Summary
Background
The molecular determinants of the severity and persistence of allergic asthma remain poorly understood. Suppressor Of Cytokine Signaling 1 (SOCS1) is a negative regulator of interleukin (IL)-4-dependent pathways in vitro and might therefore, control T helper type 2 (Th2) immunity associated traits, such as IgE levels, mucin production, IL-5 and IL-13 induction, and eosinophilic mucosal inflammation, which are implicated in allergic asthma.
Objective
To investigate the role of SOCS1 in regulating Th2-associated disease traits in a murine sub-chronic aeroallergen-driven asthma model.
Methods
Following sensitization and challenge with ovalbumin (OVA), bronchoalveolar lavage (BAL) and serum were collected from mice lacking the Socs1 gene on an interferon γ (IFN-γ) null background (Socs1−/−Ifnγ−/−). The composition of infiltrating cells in the lung, serum IgE and IgG1 levels and cytokine levels were analysed.
Results
Serum IgE levels and infiltrating eosinophils were greatly increased in the lungs of OVA-treated Socs1−/−Ifnγ−/− mice compared to Ifnγ−/− and C57BL/6 controls. Expression of the Th2 cytokines, IL-4, IL-5 and IL-13 was increased in CD4+ cells and lung tissue from OVA-treated Socs1−/−Ifnγ−/− mice. IgE, IL-5 levels and infiltrating eosinophils were also elevated in saline-treated Socs1−/−Ifnγ−/− mice, suggesting that in the absence of SOCS1, mice are already biased towards a Th2 response. It is at present unclear whether the elevated cytokine levels are sufficient to result in the exacerbated Th2-response to OVA challenge or whether enhanced intracellular signalling also contributes. Surprisingly, of the various IL-4/IL-13 responsive genes tested, only Arginase I appeared to be modestly up-regulated in the lungs of OVA-treated Socs1−/−Ifnγ−/− mice, suggesting that regulation by SOCS1 occurs primarily in hematopoietic cells and not in the airway epithelium.
Conclusions
Together these results indicate that SOCS1 is an important regulator of the Th2 response.
Keywords: SOCS1, asthma, Th2 type cytokines, bronchoalveolar lavage, eosinophils
Introduction
Allergic asthma is the most common and most widely studied of the known asthma endotypes (allergic, neutrophil variant, and paucigranulocytic) [1, 2]. It is classically characterized by eosinophil inflammation, elevated serum IgE levels and mucin production associated with Th2 immune deviation [3]. It poses an increasing health and economic problem and is prevalent in the developed nations, affecting millions of children and adults worldwide [4]. Although the etiology of all asthma subtypes remains unclear, short-term sub-chronic animal models of allergic asthma have been instrumental in demonstrating that inappropriate activation of CD4+ Th2 cells and elevated quantities of Th2 cytokines are responsible for much of the associated asthma pathology [5, 6]. Consistent with this, increased numbers of Th2-producing cells or their cytokines have been found in airway biopsies and bronchoalveolar lavage fluid from asthmatic patients [7].
The term “Th2 cell”, used here, is convenient but notional as it reflects net properties of a population of cells that may be individually heterogenous with respect to absolute cytokine profile. Accepting this caveat, Th2 cells are defined by their ability to secrete the cytokines IL-4, IL-5, IL-9 and IL-13, and the initial polarisation from naive CD4+ T helper cells is very strongly influenced by IL-4 and its signaling intermediate, the transcription factor, STAT6 [8, 9]. Depletion of CD4+ cells or deletion of the IL-4 and Stat6 genes protects mice from asthma-like symptoms [6, 10, 11]. While IL-4 is critical for initiating the early events leading to Th2-mediated lung inflammation, it is dispensable for the effector phase [12]. In contrast, IL-13 as an effector molecule, induces the local transcription of mucin, protease and chemokine genes, and is required for induction of airway hyper-responsiveness (AHR), mucin production and pulmonary fibrosis [5, 13–15]. IL-5 is essential for eosinophil proliferation and migration to the lungs [16].
