Abstract
While indices of physical mobility such as gait speed are significant predictors of future morbidity/mortality in the elderly, mechanisms of these relationships are not understood. Relevant animal models of aging and physical mobility are needed to study these relationships. The goal of this study was to develop measures of physical mobility including activity levels and gait speed in Old World monkeys which vary with age in adults. Locomotor behaviors of 21 old (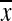 = 20 yoa) and 24 young (
= 20 yoa) and 24 young (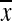 = 9 yoa) socially housed adult females of three species were recorded using focal sample and ad libitum behavior observation methods. Self-motivated walking speed was 17% slower in older than younger adults. Likewise, young adults climbed more frequently than older adults. Leaping and jumping were more common, on average, in young adults, but this difference did not reach significance. Overall activity levels did not vary significantly by age, and there were no significant age by species interactions in any of these behaviors. Of all the behaviors evaluated, walking speed measured in a simple and inexpensive manner appeared most sensitive to age and has the added feature of being least affected by differences in housing characteristics. Thus, walking speed may be a useful indicator of decline in physical mobility in nonhuman primate models of aging.
= 9 yoa) socially housed adult females of three species were recorded using focal sample and ad libitum behavior observation methods. Self-motivated walking speed was 17% slower in older than younger adults. Likewise, young adults climbed more frequently than older adults. Leaping and jumping were more common, on average, in young adults, but this difference did not reach significance. Overall activity levels did not vary significantly by age, and there were no significant age by species interactions in any of these behaviors. Of all the behaviors evaluated, walking speed measured in a simple and inexpensive manner appeared most sensitive to age and has the added feature of being least affected by differences in housing characteristics. Thus, walking speed may be a useful indicator of decline in physical mobility in nonhuman primate models of aging.
Keywords: Gait speed, Nonhuman primate, Aging, Physical mobility, Activity
Introduction
In humans, declines in physical performance as measured by such indices as muscle strength, balance, and gait speed are characteristic of aging and predictive of disability, hospitalization, and death (Era and Heikkinen 1985; Guralnik et al. 1994; Guralnik et al. 2000; Cesari et al. 2005; Cooper et al. 2010). While these measures can each contribute to the prediction of future risk, gait speed has emerged as a preferred measure because it can be reliably measured at low cost and can be easily interpreted (Cesari et al. 2005; Cooper et al. 2010). Slow gait is a pivotal dimension of physical frailty and is more common in persons with clinical and subclinical disease such as cardiovascular disease and is associated with elevated inflammatory cytokines and body composition (Ferrucci et al. 2000; Newman et al. 2006). In 2009, the International Academy on Nutrition and Aging Task Force concluded that gait speed reliably identifies community-dwelling older people at risk of disability, cognitive impairment, institutionalization, falls, and mortality, and can be used as a single-item assessment tool (Abellan van Kan et al. 2009). However, the reasons for the close association between gait speed and morbidity are not well understood.
Rodents have been used extensively as animal models of physical mobility. While this work has been helpful in understanding some of the biological factors that contribute to healthy aging, a large animal model with a central nervous system organization similar to humans would be helpful in understanding declines in motor mobility. Nonhuman primates are ideal, and macaques in particular have been used to study aging and decline primarily in fine motor performance (Irwin et al. 1994; Emborg et al. 1998; Zhang et al. 2000; Cass et al. 2007; Kastman et al. 2010). Such studies involve the assessment of performance on tasks involving retrieval of a Life Saver candy from rods of different complexity and in individualized tasks using movement assessment panels. The central mechanisms involved in motoric decline with age have also been studied in macaques. Alterations of the nigrostriatal dopaminergic system are seen with aging in macaques including decreased dopamine, decreased levels of tyrosine hydroxylase and dopamine transporter, and decreased stimulus-evoked release of dopamine (Kish et al. 1992; Bannon et al. 1992; Irwin et al. 1994; Emborg et al. 1998; Gerhardt et al. 1995, 2002), suggesting that the nigrostriatal dopaminergic system is likely involved in age-associated motor dysfunctions.
