Abstract
The health of old monkeys usually begins to deteriorate by 20 years of age, coinciding with the onset of a slowly progressing immune senescence. Changes in lymphocyte subsets and responses to several antigens have been characterized in geriatric primates, but systematic research has not been conducted on vaccination against influenza virus, a topic of considerable clinical concern for elderly humans. Antibody responses were significantly reduced to primary immunizations in old monkeys, but by administering a second vaccine at 1 month, it was possible to boost antibody titers up to the level found in young adults during their primary phase. The immune competence of unusually long-lived animals (26–37 years) was also compared to more typical aged monkeys (19–25 years). Antibody responses were low overall, although some monkeys in both age groups did respond to immunization. Among the oldest animals, the leukocytes of the responders with higher antibody titers were found to release more interleukin-2 following in vitro stimulation with an anti-CD3/anti-CD28 cocktail relative to their cellular reactions to staphylococcal enterotoxin B. The general decline in immune vigor, and the marked individual variation in how old monkeys age, provides a useful animal model for investigating factors associated with immunosenescence.
Keywords: Aging, Influenza, Monkey, Immune senescence, Immunization, Flu vaccine
Introduction
When the life span of old primates is extended in captive settings, and they are spared the natural mortality associated with predation, traumatic injury, and undernutrition, they undergo a progressive age-related decline in immune vigor (Haberthur et al. 2010). Similar to the process of immune senescence in humans, thymic size and function decrease, and there are shifts in lymphocyte populations and less effective responses to infectious pathogens (Aroeira et al. 1994; Beckman et al. 1990; Cicin-Sain et al. 2010). This geriatric decline in immune competence usually commences at the age when they would typically die under natural conditions. For example, the modal age of death for rhesus monkeys is between 20 and 25 years, which is the chronological criterion usually required for first inclusion in biomedical aging research (Bodkin et al. 2003; Smith 1982; Tigges et al. 1988). When their life span is extended, many pathological conditions associated with human aging are observed, including arthritis, atherosclerosis, cataracts, and cancer (Uno 1997), as well as the defining lymphoid features of immune senescence (Jankovic et al. 2003). The following research on responses to influenza vaccination further characterized the age-related immune changes in old rhesus monkeys.
Functional decrements have been demonstrated for many immune responses in old monkeys. A cross-sectional survey found decreased lymphocyte responsiveness using in vitro proliferation and cytolytic assays, as compared to 10–15 year-old, middle-aged monkeys (Ershler et al. 1988a). In addition, the capacity to respond to cholera toxin is diminished in old monkeys, including lower intestinal sIgA responses (Taylor et al. 1992), although reactions to other pathogens, such as plague, are better preserved (Stacy et al. 2008). It is generally believed that innate immunity remains more intact, while T cell populations shift toward senescent and less responsive memory subsets (Cicin-Sain et al. 2010). Age-related changes in antibody responses to influenza vaccination have also been reported (Ershler et al. 1988a), although the rhesus monkey has not been a popular animal model for influenza research. It can be readily infected with only certain strains of influenza (Kobasa et al. 2007; Rimmelzwaan et al. 2003), which does include the A/Sydney/5/97-like virus (H3N2) that was a component of the trivalent vaccine used in the current study (Short et al. 2010).
In addition to comparing antibody responses of old monkeys to younger animals, we determined if booster immunizations might compensate for a compromised primary response. Responsiveness to vaccination is of considerable public health relevance for humans (Goodwin et al. 2006). It is known that a substantial percent of the elderly over 65 years of age do not mount a protective antibody response, which might be corrected by administering a second immunization (Kohut et al. 2002; McElhaney et al. 1990; Nichol et al. 2007). Furthermore, we also examined particularly old monkeys to investigate if these long-lived survivors retained a unique capacity to mount antibody responses and/or maintained normal reactions to in vitro cell stimulation. The leukocytes of these simian Methusalehs over 25 years of age were cultured with two types of stimulants, an anti-CD3/CD28 antibody cocktail or staphylococcal enterotoxin B. Finally, a sufficient period of time elapsed after these immune assessments to determine if the monkeys’ responses were associated with longevity and age at death.