IL-4 and IL-13 share many overlapping functions, including upregulating the expression of MHC II molecules, the eosinophil specific chemokine eotaxin-1, and the vascular adhesion molecule VCAM-1 [17]. This functional overlap is explained by the use of shared receptor subunits, with IL-4 capable of signaling through two receptor complexes both of which converge upon STAT6. The IL-4 receptor complex I consists of the IL-4Rα subunit and the IL-2 receptor γc chain, whereas the IL-4 receptor complex II is composed of the IL-4Rα and IL-13Rα1 and is also utilised by IL-13 [17, 18]. A third subunit, the soluble IL-13Rα2 acts as a decoy receptor, sequestering IL-13, while the membrane-bound form is thought to signal through AP-1 proteins [19, 20]. Whereas the roles of IL-4 and IL-13 in allergic asthma are well established, the role of IFN-γ remains controversial. Mouse models have demonstrated that IFN-γ is likely to be involved in the termination of allergic airway inflammation, but IFN-γ is also found to be expressed in mouse models of severe asthma and human asthma where it may contribute to more aggressive forms of the disease, perhaps via macrophage activation [21].
SOCS proteins are capable of inhibiting the JAK-STAT pathway in response to a wide range of cytokines [22–24]. SOCS1 directly inhibits JAK enzymatic activity and is a critical regulator of the Th1 cytokine IFN-γ and γc-cytokine-dependent T cell homeostasis [25–27]. Mice lacking SOCS1 die within three weeks of age from a complex inflammatory condition with haematopoietic infiltration into multiple organs. When Socs1−/− mice are crossed onto either an IFN-γ or a Stat6 null background, survival is prolonged [26, 28] indicating that both IFN-γ-driven Th1 and IL-4-driven Th2 responses contribute to the observed lethality. In support of this, Socs1−/− CD4+ T cells spontaneously differentiate into Th1 and Th2 cells with enhanced production of IFN-γ and IL-4, and enhanced signaling in response to both cytokines [28, 29]. SOCS1 expression is rapidly induced in response to many cytokines, including IFN-γ and IL-4 and thus inhibits signaling both via cross-talk and in a classic negative feedback manner [22, 23, 30]. However, the in vivo consequences of SOCS1 deficiency on allergic asthma remain unknown.
SOCS3 and SOCS5 have also been implicated in regulation of the Th1/Th2 balance. SOCS3 is preferentially expressed in Th2 cells, and levels are increased in patients suffering from asthma and atopic dermatitis [31, 32]. Conditional deletion of the Socs3 gene in T cells results in Th3-like differentiation with enhanced production of IL-10 and TGFβ, and a corresponding decrease in the Th2 response [33]. SOCS5 is preferentially expressed in Th1 cells and when over-expressed can inhibit IL-4 signaling and Th2 differentiation [34]. T cell-specific expression of a SOCS5 transgene results in an augmented Th1 response in an allergic conjunctivitis model and during bacterial sepsis [35, 36]. In contrast, enhanced Th2 responses were observed when mice expressing a global SOCS5 transgene were subjected to antigen sensitization and challenge [37]. Paradoxically, SOCS5 knockout mice display normal Th1/Th2 differentiation and mount a similar immune response to control mice when infected with Leishmania [38]. Definitive physiological evidence for SOCS5 regulation of IL-4 signaling is still lacking.
In this study, we used the OVA-induced asthma model to investigate the role of SOCS1 in regulating the asthma response. On an IFN-γ null background, Th2 responses including IgE levels and lung eosinophilia were enhanced in SOCS1 knockout mice. This is in contrast to two human studies, which suggest that increased SOCS1 expression is linked to asthma susceptibility through reduction of the IFN-γ-mediated Th1 response [39, 40]. Our results indicate that SOCS1 is a physiological negative regulator of one or more Th2 cytokines, and given the importance of these cytokines in asthma pathology, suggest that augmentation of SOCS1 function may prove a useful therapeutic approach.