Evaluations of gross motoric function in association with age have been more limited. An automated video system has been used to document changes in locomotor mobility in relation to aging in nonhuman primates (Walton et al. 2006). In this study, female rhesus monkeys (Macaca mulatta) that lived in single cages were moved from their home cage to a test chamber and movement was videotaped. Adolescent (averaging 5 yoa) locomotion differed significantly from adult locomotion. However, these measures were not sensitive to differences in locomotion between middle-aged and old adults, perhaps because the monkeys normally lived in individual cages that constrain movement, which could mask smaller differences in locomotor behavior among adults of different ages. Automated video systems are expensive and limited in range, requiring constraints in the physical space in which movement can occur and its patterns documented. Thus, they are difficult to adapt to a larger space or social environment. An ideal translational assessment of nonhuman primate physical mobility would be similar to measures used in humans, inexpensive, and useful in animals housed in social groups in a variety of settings which permit less constrained locomotor behavior. The goal of the study reported here was to develop a measure of physical mobility similar to gait speed used in humans and sensitive to changes in physical mobility in adult monkeys as they age.
Methods
Subjects
Subjects were 24 young and 21 old adult females of three species of Old World monkeys: cynomolgus (Macaca fascicularis), bonnets (Macaca radiata), and African green (Chlorocebus aethiops) monkeys (Table 1). The monkeys were chosen based on age, health, and reproductive condition. To be included in the study, the monkeys had to be in good health, with normal posture and patterns of locomotion, and not known to be pregnant or to have dependent young under 9 months of age. Body weight was measured during the same time period as the behavior observations were completed.
Table 1.
Age distributions of young and old adult female nonhuman primates
| Cohort | Young Adult | Old Adult | ||||
|---|---|---|---|---|---|---|
| n | Age years (X ± SEM) | Range | n | Age years (X ± SEM) | Range | |
| Cynomolgus | 4 | 5.9 (0.25) | 5.7–6.7 | 2 | 19.3 (1.00) | 18.3–20.3 |
| Bonnet | 5 | 11.2 (0.56) | 9.8–13.1 | 5 | 18.0 (0.21) | 17.7–18.8 |
| African Green | 15 | 9.1 (0.19) | 7.8–10.0 | 14 | 20.8 (0.55) | 18-2–24.7 |
| Overall | 24 | 9.0 (0.37) | 5.7–10.0 | 21 | 20.0 (0.46) | 17.7–24.7 |
Housing
All of the monkeys lived in stable social groups. Cynomolgus monkeys were housed in groups of two to four in indoor pens that measured 2.5 (height) × 1.5 (width) × 3.0 m (length) and had outside exposure through a window. Bonnet monkeys were housed in groups of 2–11, in pens with indoor and outdoor sections each measuring 2.5 × 2.5 × 2.5 m, and were observed while enclosed in the indoor section. All indoor pens were outfitted with multiple perches running the length of the pens and had walls of square rod the monkeys routinely climb, allowing opportunity for all types of locomotor behavior reported here. Thus, the pens of cynomolgus and bonnet macaques were similar in size and configuration. African green monkeys lived in groups of 11–49 in housing units with approximately 28 m2 indoors and 111 m2 outdoors and were observed in the outdoor section.
Behavioral observations
All behaviors were recorded by multiple observers experienced in recording nonhuman primate behavior. Interobserver reliability was r ≥0.92.
Usual walking speed
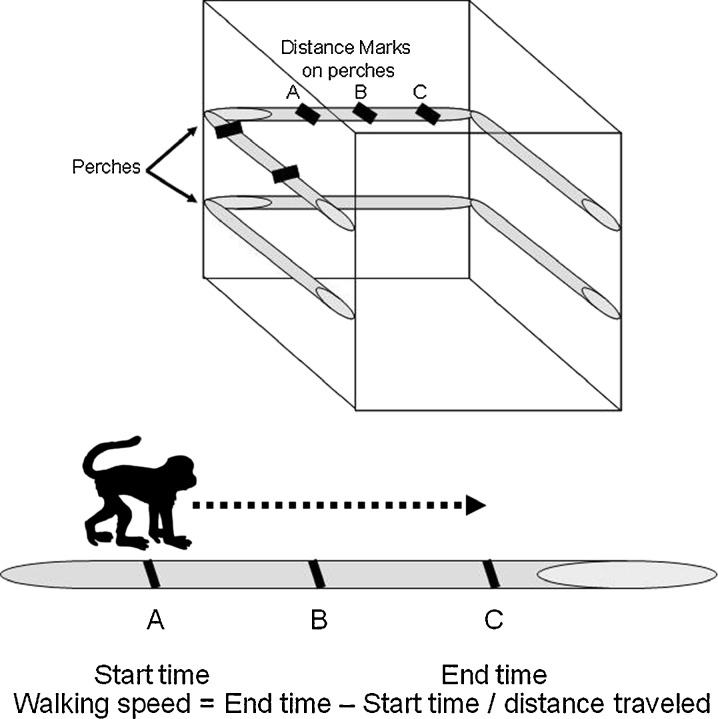
All monkeys were observed ad libitum for documentation of usual walking speed following standardized criteria (Altmann 1974). Marks were made with indelible markers at visible points on various structures throughout the home pens (Fig. 1). Each landmark was given an identification (e.g., A, B, C….), and distances between landmarks were measured. Every time a subject passed a landmark, they were timed with a stopwatch until they passed another landmark, and time and landmarks were recorded. The distance between landmarks varied from about 0.6 to 3.3 m. Walking speed was calculated as distance/time. Only instances of walking that did not include moving away after being supplanted or chased, fleeing, or moving toward a food source or other desirable objects such as a toy were recorded. Walking bouts averaged 199 cm ± 11 standard error of the mean (SEM), or about 2 m in length. Each animal was observed until a minimum of five instances of usual walking speed was recorded. For one young cynomolgus monkey, only three instances were observed; for one old bonnet monkey, only four instances were observed. Among the remaining 43 animals, recorded bouts of walking speed varied from 5–16 instances. The time required for observation varied greatly between animals from less than an hour to several hours. Observations were made early in the morning (0600–0900 hours), prior to feeding, over the course of several days, when the monkeys were most active. This approach was taken to minimize the observation time required to gather the data and the potential influence of circadian rhythms. The average of all observations and of the first five observations was calculated for analysis.
