Abstract
Rhesus monkeys provide a valuable model for studying the basis of cognitive aging because they are vulnerable to age-related decline in executive function and memory in a manner similar to humans. Some of the behavioral tasks sensitive to the effects of aging are the delayed response working memory test, recognition memory tests including the delayed nonmatching-to-sample and the delayed recognition span task, and tests of executive function including reversal learning and conceptual set-shifting task. Much effort has been directed toward discovering the neurobiological parameters that are coupled to individual differences in age-related cognitive decline. Area 46 of the dorsolateral prefrontal cortex (dlPFC) has been extensively studied for its critical role in executive function while the hippocampus and related cortical regions have been a major target of research for memory function. Some of the key age-related changes in area 46 include decreases in volume, microcolumn strength, synapse density, and α1- and α2-adrenergic receptor binding densities. All of these measures significantly correlate with cognitive scores. Interestingly, the critical synaptic subtypes associated with cognitive function appear to be different between the dlPFC and the hippocampus. For example, the dendritic spine subtype most critical to task acquisition and vulnerable to aging in area 46 is the thin spine, whereas in the dentate gyrus, the density of large mushroom spines with perforated synapses correlates with memory performance. This review summarizes age-related changes in anatomical, neuronal, and synaptic parameters within brain areas implicated in cognition and whether these changes are associated with cognitive decline.
Keywords: Aging, Area 46, Dentate gyrus, Executive function, Recognition memory, Perforated synapse
Introduction
Identification of the neurobiological mechanisms underlying age-related cognitive decline has been the subject of intense investigation. Rhesus monkeys are an ideal animal model for studying normal aging for many reasons: (1) their brain anatomy closely resembles that of humans, (2) they are vulnerable to age-related cognitive decline, (3) they can perform cognitive tests similar to those administered to humans, and (4) they do not develop neurodegenerative disorders such as Alzheimer’s disease (Squire and Zola-Morgan 1988; Gearing et al. 1996; Petrides and Pandya 1999; Kimura et al. 2003; Nagahara et al. 2010).
Executive function, including cognitive flexibility, cognitive tracking, set maintenance, divided attention, and working memory, is a cognitive domain impaired in aged monkeys and humans and is thought to be one of the first functions to decline with age (Rapp and Amaral 1989; Moore et al. 2006). Recognition memory is another function that is vulnerable to the effects of aging. As in humans, some monkeys age successfully, while others show age-related cognitive decline (Rapp and Amaral 1991). In the last two decades, quantitative neuroanatomical and molecular studies in behaviorally characterized young and aged monkeys have emerged in search of parameters that are coupled to individual differences in age-related cognitive decline. Area 46 of the dorsolateral prefrontal cortex (dlPFC) has been extensively examined for its critical role in executive function while the medial temporal lobe system including the hippocampus and related cortical areas has been the main target of research for memory function. This article will review the age-related changes (or lack thereof) in anatomical, neuronal (morphological and electrophysiological), synaptic, and molecular parameters within these brain areas and whether these changes are associated with cognitive decline.
Nonhuman primate as a model of aging
Rhesus monkeys (Macaca mulatta) are the most widely used nonhuman primate for studies of normal aging. The maximal lifespan of a rhesus monkey is 35 to 40 years of age, and the average life span of captive rhesus monkeys is under 25 years (Tigges et al. 1988). Although human age equivalence can be roughly estimated at 1:3 (Tigges et al. 1988), the ratio is not uniform across every stage of development (Voytko and Tinkler 2004). For example, menopause occurs by 27 years old, which is very late in life relative to humans (Gilardi et al. 1997; Walker and Herndon 2008).
The major advantage of using rhesus monkeys as a model of cognitive aging is that we can assess the same rich repertoire of cognitive capacities in humans using the same or similar cognitive tests (Squire et al. 1988; Nagahara et al. 2010). Age-related cognitive decline begins as early as the late teens in rhesus monkeys, although as in humans, some aged monkeys are resistant to the decline and exhibit cognitive abilities similar to those of young adults (Presty et al. 1987; Bachevalier et al. 1991; Rapp and Amaral 1991; Peters et al. 1996). Additionally, not all functional abilities are affected equally within an individual aged monkey (Voytko 1999). Some of the key cognitive measures vulnerable to age-related decline are discussed in the section below.
Another advantage of using rhesus monkeys in aging studies is that although they can accumulate some senile plaques, they do not develop the neuropathological symptoms of Alzheimer’s disease (Gearing et al. 1996; Peters et al. 1999; Kimura et al. 2003). Thus, differences observed between young and aged subjects can be generally attributed to normal aging without the confounding factors inherent to neurodegenerative diseases. In addition, experimental protocols can be made consistent to limit artifacts that affect morphology of brain specimens. Human postmortem studies, on the other hand, often include specimens of varied postmortem intervals that can affect morphological measures.
Cognitive measures vulnerable to age-related decline
Delayed response
Working memory is a type of memory that is active and relevant for a short period of time (Goldman-Rakic 1995). Delayed response (DR) is a spatial working memory test in which the subject has to remember the spatial location of a previously cued object through a delay period (Fig. 1). Aged monkeys require more trials to acquire this task, and they perform significantly and disproportionately worse at longer delay intervals compared to young monkeys (Bartus et al. 1978; Rapp and Amaral 1989; Bachevalier et al. 1991; Voytko and Tinkler 2004).
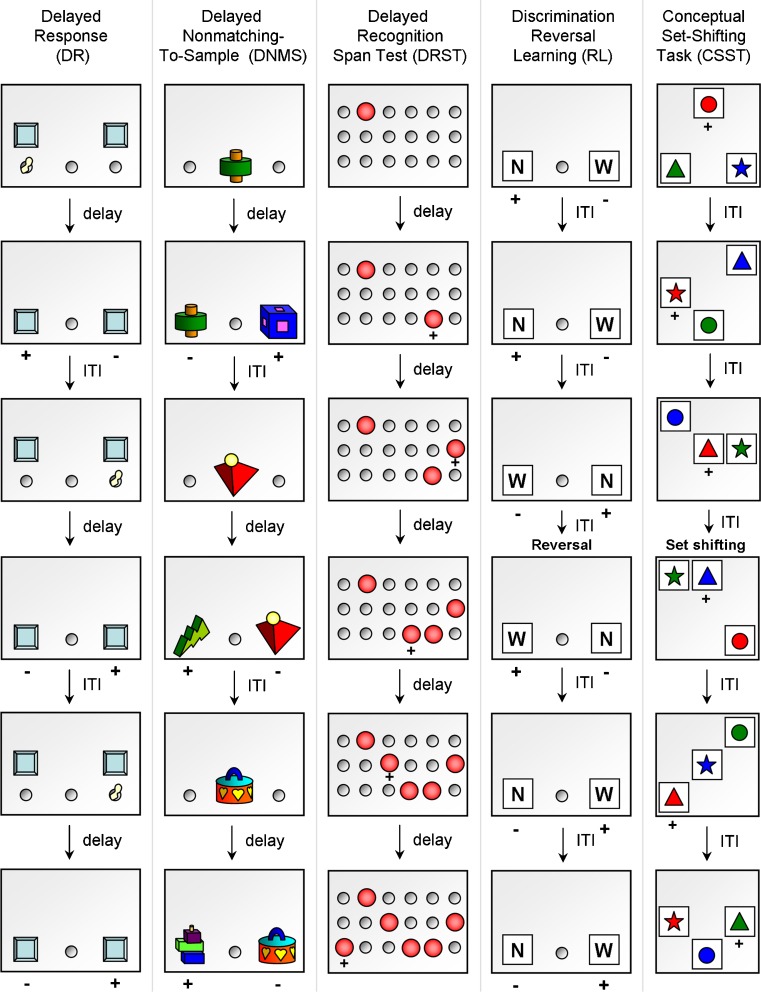
Fig. 1.
Schematic diagrams illustrating the cognitive tests sensitive to aging. DR is a spatial working memory test in which the subject has to remember the spatial location of a cued object through a delay period (during which an opaque screen hides the stimulus tray). In DNMS test of visual recognition memory, the subject is presented with a sample object, and after a delay interval, the subject must choose the novel object when presented together with the sample object. The spatial version of DRST assesses recognition and spatial working memory and requires the subject to identify a novel stimulus among an increasing array of previously presented stimuli. Discrimination RL is used to measure set-shifting ability, a component of executive function. Illustrated is the pattern version of discrimination RL, in which the subject learns to associate one of the patterns (N) with reward. After the subject achieves the accuracy criterion, discrimination reversal is initiated where the previously unrewarded stimulus (W) becomes the rewarded stimulus. CSST assesses executive functions such as abstraction and set-shifting. In CSST, the subject is rewarded when choosing the correct color (red), regardless of shape. After the subject chooses the correct stimulus consecutively, the rewarded stimulus is switched to a specific shape (triangle), regardless of color. ITI inter-trial interval, + rewarded stimulus, − unrewarded stimulus
Area 46 of the dlPFC has complex connections with somatosensory, visual, visuomotor, motor, and limbic systems and plays an important role in the mediation of executive functions, including working memory (reviewed in Luebke et al. 2010). Lesion studies have shown that area 46 is critical for performance on DR (Funahashi et al. 1993). Many neurons within area 46 show persistent spatially-tuned firing during the delay period of the DR test, and the “memory field” generated by these neurons is thought to be the cellular basis for working memory (Funahashi et al. 1989; Goldman-Rakic 1995).
In addition to the dlPFC, the medial temporal lobe also plays a role in working memory. During working memory tasks, glucose utilization in the hippocampal dentate gyrus (DG) and CA1 is significantly increased (Friedman and Goldman-Rakic 1988), and DR delay accuracy correlates with hippocampal glucose metabolism (Eberling et al. 1997). Additionally, lesions involving the medial temporal lobe structures cause deficits on DR delay accuracy in rhesus monkeys (Zola-Morgan and Squire 1985). Thus, multiple brain regions likely contribute to working memory and alterations in one or more of these regions may result in DR deficits observed in aged monkeys.
Delayed nonmatching-to-sample
Another cognitive test that is sensitive to the effects of aging is delayed nonmatching-to-sample (DNMS), which is a well-characterized visual recognition memory test (Presty et al. 1987; Moss et al. 1988; Rapp and Amaral 1989). In DNMS, a monkey is presented with a sample object, and after a delay interval, the monkey is required to pick a novel object when presented together with the sample object (Fig. 1). Aged monkeys show impairment on both the task acquisition and delay performance (Presty et al. 1987; Moss et al. 1988; Shamy et al. 2006). Although both DNMS and DR are vulnerable to age-related decline, subjects impaired on one test are not necessarily impaired on the other (Rapp and Amaral 1989; Dumitriu et al. 2010), suggesting that aging may affect recognition memory and working memory independently.
While controversy remains about the role of the hippocampus proper on recognition memory (Murray and Mishkin 1998), some lesion studies have reported that damage limited to the hippocampal region produces a significant and long-lasting impairment in DNMS delay performance (Zola-Morgan et al. 1994; Alvarez et al. 1995; Beason-Held et al. 1999). Furthermore, when the lesion is extended to the adjacent parahippocampal and perirhinal cortices in monkeys, the memory impairment is more severe (Zola-Morgan et al. 1989, 1993, 1994; Alvarez et al. 1995). These findings are consistent with clinical cases, where memory impairment is seen in a patient after a lesion limited to the hippocampal CA1 (Zola-Morgan et al. 1986), but more pronounced memory deficits are observed in patients with larger lesions within the medial temporal lobe (Corkin 1984).
