Abstract
Social decision making is arguably the most complex cognitive function performed by the human brain. This is due to two unique features of social decision making. First, predicting the behaviors of others is extremely difficult. Second, humans often take into consideration the well-beings of others during decision making, but this is influenced by many contextual factors. Despite such complexity, studies on the neural basis of social decision making have made substantial progress in the last several years. They demonstrated that the core brain areas involved in reinforcement learning and valuation, such as the ventral striatum and orbitofrontal cortex, make important contribution to social decision making. Furthermore, the contribution of brain systems implicated for theory of mind during decision making is being elucidated. Future studies are expected to provide additional details about the nature of information channeled through these brain areas.
Introduction
Decision making can be understood as the process of selecting an option that is expected to produce the most desirable outcome. In most cases, the predictions for the outcomes from alternative actions are based on the previous experience of the decision maker. In addition, decision making can be considered social, when its outcome depends jointly on the choices of multiple decision makers. For animals living in groups, including humans and other primates, purely individual decision making is rare, and most decisions are made in social settings. This review focuses on recent neurobiological findings that have begun to shed light on two important features of social decision making. First, predicting the outcomes of different actions is difficult in social settings, as the actions of other decision makers change more unpredictably than inanimate objects in the animal's environment. In this regard, the ability to infer about the intentions and knowledge of other animals, referred to as the theory of mind, is crucial. Second, social decision making in humans and other primates can be influenced by other-regarding or social preferences. A central tenet in the classical game theory is that decision makers, or players, choose their actions purely on the basis of self-interest. However, the predictions of such classic game theory often fail to predict actual human behaviors. Moreover, whether and how much the decision maker cares about the reward given to others is affected by a variety of neural and social factors [1-3].
Model-based reinforcement learning and social decision making
When humans and animals face an unfamiliar environment or their environment changes unpredictably, their decision-making strategies will be adjusted accordingly. The reinforcement learning theory provides a parsimonious account of this process for many types of decision making [4], including social decision making. In this framework, the likelihood of selecting each action is determined by a set of value functions that are adjusted according to the animal's experience. Algorithms in the reinforcement learning theory can be divided into two categories. In simple or model-free reinforcement learning, changes in the value functions are driven by the actual outcomes resulting from the actions chosen by the decision maker. In model-based reinforcement learning, value functions for multiple actions can be changed simultaneously and more flexibly according to the internal model of the decision maker about his or her environment without necessarily having to experience the outcome of each action (Figure 1).
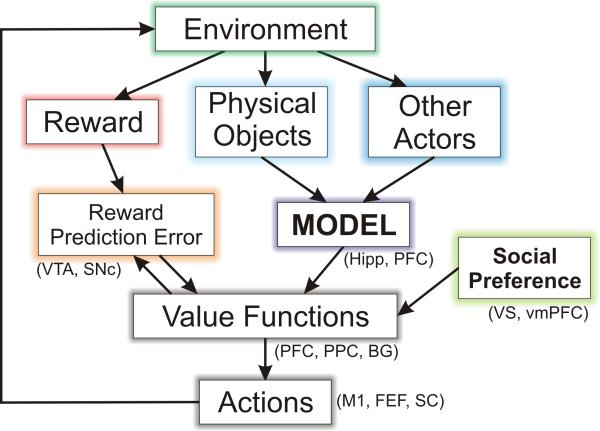
Figure 1.
Components of social decision making and their possible neural substrates. The environment of a decision maker provides 3 different types of information useful for decision making. First, it delivers reward, and its deviation from the predicted reward (reward prediction error) modifies the value functions used to select actions. Second, information about various physical objects can be used to update the decision maker's model about his or her environment. Third, observed actions of other actors can be used to update the decision maker's model about their likely future behaviors. In addition, social preference of the decision makers can also influence their value functions. FEF, frontal eye field; Hipp, hippocampus; M1, primary motor cortex; PFC, prefrontal cortex; PPC, posterior parietal cortex; SC, superior colliculus; SNc, substantia nigra pars compacta; vmPFC, ventromedial prefrontal cortex; VS, ventral striatum; VTA, ventral tegmental area.