Fig. 1.
The measurement of walking speed. Marks were made with indelible markers at visible points on various structures throughout the home pens. Each landmark was given an identification (e.g. A, B, C….), and distances between landmarks were measured. Every time a subject passed a landmark they were timed with a stopwatch until they passed another landmark, and time and landmarks were recorded. Walking speed was calculated as distance/time
Other locomotor behaviors
Subjects were observed for four 15-min focal observations (i.e., 1 h/monkey, 45 h total) balanced for time of day (Altmann 1974). Each of the four observations was done on a different day. The frequency and duration of all instances of locomotion, climbing, and leaping/jumping were recorded. Locomotion was defined as traversing space at any speed with a minimum of three continuous steps. Activity level was expressed as percent time spent locomoting. Climbing was defined as traversing space in a 45° or greater vertical plane with at least 3 ft off the ground. Leaping and jumping were recorded together (leaping/jumping) and defined as no body parts touching a surface. Climbing and leaping/jumping were expressed as total frequency per hour.
Statistical analysis
The data were analyzed using a 2 (old and young) × 3 (cynomolgus, bonnet, and African green) analysis of variance. The alpha level was set at p = 0.05. As few descriptive data are available in the literature regarding aging and types of locomotion in nonhuman primates, data are presented graphically for each behavior for each species, and main effects of age and age × species interactions are reported for all dependent variables. However, it was recognized that enclosure differences between the macaques and vervets might affect rates of climbing and leaping/jumping. Thus, main effects of species in climbing and leaping/jumping are not reported as they may be due to either species or enclosure characteristics. Age × species interaction effects are presented because any interaction of age would be important to note and follow-up for clarification. Since walking speed measurements entail only quadrupedal locomotion between two points about 2 m apart, we reasoned this measure would be very little affected by the differences in enclosures, and thus, species effects are reported for this behavior. Data are reported as mean and standard errors.
Results
Body weight
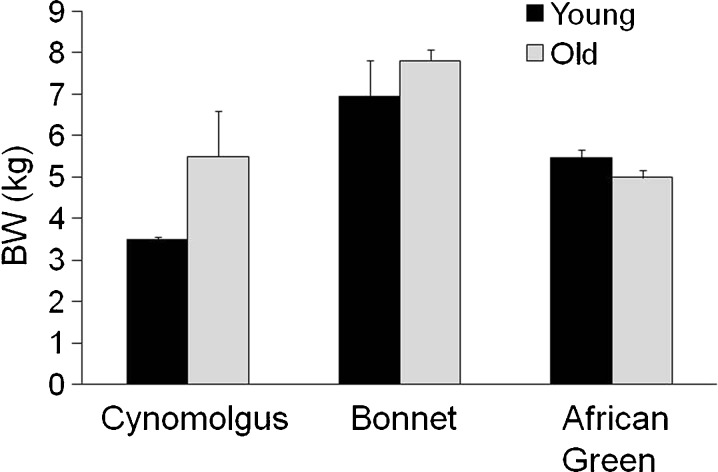
The three species differed in body weight [main effect of species, F(2,39) = 24.6, p < 0.0001] (Fig. 2). On average, old adults were heavier than young adults [main effect of age, F(1,39) = 5.3, p = 0.03], and there was a significant age by species interaction [F(2,39) = 5.1, p = 0.011]. Old macaques were heavier than younger macaques, whereas among African greens, the old adults were lighter than the younger adults.
