Abstract
Age-related bone loss is well established in humans and is known to occur in nonhuman primates. There is little information, however, on the effect of dietary interventions, such as caloric restriction (CR), on age-related bone loss. This study examined the effects of long-term, moderate CR on skeletal parameters in rhesus monkeys. Thirty adult male rhesus monkeys were subjected to either a restricted (R, n = 15) or control (C, n = 15) diet for 20 years and examined throughout for body composition and biochemical markers of bone turnover. Total body, spine, and radius bone mass and density were assessed by dual-energy X-ray absorptiometry. Assessment of biochemical markers of bone turnover included circulating serum levels of osteocalcin, carboxyterminal telopeptide of type I collagen, cross-linked aminoterminal telopeptide of type I collagen, parathyroid hormone, and 25(OH)vitamin D. Overall, we found that bone mass and density declined over time with generally higher levels in C compared to R animals. Circulating serum markers of bone turnover were not different between C and R with nonsignficant diet-by-time interactions. We believe the lower bone mass in R animals reflects the smaller body size and not pathological osteopenia.
Keywords: Caloric restriction, Bone, Aging, Osteoporosis, Dietary restriction, Dual-energy x-ray absorptiometry
Introduction
Age-related bone loss is a well-established human characteristic found in all populations despite nutritional and biomechanical differences (Garn 1975; Mazess 1982; Cauley 2011; Barrett-Connor et al. 2005; Syed and Hoey 2010). Age-related bone loss has also been reported in several macaque species (Bowden et al. 1979; Champ et al. 1996; Colman et al. 1999a, b; DeRousseau 1985; Grynpas et al. 1993; Kessler et al. 1986; Pope et al. 1989; Przybeck 1985; Smith et al. 2009; Binkley et al. 1998; Brommage 2001; Fox et al. 2007; Miller et al. 1986), although none of these model systems replicate the osteoporotic fractures found in the human disease. To date, there is little information on the effect of aging interventions on the progression of age-related bone loss.
Caloric restriction (CR), or the reduction in total energy intake without restriction of a specific nutrient, is the only environmental intervention that has been repeatedly shown in laboratory rodents to retard the rate of aging and increase maximum life span (Masoro and Austad 1996; Weindruch 1996; Weindruch and Walford 1988). The ability of CR to increase life span is also seen in spiders, fish, water fleas, and other animals (Weindruch and Walford 1988). Studies (Ingram et al. 1990; Kemnitz et al. 1993; Colman et al. 2009; Ramsey et al. 2000; Mattison et al. 2003) are currently underway exploring the effects of moderate (∼30%) CR in rhesus monkeys, but information on the skeletal effects of this intervention remains limited.
Several studies have examined the effects of varying degrees of CR on rodent skeletal systems. The earliest study (McCay et al. 1935) found that femurs removed from restricted (R) rats crumbled during dissection; however, the level of CR makes it likely that these animals were suffering from nutritional deficiencies. Later studies by Kalu et al. (1984a, b, 1988) in F344 rats under a less severe restriction found that CR resulted in delayed epiphyseal closure, bones of lower density, and no reduction in femur density in old age. Results from more recent rodent studies have been variable. Some studies have shown a reduction in bone density with CR (Sanderson et al. 1997; Talbott et al. 1998; Berrigan et al. 2005); however; among these studies, one claims this to be due entirely to decreasing body weight with CR (Sanderson et al. 1997), while another found higher bone turnover in the CR animals (Talbott et al. 1998). Contrary to this, a recent study in F344BN rats found that animals subjected to 40% CR beginning at 14 weeks of age showed a possible beneficial response to the CR diet where bone quality was maintained with bone quantity improved per unit of body mass (Westerbeek et al. 2008).
In a study of CR in rhesus monkeys, Lane et al. (1995) showed delayed skeletal maturity but no reduction in total body bone mineral density or alteration in serum levels of calcium, phosphate, or osteocalcin. However, this study included a limited number (n = 3) of older animals and was confined to males. Subsequently, a cross-sectional analysis of male rhesus macaques, calorically restricted for 11 years, found lower bone mass that was accounted for by adjustment for age and body mass, specifically lean body mass (Black et al. 2001). However, this study included animals of two broad age groups, the youngest of which were immature at the time of energy restriction. Similarly, lean body mass was found to be the strongest predictor of bone mass in female rhesus macaques calorically restricted for 6 years (Lane et al. 2001).
Two studies have examined the effects of CR on bone in humans. The first study found that 6 months of CR in young, overweight adults resulted in large changes in overall body composition but no significant bone loss (Redman et al. 2008). The second study examined the effects of long-term CR in a group of 32 middle-aged lean individuals and found that while bone mineral density (BMD) was lower at the lumbar spine and hip when compared to age- and sex-matched sedentary individuals, bone quality was not reduced (Villareal et al. 2011).
The objective of the present study is to explore longitudinally the impact of long-term CR on bone metabolism in adult male rhesus macaques, a close phylogenetic relative of humans. This study employs techniques commonly used in clinical osteoporosis research including dual-energy X-ray absorptiometry (DXA) at clinically relevant sites and biochemical markers of bone turnover. Specifically we measured both bone mineral content (BMC, total grams of bone mineral in the region of interest) and BMD (BMC divided by bone area) of the total body, lumbar spine and radius. The skeletally relevant biochemical markers available in this study included osteocalcin (a product of osteoblasts often used as a marker of bone formation), carboxyterminal telopeptide of type I collage (ICTP, a marker of bone turnover), cross-linked aminoterminal telopeptide of type I collagen (NTx, a marker of bone turnover), parathyroid hormone (PTH, a marker of calcium status), and 25 hydroxyvitamin D (25OHD, an indicator of vitamin D status).