Behaviors of human decision makers in a dynamic but non-social environment are best accounted for by hybrid learning models that combine the features of model-free and model-based reinforcement learning models [5••,6]. In a social setting, decision makers might update their beliefs about the choices of other decision makers according to their newly observed behaviors, and utilize such information to guide their subsequent choices. This is an example of model-based reinforcement learning, and is referred to as belief learning in game theory [7]. As in individual decision making, human behaviors during iterative social interactions are also more consistent with hybrid learning models than with model-free reinforcement learning models or pure belief learning models [8, 9••]. Hybrid learning models also more accurately account for the choices of non-human primates during a computer-simulated rock-paper-scissors game [10, 11•].
Much of our knowledge about the neural mechanisms of reinforcement learning is based on the results from experiments in which the observed behaviors of the subjects could not distinguish between these two different types of algorithms. For example, neurons modulating their activity according to the rewards expected from a particular state or action are widespread in the brain [12-16, 17•]. In addition, signals related to the reward prediction error, namely, the difference between actual and expected rewards are also found in multiple brain areas, including the ventral tegmental area, substantia nigra pars compacta [18], striatum [19,20], anterior cingulate cortex (ACC) [21,22•], and prefrontal cortex [23]. However, whether such signals reflect the output of model-free or model-based reinforcement learning algorithm is still not well understood.
Recently, several studies have begun to elucidate how signals related to the reward values and prediction errors computed by model-based reinforcement learning algorithms are distributed in the brain. Signals related to reward prediction errors derived from model-free and model-based reinforcement learning algorithms might be co-localized in the striatum [5••, 6]. Interestingly, during strategic social decision making, activity in the rostral ACC was related only with reward prediction errors derived from a belief learning algorithm [9••]. These results raise the possibility that model-based reward prediction errors might be processed differently in the brain depending on the social features of the behavioral task. Additional brain areas, such as the hippocampus and prefrontal cortex, might be involved in predicting the outcomes of choices according to a model-based reinforcement learning algorithm in a non-social context [6].
During social decision making, inferences about the likely behaviors of other decision makers become recursive, as a group of decision makers try to figure out how others in the group expect each other to behave. A set of brain areas associated with theory of mind, such as the medial prefrontal cortex (mPFC) and temporoparietal junction (TPJ), might be critically involved in such recursive strategic reasoning [24-26]. For example, during the beauty contest game, in which the object is to pick a number as close to 2/3 times the average of all the numbers chosen by the participants, subjects who displayed high levels of strategic reasoning also showed higher activity in the mPFC [27••]. Another study found that uncertainty in the inferences about the other decision maker's strategy during a stag-hunt game recruited the rostral medial prefrontal cortex, while activity in the DLPFC increases with the depth or level of strategic reasoning [28••]. Activity in the DLPFC was also related to the level of strategic deception during a bargaining game [29••]. These findings suggest that DLPFC activity during strategic reasoning might reflect the higher demands for working memory and cognitive control.
Single-neuron recording studies in non-human primates have identified signals related to specific conjunctions of actions and their outcomes during computer-simulated competitive games [12, 30•]. Activity related to action-outcome conjunctions provides the information necessary for updating the value functions for specific actions according to model-free reinforcement learning algorithms. Neurons involved in updating the value functions for unchosen actions according to their hypothetical outcomes are co-localized in the same brain areas that process actual outcomes and the corresponding reward prediction errors, such as the ACC [31••] and prefrontal cortex [11•]. These areas might provide converging inputs to the brain circuits responsible for updating the value functions for different actions. In particular, during a simulated rock-paper-scissors game, neurons in the orbitofrontal cortex and lateral prefrontal cortex often encode hypothetical reward that could have been obtained from a particular action, in addition to specific conjunctions of chosen action and its actual outcome [11•] (Figure 2). In both humans and monkeys, neurons in the medial frontal cortex encode specific actions produced by others, and thereby might contribute to extracting the information about hypothetical outcomes associated with the same actions [32, 33••].
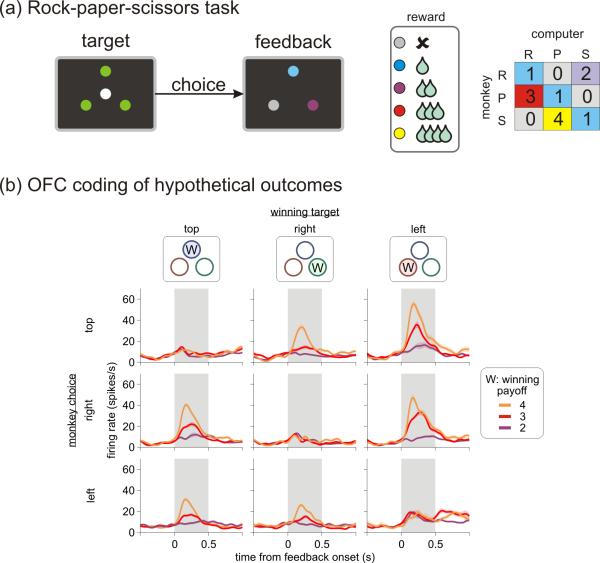
Figure 2.