Fig. 2.
Body weight (BW) of young and old adult female cynomolgus, bonnet, and African green monkeys. There was a significant age by species interaction in which older African greens appear lighter than younger, whereas older macaques appear heavier than younger macaques
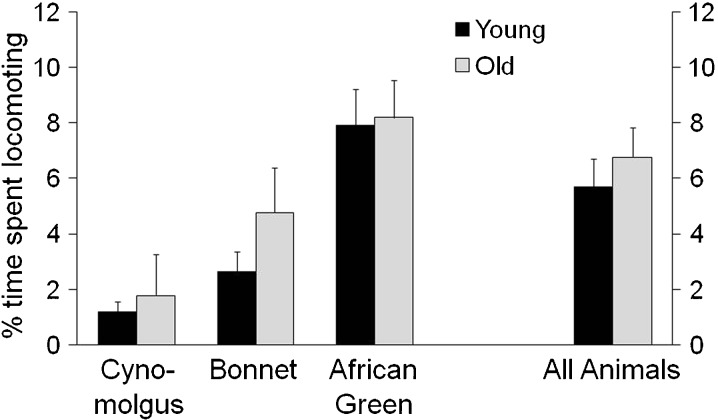
Activity levels
No difference in overall activity level was detected between old versus young monkeys [F(1,39) = 0.36, p = 0.55] nor was there a significant interaction between species and age [F(2,39) = 0.16, p = 0.85] (Fig. 3).
Fig. 3.
Activity levels of young and old adult female cynomolgus, bonnet, and African green monkeys. Activity levels were measured by the per cent of time spent locomoting (traversing space with at least three continuous steps), and did not vary significantly by age
Types of physical activity
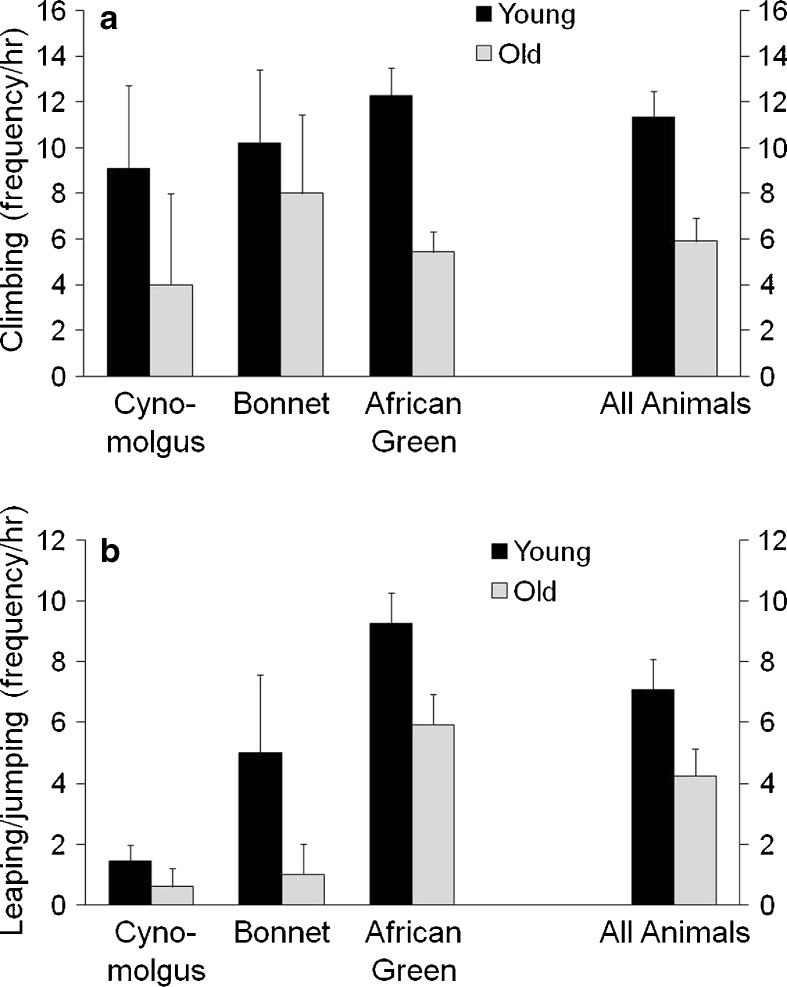
Old monkeys climbed less frequently than young monkeys [F(1,39) = 5.6, p = 0.023] (Fig. 4a, b). The age by species interaction was not significant [F(2,39) = 0.73, p = 0.49]. On average, old animals leaped and jumped less than young animals, although this difference did not reach significance [F(1,39) = 3.7, p = 0.06], and the species by age interaction was not significant [F(2,39) = 0.32, p = 0.73).