Methods
Animals and study design
The effects of aging and CR are being analyzed longitudinally in thirty adult male rhesus monkeys (Macaca mulatta), ranging in age from 8 to 14 years at study onset. More detailed descriptions of the design and methodology of this study have been reported (Colman et al. 1998; Kemnitz et al. 1993; Ramsey et al. 2000). This protocol was carried out with the approval of the Institutional Animal Care and Use Committee of the Graduate School of the University of Wisconsin, Madison.
The animals used in this study have lived their entire lives at the Wisconsin National Primate Research Center. Prior to the start of this experiment, no animals in the study group had any clinical or experimental history that would be expected to affect bone mass. All animals are individually housed, to allow for the accurate assessment of daily food intake (Colman et al. 1998; Kemnitz et al. 1993), and grouped into rooms. Within rooms, individuals have extensive visual and auditory contact with each other and are provided with an enriched environment consisting of perches, branches and small noninjurious toys that are rotated on a regular basis. Animals rooms are maintained at an approximate temperature of 21°C and humidity of 50–65%. Room lighting is automatically controlled on a 12-h light, 12-h dark schedule with no outside light reaching the animal rooms. Animals are monitored daily for general health by animal care and research staff and semi-annually by veterinary staff.
Animals were monitored for individual baseline food intakes of a purified diet (Teklad #85387, Madison, WI (15)) for a period of 3 months prior to the start of the study. Randomization in equal numbers to C and R groups was stratified by baseline food intake. Following baseline assessments, the R group food allotments were reduced by the 100 calorie wastage allowance, and for 3 successive months, a further reduction of 10% per month from individual baseline intake averages. At the time of the final food allotment reduction, R animals were switched to a caloric equivalent of a modified Teklad diet (diet #93131) which is enriched by 30% in vitamins and minerals. Food intake is measured daily for all animals. Annual differences between C and R animals in daily food intake along with the number of animals still alive at each timepoint in the study are shown in Table 1. All animals have 24-h access to tap water and are supplied with food for approximately 6–8 h per day. Following removal of food each evening, each animal receives a piece of fresh fruit.
Table 1.
Percent difference in food intake
| Years on study | Ca | Ra | %Diffb |
|---|---|---|---|
| 0 | 15 | 15 | −6.45 |
| 1 | 15 | 15 | 18.22 |
| 2 | 15 | 15 | 25.69 |
| 3 | 15 | 14 | 28.87 |
| 4 | 14 | 14 | 23.45 |
| 5 | 14 | 14 | 30.61 |
| 6 | 14 | 12 | 37.03 |
| 7 | 13 | 12 | 33.10 |
| 8 | 13 | 12 | 28.94 |
| 9 | 13 | 12 | 29.96 |
| 10 | 13 | 12 | 29.73 |
| 11 | 12 | 12 | 28.92 |
| 12 | 12 | 12 | 26.07 |
| 13 | 12 | 11 | 19.22 |
| 14 | 11 | 11 | 21.10 |
| 15 | 9 | 11 | 17.38 |
| 16 | 9 | 11 | 20.36 |
| 17 | 8 | 10 | 22.07 |
| 18 | 5 | 9 | 15.77 |
| 19 | 5 | 8 | 5.24 |
| 20 | 3 | 7 | 6.90 |
aNumber of animals alive at time of study
bPercent decrease in food intake (kcal/day) of R compared to C animals
Procedures
Bone densitometry
DXA (model DPX-L, GE/Lunar Corp., Madison, WI, USA) was used to assess total body BMC and BMD at yearly intervals from 1 through 5 years of CR and then twice yearly from 5.5 through 20 years. Additional region-specific DXA scans were performed as follows: lumbar spine scans in the posterior–anterior projection every 6 months from 6 to 8 years of CR and then annually until 20 years of CR, lumbar spine scans in the lateral projection annually from 8 to 20 years of CR, and radius scans annually from 10 to 20 years of CR. Body weights were obtained immediately prior to DXA scanning at each timepoint for all animals.
Coefficients of variation were <2.5% for each of the scans performed in this study (Colman et al. 1999b). Prior to DXA scanning, animals were fasted for approximately 16 h before sedation with ketamine HCl (up to 15 mg/kg, IM). Animals were additionally administered xylazine (0.6 mg/kg, IM) for muscle relaxation and additional ketamine HCl as needed for anesthesia maintenance. Following scan acquisition, yohimbine (0.06 mg/kg, i.v.) was administered to expedite recovery.
The standardized positioning used for bone densitometry in this study has been previously described (Champ et al. 1996; Colman et al. 1999b). Briefly, scans of the total body were performed with the animals supine on the scanner bed with foam wedges placed at the waist and a foam cylinder beneath the knees. Posterior–anterior (PA) lumbar spine scans were also performed with the animals supine with the spine parallel to the table midline and the arms abducted to 90°. Lateral lumbar spine scans were performed with the animal in left lateral recumbency. Scans of the left radius were performed with the animal in left lateral recumbency.
Total body and spine scans were acquired and analyzed using Lunar pediatric software (version 1.5e). Total body scans required no region of interest placement. Analysis of the PA lumbar spine was localized to vertebrae 2–4. Analysis of the lateral lumbar spine required manual placement of edges surrounding the bodies of vertebrae 3 and 4. Radius scans were acquired and analyzed using Lunar small animal software (version 1.0d). Radius analysis included a distal region and a shaft portion chosen to mimic the clinical “ultradistal” and “1/3” radius sites. The distal region, consisting mainly of cancellous bone, was defined as a 2 × 15 mm box located 10 mm from the distal tip of the styloid process. The shaft portion, consisting mainly of cortical bone, was defined as a 5 × 15 mm box located 47.5 mm from the distal tip of the styloid process.