In addition, ventromedial PFC (vmPFC; including areas 13, 14, 24, 25, 10, and 11) lesions also produce significant deficits in DNMS delay performance (Bachevalier and Mishkin 1986). The rule learning aspect of DNMS, however, appears to be dependent upon the integrity of the dlPFC (Peters et al. 1998a, 2008; Dumitriu et al. 2010; Shamy et al. 2010).
Delayed recognition span task
Delayed recognition span task (DRST) assesses short-term recognition memory. The task requires the subject to identify a novel stimulus among an increasing array of previously presented stimuli (Fig. 1). Different versions of this task can be administered by altering the material, space, color, or pattern of the stimulus. Because this test requires the subject to hold in memory an increasing number of stimuli, it is also considered a memory loading task (Moss et al. 1997). This test has been used in Alzheimer’s disease patients as well as in healthy, high-functioning elderly people to assess memory function (Inouye et al. 1993; Moss et al. 1986).
Aged monkeys are impaired on the spatial and color versions of DRST and make more perseverative errors (choosing the previously correct disk) than young adults (Moss et al. 1997). While the recognition memory aspect of DRST may be dependent on the medial temporal lobe system, the spatial working memory required for the spatial version of DRST has been linked to morphological alterations in the dlPFC (Peters et al. 1998a; Buckmaster et al. 2004; Cruz et al. 2004).
Reversal learning
Reversal learning (RL) paradigms can be used to investigate executive function in aged monkeys. RL requires the subject to respond to a change in reinforcement contingencies by unlearning the initial learned stimulus–reinforcement pair and then shifting to a new pair (Fig. 1). Performance on RL tasks is used as a measure of set-shifting ability (ability to shift problem-solving strategies), which is a gauge for cognitive flexibility.
Aged monkeys show no deficits in learning discrimination tasks but are significantly impaired in color, pattern, spatial, and object RL and exhibit enhanced perseverative tendencies (choosing an incorrect stimulus that would have been correct under the previous response contingency) compared to young monkeys (Bartus et al. 1979; Lai et al. 1995; Voytko 1999). Together, these studies suggest that aged monkeys are more rigid in their behavior and may be more susceptible to proactive interference (Bartus et al. 1979; Voytko 1999). Although some other studies have shown a lack of age-related impairment in RL, prior exposure to complex behavioral tasks or utilization of alternate strategies may have compensated for an underlying deficit on reversal performance (Rapp 1990).
Lesion studies in monkeys suggest that the ventromedial–orbital PFC and the striatum play an important role in RL tasks (Divac et al. 1967; McEnaney and Butter 1969; Iversen and Mishkin 1970). Human subjects with ventromedial frontal damage also exhibit impaired RL and the size of the orbitofrontal cortical lesion correlates with reversal errors (Fellows and Farah 2003). Furthermore, an imaging study in human subjects revealed that RL tasks activate the caudate nucleus and the orbitofrontal cortex (Rogers et al. 2000). Together, these studies suggest that the corticostriatal circuits may be engaged in the acquisition of stimulus–reinforcement associations (Rogers et al. 2000).
Conceptual set-shifting task
Conceptual set-shifting task (CSST) was developed to assess executive functions such as abstraction, concept formation, and set-shifting in monkeys in a way similar to the Wisconsin Card Sorting Test (WCST) used in human subjects (Haaland et al. 1987; Fristoe et al. 1997; Moore et al. 2005). On each trial of CSST, the subject is presented with stimuli that vary in shape and color (Fig. 1). During the first concept condition, the subject is rewarded when choosing the correct color, regardless of shape. Once the subject chooses the correct stimulus consecutively, the rewarded stimulus is switched to a specific shape, regardless of color.
Middle-aged (12–19 years old) and aged monkeys (20+ years old) are significantly impaired in set-shifting, as measured by the acquisition and performance on the CSST, and exhibit increased perseveration as compared to young adults (Moore et al. 2005, 2006). These studies are consistent with a study in human subjects demonstrating a marked increase in perseverative errors on the WCST by middle age (Rhodes 2004).
Marmosets with lesions in the prefrontal cortex show significant impairments in set-shifting tasks (Dias et al. 1996). In human subjects, a similar set-shifting task activates several PFC areas including the dlPFC (areas 9/46), medial frontal gyrus (area 10), and middle frontal gyrus (area 8) (Rogers et al. 2000). Thus, neurobiological changes in the dlPFC (including areas 46 and 9) are thought to underlie the age-related impairment on the CSST in monkeys (Moore et al. 2005, 2006).
PFC and its relationship with aging and cognitive decline
PFC gross anatomy
While an earlier stereological study failed to detect an effect of age on the volume of area 46 in the PFC of monkeys (O’Donnell et al. 1999), a recent magnetic resonance imaging (MRI) study that is unconfounded by variables such as shrinkage (due to perfusion and histological processing) revealed an age-related decrease in area 46 volume (Shamy et al. 2010). In addition, the decrease in area 46 volume correlates with the decline in recognition memory, as measured by DNMS acquisition and average accuracy (Shamy et al. 2010). The age-related decrease in area 46 volume may be partially due to the thinning of area 46 layer I thickness, which is also associated with lower DNMS delay accuracy (Peters et al. 1998a). While layer I of area 17 (primary visual cortex) also becomes thinner with age, there is no correlation between its thickness and cognitive scores (Peters et al. 2001). The absence of a relationship between area 17 layer I thickness and cognition is not surprising because area 17 is primarily involved in vision and is not considered to play a key role in cognition (Peters et al. 2001).
Anterior cingulate cortex (ACC; areas 24 and 32) is another PFC region whose volume decreases with age and this decrease is coupled to slower DNMS acquisition (Shamy et al. 2010). While the volumes of superior frontal gyrus (SFG; areas 9 and 8B), inferior frontal gyrus (IFG; areas 12, 45, and 47), and the vmPFC (areas 11, 13, and 14) remain unchanged with aging, IFG volume inversely correlates with DNMS acquisition (trials to criterion) while SFG and vmPFC volumes positively correlate with DNMS average accuracy (Shamy et al. 2010).
PFC neurons
For many years, it was assumed that the decline in PFC-dependent cognition with normal aging was a consequence of neuronal loss, but more recent studies using unbiased stereological techniques have shown that there is no global neuronal loss in monkey neocortex with aging (Peters et al. 1994, 1998b; Peters and Sethares 2002; Hof et al. 2000). One exception is area 8a, a prefrontal region associated with working memory, where a 32% decrease in the number of neurons has been observed in aged compared with young monkeys (Smith et al. 2004). While the number of behaviorally characterized subjects was limited, this decline in neuronal number correlated with impaired working memory performance, as measured by DR accuracy (Smith et al. 2004). The total number of neurons in dlPFC area 46, however, does not change with age and fails to correlate with cognitive performance, as measured by DNMS and DRST scores (Peters et al. 1994; Smith et al. 2004).
PFC neurons are typically arranged in microcolumns. A microcolumn consists of a vertical arrangement of neurons that possess a common set of properties and is thought to constitute a fundamental computational unit of the cerebral cortex (Mountcastle 2003). In layer III of area 46, microcolumn strength (ratio of microcolumn/total neuronal density) is reduced in aged monkeys, and this decrease correlates with the decline in cognition as measured by DNMS delay accuracy, spatial DRST memory span, and Cognitive Impairment Index (CII; normalized scores of three cognitive tests) (Cruz et al. 2004). Because total neuronal density in layer III is unaltered with aging, it is likely that subtle neuronal displacements, possibly associated with changes in dendritic and other morphological alterations (discussed below), result in the decreased strength of microcolumns and ultimately contribute to cognitive dysfunction. Other age-related morphological changes in area 46 layer II/III neurons include the accumulation of lipofuscin in the cytoplasm and the emergence of large irregular vacuoles and membranous whorls in the apical dendritic branches (Peters et al. 1994).
Electrophysiological studies have revealed a decrease in the frequency of spontaneous excitatory glutamatergic postsynaptic current (EPSC) and an increase in the spontaneous inhibitory GABAergic postsynaptic current (IPSC) in area 46 layer II/III pyramidal neurons with aging, though neither of these electrophysiological measures correlate with executive function or memory, as measured by CSST scores and CII (Luebke et al. 2004). In contrast, area 46 layers II/III pyramidal neurons of aged monkeys have increased action potential firing rates and input resistance, and these measures exhibit a U-shaped relationship with CII, DNMS acquisition (errors to criterion), DNMS delay accuracy, and spatial DRST memory span (Chang et al. 2005). These U-shaped relationships suggest that there is an optimal firing rate for normal cognitive function in aged monkeys and rates that are too low or too high are associated with poor behavioral performance (Chang et al. 2005). In addition, area 46 layer III neurons exhibit increased slow afterhyperpolarization amplitude with age, and the amplitude significantly correlates with DNMS acquisition (errors to criterion) and CII (Luebke and Amatrudo 2010).
Area 46 layer V neurons show no age effects on basic membrane and repetitive action potential firing properties, but they display significantly decreased single action potential amplitude, duration, and fall time (Luebke and Chang 2007). In contrast to layer II/III neurons, none of the electrophysiological signatures of aging among layer V neurons correlate with cognitive performance (Luebke and Chang 2007; Luebke and Amatrudo 2010). Thus, within PFC area 46, it is the layer II/III corticocortical pyramidal neurons, and not the layer V neurons that contribute to the corticostriatal–thalamic–cortical circuitry, that are thought to be crucial for cognitive capacities vulnerable to the effects of aging (Barbas and Rempel-Clower 1997; Luebke and Chang 2007; Luebke and Amatrudo 2010).
PFC synapses
Dendritic spines are the major recipients of excitatory input to cortical neurons and are key sites for synaptic plasticity. One of the earliest nonhuman primate studies examining the effects of age on PFC synapses explored area 9. While the Golgi stain method is not entirely reliable for quantitative studies, Cupp and Uemura reported that dendrites of area 9 neurons continue to grow between 7 (young adult) and 20 years old (middle-aged), but very old monkeys (27–28 years old) exhibit significantly shorter dendritic length, fewer numbers of dendritic branches, and lower spine densities (Cupp and Uemura 1980). Additionally, aged monkeys display lower synapse densities in area 9 compared to young monkeys (Uemura 1980), although this study was performed before the advent of the unbiased disector method (Sterio 1984). In contrast, an unbiased stereological study showed maintenance of synaptic density and size in lamina III and V of the human area 9 across a wide age range (20–89 years old; Scheff et al. 2001).
More recently, dlPFC area 46 has been the primary focus with respect to synaptic changes in the context of aging and cognitive decline. A study employing the unbiased disector method revealed that in layer I of monkey PFC area 46, aging results in a 30–60% decrease in synapse density (Peters et al. 1998a). This decrease is accompanied by a reduction in the number of apical dendritic branches (Peters et al. 1998a). Additionally, the loss of synapses in layer I correlates with four measures of cognitive decline: DNMS acquisition, DNMS accuracy score, spatial DRST memory span, and CII (Peters et al. 1998a). Despite these alterations in dendrites and dendritic spines, unmyelinated axons and axon terminals in layer I show no change with age (Peters et al. 1998a).
