SUMMARY
Although post-rehabilitation is routinely performed following flexor tendon repair, in some clinical scenarios post-rehabilitation must be delayed. We investigated modification of the tendon surface using carbodiimide derivatized hyaluronic acid and lubricin (cd-HA-Lub) to maintain gliding function following flexor tendon repair with postoperative immobilization in a in vivo canine model. Flexor digitorum profundus tendons from the 2nd and 5th digits of one forepaw of six dogs were transected and repaired. One tendon in each paw was treated with cd-HA-Lub; the other repaired tendon was not treated. Following tendon repair, a forearm cast was applied to fully immobilize the operated forelimb for 10 days, after which the animals were euthanized. Digit normalized work of flexion (nWOF) and tendon gliding resistance were assessed. The nWOF of the FDP tendons treated with cd-HA-Lub was significantly lower than the nWOF of the untreated tendons (p < 0.01). The gliding resistance of cd-HA-Lub treated tendons was also significantly lower than that of the untreated tendons (p < 0.05). Surface treatment with cd-HA-Lub following flexor tendon repair provides an opportunity to improve outcomes for patients in whom the post-operative therapy must be delayed after flexor tendon repair.
Keywords: Lubricin, Canine, Flexor Tendon, Immobilization, Tendon Repair
INTRODUCTION
Flexor tendon injuries are common, and primary surgical repair combined with postoperative mobilization protocols is the gold standard for treatment. However, complications are still frequent,1–4 including adhesion formation and rupture of the repaired tendon.5 Early mobilization of the repaired tendon is an effective method to improve functional outcomes post repair, but such therapy may not always be possible, due to associated injuries or the ability of the patient to cooperate with the therapy regimen.6–9 In addition, repaired tendon rupture is caused by the tensile load applied to the tendon that exceeds the repair holding strength, particular in the first days and weeks after repair when the suture strength is decreased by edema, tissue softening, and inflammation.5,10,11 Although postoperative therapy itself reduces rupture and improves motion compared to immobilization, postoperative motion can result in repair rupture due to overloaded active motion, unexpected hand reaction, or other misuse of the operated fingers.5,11,12 However, while mobilization in the first few weeks places the repaired tendon at higher risk for gap formation or rupture, immobilization, even for 10 days, places the repaired tendon at risk for adhesion formation.10,13,14 A treatment that could permit an extended initial period of tendon immobilization condition without or with minimal adhesion formation could therefore be clinically important.
Recently, tendon surface modification used in association with flexor tendon repair demonstrated promising outcomes in an animal model.15,16 Surface modification with carbodiimide derivatized hyaluronic acid plus lubricin (cd-HA-Lub) following flexor tendon repair reduced adhesions in combination with standard postoperative rehabilitation in an animal model.16 This coating may serve more as a barrier to prevent adhesions than as a lubricant to improve gliding ability.15–17
We used this novel intervention after flexor repair with immobilization to detremine if it might extend the time that tendons can be immobilized without adverse effects on tendon mobilization. We hypothesized that surface modification with cd-HA-Lub would maintain the initial post repair gliding resistance and work of flexion following short term immobilization in vivo.
MATERIALS AND METHODS
Tendon Repair and Surface Modification
6 mixed-breed adult dogs (20 to 25 kg) were used in this study, which was approved by our Institutional Animal Care and Use Committee. Tendon repair was accomplished as described previously.16 In brief, after anesthetization, one randomly selected forelimb was shaved, sterilized, and draped. The 2nd and 5th FDP tendons were exposed and lacerated at level 5 mm distal to the respectively proximal digit flexor pulley and repaired with a 2-strand modified Pennington technique reinforced with a simple running circumferential epitenon suture using 3/0 Ethibond and a 6/0 nylon (Ethicon Inc., Somerville, NJ), respectively. Following tendon repair, one tendon was randomly selected for tendon surface modification with cd-HA-gelatin plus lubricin (cd-HA-Lub) with the following protocol. First, a solution of 1% sodium hyaluronate (HA) (95%, 1.5×106MW, Acros), 10% gelatin (Sigma), 1% 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Sigma), and 1% N-hydroxysuccinimide (NHS) (Pierce), 0.1 M Mes pH 6.0 was prepared.18 The repaired tendon was coated with this compound and maintained for 5 minutes for gelation. After gelation, the excess was removed by moving the repaired tendon back and forth under the proximal pulley for five cycles. Finally, 0.2 ml of lubricin, 260 μg/ml was applied to tendon surfaces treated with cd-HA-gelatin.16 The tendon surface of the control group was rinsed with saline only. The flexor fibro-osseous sheath was not closed. The subcutaneous layer and skin were closed subcuticularly, and the paw was sterilely dressed. Once the flexor tendon was repaired, a high radial neurectomy was performed through a lateral humeral incision to prevent wrist active extension and weight bearing.19 A forearm cast was applied to fully immobilize the digit joint in neutral position and the wrist in 45° of flexion. Cage activity was allowed immediately after surgery. The cast was changed at day 3 and 7 postoperatively for wound care, and then placed back until euthanasia at day 10.