Methods
Subjects
Two experiments were conducted on young and old adult rhesus monkeys (Macaca mulatta), domestically raised with known birth dates, and housed under standardized indoor conditions at either the Harlow Primate Laboratory or Wisconsin National Primate Research Center (WNPRC). Thirty-nine animals were assessed in study 1, including eight old monkeys between 19 and 28 years of age (four male and four female). The antibody responses of the old animals were compared to 31 young adults between 4 and 7 years of age (15 male and 16 female). Study 2 contrasted the antibody responses of eight different old animals (19–25 years of age) to 14 uniquely long-lived survivors (26–37 years of age). To generate normative adult values for study 2, including the cytokine comparisons when stimulating cells in vitro, blood samples were also collected from four young adult monkeys. Serum from all animals was screened to verify low levels of influenza antibody prior to immunization, as well as to ensure they had not been exposed recently to a viral infection.
Influenza vaccination and assay
All monkeys were administered the same trivalent influenza vaccine intramuscularly (0.1 mL, 1/5 human dose given the difference in body weight), and then small blood samples (1–4 mL) were collected at 2 and 4 weeks after immunization via femoral or saphenous venipuncture. The vaccine was comprised of 45 μg viral antigen from three inactivated strains: A/Sydney/5/97-like (H3N2), A/Beijing/262/95-like (H1N1), and B/Beijing/184/93-like (Fluzone, Connaught, Wilmington, DE, USA). It was fresh and the current formulation used for seasonal immunization in humans at the time of administration (1999/2000). Then, the ultimate life span of these monkeys was tracked for another decade prior to this report. The 39 young adult and old monkeys in study 1 were also administered a second booster immunization at the 4-week time point, and secondary antibody responses were assessed 2 weeks later.
Antibody levels in the sera were determined with a customized enzyme-linked immunosorbent assay ELISA, using influenza hemagglutinin (Connaught) to coat the plates, a secondary antibody against human IgG (Sigma), and then the reacted color intensity quantified spectrophotometrically at 450 nM with a plate reader (Dynatech). The data are presented on a relative scale as “area under the curve” (AUC) based on the total amount of antibody quantified in three serial dilutions of each monkey’s sera. Three dilutions (1:100, 1:250, and 1:1,000) optimally captured differences in antibody level and binding affinity for the old monkeys. Antibody values were also detectable at 1:5,000 for young adult monkeys. The formula for a trapezoidal area was used to compute the summative antibody titer or AUC across the three dilutions for each animal as described previously (.5[Dil 1] + .5[Dil 1 + Dil 2] + .5 [Dil 2 + Dil 3] + .5[Dil 3] × 100) (Ershler et al. 1988b). Conclusions from these AUC results were comparable to findings obtained at the optimal dilution of 1:250 for old monkeys.
In vitro cell stimulation
For study 2, each monkey’s blood was diluted 1:1 in RPMI and then stimulated with either 10 μg/mL SEB (Sigma-Aldrich, St. Louis, MO, USA), a potent polyclonal activator of T and B lymphocytes, or with an anti-CD3/anti-CD28 antibody cocktail to induce a more restricted but optimal activation of the T cell receptor (1 μg/mL FN18, mouse anti-monkey CD3, BD Biosciences, San Diego, CA, USA; 1 μg/mL anti-CD28, Biosource International, Camarillo, CA, USA). CD28 is a co-stimulatory protein on the T cell surface that facilitates activation and proliferation following stimulation of the CD3 receptor (Effros et al. 1994). Cytokine levels in the supernatants were determined by ELISA both after 24 and 48 h incubation using commercial kits specifically tailored for monkey proteins [interleukin (IL)-2, interferon gamma, IL-4, IL-6, and IL-10; U-CyTech Biosciences, Utrecht, The Netherlands]. The cytokine values at the two time points were averaged, and only the IL-2 results are presented in the current report because they were most predictive of antibody responses to influenza vaccine.
Physical assessments
To evaluate the monkeys’ overall physical condition, two other indices were examined. The rate of nail growth was quantified by tracking the upward 2-week progression of a white dot, which was tattooed at the base of three fingernails under brief ketamine sedation. Prior research had demonstrated that nail growth slows by nearly 50% in old monkeys in a manner that was predictive of natural killer cell activity and ultimate longevity (Coe and Ershler 2001; Short et al. 1987). In addition, since general appearance has been reported to be associated with the benefits of health-promoting diets in old monkeys, their hair quality was scored on a 21-point scale. The hair rating scale (HRS) captures three domains, which are fur quality, patchiness, and hygiene, each given a score of 1–7, with the total ranging from a low of 3 to a maximum of 21 points.
Longevity
It was also possible to consider the ultimate life span for the old animals in study 2 because the immune assessments were conducted over a decade ago. Birth dates were known and the older animals eventually succumbed to terminal diseases or had to be euthanized for humane reasons.