Biochemical measurements
All animals were fasted overnight prior to phlebotomy. Blood was drawn into vacutainers from the saphenous or femoral vein between 0700 and 0900 hours and allowed to clot for approximately 30 min. Samples were then centrifuged and serum aliquoted into separate vials for each analyte and frozen at −80°C until analysis. Overnight urine samples were collected into a flask on ice. Samples were then centrifuged, aliquoted, and frozen at −80°C for later analysis.
The following parameters were measured (numbers represent inter- and intra-assay coefficients of variation, respectively): osteocalcin (RIA, Diagnostic systems laboratories, Webster, TX, USA, 8.28%, 4.90%, 6–8 years every 6 months then annually from 9 to 12 years), ICTP (RIA, Incstar, Stillwater, MN, USA, 6.75%, 3.08%, 6–8 years every 6 months), NTx (Ostex International Inc., Seattle, WA, USA, 4.00%, 7.63%, annually from 6 to 13 years), PTH (IRMA, Diagnostic Products Corporation, Los Angeles, CA, 10.38%, 9.45%, at 9 and 10 years), and 25OHD (RIA, Incstar, Stillwater, MN, 8.57%, 5.97%, annually from 6 to 12 years). All assays were fully validated for use in the rhesus monkey by parallelism and spike recovery.
Statistics
During the 20-year period covered by this study, 20 (12 C, 8 R) of the original 30 males have died. Animals have been included in statistical analysis and figures until the time of their death. Statistical significance was determined at the p < 0.05 level for all analyses.
Body weight and total body fat and lean masses were assessed for diet and time effects by repeated measures analysis of variance (JMP, SAS Institute, Cary, NC, USA). Bone and biochemical data were assessed using a linear mixed model (LMM) approach to estimate longitudinal trends in the data while accounting for the dependency in the data due to multiple observations per subject. LMM also accounts for missing data (under the assumption that data are missing at random) without relying on complete case data. For each bone-related outcome, an autoregressive lag-1 covariance structure over the repeated assessments was modeled in SAS PROC MIXED, and the diet and time period main effects and the diet-by-time interactions were tested while statistically controlling for fat mass and lean mass.
Results
Body weight and body composition
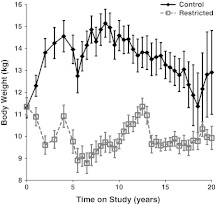
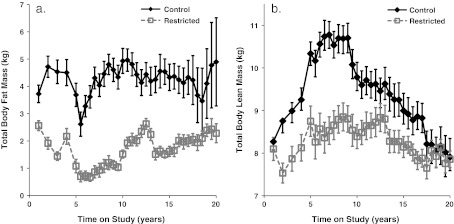
Long-term CR in this group of male rhesus macaques led to a statistically significant diet-by-time interaction (p < 0.001) for body weight (Fig. 1) explained by an increase (p < 0.001) in the C group of animals. Total body fat and lean mass both changed significantly over time and as an effect of diet with statistically significant diet-by-time interactions (p < 0.001); however, while the difference in fat mass was explained by an increase in the C group, the difference in lean mass was explained by a decrease in the C group (Fig. 2a, b).
Fig. 1.
Influence of CR on body weight. Points represent group means with standard errors for each time point. Closed diamonds and solid lines represent control (C) animals, and open squares and dashed lines represent restricted (R) animals. Diet-by-time interaction was significant (p < 0.001) with an increase (p < 0.001) in the C animals
Fig. 2.
Influence of CR on DXA-measured total body fat (a) and lean (b) mass. Points represent group means with standard errors for each time point. Closed diamonds and solid lines represent control (C) animals, and open squares and dashed lines represent restricted (R) animals. For fat mass, diet-by-time interaction was significant (p < 0.0001) explained by an increase in the C group (a). For lean mass, diet-by-time interaction was significant (p < 0.0001) explained by a decrease in the C group (b)
Bone densitometry
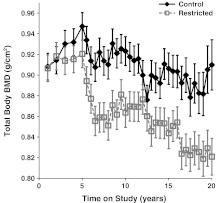
For total body BMC, the diet (p = 0.014) and time period (p < 0.001) were statistically significant factors. Total body BMC was higher in C compared to R animals; it changed over time and declined with advancing age. The diet-by-time interaction was not statistically significant (p = 0.181), indicating no differences in these longitudinal trends after controlling for the significant relationships of fat (p < 0.001) and lean mass (p < 0.001) to total body BMC. For total body BMD, the diet (p < 0.001) and time period (p < 0.001) were statistically significant. As can be seen in Fig. 3, the C animals have higher total body BMD than R animals, and it changes over time and declines with age. Furthermore, the diet-by-time interaction was statistically significant (p = 0.015) after controlling for the significant relationships of fat (p = 0.018) and lean mass (p = 0.035) to total body BMD. This indicates differential change in total body BMD over time for the two different diet conditions. Follow-up tests indicate that the C and R animals do not differ in the first 6 years of study. After year 6, the C animals have significantly higher total body BMD and the R animals experience a further decrease in total body BMD in the seventeenth year of study (Fig. 3).
Fig. 3.