Fig. 4.
Types of physical activity of young and old adult female cynomolgus, bonnet, and African green monkeys. Climbing (4a), leaping/jumping (4b) were recorded as frequency per hour. Old monkeys climbed and tended to leap and jump less than younger monkeys
Walking speed
The number of walking events from each individual ranged from 3 to 16 (Fig. 5). We analyzed the data in two ways: (1) all samples included and (2) only the first five samples collected. Because these two measures were highly correlated (r = 0.95, p < 0.0001) and yielded similar results, the analysis of the first five samples is reported. There were species differences in mean walking speed [all samples, F(2,39) = 4.9, p = 0.01; five samples, F(2,39) = 3.4, p = 0.043]; cynomolgus appeared to walk more slowly than the other two species. The walking speed of the old monkeys was 20% slower than that of the young monkeys [all samples, F(1,39) = 13.14, p = 0.0008; five samples, F(1,39) = 9.8, p = 0.003]. The species by age interaction was not significant [all samples, F(2,39) = 0.16, p = 0.85; five samples, F(2,39) = 0.08, p = 0.92]. In general, the variability in this measure was low. The difference between the first five samples and all samples was no more than 1 cm/s in each of the six species age groups; therefore, only the data for the first five samples are shown in Fig. 5.
Fig. 5.
Walking speed (cm/s) of young and old adult female cynomolgus, bonnet, and African green monkeys. The average of the first five samples of walking speed is depicted. Older monkeys walked 20% more slowly than young monkeys
Discussion
The major finding in this study was that casual, self-motivated walking speed for old adults was slower than that of younger adults in all species and all types of housing conditions considered. The data reported here represent observations of the locomotor behaviors of three species of Old World monkeys selected to represent two age cohorts: (1) reproductively and skeletally mature young to middle-aged adult monkeys and (2) old adult monkeys. The two age ranges were similarly represented. These monkeys survive in captivity approximately 25 to 35 years or about a quarter to a third of the life span of humans (Kaufman et al. 2005; Rao et al. 1998; Weigl 2005; Freimer et al. 2007; Fears et al. 2009; Oikawa et al. 2010). Therefore, the young adult group averaging 9 years of age approximates humans that are about 30 years of age, whereas the old adult group, averaging 20 years of age, approximates humans of about 70 years of age. In humans, usual gait speed remains stable through the sixth decade of life. At older ages, there is an accelerating decline with the loss of about 0.1 m/s in speed per decade (Guralnik et al. 2000; Shumway-Cook et al. 2007). While few studies have comprehensively assessed usual gait speed across as wide a proportion of the life span as studied here, data suggest that gait speeds would be approximately 15–20% lower were a similar comparison to be made between older and younger adult humans (Himann et al. 1988; Samson et al. 2001; Shumway-Cook et al. 2007). Thus, the observations reported here of a 20% decline in walking speed of the old monkeys appears to be consistent with what would be expected in humans.
Animal models of physical mobility and aging have mostly been confined to rodents. While this work has been helpful in understanding some of the biological factors that contribute to healthy aging, rodent models are limited by several key characteristics that make them dissimilar to primates and unsuitable for study of the association of physical mobility with risk of many diseases. For example, unlike primates, rodents continue to grow slowly throughout life and their skeletal metabolism differs from that of primates (Jerome 2004). Old World monkeys (e.g. macaques, baboons, African green monkeys) are the most commonly studied nonhuman primate models, and they share several characteristics and diseases associated with aging with humans. For instance, macaques are useful models for age-related bone loss (Black et al. 2001). Furthermore, percentage declines in estimated skeletal muscle mass with age in macaques are similar to those seen in humans with advancing age, making them useful models of sarcopenia (Colman et al. 2005). Like human muscle, aging macaque muscle tissue demonstrates selective upregulation of genes involved in inflammation and oxidative stress, and downregulation of genes involved in mitochondrial electron transport and oxidative phosphorylation (Kayo et al. 2001). Furthermore, Old World monkeys are preferred models for the study of whole body obesity (Kemnitz 1984) as well as some aspects of fat distribution. As in people, obesity is associated with type two diabetes in Old World monkeys (Wagner et al. 2006; Chavez et al. 2009). Central fat deposition patterns are associated with social stress as well as carotid and coronary artery atherosclerosis in cynomolgus monkeys and with cardiovascular disease in people (Shively et al. 2009). Finally, Old World monkeys are optimal models for investigations involving cognitive decline and Alzheimer’s disease due to their similarities to humans in central nervous system mobility (Gallagher and Rapp 1997; Voytko and Tinkler 2004; Kulstad et al. 2005; Zeng et al. 2006). Thus, Old World monkeys are useful models in which to study relationships between risk of many diseases important in human health and physical mobility.