Statistical analyses
Differences in the response to vaccination were evaluated with analyses of variance, considering age (young adult, old, or oldest) and gender (male and female) as between factors and antibody values over time as a repeated-measure factor (2 and 4 weeks). The old monkeys in study 2 were also categorized as minimal responders or responsive to immunization. The criterion for responsive was an AUC of >100, which was an antibody level reached during the primary phase by all 31 young adult monkeys in study 1. Associations were examined with the Pearson’s test, including the correlations between physical variables and antibody levels and between in vitro cytokine and in vivo antibody responses. Similarly, the possible linkage between immune or physical variables and age at mortality was assessed by correlation.
Results
Study 1: primary and secondary antibody responses in old monkeys
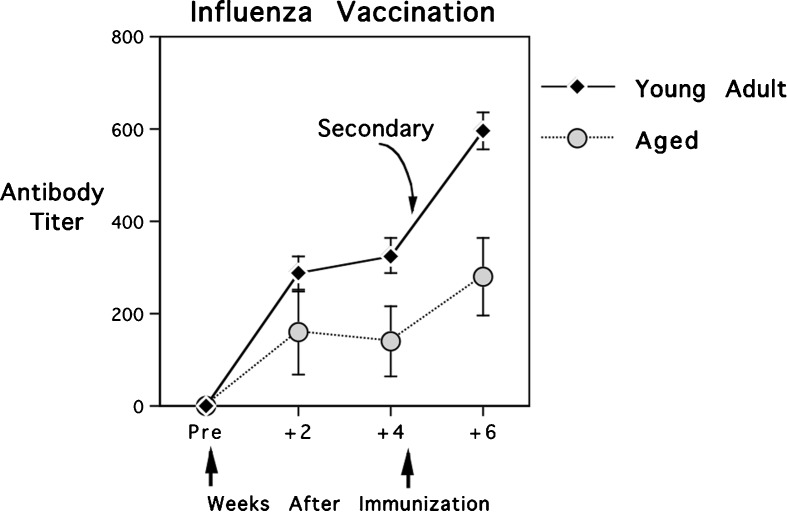
As can be seen in Fig. 1, old monkeys mounted significantly smaller antibody responses to influenza vaccination than did the young adult monkeys [F(1,37) = 6.59, p < 0.015]. This large effect of age was also evident when all monkeys were administered a booster vaccine at 1 month and assessed again 2 weeks later. Both the overall antibody levels and the increments from primary to secondary were significantly less in the old animals [F(1,37) = 8.13, p < 0.007]. However, it is noteworthy that the secondary IgG titers of old monkeys had now reached a level comparable to the primary response of young adults. That is, the values for old animals after being reimmunized were not significantly different from the antibody levels of young adults at the initial 2- or 4-week time points.
Fig. 1.
Primary and secondary antibody responses of 31 young adult and eight old rhesus monkeys to vaccination. The antibody responses of old monkeys were significantly reduced, but a booster immunization raised their secondary titers into the primary range for young adults. A relative scale is portrayed using area under the curve (AUC) based on computations of antibody titer from three serial dilutions of sera. Vertical arrows indicate when the two trivalent vaccines were administered
In keeping with expectations, both the nail growth rate and hair ratings for the old monkeys were significantly below the scores for young adult animals. The mean daily rate of nail growth for an old animal was 1.62 mm (0.28) as compared to 3.25 mm (0.21) for a young adult [F(1,37) = 13.96, p < 0.001]. Similarly, the mean HRS for old monkeys was 9.75 (0.82) as compared to 13.71 (0.65) for young animals, indicative of the sparser and more matted hair coat with less luster evinced by an older monkey [F(1,37) = 11.83, p < 0.002]. Nail growth and hair quality appeared to be statistically associated with antibody levels when both young and old monkeys were considered together in one correlational analysis. However, neither physical attribute proved to account for a significant amount of variance in antibody responses when the data were analyzed separately for the young and old cohorts. Thus, the initial suggestion of a causal relationship between these physical attributes and immunity was really due to the parallel effects of aging on each domain.