Influence of CR on DXA-measured total body BMD. Points represent group means with standard errors for each time point. Closed diamonds and solid lines represent control (C) animals, and open squares and dashed lines represent restricted (R) animals. For total body BMD, the diet (p < 0.0001) and time period (p < 0.0001) were significant. C animals have higher total body BMD than R animals, and it changes over time and declines with age
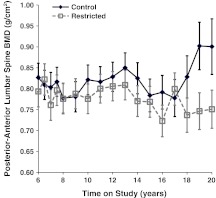
Although the diet (p = 0.073) and time (p = 0.442) main effects were not statistically significant for PA lumbar spine BMC, the diet-by-time interaction was statistically significant (p = 0.018) after controlling for the significant relationships of fat (p = 0.002) and lean mass (p < 0.001) to PA lumbar spine BMC. Follow-up analyses show that the diet groups did not differ in the first 12 years of assessment, but after that time, C animals had significantly higher lumbar spine BMC than R animals. Although C animals appear to have slightly higher levels of PA lumbar spine BMD as compared to R animals, the diet (p = 0.193) and time (p = 0.275) main effects and diet-by-time interaction (p = 0.292) were not statistically significant after controlling for the significant relationships of fat (p = 0.001) and lean mass (p < 0.001) to PA spine BMD (Fig. 4).
Fig. 4.
Influence of CR on DXA-measured PA lumbar spine BMD. Points represent group means with standard errors for each time point. Closed diamonds and solid lines represent control (C) animals, and open squares and dashed lines represent Restricted (R) animals. Main effects of diet and time as well as diet-by-time interaction were not significant
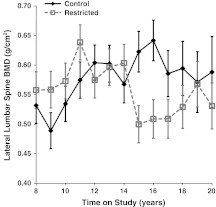
For BMC of the lumbar spine viewed in the lateral projection, although the main effect of diet was not statistically significant (p = 0.168), both the time main effect (p = 0.033) and the diet-by-time interaction (p = 0.044) were statistically significant after controlling for the significant relationships of fat (p = 0.031) and lean mass (p < 0.001). Follow-up analyses show that lateral spine BMC in C animals remained fairly constant over time, whereas the R animals displayed a decline. For BMD of the lumbar spine in the lateral projection, the diet (p = 0.303) and time (p = 0.281) main effects were not statistically significant; however, the diet-by-time interaction was statistically significant (p = 0.014) after controlling for fat (p = 0.885) and lean mass (p < 0.001). Follow-up analyses show that the diet groups did not differ in the first 7 years of assessment, but after that time, R animals had a statistically significant decline (Fig. 5).
Fig. 5.
Influence of CR on DXA-measured lateral lumbar spine BMD. Points represent group means with standard errors for each time point. Closed diamonds and solid lines represent control (C) animals, and open squares and dashed lines represent restricted (R) animals. Main effects of diet and time were not significant, however, the diet-by-time interaction was statistically significant (p = 0.0140)
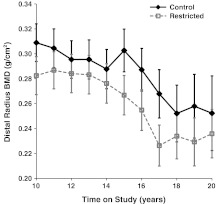
Although C animals appear to have slightly higher levels of distal radius BMC as compared to R animals, and distal radius BMC appears to decline over time, the diet (p = 0.097) and time (p = 0.442) main effects and diet-by-time interaction (p = 0.978) were not statistically significant after controlling for fat (p = 0.051) and lean mass (p < 0.001). For distal radius BMD, the diet (p = 0.034) and time (p = 0.007) were statistically significant. The C animals had higher distal radius BMD than R animals and distal radius BMD declines with age. The diet-by-time interaction was not statistically significant (p = 0.996), indicating no differences in these longitudinal trends after controlling for the significant relationships of fat (p = 0.042) and lean mass (p = 0.290) (Fig. 6).
Fig. 6.
Influence of CR on DXA-measured distal radius BMD. Points represent group means with standard errors for each time point. Closed diamonds and solid lines represent control (C) animals, and open squares and dashed lines represent restricted (R) animals. For distal radius BMD, both diet (p = 0.0340) and time (p = 0.0066) were significant factors
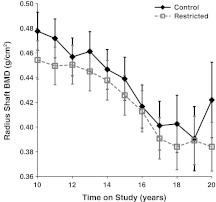
Differences between C and R animals were not statistically significant (p = 0.089) for radius shaft BMC. Although radius shaft BMC appears to decline over time, the longitudinal decline was not statistically significant (p = 0.190) nor was the diet-by-time interaction (p = 0.882) after controlling for fat (p = 0.239) and lean mass (p < 0.001). Similarly, the differences between C and R for radius shaft BMD were not statistically significant (p = 0.168); however, radius shaft BMD significantly declined over time (p < 0.001) after controlling for fat (p = 0.879) and lean mass (p < 0.001) (Fig. 7). As in the radius shaft BMC measurement, the diet-by-time interaction for radius shaft BMD was not statistically significant (p = 0.999).
Fig. 7.
Influence of CR on DXA-measured radius shaft BMD. Points represent group means with standard errors for each time point. Closed diamonds and solid lines represent control (C) animals, and open squares and dashed lines represent restricted (R) animals. C and R animals did not differ in radius shaft BMD; however, BMD significantly declined over time (p < 0.0001)
Biochemical measurements
Osteocalcin levels were extremely variable over time (p < 0.001). R animals tended to have higher levels of osteocalcin than C animals; however, this difference was not statistically significant (p = 0.062) after controlling for fat (p = 0.914) and lean mass (p = 0.390). Additionally, the diet-by-time interaction was not statistically significant (p = 0.215). Differences between the groups in ICTP (p = 0.635) and NTx (p = 0.141) were not significantly significant. ICTP significantly declined over time (p < 0.001) after controlling for fat (p = 0.009) and lean mass (p = 0.002); however, the diet-by-time interaction was not significant (p = 0.450). Similarly, NTx declined over time (p < 0.001) after controlling for fat (p = 0.392) and lean mass (p = 0.216) with a nonsignificant diet-by-time interaction (0.516). For PTH, there were only two assessment periods 1 year apart from each other. The overall differences between the groups were not significant (p = 0.351). PTH significantly increased from the first to the second assessment (p = 0.003) after controlling for fat (p = 0.457) and lean mass (p = 0.068). The diet-by-time interaction was not statistically significant (p = 0.288). Although 25OHD appears to decline over time, the diet (p = 0.312) and time (p = 0.872) main effects and diet-by-time interaction (p = 0.797) were not statistically significant after controlling for fat (p < 0.001) and lean mass (p = 0.005).