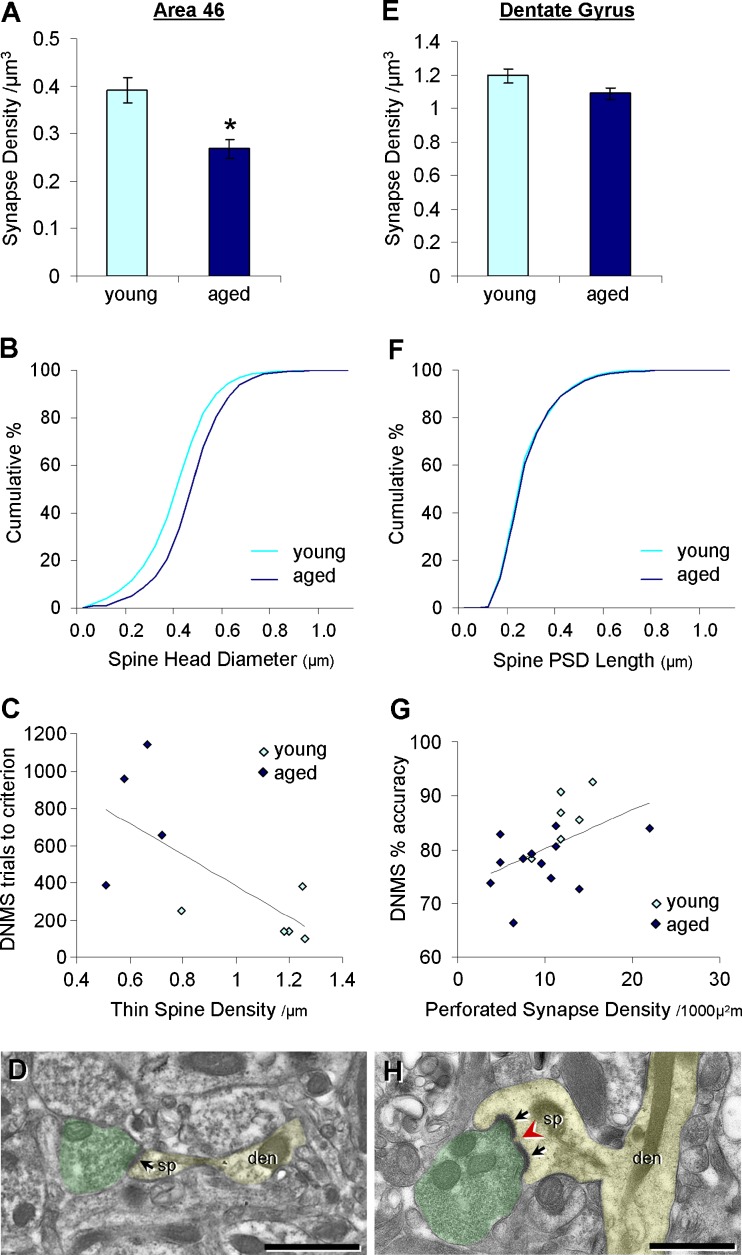
In layers II/III of PFC area 46, there is a marked 30% decrease in synapses from age 5 to 30 years, and both asymmetric (excitatory) and symmetric (inhibitory) synapses are lost at the same rate (Peters et al. 2008). Strong correlations have been found between layers II/III asymmetric synapse density and cognitive scores including DNMS acquisition and CII (Peters et al. 2008). Our group confirmed this age-related decrease in synapse density using unbiased methods and extended these findings by demonstrating an age-related loss of dendritic spines averaging 33% in monkey area 46 layer III pyramidal neurons, combined with a shift in the spine size distribution toward larger spines (Fig. 2; Dumitriu et al. 2010). Additionally, faster DNMS acquisition is strongly correlated with higher axospinous synapse density, increased thin spine density (Fig. 2), and smaller volume of thin spines on area 46 layer III neurons (Dumitriu et al. 2010). In fact, the average size of these thin spines showed the tightest correlation with DNMS acquisition (Dumitriu et al. 2010). Thus, in area 46, the class of spines selectively vulnerable to aging and essential for acquiring the nonmatching rule of the task appears to be the smallest of the thin spines, characterized by high motility, plasticity, and turnover rate (Kasai et al. 2003; Hao et al. 2007; Dumitriu et al. 2010).
Fig. 2.
Synaptic correlates of cognitive decline in the monkey dorsolateral prefrontal cortex area 46 and hippocampal dentate gyrus (DG). a, b In area 46 layer III neurons, aging results in decreased synapse density (a; p = 0.003) and a shift in the distribution of dendritic spine size (spine head diameter) toward larger spines (b; p < 0.001). c Delayed nonmatching-to-sample (DNMS) task acquisition (trials to criterion) inversely correlates with area 46 thin spine density (r = −0.69; p = 0.04). d An electron micrograph of a thin spine in area 46. The spine (sp) and dendrite (den) are shaded in yellow, while the axon terminal is shaded in green. The black arrow indicates the postsynaptic density (PSD). e–g In the DG outer molecular layer, no age effects are seen in axospinous synapse density (e) or size as measured by PSD length (f), but the density of large mushroom spines with perforated synapses correlates with DNMS memory performance (g; r = 0.49; p = 0.04). h An electron micrograph of a mushroom spine with a perforated synapse in the DG. The perforation on the PSD is indicated with a red arrowhead. For d and h, the scale bar is 1 μm. Data adapted from Dumitriu et al. (2010) and Hara et al. (2010)
Also in monkey area 46 layers II/III, axon terminals forming axosomatic and axodendritic synapses become larger with age (Soghomonian et al. 2010). In axosomatic axon terminals, the number of synaptic vesicles increases with age and correlates with cognitive impairment as measured by CII (Soghomonian et al. 2010). Thus, enhanced axosomatic GABAergic input to pyramidal neurons in the aged monkey area 46 may be another neuronal mechanism contributing to decreased cognitive performance.
In layer V of area 46, there is an age-related decrease in synapses by 20%, and this loss is due entirely to a loss of asymmetric synapses (Peters et al. 2008). In contrast to findings in layers II/III, synapse density in layer V is not correlated with cognitive measures in monkeys (Peters et al. 2008).
Age-related synaptic change in monkeys is not limited to the PFC region. Studies examining the effects of aging on dendritic trees and spines of layer III pyramidal neurons forming long corticocortical projections from the superior temporal cortex to area 46, for example, have reported a 31% decrease in apical dendritic length, a 43% loss of spines from apical dendrites, and a 27% loss of spines from the basal dendrites (Duan et al. 2003; Kabaso et al. 2009). In layer I of area 17 primary visual cortex, there is also an age-related decrease in synapse density as determined using unbiased methods, but the loss of synapses does not correlate with cognitive impairment (Peters et al. 2001). Together, multiple lines of evidence point to the importance of changes in PFC for cognitive aging.
Hippocampus and related cortical regions: relationships with aging and cognitive decline
Gross anatomy of hippocampal and related cortical regions
Patients with medial temporal lobe lesions exhibit severe memory impairment (Corkin 1984; Zola-Morgan et al. 1986). Moreover, the pattern of memory deficits observed in aged monkeys resembles that of monkeys with medial temporal lobe damage (Rapp and Amaral 1991). These findings have led to many studies in search of structures selectively vulnerable to age-related decline. Particularly, the hippocampus and adjacent cortical areas have been extensively explored.
During normal aging, the volume of the hippocampus (total or any of its subfields) remains stable in rhesus monkeys (Peters et al. 1996; Calhoun et al. 2004; Shamy et al. 2006, 2010; Makris et al. 2010). This is in contrast to a substantial number of MRI studies reporting a reliable age-related decline in hippocampal volume in healthy humans (Kaye et al. 1997; Mueller et al. 1998; Tisserand et al. 2000; Raz et al. 2004; Walhovd et al. 2009). While total hippocampal volume is unaltered with age in rhesus monkeys, it correlates with DR acquisition and average accuracy (Shamy et al. 2010), consistent with literature suggesting that the hippocampus modulates working memory (Friedman and Goldman-Rakic 1988; Eberling et al. 1997).
One of the most vulnerable circuits in normal aging is the perforant path, which originates in layer II of the entorhinal cortex (EC) and terminates in the outer molecular layer (OML) of the DG in rhesus monkeys (Witter et al. 1989; Morrison and Hof 1997; Yassa et al. 2010). The EC receives a convergence of inputs from the polysensory associational regions of the cortex and funnels the highly processed neocortical information to the DG (Squire and Zola-Morgan 1988). The DG is particularly vulnerable to normal aging, and energy metabolism in the DG, but not EC, CA1, or subiculum, is correlated with DNMS accuracy scores (Small et al. 2004).
Neurons of the hippocampus and related cortical regions
When unbiased stereological methods are applied, there are no age-dependent differences in neuronal number or density in the subiculum, CA1, CA2, CA3, DG, and EC in rhesus monkeys (West et al. 1993; Amaral 1993; Rapp 1995; Peters et al. 1996; Gazzaley et al. 1997; Merrill et al. 2000; Keuker et al. 2003). Although based on a small number of monkeys, there is no evidence for a relationship between DNMS scores and neuronal numbers in these regions (West et al. 1993; Rapp 1995).
In humans, however, some studies have shown changes in neuronal numbers with age. Using stereological techniques, West (1993) examined the hippocampus of 32 male human subjects spanning 13 to 85 years old and reported an age-related loss of neurons in the DG hilus and the subiculum. Simic et al. (1997) performed a similar quantitative analysis in 18 human subjects (16–99 years old) and found an age-associated decrease in the subiculum and CA1 neuronal numbers. In human aging studies, however, there is potential inclusion of undiagnosed Alzheimer’s disease patients. Even in very mild stages of Alzheimer’s disease, neurons are lost from the EC (35% overall, 50% in layer II) and CA1 (46%), while no significant decrease in neuronal number is observed in healthy nondemented aged individuals (Price et al. 2001). There is no convincing evidence for a significant loss of neurons from the human hippocampus during the normal, nonpathological, aging process (Peters et al. 1996).
Although there is no overt age-related loss of neurons in the monkey hippocampus, aged monkeys have fewer proliferating cells in the DG subgranular zone, and these cells also exhibit shorter and irregularly oriented dendritic processes (Aizawa et al. 2009, 2011). These studies suggest that aged monkeys have attenuated hippocampal neurogenesis (Aizawa et al. 2009), possibly due to decreased survival of immature neurons (Aizawa et al. 2011). Additionally, preliminary findings point to a potential relationship between the number of proliferating cells in the subgranular zone and learning capacity (Aizawa et al. 2009).
DG granule cells of aged monkeys have reduced vertical dendritic extents and distal dendritic branching and increased proximal dendritic branching, input resistance, and IPSC decay time constant compared to young monkeys (Luebke and Rosene 2003). However, none of these morphological and electrophysiological measures that change with aging correlate with DNMS delay performance or CII (Luebke and Rosene 2003).
