;58(2):318–328. doi: 10.2337/db08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mousavi SH, Tayarani-Najaran Z, Asghari M, Sadeghnia HR. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010;30(4):591–598. doi: 10.1007/s10571-009-9484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi SH, Tayarani NZ, Parsaee H. Protective effect of saffron extract and crocin on reactive oxygen species-mediated high glucose-induced toxicity in PC12 cells. Cell Mol Neurobiol. 2010;30(2):185–191. doi: 10.1007/s10571-009-9441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, Burk U, Onichtchouk D, Dohrmann C, Austen M. Inhibition of GSK3 promotes replication and survival of pancreatic beta cells. J Biol Chem. 2007;282(16):12030–12037. doi: 10.1074/jbc.M609637200. [DOI] [PubMed] [Google Scholar]
- Ott A, et al. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/WNL.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Chen D, Zhou J, Shaw KT, Egan JM, Greig NH. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300(3):958–966. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res. 2003;72(5):603–612. doi: 10.1002/jnr.10611. [DOI] [PubMed] [Google Scholar]
- Plastino M, et al. Effects of insulinic therapy on cognitive impairment in patients with Alzheimer disease and diabetes mellitus type-2. J Neurol Sci. 2010;288(1–2):112–116. doi: 10.1016/j.jns.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67(6):505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Qian YF, Wang H, Yao WB, Gao XD. Aqueous extract of the Chinese medicine, Danggui-Shaoyao-San, inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Cell Biol Int. 2008;32(2):304–311. doi: 10.1016/j.cellbi.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Eng J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Zvartau-Hind ME, Hosford DA, Roses AD. Rosiglitazone in Alzheimer’s Disease Study Group. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6(4):246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T (2009) Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2009.10.009 [DOI] [PubMed]
- Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43(11):1326–1333. doi: 10.2337/diabetes.43.11.1326. [DOI] [PubMed] [Google Scholar]
- Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 2010;75(22):1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107(15):7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gleichmann H. GLUT2 in pancreatic islets: crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes. 1998;47(1):50–56. doi: 10.2337/diabetes.47.1.50. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou G, Gao X, Wang Y, Yao W. Acetylcholinesterase inhibitory-active components of Rhodiola rosea L. Food Chem. 2007;105:24–27. doi: 10.1016/j.foodchem.2007.03.031. [DOI] [Google Scholar]
- Wang H, Gao X, Zhou G, Cai L, Yao W. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008;106:888–895. doi: 10.1016/j.foodchem.2007.05.068. [DOI] [Google Scholar]
- Yoon SY, Park JS, Choi JE, Choi JM, Lee WJ, Kim SW, Kim DH. Rosiglitazone reduces tau phosphorylation via JNK inhibition in the hippocampus of rats with type 2 diabetes and tau transfected SH-SY5Y cells. Neurobiol Dis. 2010;40(2):449–455. doi: 10.1016/j.nbd.2010.07.005. [DOI] [PubMed] [Google Scholar]