Discussion
This study has shown that an approximately 30% CR can be safely initiated and maintained long-term in adult male rhesus macaques. These animals are now entering old age, an exciting time for an aging intervention study. Most of the results presented here represent the impact of changing body weight on the skeleton but may also represent the metabolic effects of CR. The reported DXA results add understanding of the skeletal response to CR in a nonhuman primate. Unfortunately, the results from our biochemical markers were highly variable and not particularly informative. Perhaps, the use of more specific markers in the future will prove to be more revealing.
A growing body of evidence demonstrates a positive association between bone mineral density and body mass. A study of moderate CR in women showed a significant relationship between reduction in body mass and reduction in bone mass (Ramsdale and Bassey 1994). This is consistent with the theory that the most important influence on bone mass is the load it is required to bear (Frost 1993; Lanyon et al. 1986). Similarly, studies in both healthy men (Nuti et al. 1995) and older women (Blain et al. 2001) have found BMD to be highly related to lean body mass. In female rhesus macaque vertabrae, a weak positive correlation between bone density and weight has been reported (Grynpas et al. 1989, 1993). Contrary to this, DeRousseau (1985) found a statistically significant inverse relationship between body weight and bone mass in female rhesus macaques, possibly due to the effects of osteoarthritis (OA).
As further evidence of the effect of body weight on bone mass, decreased load on bone, or unloading, has been proven in numerous studies to cause accelerated bone loss among mammals (Wheadon and Heaney 1993; Amin 2010; Vernikos and Schneider 2010). Additionally, prolonged inactivity directly leads to bone loss at weight bearing sites (Qin et al. 2010; Habold et al. 2011). Complimentary to this, regular physical activity, resulting in increased loading, increases both the mineral content and the density of bone as well as reducing the rate of demineralization (DiGiovanna 1994; Wheadon and Heaney 1993). Furthermore, athletes have been shown to have BMD values greater than nonathletes (Eisman et al. 1991; Maimoun et al. 2011; Markou et al. 2010), and an even more dramatic example, tennis players have higher BMD in their dominant compared to their nondominant arm (Calbet et al. 1998; Haapasalo et al. 1998). Specifically regarding these mechanical-loading factors, the skeleton likely responds to mechanical stress such as increased body weight with a stimulation of osteoblast activity (Wardlaw 1996; Turner and Pavalko 1998; Mullender et al. 2004). However, in this group of animals, osteocalcin, a markers of osteoblast activity was not different between the diet groups.
A study in humans found increased BMD of the distal forearm in conjunction with obesity (Hyldstrup et al. 1993). These obesity-related alterations in BMD have also been found to a greater degree in the vertebral column. Since Hyldstrup et al. (1993) found this relationship in a peripheral bone, they argue that the effect of obesity on the skeleton is not entirely due to increased mechanical loading. Instead, they suggest that the increased fat tissue mass in obesity leads to increased estradiol levels, due to aromatization of estrogen precursors in adipocytes.
Decreased levels of estrogen and testosterone are linked to loss of bone mass as is the case in postmenopausal women and hypoandrogenic males, respectively. Since estrogen and other highly lipid soluble substances can be stored and subsequently released by body fat, it is believed that increased amounts of fat can result in higher maintenance levels of estrogen and therefore a reduction in estrogen-depletion bone loss (DeRousseau 1985). Heavier body weights are also associated with increased serum concentrations of testosterone (Edelstein and Barrett-Connor 1993). Increased fat mass is also likely to support greater conversion of androgens to estrogens. Furthermore, lower concentrations of sex hormone-binding globulin have been noted in obese individuals (Haffner and Bauer 1992) effectively increasing circulating levels of bioactive estrogen and testosterone. Although the mechanism is still unclear, a reduction in estrogen levels leads to increased skeletal remodeling perhaps via cytokine dysregulation in the bone microenvironment (Horowitz 1993).
More recently, the hormonal activity of adipose tissue has gained appreciation. Several adipose-derived hormones (adipokines) are now known to directly modulate the activity of bone cells as well as indirectly influence the skeleton through their actions on the central nervous system. The best known of these adipokines is leptin, a hormone we have previously shown to correlate directly with body fat mass in these animals (Colman et al. 1998). Locally, leptin is responsible for skeletal preservation by increasing osteoblast proliferation and differentiation (Thomas et al. 1999) and inhibiting osteoclastogenesis (Holloway et al. 2002). Indirectly, leptin may act on the skeleton through stimulation of growth hormone secretion and its effects on the hypothalamic–pituitary axis (Carro et al. 1997). Centrally, leptin has been shown to inhibit serotonin synthesis thereby inhibiting bone mass accrual (Karsenty and Oury 2010).
OA, specifically the osteophytes associated with this pathology, can lead to an increase in the amount of mineral detected by DXA technology. For this reason, it is important to account for the presence of OA in a study of DXA-measured BMC and BMD, particularly given the prevalence of OA in aging populations. We have previously shown that OA increased with age and body mass in these animals (Duncan et al. 2011). In order to account for the presence of OA, we performed lumbar spine scans in two projections, PA and lateral with the understanding that lateral scans would reduce the impact of OA on results. This effect of OA on the interpretation of PA lumbar spine scans may explain the loss of bone mass in R animals on lateral scans, while no significant loss was evident on PA scans.
In summary, male rhesus macaques subjected to long-term CR tend to have lower bone mass than age- and sex-matched C monkeys. However, bone turnover, as measured by biochemical markers is not altered by this intervention. It is believed that the lower bone mass in the R animals reflects the smaller body size of the R monkeys and not a pathological osteopenic condition.