Functional Evaluation
Following sacrifice, the 2nd and 5th digit in the operative paws and contralateral normal paws were dissected for work of flexion (WOF) evaluation based on a previously established protocol,14 including the metacarpophalangeal (MCP), proximal interphalangeal (PIP), and distal interphalangeal (DI) joints. Briefly, the MCP joint was fixed in extension by inserting a K-wire longitudinally through the metacarpal and proximal phalanx bones. Two reflective markers attached to a “T” shaped pin were affixed to the proximal, middle and distal phalanges, respectively. The K-wire was used to mount the extended phalange onto the testing device with a custom jig. The proximal FDP tendon in zone III area was connected to an actuator with a load transducer (Techniques, Temecula, CA) through a cable. The actuator pulled the tendon proximally at 2 mm/sec, causing digit flexion. Digit motion represented by the markers was recorded using two orthogonal video cameras, and marker motion was processed by motion analysis software (Motion Analysis Corp., Santa Rosa, CA). DIP joint motion was determined by changes in the angle between the “T” markers of the distal and middle phalanges, and PIP joint motion was determined by changes in the angle between the “T” markers of the middle and proximal phalanges. Work of flexion was defined as the area under the tendon displacement vs. loading curve during digit flexion. The work of flexion was calculated by force/displacement area at the DIP angle truncated at 40°. Since the PIP angle was variable, the normalized WOF (nWOF) was then calculated by dividing the sum of PIP and DIP angle based on previous reports.20
After measuring WOF, the digits were dissected further with the proximal pulley kept intact. The gliding resistance between the tendon repair site and proximal pulley was then measured using a custom tendon-pulley friction test device using techniques previously described.21 Briefly, the dissected digit including the intact proximal pulley and repaired tendon was mounted to the frame using the K-wire that fixed the MCP joint in extension. The mechanical actuator and a load transducer were attached to the proximal tendon end, and a load transducer and 500-g weight were attached to the distal end. Tendons were pulled proximally at 2 mm/sec, and the difference in force between proximal and distal transducers was used to determine the gliding resistance. Finally, tensile testing was performed using a servohydraulic testing machine (MTS Systems, Eden Prairie, MN). Tendons were clamp in place and distracted to failure at 20 mm/min. A differential variable reluctance transducer (DVRT, Microstrain, Williston, VT) was attached to the tendon by two barbed pins that were inserted into the tendon, centering the repair site between pins, to measure gap formation during distraction. Tensile force, actuator displacement and gap displacement measured by the DVRT were recorded at 20 Hz. A custom MATLAB program (Mathworks, Natick, MA ) was used to identify maximum failure load and to calculate repair stiffness (a measure of resistance to gap formation) from the slope of the linear region of the force versus gap formation curve.
Statistical Methods
One way or two-way ANOVA was used to analyze the differences in nWOF, gliding resistance, repair strength, and stiffness between treatment groups. Any p-value <0.05 was considered significant.