Gait analysis in large animal models has received an increasing amount of attention as a way to quantify both musculoskeletal diseases and also to assess the efficacy of clinical interventions (Gillette and Angle 2008; Marsh et al. 2010). In nonhuman primates, kinematic and kinetic gait analyses have been conducted using overground and treadmill walking (Courtine et al. 2005; Ogihara et al. 2010). However, we are not aware of published studies using kinematic or observational gait analysis in nonhuman primate models of aging. The ability to objectively measure these functional domains in a clinically relevant primate model will enhance the translational value of preclinical studies of aging.
We chose to present data from three primate species to inform future studies assessing aging, physical mobility, and health in nonhuman primates. Macaques and vervets were in different housing situations which might affect locomotion. Since species and enclosure type were confounded, the lack of significant species × age interactions implies a lack of enclosure × age interactions. However, since species and enclosure type could not be differentiated, species effects were not reported for locomotor behaviors that may be affected by enclosure conditions including climbing, leaping and jumping. Species effects were observed and reported for body weight and also for walking speed, since all enclosures allowed for plenty of opportunity for quadrapedal locomotion for a distance long enough to measure this behavior. Cynomolgus monkeys are the smallest of the three species and have the shortest stride which may account for their slowest walking speeds.
Data were presented from females only primarily because we had very few known aged male monkeys to study. It is useful to have these data in females as there are more older women than men, and more frail women than men (Walston and Fried 1999; Syddall et al. 2010); however, these observations need to be extended to male nonhuman primates. Although the females were presumably having menstrual cycles, observations were made without respect to time of cycle because activity levels do not vary by menstrual cycle phase in macaques or women (Barger et al. 2008).
There were age differences in the way animals moved. It is interesting that general activity levels were not significantly different by age whereas, types of movement that may require strength and balance, such as climbing and perhaps leaping/jumping, were less frequent among old adults. While there were age differences in climbing, there was more variability in this behavior than in walking speed. Walking speed was characterized by comparatively low variance and an average difference of 17% between old and younger adults. Walking speed was sensitive to age-related differences in locomotion under a variety of social and physical conditions. A limited number of observations of walking speed (about 5 per subject or 222 in all) appeared to well approximate the total number of observations (312) suggesting that limited observation time is necessary to get valid measures.
Walking speed was sensitive to age in three different nonhuman primate species, in a variety of social and physical conditions. Taken together, these data suggest that walking speed, measured in a simple, inexpensive manner, was the most promising indicator of global physial mobility among the several locomotor behaviors profiled in this report as it varied by age independent of varying social and physical conditions.. A validated global measure of physical mobility in nonhuman primates will be useful in investigating the role of declining physical mobility with age in other changes in health. For example, the relationship between muscle loss and fatty infiltration into muscle and physical mobility may be systematically studied. Likewise, the timing of declines in physical mobility in relation to other changes in health may also be assessed under controlled conditions. Thus, we may be able to determine whether loss of physical mobility precedes or follows decrements in other areas critical for late-life independence such as cognition and emotional health. We can also study factors which influence the reacquisition of physical mobility after an acute infection and whether that varies with age. A simple measure of physical mobility will allow the use of these nonhuman primates to study the biological systems leading to reductions in gait speed and underlying relationships between gait speed and morbidity and perhaps identify novel targets for interventions that promote mobility and independence in late life.
Acknowledgements
This work was supported by the Pepper Older Americans for Independence Center (P30 AG21332), the Vervet Research Colony (P40 RR019963), R01 AA013973 and intramural grants from the Translational Science Institute and the Primate Center of Wake Forest School of Medicine.