Acknowledgements
We acknowledge the excellent technical assistance provided by S. Baum, J. Christensen, J. A. Adriansjach, C. E. Armstrong, and the Animal Care and Veterinary Staff of the WNPRC. This work was supported by National Institutes of Health grants P01 AG-11915 and P51 RR000167. This research was conducted in part at the WNPRC, which received support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This research was supported in part by facilities and resources at the Wm. S. Middleton Memorial Veterans Hospital.
References
- Amin S. Mechanical factors and bone health: effects of weightlessness and neurologic injury. Curr Rheumatol Rep. 2010;12(3):170–176. doi: 10.1007/s11926-010-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20(2):185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- Berrigan D, Lavigne JA, Perkins SN, Nagy TR, Barrett JC, Hursting SD. Phenotypic effects of calorie restriction and insulin-like growth factor-1 treatment on body composition and bone mineral density of C57BL/6 mice: implications for cancer prevention. In Vivo. 2005;19(4):667–674. [PubMed] [Google Scholar]
- Binkley N, Kimmel D, Bruner J, Haffa A, Davidowitz B, Meng C, Schaffer V, Green J. Zoledronate prevents the development of absolute osteopenia following ovariectomy in adult rhesus monkeys. J Bone Miner Res. 1998;13(11):1775–1782. doi: 10.1359/jbmr.1998.13.11.1775. [DOI] [PubMed] [Google Scholar]
- Black A, Allison DB, Shapses SA, Tilmont EM, Handy AM, Ingram DK, Roth GS, Lane MA. Calorie restriction and skeletal mass in rhesus monkeys (Macaca mulatta): evidence for an effect mediated through changes in body size. J Gerontol Biol Sci. 2001;56A:B98–B107. doi: 10.1093/gerona/56.3.B98. [DOI] [PubMed] [Google Scholar]
- Blain H, Vuillemin A, Teissier A, Hanesse B, Guillemin F, Jeandel C. Influence of muscle strength and body weight and composition on regional bone mineral density in healthy women aged 60 years and over. Gerontology. 2001;47:207–212. doi: 10.1159/000052800. [DOI] [PubMed] [Google Scholar]
- Bowden DM, Teets C, Witkin J, Young DM. Long bone calcification and morphology. In: Bowden DM, editor. Aging in nonhuman primates, chapter 27. New York: Van Nostrand Reinhold; 1979. pp. 335–355. [Google Scholar]
- Brommage R. Perspectives on using nonhuman primates to understand the etiology and treatment of postmenopausal osteoporosis. J Musculoskelet Neuronal Interact. 2001;1(4):307–325. [PubMed] [Google Scholar]
- Calbet JA, Moysi JS, Dorado C, Rodriguez LP. Bone mineral content and density in professional tennis players. Calcif Tissue Int. 1998;62:491–496. doi: 10.1007/s002239900467. [DOI] [PubMed] [Google Scholar]
- Carro E, Senaris R, Considine RV, Casanueva FF, Dieguez C. Regulation of in vivo growth hormone secretion by leptin. Endocrinol. 1997;138(5):2203–2206. doi: 10.1210/en.138.5.2203. [DOI] [PubMed] [Google Scholar]
- Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469:1891–1899. doi: 10.1007/s11999-011-1863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champ JE, Binkley N, Havighurst T, Colman RJ, Kemnitz JW, Roecker EB. The effect of advancing age on bone mineral content of the female rhesus monkey. Bone. 1996;19(5):485–492. doi: 10.1016/S8756-3282(96)00243-8. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Roecker EB, Ramsey JJ, Kemnitz JW. The effect of dietary restriction on body composition in adult male and female rhesus macaques. Aging Clin Exp Res. 1998;10(2):83–92. doi: 10.1007/BF03339642. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Binkley N, Lane MA, Abbott DH, Kemnitz JW. Skeletal effects of aging and menopausal status in female rhesus macaques. J Clin Endocrinol Metab. 1999;84(11):4144–4148. doi: 10.1210/jc.84.11.4144. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Lane MA, Binkley N, Wegner FH, Kemnitz JW. Skeletal effects of aging in male rhesus monkeys. Bone. 1999;24(1):17–23. doi: 10.1016/S8756-3282(98)00147-1. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRousseau CJ. Aging in the musculoskeletal system of rhesus monkeys: III. Bone loss. Am J Phys Anthropol. 1985;68:157–167. doi: 10.1002/ajpa.1330680203. [DOI] [PubMed] [Google Scholar]
- DiGiovanna AG (1994) Skeletal system. Human aging: biological perspectives. McGraw-Hill, New York, pp 165–183
- Duncan AE, Colman RJ, Kramer PA. Longitudinal study of radiographic spinal osteoarthritis in a macaque model. J Orthop Res. 2011;29:1152–1160. doi: 10.1002/jor.21390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138(3):160–169. doi: 10.1093/oxfordjournals.aje.a116842. [DOI] [PubMed] [Google Scholar]
- Eisman JA, Sambrook PN, Kelly PJ, Pocock NA. Exercise and its interaction with genetic influences in the determination of bone mineral density. Am J Med. 1991;91:5S–9S. doi: 10.1016/0002-9343(91)90239-T. [DOI] [PubMed] [Google Scholar]
- Fox J, Miller MA, Newman MK, Turner CH, Recker RR, Smith SY. Treatment of skeletally mature ovariectomized rhesus monkeys with PTH(1-84) for 16 months increases bone formation and density and improves trabecular architecture and biomechanical properties at the lumbar spine. J Bone Miner Res. 2007;22(2):260–273. doi: 10.1359/jbmr.061101. [DOI] [PubMed] [Google Scholar]