RESULTS
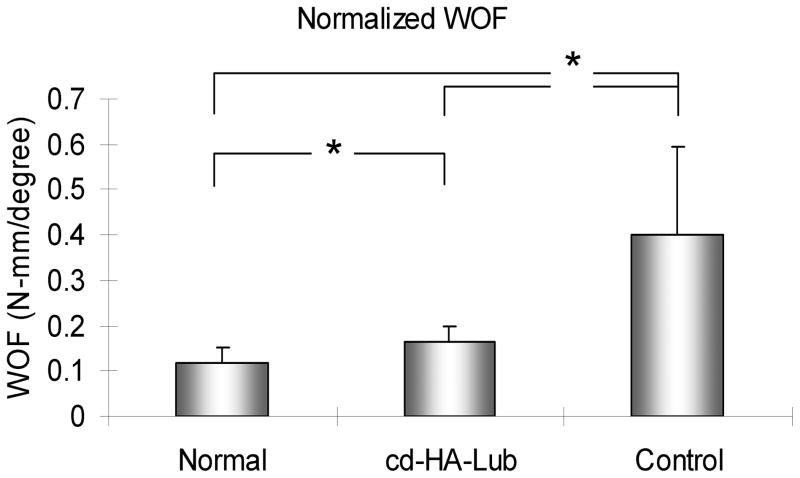
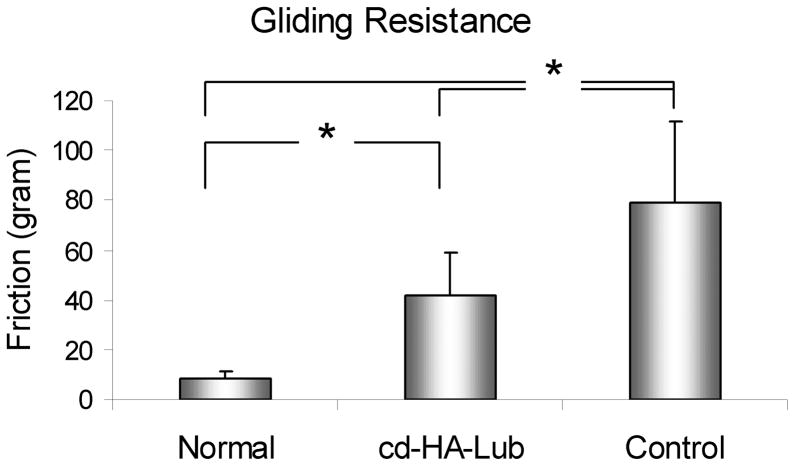
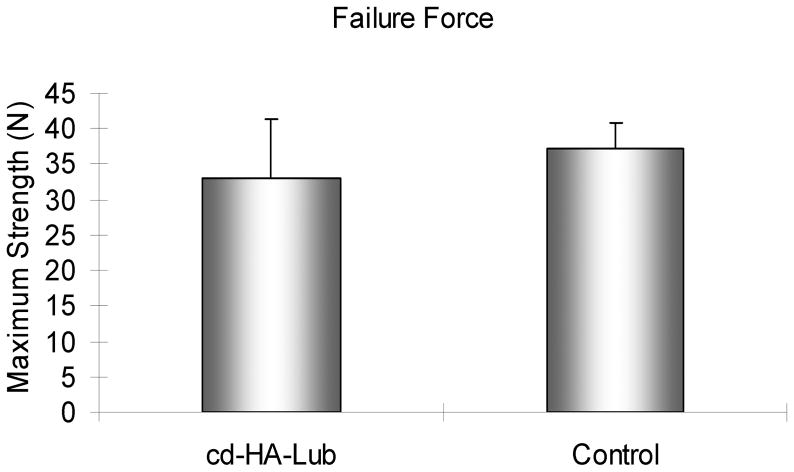
The nWOF of the normal digits from the non-surgical contralateral paw was significantly lower than the surgical digits, regardless of treatment (p < 0.05). The nWOF of the FDP tendons treated with cd-HA-Lub was significantly lower than the control repaired FDP tendons (p < 0.05) (Fig. 1). The gliding resistance of the normal FDP tendons was significantly lower than that of the repaired FDP tendons regardless of treatment (p < 0.05). The gliding resistance of cd-HA-Lub treated tendons was significantly lower than that of the control group (p < 0.05) (Fig. 2). No significant difference was found in maximum failure strength or stiffness when comparing repaired tendons with and without cd-HA-Lub treatment (Fig. 3).