Hippocampal synapses
The DG molecular layer consists of the OML and the inner molecular layer (IML), which receive perforant path input from the EC (Witter et al. 1989) and intrinsic projections from the DG hilus (Kondo et al. 2008), respectively. In contrast to the age-related changes observed in dlPFC synapses (Peters et al. 2008; Dumitriu et al. 2010), axospinous synapse density, as measured by the disector method, and postsynaptic density (PSD) length in the monkey DG OML (Fig. 2) and IML are unchanged with aging and fail to correlate with DNMS acquisition or accuracy (Tigges et al. 1996; Hara et al. 2010). Aged monkeys do exhibit lower axodendritic synapse density in the OML, but because axospinous synapses predominate in the DG molecular layer, this age-related decrease constitutes a mere 3% reduction in total synapses (Tigges et al. 1995).
Perforated synapses, morphologically characterized by a discontinuity in the PSD, are large, stable synapses implicated in memory-related plasticity and are proposed to be a structural correlate of enhanced synaptic efficacy (Peters and Kaiserman-Abramof 1969; Greenough et al. 1978; Geinisman et al. 1986, 1991; Toni et al. 2001). While perforated synapse density is unaltered with normal aging in the monkey OML and IML, OML perforated synapse density correlates with recognition accuracy on the delay portion of DNMS (Fig. 2; Hara et al. 2010). Unlike in dlPFC, where the highly plastic thin spines are most tightly linked to variability in cognitive function, large stable perforated synapses appear to be the key morphological correlate of memory capacity in the DG (Fig. 2).
Because no age-related changes were observed in the DG postsynaptic dendritic spines, we sought to determine whether synaptic characteristics of OML axonal boutons are coupled to age (Hara et al. 2011). The frequencies of single-synapse boutons, multiple-synapse boutons (MSBs), and boutons with no apparent synaptic contacts (nonsynaptic boutons, NSBs) in the OML were determined using serial section electron microscopy. MSBs are presynaptic boutons synaptically connected to more than one dendritic spine and potentially act as a morphological substrate to increase coupling between presynaptic and postsynaptic neurons (Harris 1995; Toni et al. 1999; Geinisman et al. 2001; Yankova et al. 2001). MSBs are implicated in structural plasticity and hippocampus-dependent associative learning (Jones et al. 1999; Toni et al. 1999; Geinisman et al. 2001). While there are no age effects on bouton density or average size in the DG OML, the proportion of NSBs is doubled in aged compared to young monkeys (Hara et al. 2011), suggesting that aging results in a lower turnover or higher retraction of spines, leading to a greater proportion of boutons that fail to make or maintain a synaptic contact. Most notably, the proportions of NSBs correlate with both DNMS acquisition and delay accuracy such that individuals with high NSBs are slow to learn the task initially and they exhibit worse memory (Hara et al. 2011). Aged monkeys also display a lower frequency of MSBs and fewer synaptic contacts per MSB. Indeed, the latter parameter inversely correlates with DNMS acquisition rates (trials to criterion) (Hara et al. 2011). Together, these observations suggest that increased NSB density in the OML interferes with both task acquisition and memory, while boutons with many synaptic contacts facilitate learning.
The subiculum is the only other hippocampal brain region where synaptic changes in the context of normal aging have been examined in monkeys. Apical dendrites continue to grow between 7 (young adult) and 20 years old (middle-aged), but very old monkeys (27–28 years old) exhibit markedly shorter dendritic length and branches in both apical and basal dendrites (Uemura 1985a). Although no reliable difference has been detected from young adulthood to middle age, subiculum pyramidal neurons in old subjects display significantly lower spine and synapse density compared to those of young (by 19%) and middle-aged (by 13%) rhesus monkeys (Uemura 1985b).
Molecular markers and mechanisms of aging
Glutamate
Glutamate is the major excitatory neurotransmitter in cortical and hippocampal synapses, and glutamate receptors are key determinants of synaptic strength and plasticity (Nusser et al. 1998; Heynen et al. 2000; Kessels and Malinow 2009). Glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors are the primary mediators of fast excitatory transmission, while N-methyl-d-aspartate (NMDA) receptors play a pivotal role in synaptic plasticity and synaptogenesis (Bliss and Collingridge 1993; Woolley and McEwen 1994; Kessels and Malinow 2009). Both AMPA and NMDA receptors undergo dynamic trafficking into and out of the synapse in a tightly regulated and activity-dependent manner (Carroll and Zukin 2002; Groc and Choquet 2006; Kessels and Malinow 2009). Thus, there is considerable interest in the effects of normal aging on glutamate receptor profiles.
Compared to young adults, aged monkeys exhibit a significant decrease in the expression of the essential NR1 subunit of the NMDA receptor (by 30%), selectively in the DG OML (Gazzaley et al. 1996), where the perforant path input terminates (Witter et al. 1989). In contrast, no age-related changes in the AMPA receptor GluR2/3 subunits or kainate receptor GluR5/6/7 subunits are observed in the monkey DG molecular layer (Gazzaley et al. 1996). Because the number of EC layer II neurons remains unaltered with aging (Gazzaley et al. 1997), the selective decrease in OML NR1 expression is unlikely to be a consequence of perforant path degeneration, but instead may represent a molecular mechanism of aging (Gazzaley et al. 1996).
An in situ hybridization study showed that mRNA for the NR2B subunit of NMDA receptor is decreased with age in the monkey PFC, but not in the subiculum, CA1, CA3, CA4, DG, or the parahippocampal gyrus (Bai et al. 2004). However, a trend for an age-related decrease in NR2B expression exists in the DG granule cell layer and CA3 pyramidal cell layer (Bai et al. 2004).
Aging results in a significant reduction of GluR2 and NR1 expression on neurons that project to area 46 (from the superior temporal sulcus, area 7a, and the contralateral area 46) in monkeys (Hof et al. 2002). Additionally, GluR2 expression on corticocortical neurons within area 46 is also decreased with age in monkeys (Hof et al. 2002). Despite these age-related changes in glutamate receptor subunits, none of them have been studied in behaviorally characterized monkeys, which will be fruitful avenues of research.
Monoamines
Monoamine neurotransmitter systems have complex and complementary roles in the cognitive functions associated with area 46. Area 46 receives dopaminergic fibers from the ventral tegmental area, noradrenergic fibers from the locus coeruleus, and serotonergic fibers from the dorsal raphe nucleus (Porrino and Goldman-Rakic 1982).
Dopamine
Monkeys lose about 56% of dopamine from age 2 to 18 in the PFC, including area 46 (Goldman-Rakic and Brown 1981). Reductions in dopamine can have grave consequences for cognitive function as dopamine depletion in area 46 produces a profound impairment in working memory (Brozoski et al. 1979). Dopamine exerts its effect on working memory through actions at the dopamine D1 receptor (D1R) family. D1R agonists rescue DR performance in aged monkeys with naturally low catecholamines (dopamine and norepinephrine) and also in young monkeys with surgically-depleted catecholamines (Arnsten et al. 1994). At the cellular level, D1R agonists exhibit an inverted U-shaped dose response influence on area 46 neurons such that moderate levels enhance spatial tuning in DR by suppressing responses to nonpreferred directions (reducing “noise”), whereas high levels reduce delay-related firing for all directions (Arnsten 2006; Vijayraghavan et al. 2007).
While the D1R family is critically involved in working memory, the D2/D3 receptors have been implicated in RL. In vervet monkeys, blockade of D2/D3 receptors, but not the D1/D5 receptors, increases the number of errors and trials required to reach criterion in a visual RL task (Lee et al. 2007).
Although an autoradiographic receptor binding study showed that densities of D1R and dopamine transporter are unaltered with age in monkey area 46, D1R binding in PFC positively correlates with object DRST memory span (Moore et al. 2005). Additionally, a significant inverse correlation is seen between dopamine transporter density in the monkey PFC and DNMS acquisition (errors to criterion) (Moore et al. 2005). These relationships are consistent with the critical roles of dopamine in PFC-dependent cognitive tasks. No age-related changes in monoamine content (dopamine, norepinephrine, and serotonin) are observed in the monkey hippocampus (Goldman-Rakic and Brown 1981), suggesting that the role of monoamines in cognition may be more prominent in the PFC than in the hippocampus.
Norepinephrine
Cognitive functions subserved by the PFC are thought to be modulated in part by norepinephrine, which is implicated in reducing interference and improving attention during cognitive tasks (Arnsten and Contant 1992; Arnsten et al. 1998; Arnsten 2006). Norepinephrine exerts its influence on cognition through actions at α1- (α1-AR) and α2-adrenergic receptors (α2-AR), which impair and improve working memory, respectively (Arnsten et al. 1998; Arnsten 2006).
The α2-AR agonist, clonidine, improves DR performance in surgically intact aged monkeys with naturally occurring decline in norepinephrine as well as in young monkeys with experimentally induced norepinephrine depletion within the principal sulcus (including area 46) (Arnsten and Goldman-Rakic 1985). Clonidine’s potency in restoring DR performance in young monkeys with norepinephrine depletion is directly related to the degree of depletion in this region (Arnsten and Goldman-Rakic 1985). Clonidine fails to rescue DR performance in monkeys with principal sulcus ablations, suggesting that the dlPFC is one of the key sites of action for the cognitive-enhancing effects of α2-AR agonists (Arnsten and Goldman-Rakic 1985). At the cellular level, stimulation of α2-AR in area 46 increases firing of spatially-tuned neurons during the delay period of DR, hence increasing “signals” in the PFC (Li et al. 1999; Arnsten 2006). Activation of α2-AR also significantly improves object discrimination RL (Steere and Arnsten 1997) and modestly improves DNMS performance (Arnsten and Goldman-Rakic 1990) in aged rhesus monkeys.
Catecholamine biosynthesis, as measured by l-DOPA, is reduced by more than 60% in the PFC of aged rhesus monkeys (Goldman-Rakic and Brown 1981). Autoradiographic receptor binding studies have shown that in the superficial layers (I–III) of monkey area 46, α1- and α2-AR binding decreases with age, whereas densities of β-AR and norepinephrine transporter are unchanged (Bigham and Lidow 1995; Moore et al. 2005). The density of α1-AR binding in the monkey PFC correlates with DNMS acquisition impairment (errors to criterion) and perseverative responding on CSST (Moore et al. 2005). Significant inverse correlations are also observed between α2-AR binding density and total and perseverative errors on CSST (Moore et al. 2005), consistent with a procognitive benefit of α2-AR activation (Arnsten and Goldman-Rakic 1985). While converging evidence suggests that norepinephrine improves cognitive function through actions at postsynaptic α2-ARs (Aoki et al. 1998; Arnsten and Li 2005), the critical synaptic subtypes involved have yet to be determined.
Serotonin
Serotonin (5-HT) dysregulation in the PFC is implicated in cognitive inflexibility. In marmosets, 5-HT depletion within the orbitofrontal cortex produces increased perseverative responding in a serial discrimination RL paradigm (Clarke et al. 2004, 2007), while leaving set-shifting abilities intact (Clarke et al. 2005). In contrast to the marked effect of dopamine, 5-HT depletion in area 46 has no effect on working memory in monkeys (Brozoski et al. 1979).