Orbitofrontal cortex encodes hypothetical outcomes during a competitive game. (a) Simulated rock-paper-sciossors task performed by monkeys (adapted from 11•). The animal was required to shift its gaze towards one of the 3 peripheral targets when the white central target was extinguished. After a short delay, the peripheral targets all changed their colors and indicated the amount of reward available from each location (e.g., purple = 2 drops of fruit juice), which was determined by the payoff matrix of a biased rock-paper-scissors game shown on the right (R, rock; P, paper; S, scissors). (b) A single neuron in the orbitofrontal cortex that encoded how much reward could have been earned from an unchosen winning target (indicated by ‘W’ in the top panels). The spike density function of this neuron is shown separately according to the position of the winning target (columns), the position of the target chosen by the animal (rows), and the magnitude of the reward available from the winning target (colors).
Neural basis of social preference
The game theory, originally developed by von Neunman and Morgenstein [34], seeks to identify a set of strategies expected for a group of rational and selfish decision makers, and can provide useful approximations to human behaviors observed in a broad range of social interactions [7]. However, there are many counter-examples violating the assumption of purely self-interested homo economicus. Not only are people often willing to give up some of their incomes to benefit others, but they can also choose costly actions to punish others acting unfairly. The neural correlates of such prosocial preferences are increasingly better understood.
Charitable donations are common in human societies, and sharing valuable resources or donating them to others induces activity patterns in the brain that resemble those resulting from individual gains. For example, decisions to donate to charitable organizations increase the activity in the ventral striatum, whereas decisions to oppose such donations activate the lateral orbitofrontal cortex [35]. Furthermore, the ventral striatum and caudate nucleus showed greater activations when the monetary transfer to the charity was voluntary compared to when it was forced or taxed [36]. The level of activity in the ventral striatum and ventromedial prefrontal cortex (vmPFC) is also correlated with the subjective value of donation to others [37, 38]. Activity in these two areas is temporally correlated with the activity in other cortical areas implicated in empathy and agency perception, such as the ACC and posterior superior temporal cortex [37,38], suggesting that they might constitute a network of brain areas responsible for modulating social preference according to the nature of interpersonal relationship.
A number of studies have used ultimatum games to investigate the nature of neurobiological correlates of fairness norms. During an ultimatum game, a proposer receives a fixed amount of money and offers a proportion of it to the responder, who then chooses to accept or reject the offer. Although game theory predicts that a self-interested rational proposer would offer the smallest amount possible, such small offers are frequently rejected when humans play this game. Unfair offers leads to activation in the anterior insula, DLPFC, ACC, and amygdala of the responder [39, 40]. Among these areas, DLPFC is implicated for enforcing costly actions with the aim of achieving fair outcomes in the long run. For example, disrupting the DLPFC activity in the responder with repetitive transcranial stimulation (rTMS) during an ultimatum game makes it more likely for unfair offers to be accepted [41], and also reduces the activity related to unfair offers in the vmPFC [42••]. Similarly, the baseline activity of the lateral prefrontal cortex of the responder predicts the rate of acceptance [43]. Faces of proposers that are judged to be trustworthy also increase the acceptance rate, and this might be mediated by the lateral orbitofrontal cortex and its connections with the amygdala and insula [44].
Non-selfish behaviors, including altruistic donations or punishment, can be accounted for by a model of inequity aversion [45]. In this model, the utility for a particular distribution of wealth among the members in a group is diminished by both advantageous and disadvantageous inequity that is related to guilt and envy, respectively. This model has been corroborated and further elaborated by the results from neuroimaging studies. For example, inequitable monetary transfer activates the anterior insula [46], and decreases the activity in the ventral striatum and vmPFC [47••]. Envy and its dissolution (schadenfreude) also activate ACC and ventral striatum, respectively [48]. In addition, individual variability in the strength of social preference is correlated with the activation related to inequity in the amygdala [49]. These results illustrate how effects of multiple contextual factors on social preference might be mediated by a network of brain areas. Both pro-social and anti-social preference has been demonstrated in non-human primates [50, 51••], so this remains an important topic for further research. In addition, activity of neurons in the orbitofrontal cortex of monkeys is influenced by the reward given to another monkey, suggesting that the neural mechanisms underlying social preference in humans and other primates might overlap [51••].