Figure 1.
The work of flexion normalized by PIP and DIP angle (nWOF) in repaired tendons with and without cd-HA-Lub treatment and the contralateral normal digit after 10 days of immobilization. Asterisk denotes a significant difference (p < 0.05).
Figure 2.
The gliding resistance in repaired tendons with and without cd-HA-Lub treatment and the contralateral normal FDP tendon. Asterisk denotes a significant difference (p < 0.05).
Figure 3.
Tensile strength of the repaired tendons with and without cd-HA-Lub.
DISCUSSION
Until the early 1970’s, postoperative immobilization for a few weeks was a standard method following flexor tendon primary repair in zone II , in which two flexor tendons pass through a narrow osseofibrous tunnel in fingers. This standard treatment was based on canine research of Mason and Allen,22 who had shown that the tensile strength of canine flexor tendon repairs was decreased up to three weeks postoperatively. However, adhesion formation following immobilization interfered with tendon gliding, impaired hand function, and resulted in poor outcomes in many cases. Subsequent improvement in surgical methods, repair techniques, suture materials, and rehabilitation methods allowed earlier mobilization with improved clinical outcomes.1,23–27 Currently, the clinical standard is for postoperative mobilization after flexor tendon primary repair, whenever this is clinically possible.4,28,29 However, complete immobilization postoperatively is the most conservative approach and still holds a place in hand rehabilitation for patients who cannot cooperate with therapy or have associated injuries which preclude motion8. Stewart and van Strien stated that “no matter how sophisticated our therapeutic and surgical care becomes, there probably will always be need for immobilization of flexor tendon repairs in some circumstances”.30 In such cases, an alternative method to manage flexor tendon injuries postoperatively, which does not require mobilization but preserves benefits of mobilization in terms of improved tendon gliding, would be useful.
The flexor tendon surface is covered by a thin layer of epitenon that contains several layers of epitenon cells embedded within an extracellular matrix including hyaluronic acid, proteoglycans, and phospholipids. This composite of extracellular matrix functions as a tendon ‘skin’ to protect the tendon from abrasion wear, decrease tendon friction, and prevent adhesions.31–33 However, after flexor tendon injury the integrity of the epitenon is damaged, especially at the repair site, which allows cells from surrounding tissues to attach to the tendon and form adhesions. Increased frictional force after tendon injury and repair further jeopardizes gliding and promotes adhesion formation.34 Recently, Taguchi et al. used two substances in epitenon, hyaluronic acid and lubricin, chemically crosslinked to the tendon surface, to restore the epitenon skin-like function. They found that repaired flexor tendons treated with this surface modification had significantly decreased gliding resistance and increased durability of surface integrity.15 More recently, Zhao, et al. applied this technique in a canine flexor tendon repair model in vivo and found that adhesions were noticeably decreased and digit function was significantly improved compared to repaired tendons without surface treatment.16 These findings provide the rationale to investigate the possibility that surface treatment might be of benefit in those cases where repaired tendons must be immobilized postoperatively. Our current results indeed showed that the repaired tendons treated with cd-HA-Lub had significantly better digit function and gliding resistance compared to the untreated tendons following 10 days of immobilization. This stands in contrast to the previous study by Gelberman et al. that reported that adhesions between the repaired tendon and flexor sheath were observed with 10 days of immobilization after flexor tendon repair in a canine model.10,35 Recently, Zhao et al. also demonstrated increased adhesions and reduced gliding function after 10 days of immobilization, compared to mobilized tendons.14 Our results suggest that the surface modification that we used in the current study may play a role equivalent to postoperative therapy in maintaining tendon gliding ability, at least within 10 days of surgery.