- Frost H. Suggested fundamental concepts in skeletal physiology. Calcif Tissue Int. 1993;52:1–4. doi: 10.1007/BF00675618. [DOI] [PubMed] [Google Scholar]
- Garn SM. Bone-loss and aging. In: Goldman R, Rockstein M, editors. The physiology and pathology of human aging. New York: Academic; 1975. pp. 39–57. [Google Scholar]
- Grynpas MD, Huckell B, Protzker KPH, Hancock RGV, Kessler MJ. Bone mineral and osteoporosis in aging rhesus monkeys. PR Health Sci J. 1989;8(1):197–204. [PubMed] [Google Scholar]
- Grynpas MD, Huckell CB, Reichs KJ, DeRousseau CJ, Greenwood C, Kessler MJ. Effect of age and osteoarthritis on bone mineral in rhesus monkey vertebrae. J Bone Miner Res. 1993;8(8):909–917. doi: 10.1002/jbmr.5650080803. [DOI] [PubMed] [Google Scholar]
- Haapasalo H, Kannus P, Sievanen H, Pasanen M, Uusi-Rasi K, Heinonen A, Oja P, Vuori I. Effect of long-term unilateral activity on bone mineral density of female junior tennis players. J Bone Miner Res. 1998;13:310–319. doi: 10.1359/jbmr.1998.13.2.310. [DOI] [PubMed] [Google Scholar]
- Habold C, Momken I, Ouadi A, Bekaert V, Brasse D. Effect of prior treatment with resveratrol on density and structure of rat long bones under tail-suspension. J Bone Miner Metab. 2011;29(1):15–22. doi: 10.1007/s00774-010-0187-y. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Bauer RL. Excess androgenicity only partially explains the relationship between obesity and bone density in premenopausal women. Int J Obes. 1992;16:869–874. [PubMed] [Google Scholar]
- Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, Gough TJ, Collier GR, Nicholson GC. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17(2):200–209. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- Horowitz MC. Cytokines and estrogen in bone: anti-osteoporotic effects. Science. 1993;260:626–627. doi: 10.1126/science.8480174. [DOI] [PubMed] [Google Scholar]
- Hyldstrup L, Andersen T, McNair P, Breum L, Transbol I. Bone metabolism in obesity: changes related to severe overweight and dietary weight reduction. Acta Endocrinol. 1993;129:393–398. doi: 10.1530/acta.0.1290393. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Cutler RG, Wendruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, Roth GS. Dietary restriction and aging: the initiation of a primate study. J Gerontol Biol Sci. 1990;45(5):B148–B163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- Kalu DN, Hardin RH, Cockerham R, Yu BP. Aging and dietary modulation of rat skeleton and parathyroid hormone. Endocrinol. 1984;115(4):1239–1247. doi: 10.1210/endo-115-4-1239. [DOI] [PubMed] [Google Scholar]
- Kalu DN, Hardin RR, Cockerham R, Yu BP, Norling BK, Egan JW. Lifelong food restriction prevents senile osteopenia and hyperparathyroidism in F344 rats. Mech Age Dev. 1984;26:103–112. doi: 10.1016/0047-6374(84)90169-6. [DOI] [PubMed] [Google Scholar]
- Kalu DN, Masoro EJ, Yu BP, Hardin RR, Hollis BW. Modulation of age-related hyperparathyroidism and senile bone loss in fischer rats by soy protein and food restriction. Endocrinol. 1988;122(5):1847–1854. doi: 10.1210/endo-122-5-1847. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Oury F. The central regulation of bone mass, the first link between bone remodeling and energy metabolism. J Clin Endocrinol Metab. 2010;95(11):4795–4801. doi: 10.1210/jc.2010-1030. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Weindruch R, Roecker EB, Crawford K, Kaufman PL, Ershler WB. Dietary restriction of adult male rhesus monkeys: design, methodology, and preliminary findings from the first year of study. J Gerontol Biol Sci. 1993;48(1):B17–B26. doi: 10.1093/geronj/48.1.b17. [DOI] [PubMed] [Google Scholar]
- Kessler MJ, Turnquist JE, Pritzker KPH. Reduction of passive extension and radiographic evidence of degenerative knee joint diseases in cage-raised and free-ranging aged rhesus monkeys (Macaca mulatta) J Med Primatol. 1986;15:1–9. [PubMed] [Google Scholar]
- Lane MA, Reznick AZ, Tilmont EM, Lanir A, Ball SS, Read V, Ingram DK, Cutler RG, Roth GS. Aging and food restriction alter some indices of bone metabolism in male rhesus monkeys (Macaca mulatta) J Nutr. 1995;125:1600–1610. doi: 10.1093/jn/125.6.1600. [DOI] [PubMed] [Google Scholar]
- Lane MA, Black A, Handy AM, Shapses SA, Tilmont EM, Kiefer TL, Ingram DK, Roth GS. Energy restriction does not alter bone mineral metabolism or reproductive cycling and hormones in female rhesus monkeys. J Nutr. 2001;131:820–827. doi: 10.1093/jn/131.3.820. [DOI] [PubMed] [Google Scholar]
- Lanyon LE, Rubin CT, Baust G. Modulation of bone loss during calcium insufficiency by controlled dynamic loading. Calcif Tissue Int. 1986;38:209–216. doi: 10.1007/BF02556712. [DOI] [PubMed] [Google Scholar]
- Maimoun L, Coste O, Mariano-Goulart D, Galtier F, Mura T, Philibert P, Briot K, Paris F, Sultan C. In peripubertal girls, artistic gymnastics improves areal bone mineral density and femoral bone geometry without affecting serum OPG/RANKL levels. Osteoporos Int. 2011;22:3055–3066. doi: 10.1007/s00198-011-1541-1. [DOI] [PubMed] [Google Scholar]