Conclusions
The results from the studies summarized above suggest that the brain regions involved in the valuation of different options during individual decision making, such as the ventral striatum and vmPFC, might perform similar functions during social decision making. Other areas, such as the amygdala and insula, might also contribute to the emotional aspect of decision making in both social and non-social context. Furthermore, areas involved in specific aspects of social perception and cognition, such as the TPJ, might be additionally recruited during social decision making, as when decision makers are engaged in recursive reasoning to predict the actions of others. Recent studies showed that many of these areas thought to be important for social decision making are enlarged when the size of the social group increases [52••, 53], suggesting that additional processing power in these brain areas must be beneficial during complex social interaction. We expect that the future studies will provide more detailed insights into the features of our brain that make us socially competent.
Highlights.
Human social decision making is characterized by hybrid learning and non-selfish preference.
Striatum and prefrontal cortex underlie learning and inference during social decision making.
Orbitofrontal cortex might be a hub of brain areas that determines social preference.
Acknowledgements
The authors are supported by the National Institute of Health grants (DA024855, DA029330, DA027844).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Herrmann B, Thöni C, Gächter S. Antisocial punishment across societies. Science. 2008;319:1362–1367. doi: 10.1126/science.1153808. [DOI] [PubMed] [Google Scholar]
- 2.De Dreu CKW, Greer LL, Handgraaf MJJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SWW. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 3.De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci USA. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton RS, Barto AG. Reinforcement Learning: an Introduction. MIT Press; 1998. [Google Scholar]
- 5••.Daw ND, Gershman SJ, Seymour B, Dayan P, Dolan RJ. Model-based influences on humans’ choices and striatal prediction errors. Neuron. 2011;69:1204–1215. doi: 10.1016/j.neuron.2011.02.027. [This study showed that the reward prediction errors computed according to model-free and model-based reinforcement learning algorithms are both encoded in the ventral striatum, suggesting that some brain areas might be utilized for both types of reinforcement learning.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simon DA, Daw ND. Neural correlates of forward planning in a spatial decision task in humans. J Neurosci. 2011;31:5526–5539. doi: 10.1523/JNEUROSCI.4647-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camerer CF. Behavioral Game Theory. Princeton University Press; 2003. [Google Scholar]
- 8.Camerer C, Ho TH. Experience-weighted attraction learning in normal form games. Econometrica. 1999;67:827–874. [Google Scholar]
- 9••.Zhu L, Mathewson KE, Hsu M. Dissociable neural representations of reinforcement and belief prediction errors underlie strategic learning. Proc Natl Acad Sci USA. 2012;109:1419–1424. doi: 10.1073/pnas.1116783109. [This study showed that during an iterative competitive game, reward prediction errors estimated using a simple, model-free reinforcement learning algorithm and those estimated using a belief learning algorithm are separately localized in the ventral striatum and rostral anterior cingulate cortex.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D, McGreevy BP, Barraclough DJ. Learning and decision making in monkeys during a rock-paper-scissors game. Cogn Brain Res. 2005;25:416–430. doi: 10.1016/j.cogbrainres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11•.Abe H, Lee D. Distributed coding of actual and hypothetical outcomes in the orbital and dorsolateral prefrontal cortex. Neuron. 2011;70:731–741. doi: 10.1016/j.neuron.2011.03.026. [This study demonstrated that the behaviors of monkeys during a simulated rock-paper-scissors game are better accounted for by a hybrid learning model than by a simple reinforcement or belief learning model. It also showed that neurons in the orbital and lateral prefrontal cortex often encode specific conjunctions of counterfactual (unchosen) actions and hypothetical outcomes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barraclough DJ, Conroy ML, Lee D. Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci. 2004;7:404–410. doi: 10.1038/nn1209. [DOI] [PubMed] [Google Scholar]