While age-related reductions in 5-HT have been observed in the PFC frontal pole (Wenk et al. 1989), the motor cortex (Goldman-Rakic and Brown 1981), and the occipital pole (Beal et al. 1991), no significant age effects are seen in the PFC (as a whole), the temporal cortex, or the hippocampus of rhesus monkeys (Goldman-Rakic and Brown 1981). With age, 5-HT1 receptor binding density decreases in monkey somatosensory cortex (area 1), but not in area 46, area 4 (motor cortex), or area 17 (visual cortex) (Bigham and Lidow 1995). In addition, 5-HT2 receptor binding sites decrease with age in the frontal pole and motor, parietal, and occipital cortices (Wenk et al. 1989; Bigham and Lidow 1995). At very old ages (28–37 years old), a trend for a reduction in 5-HT2 receptor binding density is also seen in the monkey dlPFC (Wenk et al. 1989). More research is needed to understand whether age-related changes in the 5-HT system are associated with cognitive decline or occur independently from it.
Acetylcholine
The basal forebrain cholinergic system has been widely explored in aging and Alzheimer’s disease studies. One of the earliest monkey studies assessing age effects on the cholinergic system examined the levels of choline acetyl-transferase (ChAT; the rate-limiting enzyme for acetylcholine synthesis) in cortical regions. While ChAT levels are stable across age in most cortical areas, an age-related reduction is observed in the PFC frontal pole (Wenk et al. 1989).
The nucleus basalis of Meynert in the basal forebrain is the major source of cholinergic input to the cerebral cortex (Mesulam et al. 1983; Saper 1984). Nucleus basalis cholinergic neurons increase in size with age in rhesus monkeys (Voytko et al. 1995). Additionally, aging results in a 50% decrease in the number of cholinergic neurons in the intermediate nucleus basalis (which projects to area 8a among other regions) and a 23% decrease in the cholinergic axon density within area 8a (Mesulam et al. 1983; Smith et al. 2004). Controlling for age, DR delay performance correlates with cholinergic neuronal number in this region of the nucleus basalis (Voytko et al. 1995). Conversely, no age effects are evident in the number of anterior nucleus basalis cholinergic neurons (that innervate area 46 among other regions) or in the cholinergic axon density within area 46 (Smith et al. 2004).
The neurons in the medial septal nucleus provide the major cholinergic input to the hippocampal formation (Mesulam et al. 1983). In aged monkeys, there is a significant loss of these cholinergic neurons, especially in the caudal region where the loss amounts to 41% (Stroessner-Johnson et al. 1992). The degree of neuronal loss, however, is similar between aged subjects impaired on the DNMS test and those that are not (Stroessner-Johnson et al. 1992). In contrast, rostral levels of the medial septal nucleus show minimal neuronal loss, but the mean size of cholinergic neurons is significantly larger in aged subjects with impaired DNMS performance compared to young and aged unimpaired monkeys (Stroessner-Johnson et al. 1992).
More recently, vesicular acetylcholine transporter immunolabeling and an unbiased stereological technique were used to estimate total cholinergic fiber length in hippocampal subregions of behaviorally characterized young and aged rhesus monkeys (Calhoun et al. 2004). In the DG, CA2/3, and CA1, the length of cholinergic fibers decreases with age, but this age-related decline does not correlate with recognition memory deficits as measured by DNMS scores (Calhoun et al. 2004). Despite converging evidence for age-dependent vulnerability of the cholinergic system, the decline in hippocampal cholinergic innervation is not sufficient to account for DNMS impairment (Calhoun et al. 2004). Thus, the functional and cognitive consequences of these morphological changes remain to be defined.
Estrogen
Women undergoing the menopausal transition report worsening of memory. It is becoming increasingly evident that there is a strong interaction between age-related cognitive decline and endocrine changes (Hao et al. 2007; Henderson 2008). Female macaque monkeys are valuable models of menopause because they share many reproductive and endocrine features with women and also undergo menopause in a similar manner to women (Gilardi et al. 1997; Walker and Herndon 2008).
Interestingly, the menopause-related cognitive impairment seen in women is also evident in female nonhuman primates. For example, peri-/post-menopausal female monkeys show significant impairments in DR and DNMS performance compared to premenopausal monkeys after accounting for the effect of age (Roberts et al. 1997; Hara et al. 2010).
While cognitive deficits associated with menopause in women are multifactorial in origin, estrogen deficiency is thought to be one of the culprits. Some of these effects are attenuated by hormone replacement therapy in nonhuman primate models of menopause. For example, in aged ovariectomized female monkeys, cyclic estradiol replacement consisting of a single injection every 3 weeks (closely mimicking the natural fluctuations of estrogen in premenopausal monkeys) reverses the age-related impairment in DR and modestly improves DNMS scores (Rapp et al. 2003). Estrogen replacement also improves CSST performance in middle-aged ovariectomized monkeys (Voytko et al. 2009).
Improvements in cognitive function with cyclic estradiol treatment occur concomitantly with alterations in area 46 neuronal morphology. We have examined the effect of estrogen on area 46 pyramidal neurons in a cohort of young and aged ovariectomized female monkeys receiving cyclic estradiol or vehicle treatments (Hao et al. 2007). In the DR test, young monkeys receiving estradiol or vehicle and aged monkeys receiving estradiol perform equally well (Hao et al. 2007). Reconstructions of pyramidal neurons in PFC area 46 showed that estradiol increases the density of the highly plastic and motile small spines in both young and aged monkeys (Hao et al. 2007). Although young vehicle-treated monkeys exhibit lower spine density, their dendritic length and branch numbers are increased such that the total number of spines per neuron is equivalent to the young estradiol-treated group (Hao et al. 2007). This dendritic expansion may be a compensatory mechanism that protects cognitive function in young monkeys deprived of estrogen. In contrast, only vehicle-treated aged monkeys exhibit significant DR impairment, and this deficit occurs concomitantly with a significant decrease in area 46 layer III spine density in the absence of a compensatory extension of dendrites (Hao et al. 2007).
We have begun to probe the molecular mechanisms underlying the beneficial effects of estrogen on cognition in aged monkeys. The synaptic distribution of estrogen receptor α (ERα) in area 46 has been examined with electron microscopy in the same behaviorally characterized young and aged ovariectomized monkeys (Wang et al. 2010). While ERα levels remain stable across age and hormone treatment groups, the abundance of ERα within the PSD correlates with DR delay performance exclusively in aged monkeys receiving cyclic estradiol (Wang et al. 2010). Hence, the cognitive benefits observed in aged monkeys with estradiol may be mediated by the activation of ERα in a synaptic domain coupled to signaling cascades involved in spine/synapse formation and stabilization (Spencer et al. 2008).
Plaques and Tangles
Aged monkeys do not express the full histopathological profile characteristic of Alzheimer’s disease. For example, neurofibrillary tangles do not occur in the aged monkey neocortex (Kimura et al. 2003). Although amyloid plaques are frequently observed, in contrast to Alzheimer’s disease, the ratio of amyloid β peptide 40 and 42 in the aged monkeys favors the shorter, less pathogenic form (Gearing et al. 1996). While the accumulation of amyloid β plaques increases after age 25 in rhesus monkeys, the plaque content does not correlate with cognitive dysfunction, as measured by a standardized score derived from DNMS, spatial/color DRST, and spatial/object RL (Sloane et al. 1997). The number of amyloid β plaques also fails to correlate with DR performance (Cork 1993). Therefore, aged monkeys provide a valuable model for normal aging devoid of these signatures of Alzheimer’s disease that reflect pathological aging.
Conclusions
The anatomical, neuronal, synaptic, and molecular findings in the context of aging and/or cognitive decline in the monkey PFC and hippocampus are summarized, respectively, in Tables 1 and 2. While the number of subjects available for analysis is typically limited in nonhuman primate studies and the presence or lack of correlations should be interpreted with caution, the findings provide important clues for identifying the mechanisms involved in age-related cognitive decline.
Table 1.
Anatomical, neuronal, synaptic, and molecular parameters in the monkey prefrontal cortex in the context of chronological and cognitive aging
| Brain area | Measure | Change with aging | Correlation with behavior | Citation |
|---|---|---|---|---|
| PFC | Volume | No change | DNMS accuracy | Shamy et al. 2010 |
| Amyloid plaque | ↑ | Not significant (DR, DNMS, DRST, RL) | Cork 1993; Sloane et al. 1997 | |
| Dopamine | ↓ by 56% | n/a | Goldman-Rakic and Brown 1981 | |
| Norepinephrine | No change | n/a | Goldman-Rakic and Brown 1981 | |
| Catecholamine biosynthesis | ↓ by 60% | n/a | Goldman-Rakic and Brown 1981 | |
| Serotonin | ↓/no change | n/a | Goldman-Rakic and Brown 1981 | |
| 5HT2 R binding | ↓ | n/a | Wenk et al. 1989 | |
| ChAT activity | ↓ | n/a | Wenk et al. 1989 | |
| NR2B mRNA | ↓ | n/a | Bai et al. 2004 | |
| Area 46 (dlPFC) | Volume | ↓ by 10% | DNMS acquisition, accuracy | Shamy et al. 2010 |
| Neuron number | No change | Not significant (DNMS, DRST) | Peters et al. 1994; Smith et al. 2004 | |
| Lipofuscin | ↑ | n/a | Peters et al. 1994 | |
| Dopamine D1 R binding | No change | object DRST | Moore et al. 2005 | |
| Dopamine transporter | No change | DNMS acquisition | Moore et al. 2005 | |
| Norepinephrine transporter | No change | Not significant (DNMS, DRST, CSST) | Moore et al. 2005 | |
| α-1 Adrenergic R binding | ↓ | CSST perseveration, DNMS acquisition | Moore et al. 2005 | |
| α-2 Adrenergic R binding | ↓ | CSST errors, perseveration | Moore et al. 2005 | |
| β Adrenergic R binding | No change | n/a | Bigham and Lidow 1995 | |
| 5HT1, 5HT2 R binding | No change | n/a | Bigham and Lidow 1995 | |
| Cholinergic axon density | No change | n/a | Smith et al. 2004 | |
| Area 46 layer I | Volume | No change | Not significant (DR) | O’Donnell et al. 1999 |
| Layer thickness | ↓ | DNMS accuracy | Peters et al. 1998a | |
| # of dendritic profiles | ↓ | Not significant (DNMS, DRST, CII) | Peters et al. 1998a | |
| Synapse density | ↓ by 30–60% | DNMS acquisition, accuracy, spatial DRST, and CII | Peters et al. 1998a | |
| Axons and axon terminals | No change | Not significant (DNMS, DRST, CII) | Peters et al. 1998a | |
| α-2 Adrenergic R binding | ↓ | n/a | Bigham and Lidow 1995 | |
| Area 46 layers II/III | Vacuoles and membrane whorls in dendrites | ↑ | n/a | Peters et al. 1994 |
| Asymmetric synapse density | ↓ by 30% | DNMS acquisition, CII | Peters et al. 2008; Dumitriu et al. 2010 | |
| Symmetric synapse density | ↓ by 30% | DNMS acquisition | Peters et al. 2008 | |
| Axosomatic terminal size | ↑ | Not significant (CII) | Soghomonian et al. 2010 | |
| Axodendritic terminal size | ↑ | Not significant (CII) | Soghomonian et al. 2010 | |
| Axosomatic terminal vesicles | ↑ by 20% | CII | Soghomonian et al. 2010 | |
| AP firing rate | ↑ | U-shaped relationship with DNMS, DRST, and CII | Chang et al. 2005 | |
| Input resistance | ↑ | U-shaped relationship with DNMS, DRST, and CII | Chang et al. 2005 | |
| AP amplitude, fall time | ↓ | Not significant (DNMS, DRST, CII) | Chang et al. 2005 | |
| Slow AHP amplitude | ↑ | DNMS acquisition, CII | Luebke and Amatrudo 2010 | |
| Spontaneous EPSC frequency | ↓ | Not significant (DNMS, DRST, CII, CSST) | Luebke et al. 2004 | |
| Spontaneous IPSC frequency | ↑ | Not significant (DNMS, DRST, CII, CSST) | Luebke et al. 2004 | |
| Area 46 layer III | Microcolumn strength | ↓ | DNMS accuracy, spatial DRST, CII | Cruz et al. 2004 |
| Dendritic spine head volume | ↑ by 27% | DNMS acquisition | Dumitriu et al. 2010 | |
| Thin spine density | ↓ by 46% | DNMS acquisition | Dumitriu et al. 2010 | |
| Mushroom spine density, size | No change | Not significant (DNMS, DR) | Dumitriu et al. 2010 | |
| GluR2 expression | ↓ | n/a | Hof et al. 2002 | |
| Area 46 layer V | Synapse density | ↓ by 20% | Not significant (DNMS, DRST, CII) | Peters et al. 2008 |
| Single AP amplitude, duration, fall time | ↓ | Not significant (DNMS, DRST) | Luebke and Chang 2007 | |
| Slow AHP amplitude | No change | Not significant (DNMS, DRST, CII) | Luebke and Amatrudo 2010 | |
| Areas 9, 8B (SFG) | Volume | No change | DNMS accuracy | Shamy et al. 2010 |
| Area 9 layers III/IV | Dendritic length, branches | ↑ until middle-age, then ↓ | n/a | Cupp and Uemura 1980 |
| Synapse density | ↓ | n/a | Uemura 1980 | |
| Area 8A | Volume | No change | n/a | Smith et al. 2004 |
| Neuron number | ↓ by 32% | DR accuracy | Smith et al. 2004 | |
| Cholinergic axon density | ↓ by 23% | n/a | Smith et al. 2004 | |
| Areas 24, 32 (ACC) | Volume | ↓ | DNMS acquisition | Shamy et al. 2010 |
| Areas 12, 45, 47 (IFG) | Volume | No change | DNMS acquisition | Shamy et al. 2010 |
| Areas 11, 13, 14 (vmPFC) | Volume | No change | DNMS accuracy | Shamy et al. 2010 |
AHP afterhyperpolarization, ACC anterior cingulate cortex, AP action potential, ChAT choline acetyl-transferase, CII Cognitive Impairment Index, CSST conceptual set-shifting task, dlPFC dorsolateral prefrontal cortex, DNMS delayed nonmatching-to-sample, DR delayed response, DRST delayed recognition span test, EPSC excitatory postsynaptic current, IFG inferior frontal gyrus, IPSC inhibitory postsynaptic current, PFC prefrontal cortex, R receptor, RL reversal learning, SFG superior frontal gyrus, vmPFC ventromedial prefrontal cortex
Table 2.