Study 2: immunity in old and very old monkeys
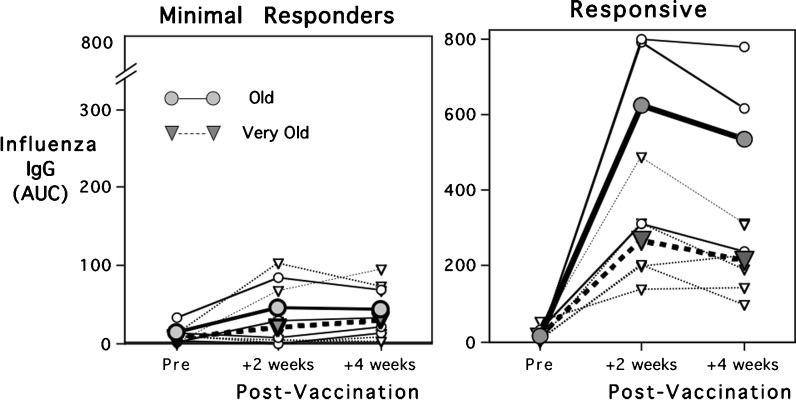
The finding of low antibody responses in old monkeys to primary immunization was replicated when additional animals were assessed in the second experiment. Antibody levels at 2 and 4 weeks were further reduced in the very aged monkeys of 26–37 years [F(1,18) = 12.29, p < 0.003]. However, it was evident that some animals in both the old and oldest cohorts should be considered in categorical manner as either minimal responders or responsive to vaccination (Fig. 2). The criterion for responsive was based on attaining an AUC >100 (a value exceeded by all young adults in study 1) or showing an incremental rise in antibody titer from weeks 2 to 4. The responsive monkeys that generated larger antibody responses did not differ in age, the two assessed physical indices (nail growth, HRS) or ultimate longevity (Table 1). However, it was notable that the cultured leukocytes of the responsive animals in the very old group released more IL-2 following T cell receptor stimulation (anti-CD3/CD28) relative to their IL-2 responses when SEB was the stimulant [F(1,18) = 4.9, p < 0.04). For those mounting larger antibody responses to vaccination, the IL-2 levels were also more comparable to young adults (Table 1). None of these age-related effects on cytokine levels were attributable to differences in total white blood cell counts or the percentages of lymphocytes in the specimens.
Fig. 2.
Primary antibody responses of eight old and 14 very old rhesus monkeys to influenza vaccination. Antibody levels were low overall and significantly lower in the oldest animals, although responders were found in both age groups. Note that the scale for minimal responders is expanded (2x) to illustrate their low responses. Responder status was based on attaining an AUC >100, a criterion generated from the young adult monkeys in study 1. Circles illustrate data for the old monkeys; triangles connote the older ones. Mean responses for the group are shown with bold lines and larger symbols; lighter lines indicate individual responses. Additional information on these monkeys is provided in Table 1
Table 1.
Age, immune, and physical attributes of the old and very old monkeys in study 2, which were categorized as minimally responsive or responders to influenza vaccination
| Age (year) | Deathc (year) | AUCd (4 weeks) | NG (mm) | HRS (3–21) | CD3/CD28 (IL-2, ng/mL) | SEB (IL-2, ng/mL) | IL-2d ratio | WBC (k/μL) | Lymph (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Old (<25 years) | ||||||||||
| Minimal responders | 21.1 (0.9) | 28.5 (1.4) | 41.5 (12.1) | 1.7 (0.2) | 11.6 (1.2) | 0.34 (0.15) | 15.96 (4.61) | .037 (0.02) | 7.46 (.46) | 44.2 (7.9) |
| Respondera | 20.3 (0.9) | 26.2 (0.5) | 534.2 (171.3) | 1.5 (0.2) | 11.0 (2.3) | 0.26 (0.19) | 18.13 (2.45) | .018 (.014) | 7.17 (1.0) | 39.3 (3.2) |
| Oldest (>26 years) | ||||||||||
| Minimal responders | 29.9 (0.8) | 34.3 (1.3) | 27.7 (12.1) | 1.5 (0.2) | 8.4 (1.5) | 0.51 (0.08) | 12.81 (0.72) | .043 (.001) | 7.64 (0.79) | 38.1 (3.9) |
| Responder | 28.7 (1.7) | 30.9 (1.5) | 212.1 (36.1) | 1.6 (0.2) | 9.5 (1.8) | 1.07 (0.23 | 9.61 (1.80) | .138 (.038) | 9.27 (0.71) | 46.1 (9.8) |
| Young adult referenceb | 3.3 (0.2) | 13.7 (0.7) | 0.79 (0.16) | 9.18 (1.22) | .090 (.01) | 12.7 (0.71) | 43.7 (9.8) | |||
aResponders to the vaccine (9 of 22, 41%) met the antibody criterion of AUC >100
bReference values generated from four younger adult monkeys assessed contemporaneously in study 2. Young adults exhibited faster nail growth and better hair quality than old monkeys. The IL-2 values of the oldest monkeys that still responded to the vaccine were more similar to those of the young adults
cAge at natural death subsequent to the vaccine study was used as the measure of longevity
dSignificant difference between old and oldest monkeys as well as with respect to vaccine responsiveness
Longevity and mortality
The mean age at death for monkeys in study 2 was 31.5 years (range, 26.1–41.2), with the survival period subsequent to the vaccine phase averaging 4.6 years (range, 1.0–11.2). Neither survival nor terminal age was associated with the monkeys’ prior antibody responses. As anticipated, old monkeys in both studies exhibited slower nail growth rate and poorer hair quality, but these indices were not predictive of life span or the age-related decrement in antibody responses (Table 1).