References
- Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974X00534. [DOI] [PubMed] [Google Scholar]
- Bannon MJ, Poosch MS, Xia Y, Goebel DJ, Cassin B, Kapatos G. Dopamine transporter mRNA content in human substantia nigra decreases precipitously with age. Proc Natl Acad Sci U S A. 1992;89(15):7095–7099. doi: 10.1073/pnas.89.15.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger LK, Hoban-Higgins TM, Fuller CA. Assessment of circadian rhythms throughout the menstrual cycle of female rhesus monkeys. Am J Primatol. 2008;70(1):19–25. doi: 10.1002/ajp.20451. [DOI] [PubMed] [Google Scholar]
- Black A, Tilmont EM, Handy AM, et al. A nonhuman primate model of age-related bone loss: a longitudinal study in male and premenopausal female rhesus monkeys. Bone. 2001;28(3):295–302. doi: 10.1016/S8756-3282(00)00452-X. [DOI] [PubMed] [Google Scholar]
- Cass WA, Grondin R, Andersen AH, et al. Iron accumulation in the striatum predicts aging-related decline in motor function in rhesus monkeys. Neurobiol Aging. 2007;28(2):258–271. doi: 10.1016/j.neurobiolaging.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Chavez AO, Gastaldelli A, Guardado-Mendoza R, et al. Predictive models of insulin resistance derived from simple morphometric and biochemical indices related to obesity and the metabolic syndrome in baboons. Cardiovasc Diabetol. 2009;8:22. doi: 10.1186/1475-2840-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, McKiernan SH, Aiken JM, Weindruch R. Muscle mass loss in rhesus monkeys: age of onset. Exp Gerontol. 2005;40:573–581. doi: 10.1016/j.exger.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Cooper R, Kuh D, Hardy R, Mortality Review Group. FALCon and HALCyon Study Teams Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Roy RR, Hodgson J, et al. Kinematic and EMG determinants in quadrupedal locomotion of a non-human primate (Rhesus) J Neurophysiol. 2005;93(6):3127–3145. doi: 10.1152/jn.01073.2004. [DOI] [PubMed] [Google Scholar]
- Emborg ME, Ma SY, Mufson EJ, et al. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401(2):253–265. doi: 10.1002/(SICI)1096-9861(19981116)401:2<253::AID-CNE7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Era P, Heikkinen E. Postural sway during standing and unexpected disturbance of balance in random samples of men of different ages. J Gerontol. 1985;40(3):287–295. doi: 10.1093/geronj/40.3.287. [DOI] [PubMed] [Google Scholar]
- Fears SC, Melega WP, Service SK, et al. Identifying heritable brain phenotypes in an extended pedigree of vervet monkeys. J Neurosci. 2009;29(9):2867–2875. doi: 10.1523/JNEUROSCI.5153-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Penninx BW, Leveille SG, et al. Characteristics of nondisabled older persons who perform poorly in objective tests of lower extremity function. J Am Geriatr Soc. 2000;48(9):1102–1110. doi: 10.1111/j.1532-5415.2000.tb04787.x. [DOI] [PubMed] [Google Scholar]
- Freimer NB, Service SK, Ophoff RA, et al. A quantitative trait locus for variation in dopamine metabolism mapped in a primate model using reference sequences from related species. Proc Natl Acad Sci U S A. 2007;104(40):15811–15816. doi: 10.1073/pnas.0707640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Henson M, et al. Age-related changes in potassium-evoked overflow of dopamine in the striatum of the rhesus monkey. Neurobiol Aging. 1995;16(6):939–946. doi: 10.1016/0197-4580(95)02013-6. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Cass WA, Yi A, Zhang Z, Gash DM. Changes in somatodendritic but not terminal dopamine regulation in aged rhesus monkeys. J Neurochem. 2002;80(1):168–477. doi: 10.1046/j.0022-3042.2001.00684.x. [DOI] [PubMed] [Google Scholar]
- Gillette RL, Angle TC. Recent developments in canine locomotor analysis: a review. Vet J. 2008;178(2):165–176. doi: 10.1016/j.tvjl.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.M221. [DOI] [PubMed] [Google Scholar]
- Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Med Sci Sports Exerc. 1988;20(2):161–166. doi: 10.1249/00005768-198820020-00010. [DOI] [PubMed] [Google Scholar]
- Irwin I, DeLanney LE, McNeill T, et al. Aging and the nigrostriatal dopamine system: a non-human primate study. Neurodegeneration. 1994;3(4):251–265. [PubMed] [Google Scholar]
- Jerome CP. Hormonal therapies and osteoporosis. ILAR J. 2004;45:170–178. doi: 10.1093/ilar.45.2.170. [DOI] [PubMed] [Google Scholar]