Anatomical, neuronal, synaptic, and molecular parameters in the monkey hippocampus and related cortical regions in the context of chronological and cognitive aging
| Brain area | Measure | Change with aging | Correlation with behavior | Citation |
|---|---|---|---|---|
| Hippocampus | Total volume | No change | DR acquisition, accuracy | Shamy et al. 2010 |
| Glucose metabolism | No change | DR accuracy | Eberling et al. 1997 | |
| Dopamine | No change | n/a | Goldman-Rakic and Brown 1981 | |
| Norepinephrine | No change | n/a | Goldman-Rakic and Brown 1981 | |
| Serotonin | No change | n/a | Goldman-Rakic and Brown 1981 | |
| DG | Volume | No change | n/a | Calhoun et al. 2004 |
| Neuron number | No change | n/a | West et al. 1993; Keuker et al. 2003 | |
| Cerebral blood volume | ↓ | DNMS accuracy | Small et al. 2004 | |
| Vertical dendritic extent | ↓ | Not significant (DNMS, CPI) | Luebke and Rosene 2003 | |
| Dendritic branching | ↓ distal, ↑ proximal | Not significant (DNMS, CPI) | Luebke and Rosene 2003 | |
| Input resistance | ↑ | Not significant (DNMS, CPI) | Luebke and Rosene 2003 | |
| IPSC decay time constant | ↑ | Not significant (DNMS, CPI) | Luebke and Rosene 2003 | |
| NR2B mRNA | No change (trend for ↓) | n/a | Bai et al. 2004 | |
| Cholinergic fiber length | ↓ by 27% | Not significant (DNMS) | Calhoun et al. 2004 | |
| DG OML | Axospinous synapse density | No change | Not significant (DNMS) | Hara et al. 2010 |
| Axodendritic synapse density | ↓ | n/a | Tigges et al. 1995 | |
| PSD length | No change | Not significant (DNMS) | Hara et al. 2010 | |
| Perforated synapse density | No change | DNMS accuracy | Hara et al. 2010 | |
| Axon terminal size | No change | Not significant (DNMS) | Hara et al. 2011 | |
| Nonsynaptic bouton | ↑ | DNMS acquisition, accuracy | Hara et al. 2011 | |
| Multiple-synaptic bouton | ↓ | DNMS acquisition | Hara et al. 2011 | |
| NR1 expression | ↓ by 30% | n/a | Gazzaley et al. 1996 | |
| GluR2/3, GluR5/6/7 expression | No change | n/a | Gazzaley et al. 1996 | |
| DG IML | Synapse density | No change | Not significant (DNMS) | Hara et al. 2010 |
| PSD length | No change | Not significant (DNMS) | Hara et al. 2010 | |
| Perforated synapse density | No change | Not significant (DNMS) | Hara et al. 2010 | |
| Axon terminal size | No change | n/a | Tigges et al. 1996 | |
| DG SGZ | Proliferating cells | ↓ | Visual pattern discrimination | Aizawa et al. 2009 |
| CA3 | Volume | No change | n/a | Calhoun et al. 2004 |
| Neuron number | No change | n/a | West et al. 1993; Keuker et al. 2003 | |
| NR2B mRNA | No change (trend for ↓) | n/a | Bai et al. 2004 | |
| CA2/3-hilus | Cholinergic fiber length | ↓ by 18% | Not significant (DNMS) | Calhoun et al. 2004 |
| CA1 | Volume | No change | n/a | Calhoun et al. 2004 |
| Neuron number | No change | n/a | West et al. 1993; Keuker et al. 2003 | |
| Cerebral blood volume | No change | Not significant (DNMS) | Small et al. 2004 | |
| NR2B mRNA | No change | n/a | Bai et al. 2004 | |
| Cholinergic fiber length | ↓ by 26% | Not significant (DNMS) | Calhoun et al. 2004 | |
| Subiculum | Cerebral blood volume | No change | Not significant (DNMS) | Small et al. 2004 |
| Neuron number | No change | n/a | West et al. 1993; Keuker et al. 2003 | |
| Dendritic length, branches | ↑ until middle aged, then ↓ | n/a | Uemura 1985a | |
| Synapse density | ↓ by 19% | n/a | Uemura 1985b | |
| NR2B mRNA | No change | n/a | Bai et al. 2004 | |
| EC | Neuron number | No change | n/a | Merrill et al. 2000 |
| Cerebral blood volume | No change | Not significant (DNMS) | Small et al. 2004 | |
| EC layer II | Neuron number | No change | n/a | Gazzaley et al. 1997; Merrill et al. 2000 |
| Neuronal size | No change | n/a | Gazzaley et al. 1997; Merrill et al. 2000 | |
| Parahippocampal gyrus | NR2B mRNA | No change | n/a | Bai et al. 2004 |
CPI Cognitive Performance Index, DG dentate gyrus, DNMS delayed nonmatching-to-sample, DR delayed response, EC entorhinal cortex, IML inner molecular layer, IPSC inhibitory postsynaptic current, OML outer molecular layer, PSD postsynaptic density, SGZ subgranular zone
Some parameters are altered with aging, but are not associated with cognitive decline. For example in area 46, aging leads to a decreased number of dendritic profiles, increased axon terminal size, reduced action potential amplitude, and changes in spontaneous EPSC and IPSC frequencies. Other measures remain stable with aging but correlate with behavioral scores. These include cortical volumes (PFC, SFG, IFG, and vmPFC), hippocampal volume and metabolism, and densities of D1R and dopamine transporter in area 46. Perhaps the most intriguing are parameters that are coupled to both chronological and cognitive aging. Some examples of these within area 46 include the total volume, microcolumn strength, synapse density, action potential firing rate, input resistance, and α1- and α2-adrenergic receptor binding densities. Examples from other brain regions include ACC volume, DG metabolism, and the frequency of NSBs in the DG.
Importantly, the critical synaptic subtypes coupled to cognitive decline appear to be different across brain regions and dependent on the types of cognitive function. For example, the dendritic spine subtype most tightly coupled to rule learning in the DNMS task and vulnerable to aging in the dlPFC area 46 is the thin spine, whereas in the DG, the density of large mushroom spines with perforated synapses correlates with DNMS memory performance (Dumitriu et al. 2010; Hara et al. 2010). These findings are consistent with observations that scores across multiple cognitive tests do not correlate with one another (Lai et al. 1995), suggesting that different cognitive domains age independently, mediated by distinct mechanisms.
Molecular targets explored thus far in the context of aging and cognition in monkeys have been studied primarily in light microscopy and autoradiography with little or no subcellular resolution. Electron microscopic studies on synaptic distributions of proteins in behaviorally characterized monkeys are underway and should help elucidate the molecular mechanisms underlying age-related cognitive decline.
Acknowledgments
Original research and manuscript preparation was supported by National Institute on Aging grants R37 AG06647, R01 AG010606, P01 AG16765 to J.H.M. and in part by the Intramural Research Program of the National Institute on Aging. This manuscript was prepared while Y.H. was an Ellison Medical Foundation/AFAR Postdoctoral Fellow. We thank Erik Bloss for critical discussion and helpful comments on the manuscript.