The mechanism by which the surface modification maintains gliding function despite immobilization may be related to both physical and biological effects. cd-HA-Lub can serve as a lubricant to reduce friction and increase the durability of the tendon by protecting tenocytes from abrasive injury. This coating also provides a physical barrier to prevent adhesion formation. Recent studies also demonstrated that lubricin has anti-adhesive properties that prohibit cell attachment.36,37 Simple application of these substances alone or combination, without chemical modification, does not show any benefits to tendon lubrication,15,18,38 but this may due to the poor efficiency of substance attachment or the short half-life of hyaluronic acid and lubricin in vivo.39,40 The carbodiimide crosslinking reagent significantly increases the binding efficiency of hyaluronic acid and increases hyaluronic acid half-life on the tendon surface from 3 days to as long as 3 weeks.41
Immobilization following flexor tendon primary repair not only causes adhesion formation, but also weakens tendon strength, especially when immobilization continues for 3 weeks.10,35,42 Although it is not fully understood why the repaired tendon is weak with immobilization, histological findings showed altered tendon structure and delayed tissue remodeling, which impairs the normal healing process.43,44 However, in the current study, the repair strength at 10 days was not significantly affected by the mobilization status. This might indicate that tissue remodeling was not involved at 10 days. Similar findings were also reported from other experimental studies in which mobilization initiated on day 1, 3, 5, or 7 did not alter the mechanical strength within 10 days of repair.13,14 However, mobilization started the day after repair resulted in more ruptures compared to mobilization starting at day 5.14 The current study suggests that repaired tendon could be further immobilized until 10 days without risk of adhesions if a surface modification is included. This short-term immobilization may provide a beneficial window for the coupled tissue injuries to become tolerant for mobilization later. It may also offer time for the patient to rest physically and psychologically following trauma, and potentially reduce pain when mobilization does begin. However, mobilization is necessary at some point to increase the tendon healing quality and hand function.
This work has several limitations. First, the sample size is small. However, the small standard deviation and relatively large differences did produce significant differences. Second, we only focused on digit function and tendon gliding. Other dependent variables such as adhesion status, measurement of the residual hyaluronic acid and lubricin, histological evaluation of healing, and the effect of cell viability were not studied. We wanted to first establish whether there was a protective effect on friction and digit function. Third, this study was performed at only one time point. Therefore, the long term effect of cd-HA-Lub on adhesion and healing remains unknown. Again, our rationale was to assess a basic effect, and then to pursue the mechanism in more animals only if an effect was noted. This strategy should minimize the number of experimental animals needed for the overall analysis. Based on our findings, we plan to conduct additional studies with more outcome measures, with larger sample size, and with additional long-term time points.
In summary, we investigated the possibility to protect repaired flexor tendons from known adverse effects of immobilization, using a cd-HA-Lub surface modification. The results encouragingly showed that this surface modification maintained digit function and tendon gliding capability. If validated by future studies as outlined above, surface treatment may become a useful adjunct for patients who are not candidates for early mobilization.
Acknowledgments
This study was funded by a grant from NIH/NIAMS (AR44391).
References