- Markou KB, Theodoropoulou A, Tsekouras A, Vagenakis AG, Georgopoulos NA. Bone acquisition during adolescence in athletes. Ann N Y Acad Sci. 2010;1205:12–16. doi: 10.1111/j.1749-6632.2010.05675.x. [DOI] [PubMed] [Google Scholar]
- Masoro EJ, Austad SN. The evolution of the antiaging action of dietary restriction: a hypothesis. J Gerontol Biol Sci. 1996;51A:B387–B397. doi: 10.1093/gerona/51A.6.B387. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38(1–2):35–46. doi: 10.1016/S0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Mazess RB. On aging bone loss. Clin Orthop. 1982;165:239–252. [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of the lifespan and upon ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Miller LC, Weaver DS, McAlister JA, Koritnik DR. Effects of ovariectomy on vertebral trabecular bone in the cynomolgus monkey (Macaca fascicularis) Calcif Tissue Int. 1986;38(1):62–65. doi: 10.1007/BF02556597. [DOI] [PubMed] [Google Scholar]
- Mullender M, El Haj AJ, Yang Y, Duin MA, Burger EH, Klein-Nulend J. Mechanotransduction of bone cells in vitro: mechanobiology of bone tissue. Med Biol Eng Comput. 2004;42(1):14–21. doi: 10.1007/BF02351006. [DOI] [PubMed] [Google Scholar]
- Nuti R, Martini G, Gennari C. Age-related changes of whole skeleton and body composition in healthy men. Calcif Tissue Int. 1995;57:336–339. doi: 10.1007/BF00302068. [DOI] [PubMed] [Google Scholar]
- Pope NS, Gould KG, Anderson DC, Mann DR. Effects of age and sex on bone density in the rhesus monkey. Bone. 1989;10:109–112. doi: 10.1016/8756-3282(89)90007-0. [DOI] [PubMed] [Google Scholar]
- Przybeck TR. Histomorphology of the rib: bone mass and cortical remodeling. In: Davis RT, Leatleus CW, editors. Behavior and pathology of aging in rhesus monkeys. New York: Alan R. Liss; 1985. pp. 303–326. [Google Scholar]
- Qin W, Bauman WA, Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci. 2010;1211:66–84. doi: 10.1111/j.1749-6632.2010.05806.x. [DOI] [PubMed] [Google Scholar]
- Ramsdale SJ, Bassey EJ. Changes in bone mineral density associated with dietary-induced loss of body mass in young women. Clin Sci. 1994;87:343–348. doi: 10.1042/cs0870343. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol. 2000;35(9–10):1131–1149. doi: 10.1016/S0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008;168(17):1859–1866. doi: 10.1001/archinte.168.17.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JP, Binkley N, Roecker EB, Champ JE, Pugh TD, Aspnes L, Weindruch R. Influence of fat intake and caloric restriction on bone in aging male rats. J Gerontol Biol Sci. 1997;52A(1):B20–B25. doi: 10.1093/gerona/52A.1.B20. [DOI] [PubMed] [Google Scholar]
- Smith SY, Jolette J, Turner CH. Skeletal health: primate model of postmenopausal osteoporosis. Am J Primatol. 2009;71(9):752–765. doi: 10.1002/ajp.20715. [DOI] [PubMed] [Google Scholar]
- Syed FA, Hoey KA. Integrative physiology of the aging bone: insights from animal and cellular models. Ann N Y Acad Sci. 2010;1211:95–106. doi: 10.1111/j.1749-6632.2010.05813.x. [DOI] [PubMed] [Google Scholar]
- Talbott SM, Rothkopf MM, Shapses SA. Dietary restriction of energy and calcium alters bone turnover and density in younger and older female rats. J Nutr. 1998;128:640–645. doi: 10.1093/jn/128.3.640. [DOI] [PubMed] [Google Scholar]
- Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinol. 1999;140(4):1630–1638. doi: 10.1210/en.140.4.1630. [DOI] [PubMed] [Google Scholar]
- Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci. 1998;3(6):346–355. doi: 10.1007/s007760050064. [DOI] [PubMed] [Google Scholar]
- Vernikos J, Schneider VS. Space, gravity and the physiology of aging: parallel or convergent disciplines? A mini-review. Gerontology. 2010;56(2):157–166. doi: 10.1159/000252852. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Kotyk JJ, Armamento-Villareal RC, Kenguva V, Seaman P, Shahar A, Wald MJ, Kleerekoper M, Fontana L. Reduced bone mineral density is not associated with significantly reduced bone quality in men and women practicing long-term calorie restriction with adequate nutrition. Aging Cell. 2011;10(1):96–102. doi: 10.1111/j.1474-9726.2010.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw GM. Putting body weight and osteoporosis into perspective. Am J Clin Nutr. 1996;63(Supplement):433S–436S. doi: 10.1093/ajcn/63.3.433. [DOI] [PubMed] [Google Scholar]
- Weindruch R (1996) Caloric restriction and aging. Sci Am 274:46–52 [DOI] [PubMed]
- Weindruch R, Walford RL. The retardation of aging and disease by dietary restriction. Springfield: Charles C. Thomas; 1988. [Google Scholar]
- Westerbeek ZW, Hepple RT, Zernicke RF. Effects of aging and caloric restriction on bone structure and mechanical properties. J Gerontol A Biol Sci Med Sci. 2008;63(11):1131–1136. doi: 10.1093/gerona/63.11.1131. [DOI] [PubMed] [Google Scholar]
- Wheadon GD, Heaney RP. Effects of physical inactivity, paralysis, and weightlessness on bone growth. In: Hall BK, editor. Bone growth, vol 7. Boca Raton: CRC; 1993. [Google Scholar]