References
- Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim. 2009;58(4):403–407. doi: 10.1538/expanim.58.403. [DOI] [PubMed] [Google Scholar]
- Aizawa K, Ageyama N, Terao K, Hisatsune T. Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiol Aging. 2011;32(1):140–150. doi: 10.1016/j.neurobiolaging.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Zola-Morgan S, Squire LR. Damage limited to the hippocampal region produces long-lasting memory impairment in monkeys. J Neurosci. 1995;15(5 Pt 2):3796–3807. doi: 10.1523/JNEUROSCI.15-05-03796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. Morphological analyses of the brains of behaviorally characterized aged nonhuman primates. Neurobiol Aging. 1993;14(6):671–672. doi: 10.1016/0197-4580(93)90066-k. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go CG, Forman R, Kurose H. Cellular and subcellular sites for noradrenergic action in the monkey dorsolateral prefrontal cortex as revealed by the immunocytochemical localization of noradrenergic receptors and axons. Cereb Cortex. 1998;8(3):269–277. doi: 10.1093/cercor/8.3.269. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Fundamentals of attention-deficit/hyperactivity disorder: circuits and pathways. J Clin Psychiatry. 2006;67(Suppl 8):7–12. [PubMed] [Google Scholar]
- Arnsten AF, Contant TA. Alpha-2 adrenergic agonists decrease distractibility in aged monkeys performing the delayed response task. Psychopharmacology (Berl) 1992;108(1–2):159–169. doi: 10.1007/BF02245302. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230(4731):1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. Analysis of alpha-2 adrenergic agonist effects on the delayed nonmatch-to-sample performance of aged rhesus monkeys. Neurobiol Aging. 1990;11(6):583–590. doi: 10.1016/0197-4580(90)90021-q. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57(11):1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116(2):143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Steere JC, Jentsch DJ, Li BM. Noradrenergic influences on prefrontal cortical cognitive function: opposing actions at postjunctional alpha 1 versus alpha 2-adrenergic receptors. Adv Pharmacol. 1998;42:764–767. doi: 10.1016/s1054-3589(08)60859-5. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20(3):249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, Cork LC. Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiol Aging. 1991;12(2):99–111. doi: 10.1016/0197-4580(91)90048-o. [DOI] [PubMed] [Google Scholar]
- Bai L, Hof PR, Standaert DG, Xing Y, Nelson SE, Young AB, Magnusson KR. Changes in the expression of the NR2B subunit during aging in macaque monkeys. Neurobiol Aging. 2004;25(2):201–208. doi: 10.1016/s0197-4580(03)00091-5. [DOI] [PubMed] [Google Scholar]
- Barbas H, Rempel-Clower N. Cortical structure predicts the pattern of corticocortical connections. Cereb Cortex. 1997;7(7):635–646. doi: 10.1093/cercor/7.7.635. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Fleming D, Johnson HR. Aging in the rhesus monkey: debilitating effects on short-term memory. J Gerontol. 1978;33(6):858–871. doi: 10.1093/geronj/33.6.858. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Fleming DL. Aging in the rhesus monkey: effects on visual discrimination learning and reversal learning. J Gerontol. 1979;34(2):209–219. doi: 10.1093/geronj/34.2.209. [DOI] [PubMed] [Google Scholar]
- Beal MF, Walker LC, Storey E, Segar L, Price DL, Cork LC. Neurotransmitters in neocortex of aged rhesus monkeys. Neurobiol Aging. 1991;12(5):407–412. doi: 10.1016/0197-4580(91)90065-r. [DOI] [PubMed] [Google Scholar]
- Beason-Held LL, Rosene DL, Killiany RJ, Moss MB. Hippocampal formation lesions produce memory impairment in the rhesus monkey. Hippocampus. 1999;9(5):562–574. doi: 10.1002/(SICI)1098-1063(1999)9:5<562::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Bigham MH, Lidow MS. Adrenergic and serotonergic receptors in aged monkey neocortex. Neurobiol Aging. 1995;16(1):91–104. doi: 10.1016/0197-4580(95)80012-g. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205(4409):929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Buckmaster CA, Eichenbaum H, Amaral DG, Suzuki WA, Rapp PR. Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. J Neurosci. 2004;24(44):9811–9825. doi: 10.1523/JNEUROSCI.1532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun ME, Mao Y, Roberts JA, Rapp PR. Reduction in hippocampal cholinergic innervation is unrelated to recognition memory impairment in aged rhesus monkeys. J Comp Neurol. 2004;475(2):238–246. doi: 10.1002/cne.20181. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Zukin RS. NMDA-receptor trafficking and targeting: implications for synaptic transmission and plasticity. Trends Neurosci. 2002;25(11):571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex. 2005;15(4):409–418. doi: 10.1093/cercor/bhh144. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304(5672):878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Crofts HS, Dalley JW, Robbins TW, Roberts AC. Prefrontal serotonin depletion affects reversal learning but not attentional set shifting. J Neurosci. 2005;25(2):532–538. doi: 10.1523/JNEUROSCI.3690-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17(1):18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Cork LC. Plaques in prefrontal cortex of aged, behaviorally-tested rhesus monkeys: incidence, distribution, and relationship to task performance. Neurobiol Aging. 1993;14(6):675–676. doi: 10.1016/0197-4580(93)90068-m. [DOI] [PubMed] [Google Scholar]
- Corkin S. Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in H.M. Semin Neurol. 1984;4:249–259. [Google Scholar]
- Cruz L, Roe DL, Urbanc B, Cabral H, Stanley HE, Rosene DL. Age-related reduction in microcolumnar structure in area 46 of the rhesus monkey correlates with behavioral decline. Proc Natl Acad Sci USA. 2004;101(45):15846–15851. doi: 10.1073/pnas.0407002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp CJ, Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: quantitative analysis of dendritic branching patterns. Exp Neurol. 1980;69(1):143–163. doi: 10.1016/0014-4886(80)90150-8. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110(5):872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63(2):184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13(9):950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30(22):7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Roberts JA, Rapp PR, Tuszynski MH, Jagust WJ. Cerebral glucose metabolism and memory in aged rhesus macaques. Neurobiol Aging. 1997;18(4):437–443. doi: 10.1016/s0197-4580(97)00040-7. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126(Pt 8):1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Friedman HR, Goldman-Rakic PS. Activation of the hippocampus and dentate gyrus by working-memory: a 2-deoxyglucose study of behaving rhesus monkeys. J Neurosci. 1988;8(12):4693–4706. doi: 10.1523/JNEUROSCI.08-12-04693.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristoe NM, Salthouse TA, Woodard JL. Examination of age-related deficits on the Wisconsin Card Sorting Test. Neuropsychology. 1997;11(3):428–436. doi: 10.1037//0894-4105.11.3.428. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci. 1993;13(4):1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley AH, Siegel SJ, Kordower JH, Mufson EJ, Morrison JH. Circuit-specific alterations of N-methyl-D-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc Natl Acad Sci USA. 1996;93(7):3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley AH, Thakker MM, Hof PR, Morrison JH. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol Aging. 1997;18(5):549–553. doi: 10.1016/s0197-4580(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Gearing M, Tigges J, Mori H, Mirra SS. A beta40 is a major form of beta-amyloid in nonhuman primates. Neurobiol Aging. 1996;17(6):903–908. doi: 10.1016/s0197-4580(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Toledo-Morrell L, Morrell F. Aged rats need a preserved complement of perforated axospinous synapses per hippocampal neuron to maintain good spatial memory. Brain Res. 1986;398(2):266–275. doi: 10.1016/0006-8993(86)91486-1. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991;566(1–2):77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Berry RW, Disterhoft JF, Power JM, Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci. 2001;21(15):5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57(2):335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14(3):477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6(2):177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Greenough WT, West RW, DeVoogd TJ. Subsynaptic plate perforations: changes with age and experience in the rat. Science. 1978;202(4372):1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking: multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326(2):423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Vranes LF, Goodwin JS, Garry PJ. Wisconsin Card Sort Test performance in a healthy elderly population. J Gerontol. 1987;42(3):345–346. doi: 10.1093/geronj/42.3.345. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2007;104(27):11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Roberts MT, Morrison JH, Rapp PR (2010) Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2010.09.014 [DOI] [PMC free article] [PubMed]
- Hara Y, Park CS, Janssen WG, Punsoni M, Rapp PR, Morrison JH. Synaptic characteristics of dentate gyrus axonal boutons and their relationships with aging, menopause, and memory in female rhesus monkeys. J Neurosci. 2011;31(21):7737–7744. doi: 10.1523/JNEUROSCI.0822-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM. How multiple-synapse boutons could preserve input specificity during an interneuronal spread of LTP. Trends Neurosci. 1995;18(8):365–369. doi: 10.1016/0166-2236(95)93930-v. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Cognitive changes after menopause: influence of estrogen. Clin Obstet Gynecol. 2008;51(3):618–626. doi: 10.1097/GRF.0b013e318180ba10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, Quinlan EM, Bae DC, Bear MF. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron. 2000;28(2):527–536. doi: 10.1016/s0896-6273(00)00130-6. [DOI] [PubMed] [Google Scholar]
- Hof PR, Nimchinsky EA, Young WG, Morrison JH. Numbers of Meynert and layer IVB cells in area V1: a stereologic analysis in young and aged macaque monkeys. J Comp Neurol. 2000;420(1):113–126. doi: 10.1002/(sici)1096-9861(20000424)420:1<113::aid-cne8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Hof PR, Duan H, Page TL, Einstein M, Wicinski B, He Y, Erwin JM, Morrison JH. Age-related changes in GluR2 and NMDAR1 glutamate receptor subunit protein immunoreactivity in corticocortically projecting neurons in macaque and patas monkeys. Brain Res. 2002;928(1–2):175–186. doi: 10.1016/s0006-8993(01)03345-5. [DOI] [PubMed] [Google Scholar]
- Inouye SK, Albert MS, Mohs R, Sun K, Berkman LF. Cognitive performance in a high-functioning community-dwelling elderly population. J Gerontol. 1993;48(4):M146–M151. doi: 10.1093/geronj/48.4.m146. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11(4):376–386. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19(22):10153–10163. doi: 10.1523/JNEUROSCI.19-22-10153.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabaso D, Coskren PJ, Henry BI, Hof PR, Wearne SL. The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging. Cereb Cortex. 2009;19(10):2248–2268. doi: 10.1093/cercor/bhn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure–stability–function relationships of dendritic spines. Trends Neurosci. 2003;26(7):360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kaye JA, Swihart T, Howieson D, Dame A, Moore MM, Karnos T, Camicioli R, Ball M, Oken B, Sexton G. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48(5):1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61(3):340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24(1):157–165. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Kimura N, Tanemura K, Nakamura S, Takashima A, Ono F, Sakakibara I, Ishii Y, Kyuwa S, Yoshikawa Y. Age-related changes of Alzheimer’s disease-associated proteins in cynomolgus monkey brains. Biochem Biophys Res Commun. 2003;310(2):303–311. doi: 10.1016/j.bbrc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Kondo H, Lavenex P, Amaral DG. Intrinsic connections of the macaque monkey hippocampal formation: I. Dentate gyrus. J Comp Neurol. 2008;511(4):497–520. doi: 10.1002/cne.21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol Aging. 1995;16(6):947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32(10):2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Li BM, Mao ZM, Wang M, Mei ZT. Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology. 1999;21(5):601–610. doi: 10.1016/S0893-133X(99)00070-6. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Amatrudo JM (2010) Age-related increase of sI(AHP) in prefrontal pyramidal cells of monkeys: relationship to cognition. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2010.07.002 [DOI] [PMC free article] [PubMed]