- 1.Silfverskiold KL, May EJ, Oden A. Factors affecting results after flexor tendon repair in zone II: a multivariate prospective analysis. J Hand Surg [Am] 1993;18:654–662. doi: 10.1016/0363-5023(93)90312-Q. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez JD, Stern PJ. Complex injuries including flexor tendon disruption. Hand Clin. 2005;21:187–197. doi: 10.1016/j.hcl.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Groth GN. Current practice patterns of flexor tendon rehabilitation. J Hand Ther. 2005;18:169–174. doi: 10.1197/j.jht.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Chesney A, Chauhan A, Kattan A, et al. Systematic review of flexor tendon rehabilitation protocols in zone II of the hand. Plast Reconstr Surg. 2011;127:1583–1592. doi: 10.1097/PRS.0b013e318208d28e. [DOI] [PubMed] [Google Scholar]
- 5.Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clin. 2005;21:199–210. doi: 10.1016/j.hcl.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Strickland JW, Glogovac SV. Digital function following flexor tendon repair in Zone II: A comparison of immobilization and controlled passive motion techniques. J Hand Surg [Am] 1980;5:537–543. doi: 10.1016/s0363-5023(80)80101-8. [DOI] [PubMed] [Google Scholar]
- 7.Sturm JT, Cicero JJ. Emergency management of hand injuries. When to repair, when to refer. Postgrad Med. 1986;80:97–103. doi: 10.1080/00325481.1986.11699559. [DOI] [PubMed] [Google Scholar]
- 8.Vucekovich K, Gallardo G, Fiala K. Rehabilitation after flexor tendon repair, reconstruction, and tenolysis. Hand Clin. 2005;21:257–265. doi: 10.1016/j.hcl.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong MB, Adeogun O. Tendon injuries in the pediatric hand. J Craniofac Surg. 2009;20:1005–1010. doi: 10.1097/SCS.0b013e3181abb141. [DOI] [PubMed] [Google Scholar]
- 10.Gelberman RH, Manske PR, Akeson WH, et al. Flexor tendon repair. J Orthop Res. 1986;4:119–128. doi: 10.1002/jor.1100040116. [DOI] [PubMed] [Google Scholar]
- 11.Harris SB, Harris D, Foster AJ, Elliot D. The aetiology of acute rupture of flexor tendon repairs in zones 1 and 2 of the fingers during early mobilization. J Hand Surg [Br] 1999;24:275–280. doi: 10.1054/jhsb.1998.0212. [DOI] [PubMed] [Google Scholar]
- 12.Elliot D, Moiemen NS, Flemming AF, et al. The rupture rate of acute flexor tendon repairs mobilized by the controlled active motion regimen. J Hand Surg [Br] 1994;19:607–612. doi: 10.1016/0266-7681(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhao C, Amadio PC, Paillard P, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2004;86-A:320–327. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Zhao C, Amadio PC, Tanaka T, et al. Short-term assessment of optimal timing for postoperative rehabilitation after flexor digitorum profundus tendon repair in a canine model. J Hand Ther. 2005;18:322–329. doi: 10.1197/j.jht.2005.04.017. quiz 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taguchi M, Sun Y, Zhao C, et al. Lubricin surface modification improves tendon gliding after tendon repair in a canine model in vitro. J Orthop Res. 2009;27:257–263. doi: 10.1002/jor.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao C, Sun Y-L, Kirk RL, et al. Effects of a lubricin-containing compound on the results of flexor tendon repair in a canine model in vivo. J Bone Joint Surg Am. 2010;92:1453–1461. doi: 10.2106/JBJS.I.00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C, Amadio P, Sun Y, et al. Tendon Surface Modification by Chemically Modified HA Coating after Flexor Digitorum Profundus Tendon Repair. Transactions of the 49th Annual Meeting of Orthopedic Research Society; New Orleans, LA.. 2003. [DOI] [PubMed] [Google Scholar]
- 18.Sun YL, Yang C, Amadio PC, et al. Reducing friction by chemically modifying the surface of extrasynovial tendon grafts. J Orthop Res. 2004;22:984–989. doi: 10.1016/j.orthres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Bishop AT. Functioning free-muscle transfer for brachial plexus injury. Hand Clin. 2005;21:91–102. doi: 10.1016/j.hcl.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Zhao C, Sun Y-L, Ikeda J, et al. Improvement of flexor tendon reconstruction with carbodiimide-derivatized hyaluronic acid and gelatin-modified intrasynovial allografts: study of a primary repair failure model. J Bone Joint Surg Am. 2010;92:2817–2828. doi: 10.2106/JBJS.I.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchiyama S, Amadio PC, Coert JH, et al. Gliding resistance of extrasynovial and intrasynovial tendons through the A2 pulley. J Bone Joint Surg Am. 1997;79:219–224. doi: 10.2106/00004623-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Mason M, Allen H. The rate of healing of tendons. An excperimental study of tensile strength. Ann Surg. 1941;113:424–459. doi: 10.1097/00000658-194103000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verdan CE. Half a century of flexor-tendon surgery. Current status and changing philosophies. J Bone Joint Surg Am. 1972;54:472–491. [PubMed] [Google Scholar]