Discussion
These two studies of old rhesus monkeys demonstrated that antibody responses to influenza vaccination are markedly reduced below those of young adults. Further decrements were evident in the oldest animals assessed in the second study, and many old monkeys should be categorized as non-responders. The poor vaccine response is not due to low antibody production in general, because overall immunoglobulin in circulation, especially IgA, tends to rise slightly in older monkeys (Stone et al. 1994). In part, the inadequate responses were due to our focus on primary immunizations, and the absence of prior influenza infections in modern animal facilities following BSL-2 practices and using protective garb. Some improvement in vaccine responses could be elicited by inducing secondary responses with a booster immunization, even in the monkeys older than 20 years of age. Other researchers have also utilized repeat exposures to influenza virus and/or vaccine to induce more effective immune responses in monkeys (Bergman et al. 1986; Rimmelzwaan et al. 1997; Saslaw and Carlisle 1965). Given that many elderly humans do not mount protective responses following influenza vaccination, it may be worthwhile to consider a dual vaccine regimen for those likely to have inadequate responses.
Some papers have also highlighted the rapidity with which vaccine-enhanced immunity against influenza declines in the elderly, although the more critical issue seems to be the robustness of the primary response (Skowronski et al. 2008). In monkeys, the primary response may be relatively short-lived, as was evident in those animals that showed a decrement even between weeks 2 and 4. The half-life of IgG in monkeys once generated is comparatively short, only 8 days in circulation as compared to the average 21 days for humans (Challacombe and Russell 1979).
These old monkeys were unique in a number of ways, including not showing serological evidence of a recent history of flu infections in the baseline blood sample, and many may never have been infected with an influenza virus. They were also selected on the basis of appearing to be overtly healthy, except for the unavoidable geriatric conditions like osteoarthritis and cataracts, and were not receiving any chronic medications. All research on aging, whether with animals or humans, necessitates taking this type of inclusion/exclusion criteria into consideration. If only the healthiest of old animals are evaluated, then immune responses tend to look more like middle-aged animals. In this regard, the leukocyte IL-2 responses of the old monkeys that were responsive to vaccine tended to look more like those of young adult animals. Similar issues regarding the difference between healthy and pathological aging have been discussed for studies of human aging, especially with respect to employing the health criteria of the SENIEUR protocol (Castle et al. 2001). Because one of our aims was to evaluate particularly old animals, the mean life span of 31.5 years was unusually long. The oldest monkey in this cohort survived until 41.2 years of age. In contrast, the median survival for old rhesus monkeys in most colonies, and when ranging under semi-natural conditions on the food-provisioned island of Cayo Santiago, is 25 years of age (Bodkin et al. 2003; Uno 1997). Our capacity to determine age at death was due to the fact that the initial immunization phase had been conducted more than a decade before the current report.
Despite the novel aspects of this research, several limitations should be acknowledged. Our choice to use ELISA methods to assess antibody responses rather than the traditional but more labor-intensive test of hemagglutination inhibition (HI) limits the conclusions. This quantification of just antibody bound to influenza antigen on the coated plates precluded us from verifying virus neutralization and protection, but there have been many publications both in animals and humans showing that ELISA methods are adequate for documenting seroconversion post-vaccination (Fazlani et al. 2010; Grund et al. 2011; Skibe et al. 2004; Turner et al. 1982; Yoon et al. 2004; Zarkov 2006). In general, the results are usually consonant with HI assays in terms of both sensitivity and specificity. Another caveat is that our assay did not distinguish between responses to each antigen in the trivalent vaccine, although it is likely that monkeys responded primarily to the influenza A strains. Furthermore, one ideally might follow the immunization phase with a viral challenge, but that could not be done with these precious old primates without isolating them in quarantine conditions. However, from other experiments involving influenza infection in our laboratory, we know that primary immunizations are not as effective as dual vaccination regimens for providing protection, even in younger monkeys.