- 13.Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310:1337–1340. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- 14.Lau B, Glimcher PW. Value representations in the primate striatum during matching behavior. Neuron. 2008;58:451–463. doi: 10.1016/j.neuron.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wunderlich K, Rangel A, O'Doherty JP. Neural computations underlying action-based decision making in the human brain. Proc Natl Acad Sci USA. 2009;106:17199–17204. doi: 10.1073/pnas.0901077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Vickery TJ, Chun MM, Lee D. Ubiquity and specificity of reinforcement signals throughout the human brain. Neuron. 2011;72:166–177. doi: 10.1016/j.neuron.2011.08.011. [Using a multi-voxel pattern analysis, this study demonstrated that the blood-oxygen-level-dependent signals related to decision outcomes are distributed throughout the entire human brain.] [DOI] [PubMed] [Google Scholar]
- 18.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Sul JH, Huh N, Lee D, Jung MW. Role of striatum in updating values of chosen actions. J Neurosci. 2009;29:14701–14712. doi: 10.1523/JNEUROSCI.2728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyama K, Hernadi I, Iijima T, Tsutsui KI. Reward prediction error coding in dorsal striatal neurons. J Neurosci. 2010;30:11447–11457. doi: 10.1523/JNEUROSCI.1719-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo H, Lee D. Temporal filtering of reward signals in the dorsal anterior cingulate cortex during a mixed-strategy game. J Neurosci. 2007;27:8366–8377. doi: 10.1523/JNEUROSCI.2369-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Kennerley SW, Behrens TE, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. 2011;14:1581–1589. doi: 10.1038/nn.2961. [This study showed that neurons in the anterior cingulate cortex tend to encode the reward prediction errors while those in the orbitofrontal cortex tend to encode the value of current reward relative to the animal's reward history.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asaad WF, Eskandar EN. Encoding of both positive and negative reward prediction errors by neurons of the primate lateral prefrontal cortex and caudate nucleus. J Neurosci. 2011;31:17772–17787. doi: 10.1523/JNEUROSCI.3793-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 25.Hampton AN, Bossaerts P, O'Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc Natl Acad Sci USA. 2008;105:6741–6746. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behrens TE, Hunt LT, Woolrich MW, Rushworth MF. Associative learning of social value. Nature. 2008;456:245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Coricelli G, Nagel R. Neural correlates of depth of strategic reasoning in medial prefrontal cortex. Proc Natl Acad Sci USA. 2009;106:9163–9168. doi: 10.1073/pnas.0807721106. [This study found that the degree of recursive strategic reasoning during a beauty-contest game is correlated with the activity in the medial fronal cortex.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Yoshida W, Seymour B, Firston KJ, Dolan RJ. Neural mechanisms of belief inference during cooperative games. J Neurosci. 2010;30:10744–10751. doi: 10.1523/JNEUROSCI.5895-09.2010. [This study showed that during a stag hunt game, the uncertainty of inference regarding the strategy of other players and the depth of recursive strategy used to make such inference are reflected in the activity of medial and lateral prefrontal cortex, respectively.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Bhatt MA, Lohrenz T, Camerer CF, Montague PR. Neural signatures of strategic types in a two-person bargaining game. Proc Natl Acad Sci USA. 2010;107:19720–19725. doi: 10.1073/pnas.1009625107. [This study showed that the dorsolateral prefrontal cortex and Brodmann area 10 might be involved in strategic deception during a sender-receive game. In addition, it also found that the activity in the temporoparietal junction reflected the expected outcome of a deceptive strategy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Seo H, Lee D. Behavioral and neural changes after gains and losses of conditioned reinforcers. J Neurosci. 2009;29:3627–3641. doi: 10.1523/JNEUROSCI.4726-08.2009. [This study showed that during a computer-simulated biased matching pennies game, neurons in the medial frontal cortex often encoded the positive or negative outcomes resulting from specific choices.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Hayden BY, Pearson JM, Platt ML. Fictive reward signals in the anterior cingulate cortex. Science. 2009;324:948–950. doi: 10.1126/science.1168488. [This study provided the first evidence that signals related to hypothetical rewards from unchosen actions are encoded by single neurons in the anterior cingulate cortex.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr Biol. 2010;20:750–756. doi: 10.1016/j.cub.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33••.Yoshida K, Saito N, Iriki A, Isoda M. Representation of others’ action by neurons in monkey medial frontal cortex. Curr Biol. 2011;21:249–253. doi: 10.1016/j.cub.2011.01.004. [This study showed that neurons in the anterior cingulate cortex differentially encode actions executed by the self and others.] [DOI] [PubMed] [Google Scholar]