- 24.Kleinert HE, Kutz JE, Atasoy E, Stormo A. Primary repair of flexor tendons. Orthop Clin North Am. 1973;4:865–876. [PubMed] [Google Scholar]
- 25.Lister GD, Kleinert HE, Kutz JE, Atasoy E. Primary flexor tendon repair followed by immediate controlled mobilization. J Hand Surg [Am] 1977;2:441–451. doi: 10.1016/s0363-5023(77)80025-7. [DOI] [PubMed] [Google Scholar]
- 26.Duran RJ, Houser RG, Stover MG. Rehabilitation of the Hand. CV Mosby; St. Louis, MO: 1978. [Google Scholar]
- 27.Strickland JW. Flexor tendon surgery. Part 1: Primary flexor tendon repair.[see comment] J Hand Surg [Br] 1989;14:261–272. doi: 10.1016/0266-7681_89_90079-x. [DOI] [PubMed] [Google Scholar]
- 28.Boyer MI. Flexor tendon biology. Hand Clin. 2005;21:159–166. doi: 10.1016/j.hcl.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Khanna A, Friel M, Gougoulias N, et al. Prevention of adhesions in surgery of the flexor tendons of the hand: what is the evidence? Br Med Bull. 2009;90:85–109. doi: 10.1093/bmb/ldp013. [DOI] [PubMed] [Google Scholar]
- 30.Stewart MK, van Strien G. Postoperative management of flexor tendon injuries. In: Hunter JM, Mackin EJ, Callahan AD, editors. Rehabilitation of the hand. 2002. p. 439. [Google Scholar]
- 31.Manske PR, Gelberman RH, Lesker PA. Flexor tendon healing. Hand Clin. 1985;1:25–34. [PubMed] [Google Scholar]
- 32.Zhao C, Amadio PC, Momose T, et al. Remodeling of the gliding surface after flexor tendon repair in a canine model in vivo. J Orthop Res. 2002;20:857–862. doi: 10.1016/S0736-0266(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Chen M-Y, Zhao C, et al. The effect of hyaluronidase, phospholipase, lipid solvent and trypsin on the lubrication of canine flexor digitorum profundus tendon. J Orthop Res. 2008;26:1225–1229. doi: 10.1002/jor.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao C, Amadio PC, Momose T, et al. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma. 2001;51:917–921. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Gelberman RH, Woo SL, Lothringer K, et al. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg [Am] 1982;7:170–175. doi: 10.1016/s0363-5023(82)80083-x. [DOI] [PubMed] [Google Scholar]
- 36.Englert C, McGowan KB, Klein TJ, et al. Inhibition of integrative cartilage repair by proteoglycan 4 in synovial fluid. Arthritis & Rheumatism. 2005;52:1091–1099. doi: 10.1002/art.20986. [DOI] [PubMed] [Google Scholar]
- 37.Chang DP, Abu-Lail NI, Guilak F, et al. Conformational mechanics, adsorption, and normal force interactions of lubricin and hyaluronic acid on model surfaces. Langmuir. 2008;24:1183–1193. doi: 10.1021/la702366t. [DOI] [PubMed] [Google Scholar]
- 38.Taguchi M, Sun YL, Zhao C, et al. Lubricin surface modification improves extrasynovial tendon gliding in a canine model in vitro. J Bone Joint Surg Am. 2008;90:129–135. doi: 10.2106/JBJS.G.00045. [DOI] [PubMed] [Google Scholar]
- 39.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 40.Glasson SS, Rivera-Bermudez M, Tejada J, et al. Intra-articular Lubricin Supplementation Modifies Disease Progression and Ameliorates Pain in a Rat Model of Osteoarthritis. 5th Annual Meeting of the Orthopaedic Research Society Poster No. 1116.2009. [Google Scholar]
- 41.Zhao C, Sun Y-L, Amadio PC, et al. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic Acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88:2181–2191. doi: 10.2106/JBJS.E.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo SL, Gelberman RH, Cobb NG, et al. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981;52:615–622. doi: 10.3109/17453678108992156. [DOI] [PubMed] [Google Scholar]
- 43.Gelberman RH, Vande Berg JS, Lundborg GN, Akeson WH. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Joint Surg Am. 1983;65:70–80. [PubMed] [Google Scholar]
- 44.Gelberman RH, Menon J, Gonsalves M, Akeson WH. The effects of mobilization on the vascularization of healing flexor tendons in dogs. Clin Orthop. 1980:283–289. [PubMed] [Google Scholar]