One physical measure found previously to be diagnostic of health status in geriatric animals, nail growth rate, was clearly affected in these old monkeys, but it did not accurately predict immune responses or life span as reported in an earlier study (Coe and Ershler 2001). The focus on IL-2 release, especially following T cell receptor stimulation, provided some preliminary data in support of the conclusion that T cell function and IL-2 are useful metrics of the immune status in old monkeys, as demonstrated in research on human aging (Andersson and Sander 1989; Effros et al. 1994; Jankovic et al. 2003; McElhaney et al. 1990). Decreased responsiveness to IL-2 with age is also associated with increased shedding of the soluble IL-2 receptor in circulation (Saadeh et al. 1986; Hallgren et al. 1988). Moreover, enhanced lymphocyte responsiveness to IL-2 seems to be one of the collateral benefits of health-promoting nutriture, including calorie-restricted diets in rodent models (Pahlavanni and Vargas 2001). SEB was employed as a reference stimulant, and even the oldest monkeys continued to mount strong cytokine responses to this potent superantigen. However, it is known that containment of staphylococcal infection in vivo does decline with age in old monkeys (Aroeira et al. 1994).
In sum, when rhesus monkeys reach 20 years of age, which is the end of their life span under natural conditions, they undergo many immune changes similar to the process of immune senescence in elderly humans (DeGreef et al. 1992). Some primate researchers have argued that life-long efforts to contain latent Herpes viruses, including the macaque version of cytomegalovirus (CMV), contribute to this progressive aging of the immune system (Haberthur et al. 2010). While we did not determine the CMV status of our animals, it is likely that most were latently infected, given that it is endemic in primate colonies. Many monkeys ultimately die of gastrointestinal or cardiovascular disease (Bodkin et al. 2003), although terminal pneumonia and cancerous conditions also become more common after 25 years of age (Uno 1997). For the unusually long-lived survivors that have not yet succumbed by their mid-20s, which comprise about 10% of the population, immune vigor may remain intact, analogous to findings on some centenarian humans (Argentati et al. 2002; Sansoni et al. 2008). Their longevity, and this individual variation in the rate of aging, makes the monkey a useful model for investigating immunobiology in the aged host.
Acknowledgements
This study was supported by grant awards from the National Institute of Allergy and Infectious Disease to CLC (AI46521, AI607517), with additional primate resources from the Wisconsin National Primate Research Center (P51 RR000167). Special acknowledgements are also due to J. Karaszewski and A. Slukvina, and belatedly to H. Crispen, for their assistance with the collection of samples and assays.
References
- Andersson U, Sander B. Detection of individual interleukin 2-producing cells after anti-CD3 antibody activation. Immunol Lett. 1989;20(2):115–120. doi: 10.1016/0165-2478(89)90095-3. [DOI] [PubMed] [Google Scholar]
- Argentati K, Re F, Donni A, Tucci MG, Franceschi C, Bartozzi B, Bernardini G, Provinciali M. Numerical and functional alterations of circulating gamma-delta T lymphocytes in aged people and centenarians. J Leuk Biol. 2002;72:65–71. [PubMed] [Google Scholar]
- Aroeira LS, Williams O, Lozano EG, Martinez AC. Age-dependent changes in the response to staphylococcal enterotoxin B. Intern Immunol. 1994;6(10):1555–1560. doi: 10.1093/intimm/6.10.1555. [DOI] [PubMed] [Google Scholar]
- Beckman I, Dimopoulos K, Xiaoning X, Bradley J, Henschke JP, Ahern M. T cell activation in the elderly: evidence for specific deficiencies in T cell/accessory cell interactions. Mech Ageing Devel. 1990;51(3):265–276. doi: 10.1016/0047-6374(90)90076-R. [DOI] [PubMed] [Google Scholar]
- Bergman KC, Waldman RH, Norheim NHT, Huynh PL, Tischner H. Remote-site stimulation of influenza antibody in monkeys following intestinal stimulation. Immunol Lett. 1986;12(2–3):65–67. doi: 10.1016/0165-2478(86)90084-2. [DOI] [PubMed] [Google Scholar]
- Bodkin NL, Alexander TM, Ortmeyer HK, Johnson E, Hansen BC. Mortality and morbidity in laboratory-maintained rhesus monkeys and effects of long-term dietary restriction. J Gerontol: Biol Sci. 2003;58A(3):212–219. doi: 10.1093/gerona/58.3.B212. [DOI] [PubMed] [Google Scholar]