- Kastman EK, Willette AA, Coe CL, et al. A calorie-restricted diet decreases brain iron accumulation and preserves motor performance in old rhesus monkeys. J Neurosci. 2010;30(23):7940–7947. doi: 10.1523/JNEUROSCI.0835-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D, Smith ELP, Gohil BC, et al. Early appearance of metabolic syndrome in socially reared bonnet macaques. J Clin Endocrinol Metab. 2005;90(1):404–408. doi: 10.1210/jc.2004-0452. [DOI] [PubMed] [Google Scholar]
- Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc Natl Acad Sci U S A. 2001;98:5093–5098. doi: 10.1073/pnas.081061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW. Obesity in macaques: spontaneous and induced. Adv Vet Sci Comp Med. 1984;28:81–114. doi: 10.1016/b978-0-12-039228-5.50009-7. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Robitaille Y, el-Awar M, et al. Striatal monoamine neurotransmitters and metabolites in dominantly inherited olivopontocerebellar atrophy. Neurology. 1992;42(8):1573–1577. doi: 10.1212/WNL.42.8.1573. [DOI] [PubMed] [Google Scholar]
- Kulstad JJ, McMillan PJ, Leverenz JB, et al. Effects of chronic glucocorticoid administration on insulin-degrading enzyme and amyloid-beta peptide in the aged macaque. J Neuropathol Exp Neurol. 2005;64(2):139–146. doi: 10.1093/jnen/64.2.139. [DOI] [PubMed] [Google Scholar]
- Marsh AP, Eggebeen JD, Kornegay JN, Markert CD, Childers MK. Kinematics of gait in golden retriever muscular dystrophy. Neuromuscul Disord. 2010;20(1):16–20. doi: 10.1016/j.nmd.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- Ogihara N, Makishima H, Nakatsukasa M. Three-dimensional musculoskeletal kinematics during bipedal locomotion in the Japanese macaque, reconstructed based on an anatomical model-matching method. J Hum Evol. 2010;58(3):252–261. doi: 10.1016/j.jhevol.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Oikawa N, Kimura N, Yanagisawa K. Alzheimer-type tau pathology in advanced aged nonhuman primate brains harboring substantial amyloid deposition. Brain Res. 2010;1315:137–149. doi: 10.1016/j.brainres.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Rao AJ, Ramesh V, Ramachadra SG, Krishnamurthy HN, Ravindranath N, Moudgal NR. Growth and reproductive parameter of bonnet monkey (Macaca radiata) Primates. 1998;39(1):97–107. doi: 10.1007/BF02557748. [DOI] [Google Scholar]
- Samson MM, Crowe A, Vreede PL, Dessens JA, Duursma SA, Verhaar HJ. Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging (Milano) 2001;13(1):16–21. doi: 10.1007/BF03351489. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis in female primates. Obesity (Silver Spring) 2009;17(8):1513–1520. doi: 10.1038/oby.2009.74. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc. 2007;55(1):58–65. doi: 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syddall H, Roberts HC, Evandrou M, Cooper C, Bergman H, Aihie Sayer A. Prevalence and correlates of frailty among community-dwelling older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2010;39(2):197–203. doi: 10.1093/ageing/afp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Tinkler GP. Cognitive function and its neural mechanisms in nonhuman primate models of aging, Alzheimer disease, and menopause. Front Biosci. 2004;9:1899–1914. doi: 10.2741/1370. [DOI] [PubMed] [Google Scholar]
- Wagner JD, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJ, Jr, Kaplan JR. Old World nonhuman primate models of type 2 diabetes mellitus. ILAR J. 2006;47(3):259–271. doi: 10.1093/ilar.47.3.259. [DOI] [PubMed] [Google Scholar]
- Walston J, Fried LP. Frailty and the older man. Med Clin North Am. 1999;83(5):1173–1194. doi: 10.1016/S0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- Walton A, Branham A, Gash DM, Grondin R. Automated video analysis of age-related motor deficits in monkeys using EthoVision. Neurobiol Aging. 2006;27(10):1477–1483. doi: 10.1016/j.neurobiolaging.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Weigl R (2005) Longevity of mammals in captivity; from the living collections of the world. Kleine Senckenberg-Reihe 48: Stuttgart
- Zeng F, Southerland JA, Voll RJ, et al. Synthesis and evaluation of two 18F-labeled imidazo[1,2-a]pyridine analogues as potential agents for imaging beta-amyloid in Alzheimer’s disease. Bioorg Med Chem Lett. 2006;16(11):3015–3058. doi: 10.1016/j.bmcl.2006.02.055. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Andersen A, Smith C, Grondin R, Gerhardt G, Gash D. Motor slowing and parkinsonian signs in aging rhesus monkeys mirror human aging. J Gerontol A Biol Sci Med Sci. 2000;55(10):B473–B480. doi: 10.1093/gerona/55.10.B473. [DOI] [PubMed] [Google Scholar]