- Luebke JI, Chang YM. Effects of aging on the electrophysiological properties of layer 5 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2007;150(3):556–562. doi: 10.1016/j.neuroscience.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke JI, Rosene DL. Aging alters dendritic morphology, input resistance, and inhibitory signaling in dentate granule cells of the rhesus monkey. J Comp Neurol. 2003;460(4):573–584. doi: 10.1002/cne.10668. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125(1):277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Luebke J, Barbas H, Peters A. Effects of normal aging on prefrontal area 46 in the rhesus monkey. Brain Res Rev. 2010;62(2):212–232. doi: 10.1016/j.brainresrev.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, Boriel DL, Rosene DL (2010) Methods of MRI-based structural imaging in the aging monkey. Methods 50(3):166–177. doi:10.1016/j.ymeth.2009.06.007 [DOI] [PMC free article] [PubMed]
- McEnaney KW, Butter CM. Perseveration of responding and nonresponding in monkeys with orbital frontal ablations. J Comp Physiol Psychol. 1969;68(4):558–561. doi: 10.1037/h0027639. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Roberts JA, Tuszynski MH. Conservation of neuron number and size in entorhinal cortex layers II, III, and V/VI of aged primates. J Comp Neurol. 2000;422(3):396–401. doi: 10.1002/1096-9861(20000703)422:3<396::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214(2):170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Herndon JG, Luebke JI, Moss MB, Rosene DL. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behav Brain Res. 2005;160(2):208–221. doi: 10.1016/j.bbr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging. 2006;27(10):1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278(5337):412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Moss MB, Albert MS, Butters N, Payne M. Differential patterns of memory loss among patients with Alzheimer’s disease, Huntington’s disease, and alcoholic Korsakoff’s syndrome. Arch Neurol. 1986;43(3):239–246. doi: 10.1001/archneur.1986.00520030031008. [DOI] [PubMed] [Google Scholar]
- Moss MB, Rosene DL, Peters A. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging. 1988;9(5–6):495–502. doi: 10.1016/s0197-4580(88)80103-9. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ, Lai ZC, Rosene DL, Herndon JG. Recognition memory span in rhesus monkeys of advanced age. Neurobiol Aging. 1997;18(1):13–19. doi: 10.1016/s0197-4580(96)00211-4. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. Introduction. Computation in cortical columns. Cereb Cortex. 2003;13(1):2–4. doi: 10.1093/cercor/13.1.2. [DOI] [PubMed] [Google Scholar]
- Mueller EA, Moore MM, Kerr DC, Sexton G, Camicioli RM, Howieson DB, Quinn JF, Kaye JA. Brain volume preserved in healthy elderly through the eleventh decade. Neurology. 1998;51(6):1555–1562. doi: 10.1212/wnl.51.6.1555. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18(16):6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Bernot T, Tuszynski MH. Age-related cognitive deficits in rhesus monkeys mirror human deficits on an automated test battery. Neurobiol Aging. 2010;31(6):1020–1031. doi: 10.1016/j.neurobiolaging.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21(3):545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- O’Donnell KA, Rapp PR, Hof PR. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Exp Neurol. 1999;160(1):300–310. doi: 10.1006/exnr.1999.7192. [DOI] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The synapses upon dendritic spines. Z Zellforsch Mikrosk Anat. 1969;100(4):487–506. doi: 10.1007/BF00344370. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. The effects of age on the cells in layer 1 of primate cerebral cortex. Cereb Cortex. 2002;12(1):27–36. doi: 10.1093/cercor/12.1.27. [DOI] [PubMed] [Google Scholar]
- Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb Cortex. 1994;4(6):621–635. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55(8):861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8(4):295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8(8):671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Peters A, Jones EG, Morrison JH. Cerebral cortex: neurodegenerative and age-related changes in structure and function of cerebral cortex. New York: Springer; 1999. [Google Scholar]
- Peters A, Moss MB, Sethares C. The effects of aging on layer 1 of primary visual cortex in the rhesus monkey. Cereb Cortex. 2001;11(2):93–103. doi: 10.1093/cercor/11.2.93. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152(4):970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Goldman-Rakic PS. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol. 1982;205(1):63–76. doi: 10.1002/cne.902050107. [DOI] [PubMed] [Google Scholar]
- Presty SK, Bachevalier J, Walker LC, Struble RG, Price DL, Mishkin M, Cork LC. Age differences in recognition memory of the rhesus monkey (Macaca mulatta) Neurobiol Aging. 1987;8(5):435–440. doi: 10.1016/0197-4580(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58(9):1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behav Neurosci. 1990;104(6):876–884. doi: 10.1037//0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- Rapp PR. Emotion, memory and behavior: studies on human and nonhuman primates, vol 18. Cognitive neuroscience perspectives on aging in nonhuman primates. Tokyo: Japan Scientific Societies; 1995. [Google Scholar]
- Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9(10):3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging. 1991;12(5):481–486. doi: 10.1016/0197-4580(91)90077-w. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23(13):5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Head D, Kennedy KM, Acker JD. Differential aging of the medial temporal lobe: a study of a five-year change. Neurology. 2004;62(3):433–438. doi: 10.1212/01.wnl.0000106466.09835.46. [DOI] [PubMed] [Google Scholar]
- Rhodes MG. Age-related differences in performance on the Wisconsin Card Sorting Test: a meta-analytic review. Psychol Aging. 2004;19(3):482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Gilardi KV, Lasley B, Rapp PR. Reproductive senescence predicts cognitive decline in aged female monkeys. Neuroreport. 1997;8(8):2047–2051. doi: 10.1097/00001756-199705260-00048. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12(1):142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Magnocellular basal nucleus. J Comp Neurol. 1984;222(3):313–342. doi: 10.1002/cne.902220302. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Sparks DL. Quantitative assessment of possible age-related change in synaptic numbers in the human frontal cortex. Neurobiol Aging. 2001;22(3):355–365. doi: 10.1016/s0197-4580(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Buonocore MH, Makaron LM, Amaral DG, Barnes CA, Rapp PR. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol Aging. 2006;27(10):1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Habeck C, Hof PR, Amaral DG, Fong SG, Buonocore MH, Stern Y, Barnes CA, Rapp PR (2010) Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cereb Cortex. doi:10.1093/cercor/bhq210 [DOI] [PMC free article] [PubMed]
- Simic G, Kostovic I, Winblad B, Bogdanovic N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J Comp Neurol. 1997;379(4):482–494. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Pietropaolo MF, Rosene DL, Moss MB, Peters A, Kemper T, Abraham CR. Lack of correlation between plaque burden and cognition in the aged monkey. Acta Neuropathol. 1997;94(5):471–478. doi: 10.1007/s004010050735. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci USA. 2004;101(18):7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24(18):4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soghomonian JJ, Sethares C, Peters A. Effects of age on axon terminals forming axosomatic and axodendritic inhibitory synapses in prefrontal cortex. Neuroscience. 2010;168(1):74–81. doi: 10.1016/j.neuroscience.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. Memory: brain systems and behavior. Trends Neurosci. 1988;11(4):170–175. doi: 10.1016/0166-2236(88)90144-0. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S, Chen KS. Human amnesia and animal models of amnesia: performance of amnesic patients on tests designed for the monkey. Behav Neurosci. 1988;102(2):210–221. doi: 10.1037//0735-7044.102.2.210. [DOI] [PubMed] [Google Scholar]
- Steere JC, Arnsten AF. The alpha-2A noradrenergic receptor agonist guanfacine improves visual object discrimination reversal performance in aged rhesus monkeys. Behav Neurosci. 1997;111(5):883–891. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134(Pt 2):127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Stroessner-Johnson HM, Rapp PR, Amaral DG. Cholinergic cell loss and hypertrophy in the medial septal nucleus of the behaviorally characterized aged rhesus monkey. J Neurosci. 1992;12(5):1936–1944. doi: 10.1523/JNEUROSCI.12-05-01936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Gordon TP, McClure HM, Hall EC, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes regional primate research center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- Tigges J, Herndon JG, Rosene DL. Mild age-related changes in the dentate gyrus of adult rhesus monkeys. Acta Anat (Basel) 1995;153(1):39–48. doi: 10.1159/000147713. [DOI] [PubMed] [Google Scholar]
- Tigges J, Herndon JG, Rosene DL. Preservation into old age of synaptic number and size in the supragranular layer of the dentate gyrus in rhesus monkeys. Acta Anat (Basel) 1996;157(1):63–72. doi: 10.1159/000147867. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Visser PJ, Boxtel MP, Jolles J. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging. 2000;21(4):569–576. doi: 10.1016/s0197-4580(00)00133-0. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature. 1999;402(6760):421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Povilaitite P, Parisi L, Muller D. Remodeling of synaptic membranes after induction of long-term potentiation. J Neurosci. 2001;21(16):6245–6251. doi: 10.1523/JNEUROSCI.21-16-06245.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: synaptic density. Exp Neurol. 1980;69(1):164–172. doi: 10.1016/0014-4886(80)90151-x. [DOI] [PubMed] [Google Scholar]
- Uemura E. Age-related changes in the subiculum of Macaca mulatta: dendritic branching pattern. Exp Neurol. 1985;87(3):412–427. doi: 10.1016/0014-4886(85)90172-4. [DOI] [PubMed] [Google Scholar]
- Uemura E. Age-related changes in the subiculum of Macaca mulatta: synaptic density. Exp Neurol. 1985;87(3):403–411. doi: 10.1016/0014-4886(85)90171-2. [DOI] [PubMed] [Google Scholar]
- Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat Neurosci. 2007;10(3):376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Impairments in acquisition and reversals of two-choice discriminations by aged rhesus monkeys. Neurobiol Aging. 1999;20(6):617–627. doi: 10.1016/s0197-4580(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Tinkler GP. Cognitive function and its neural mechanisms in nonhuman primate models of aging, Alzheimer disease, and menopause. Front Biosci. 2004;9:1899–1914. doi: 10.2741/1370. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Sukhov RR, Walker LC, Breckler SJ, Price DL, Koliatsos VE. Neuronal number and size are preserved in the nucleus basalis of aged rhesus monkeys. Dementia. 1995;6(3):131–141. doi: 10.1159/000106936. [DOI] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J Neurosci. 2009;29(33):10362–10370. doi: 10.1523/JNEUROSCI.1591-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM (2009) Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2009.05.013 [DOI] [PMC free article] [PubMed]
- Walker ML, Herndon JG. Menopause in nonhuman primates? Biol Reprod. 2008;79(3):398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30(38):12770–12776. doi: 10.1523/JNEUROSCI.3192-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiol Aging. 1989;10(1):11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14(4):287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West MJ, Amaral DG, Rapp PR. Preserved hippocampal cell number in aged monkeys with recognition memory deficits. Soc for Neurosci Abstr. 1993;19:599. [Google Scholar]
- Witter MP, Hoesen GW, Amaral DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci. 1989;9(1):216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14(12):7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci USA. 2001;98(6):3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc Natl Acad Sci USA. 2010;107(28):12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Medial temporal lesions in monkeys impair memory on a variety of tasks sensitive to human amnesia. Behav Neurosci. 1985;99(1):22–34. doi: 10.1037//0735-7044.99.1.22. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. J Neurosci. 1989;9(12):4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Clower RP, Rempel NL. Damage to the perirhinal cortex exacerbates memory impairment following lesions to the hippocampal formation. J Neurosci. 1993;13(1):251–265. doi: 10.1523/JNEUROSCI.13-01-00251.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Ramus SJ. Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus. 1994;4(4):483–495. doi: 10.1002/hipo.450040410. [DOI] [PubMed] [Google Scholar]