- Castle SC, Uyemura K, Makinodan T. The SENIEUR protocol after 16 years: a need for a paradigm shift. Mech Ageing Devel. 2001;122(2):127–130. doi: 10.1016/S0047-6374(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Challacombe SJ, Russell MW. Estimation of the intravascular half-lives of normal rhesus monkey IgG, IgA and IgM. Immunol. 1979;36:331–338. [PMC free article] [PubMed] [Google Scholar]
- Cicin-Sain L, Smyk-Paerson S, Currier N, Byrd L, Koudelka C, Robinson T, Swarbrick G, Tackitt S, Legasse A, Fischer M, Nikolich-Zugh D, Park B, Hobbs T, Doane CJ, Mori M, Axhelm M, Lewinsohn DA, Nikolich-Zugich J. Loss of naïve T cells and repertoire constriction predict poor response to vaccination in old primates. J Immunol. 2010;184:6739–6745. doi: 10.4049/jimmunol.0904193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Ershler WB. Intrinsic and environmental influences on immune senescence in the aged monkey. Physiol Beh. 2001;73:379–384. doi: 10.1016/S0031-9384(01)00491-7. [DOI] [PubMed] [Google Scholar]
- De Greef DE, Van Staalduinen GJ, Van Doorninck H, Van Tol MJD, Hijmans W (1992) Agerelated changes of the antigen-specific antibody formation and PHA-induced T-cell proliferation in individuals who met the health criteria of the Senieur protocol. Mech Ageing Devel 66(1):1–14 [DOI] [PubMed]
- Effros RB, Boucher N, Porter V, Zhu X, Spaulding C, Walford R, Kronenberg M, Cohen D, Schacter F. Decline in CD28+ T cells in centenarians and in long-term T cell cultures. A cause for both in vivo and in vitro immunosenescence. Exp Gerontol. 1994;29(6):601–609. doi: 10.1016/0531-5565(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Coe CL, Gravenstein S, Klopp RG, Meyer M, Houser WD. Aging and immunity in nonhuman primates. I. Effects of age and gender on cellular immune function in rhesus monkeys (Macaca mulatta) Am J Primatol. 1988;15:181–188. doi: 10.1002/ajp.1350150210. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Coe CL, Gravenstein S, Klopp RG. Specific antibody synthesis in vitro. IV. The correlation of in vitro and in vivo antibody response to influenza vaccine in rhesus monkeys. Clin Exp Immunol. 1988;73:355–359. [PMC free article] [PubMed] [Google Scholar]
- Fazlani SA, Abubakar M, Shahid F, Irfan M, Arshed MJ. A comparative study on efficiency of enzyme linked immunosorbent assay and hemagglutination inhibition test in detecting serum antibody against avian influenza viruses in commercial broilers. Vet Scan. 2010;5(2):64. [Google Scholar]
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly; a quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Grund S, Adams O, Wahlisch S, Schweiger B. Comparison of hemagglutination inhibition assay, an ELISA-based micro-neutralization assay and colorimetric micro-neutralization assay to detect antibody responses to vaccination against influenza A H1N1 2009 virus. J Virol Methods. 2011;171:369–373. doi: 10.1016/j.jviromet.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Haberthur K, Engelman F, Barron A, Messaoudi I. Immune senescence in aged nonhuman primates. Exp Geront. 2010;45(9):655–661. doi: 10.1016/j.exger.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren HM, Bergh N, Rodysil KJ. Lymphocyte proliferative response to PHA and anti-CD3/Ti monoclonal antibodies, T cell surface marker expression, and serum IL-2 receptor levels as biomarkers of age and health. Mech Ageing Devel. 1988;43(2):175–185. doi: 10.1016/0047-6374(88)90045-0. [DOI] [PubMed] [Google Scholar]
- Jankovic V, Messaoudi I, Nikolich-Zugich J. Phenotypic and functional T-cell aging in rhesus macaques (Macaca mulatta): differential behavior of CD4 and CD8 subsets. Blood. 2003;102:3244–3251. doi: 10.1182/blood-2003-03-0927. [DOI] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Felmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Kohut ML, Cooper MM, Nickolluas MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol: Med Sci. 2002;57A:M557–M562. doi: 10.1093/gerona/57.9.M557. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Beattie BL, Devine R, Grynoch R, Toth EL, Bleackley RC. Age- related decline in interleukin 2 production in response to influenza vaccine. J Am Geriatr Soc. 1990;38:652–658. doi: 10.1111/j.1532-5415.1990.tb01424.x. [DOI] [PubMed] [Google Scholar]