- 34.von Neumann J, Morgenstern O. Theory of Games and Economic Behavior. Princeton University Press; 1944. [Google Scholar]
- 35.Moll J, Krueger F, Zahn R, Pardini M, de Oliveira-Souza R, Grafman J. Human frontomesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103:15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harbaugh WT, Mayr U, Burghart DR. Neural responses to taxation and voluntary giving reveal motives for charitable donations. Science. 2007;316:1622–1625. doi: 10.1126/science.1140738. [DOI] [PubMed] [Google Scholar]
- 37.Mobbs D, Yu R, Meyer M, Passmonti L, Seymour B, Calder AJ, Schweizer S, Frith CD, Dalgleish T. A key role for similarity in vicarious reward. Science. 2009;324:900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hare T, Camerer CF, Knoepfle DT, O'Doherty JP, Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J Neurosci. 2010;30:583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 40.Gospic K, Mohlin E, Fransson P, Petrovic P, Johannesson M, Ingvar M. Limbic justice - amygdala involvement in immediate rejection in the ultimatum game. PLoS Biol. 2011;9:e1001054. doi: 10.1371/journal.pbio.1001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disruping the right prefrontal cortex. Science. 2006;314:829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 42••.Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nat Neurosci. 2011;14:1468–1474. doi: 10.1038/nn.2933. [This study demonstrated that repetitive transcranial magnetic stimulation applied to the right dorsolateral prefrontal cortex of responders in an ultimatum game subsequently reduce their rejection rate, and also diminished the activity in the same area and that in the ventromedial prefrontal cortex.] [DOI] [PubMed] [Google Scholar]
- 43.Knoch D, Gianotti LRR, Baumgartner T, Fehr E. A neural marker of costly punishment behavior. Psychol Sci. 2010;21:337–342. doi: 10.1177/0956797609360750. [DOI] [PubMed] [Google Scholar]
- 44.Kim H, Choi MJ, Jang IJ. Lateral OFC activity predicts decision bias due to first impressions during ultimatum games. J Cogn Neurosci. 2012;24:428–439. doi: 10.1162/jocn_a_00136. [DOI] [PubMed] [Google Scholar]
- 45.Fehr E, Schmidt KM. A theory of fairness, competition, and cooperation. Q J Econ. 1999;114:817–868. [Google Scholar]
- 46.Zaki J, Mitchell JP. Equitable decision making is associated with neural markers of intrinsic value. Proc Natl Acad Sci USA. 2011;108:19761–19766. doi: 10.1073/pnas.1112324108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47••.Tricomi E, Rangel A, Camerer CF, O'Doherty JP. Neural evidence for inequality-averse social preferences. Nature. 2010;463:1089–1091. doi: 10.1038/nature08785. [This study showed that monetary transfer increases the activity in the ventral striatum and ventromedial prefrontal cortex when it reduces the inequity between self and others.] [DOI] [PubMed] [Google Scholar]
- 48.Takahashi H, Kato M, Matsuura M, Mobbs D, Suhara T, Okubo Y. When your gain is my pain and your pain is my gain: neural correlates of envy and schadenfreude. Science. 2009;323:937–939. doi: 10.1126/science.1165604. [DOI] [PubMed] [Google Scholar]
- 49.Haruno M, Frith CD. Activity in the amygdala elicited by unfair divisions predicts social value orientation. Nat Neurosci. 2010;13:160–161. doi: 10.1038/nn.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Chang SWC, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (Macaca mulatta). Front Neurosci. 2011;5:27. doi: 10.3389/fnins.2011.00027. [This study evaluated the social preference of monkeys during a choice task, and found that the animal's subjective value of reward received by another animal changed depending on whether the animal making the choice receives reward or not.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Azzi JC, Sirigu A, Duhamel JR. Modulation of value representation by social cotext in the primate orbitofrontal cortex. Proc Natl Aca Sci USA. 2012;109:2126–2131. doi: 10.1073/pnas.1111715109. [This study found that the monkeys were more willing to work to obtain reward only for themselves compared to when the reward was given to themselves and another monkey. In addition, it also showed that the activity of neurons in the orbitofrontal cortex reflected the animal's social preference.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52••.Sallet J, Mars RB, Noonan MP, Andersson JL, O'Reilly JX, Jbabdi S, Croxson PL, Jenkinson M, Miller KL, Rushworth MFS. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [This study found that the size of gray matter in several brain areas implicated in social cognition, such as the superior temporal sulcus and rostral prefrontal cortex, increases in animals housed in a large social group.] [DOI] [PubMed] [Google Scholar]
- 53.Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RIM. Ventromedial prefrontal volume predicts understanding of others and social network size. NeuroImage. 2011;57:1624–1629. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]