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community dwelling elderly. N Engl J Med. 2007;357:1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- Pahlavanni MA, Vargas DA. Activation-induced apoptosis in T cells: effect of age and caloric restriction. Iran Biomed J. 2001;5(1):1–9. [Google Scholar]
- Rimmelzwaan EF, Baars M, Beek R, Amerongen G, Lovgren-Bengtsson K, Claas ECJ, Osterhause ADME. Induction of protective immunity against influenza virus in a macaque model: comparison of conventional and iscom vaccines. J Gen Virol. 1997;78:757–765. doi: 10.1099/0022-1317-78-4-757. [DOI] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Kuiken T, Amerogen G, Bestebroer TM, Fouchier TA, Osterhouse AD. A primate model to study the pathogenesis of influenza A (H5N1) virus infection. Avian Dis. 2003;47:931–933. doi: 10.1637/0005-2086-47.s3.931. [DOI] [PubMed] [Google Scholar]
- Saadeh C, Auzenne D, Nelson D, Orson F. Sera from the aged contain higher levels of the IL-2 receptor compared to young adults. Fed Proc. 1986;45:1298. [Google Scholar]
- Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Llucchini G, Paseri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43(2):61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Saslaw S, Carlisle HN. Aerosol exposure of monkeys to influenza virus. Exp Bio Med. 1965;119:838–843. doi: 10.3181/00379727-119-30315. [DOI] [PubMed] [Google Scholar]
- Short R, Williams DD, Bowden DM. Cross-sectional evaluation of potential biomarkers of aging in pigtailed macaques: effects of age, sex, and diet. J Geront. 1987;42:644–654. doi: 10.1093/geronj/42.6.644. [DOI] [PubMed] [Google Scholar]
- Short SJ, Lubach GR, Karasin AI, Olsen CW, Styner M, Knickmeyer RC, Gilmore JH, Coe CL. Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiat. 2010;67:965–973. doi: 10.1016/j.biopsych.2009.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibe D, Zhou E-M, Janke BH. Comparison of a commercial enzyme-linked immunosorbent assay with hemagglutination inhibition assay for serodiagnosis of swine influenza virus (H1N1) infection. J Vet Diagn Invest. 2004;16:86–89. doi: 10.1177/104063870401600116. [DOI] [PubMed] [Google Scholar]
- Skowronski DRM, Tweed SA, Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: Is it real or is it relevant? J Inf Dis. 2008;197:490–502. doi: 10.1086/524146. [DOI] [PubMed] [Google Scholar]
- Smith DA. A comparison of the demographic structure and growth of free-ranging and captive groups of rhesus monkeys (Macaca mulatta) Primates. 1982;23:24–30. doi: 10.1007/BF02381435. [DOI] [Google Scholar]
- Stacy S, Pasquali A, Sexton VL, Cantwell AM, Kraig E, Dube PH. An age-old paradigm challenged: old baboons generate vigorous humoral immune responses to LcrV, a plague antigen. J Immunol. 2008;181:109–115. doi: 10.4049/jimmunol.181.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WH, Saphire DR, Hackleman SM, Braun AM, Pennington P, Scheffler J, Wigle JC, Cox AB. Effects of radiation and age on immunoglobulin levels in rhesus monkeys (Macaca mulatta) Radiat Res. 1994;138(3):401–408. doi: 10.2307/3578689. [DOI] [PubMed] [Google Scholar]
- Taylor LD, Ck D, Schmucker DL. Ageing compromises gastrointestinal mucosal immune responses in the rhesus monkey. Immunol. 1992;75(4):614–618. [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Gordon T, McClure H, Hall E, Peters A. Survival rate and life span of rhesus monkeys at the Yerkes Regional Primate Research Center. Am J Primatol. 1988;15:263–273. doi: 10.1002/ajp.1350150308. [DOI] [PubMed] [Google Scholar]
- Turner R, Lathey JL, Voris LP, Belshe RB. Serological diagnosis of influenza B virus infection: comparison of an enzyme-linked immunosorbent assay and the hemagglutination inhibition test. J Clin Microbio. 1982;15(5):8240828. doi: 10.1128/jcm.15.5.824-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H. Age-related pathology and biosenescent markers in captive rhesus macaques. Age. 1997;20(1):1–13. doi: 10.1007/s11357-997-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K-J, Janke BH, Swalla RW, Erickson G. Comparison of a commercial H1N1 enzyme-linked immunosorbent assay and hemagglutination inhibition test in detecting serum antibody against swine influenza viruses. J Vet Diagn Invest. 2004;16:197–201. doi: 10.1177/104063870401600304. [DOI] [PubMed] [Google Scholar]
- Zarkov IS. Comparison of haemagglutination inhibition (HI), immunodiffusion (ID) and ELISA tests for detecting anti-avian influenza antibodies in chicken after inoculations with inactivated antigen. Revue Med Vet. 2006;157(6):336–340. [Google Scholar]