Materials and Methods
Mice
Mice with a homozygous deletion of both the Socs1 and Ifn-γ genes (Socs1−/−Ifnγ−/−) have been described previously [26] and were maintained on a C57BL/6 background. Mice with a homozygous deletion of the Ifn-γ gene (Ifnγ−/−) were obtained from The Jackson Laboratory. All mice were bred at the animal facilities of The Walter and Elisa Hall Institute of Medical Research. Ethics approval was obtained from the Animal Ethics Committee at the Walter and Eliza Hall Institute of Medical Research.
Antigen sensitization and challenge
Six to eight week-old female mice were treated with either OVA (Grade V, Sigma-Aldrich, St Louis, MO, USA) or saline. OVA-treated mice were sensitized with 20µg OVA/1.3mg aluminium hydroxide (Alum, Sigma-Aldrich, St Louis, MO, USA) in 200µl saline on days 0 and 14 via intraperitoneal injection. On days 26, 27, 28 mice were challenged with 20ml of OVA in saline (50mg/ml) via an ultrasonic nebuliser (DeVilbiss, Sunrise Medical, Pty. Ltd., NSW, Australia) for 20 min/day. Saline-treated mice received 1.3mg Alum in 200µl saline on days 0 and 14, and on days 26, 27, 28 received 20ml of nebulized saline for 20 min/day. All procedures were performed aseptically.
Serum Collection
200µl of blood was collected by retro-orbital bleeding prior to the first injection and immediately before mice were sacrificed on day 29. Bloods were allowed to clot at 4°C overnight and blood-free serum collected. Blood from Socs1Lck-Cre and littermate control mice was provided by Prof. T. Kay (St Vincent’s Research Institute, Melbourne, Australia).
Bronchoalveolar Lavage (BAL)
Mice were anaesthetised with a lethal intraperitoneal injection of Xylazine/Ketamine or sodium pentobarbitone (Xylazine: Troy Laboratories, Smithfield; Ketamine: Parnell Laboratories, Alexandra, NSW, Australia). Once the mice ceased breathing the trachea was cannulated with a shortened and blunt-ended 21G needle held in placed by a 5mm artery clamp, and the lungs lavaged with 1× 0.4ml, and then 3× 0.3ml saline (recovery volume approximately 85%). Samples were centrifuged, and the supernatant stored at −70°C for cytokine analysis by ELISA. The cell pellet was resuspended in FACS buffer (buffered saline solution containing 5% fetal calf serum), and cell number and viability determined by vital dye exclusion (trypan blue, Sigma-Aldrich) using a Neubauer haemocytometer.
Cytospin Preparations
5×104 BAL recovered cells/200µl FACS buffer were centrifuged onto slides (Cytospin 3, Shandon, USA) and stained with May-Grunswald and Giemsa. BAL cell composition was determined by differential cell counts (Nikon Microscope E600, 200×, at least 100 cells/slide).
Pulmonary infusion fixation and histology
Tracheas were cannulated as in BAL. The lungs were then infused with freshly prepared 4% paraformaldehyde (w/v) with MTPBS and 0.5% gluteraldehyde. Inflation-fixed, 3µm paraffin-embedded transverse sections of the lungs were stained with haematoxylin and eosin to examine peri-bronchial, peri-vascular and alveolar inflammation. Slides were observed using a Zeiss Axioplan 2 microscope and images were obtained using a Zeiss Axiocam digital camera.
CD4+ T cell cultures
CD4+ T cells were purified from pooled, draining bronchiolar lymph nodes using a T cell isolation kit (MACS:Miltenyi Biotec) according to the manufacturer’s instructions. CD4+ purification was confirmed by flow cytometry. 2×106 cells were cultured with plate-bound anti-CD3 (16µg/ml) in 500µl RPMI supplemented with 10% heat-inactivated fetal calf serum (Bovagen Biologicals, Melbourne, Australia) and 50µM 2-mercaptoethanol (Sigma-Aldrich) at 37°C in a humidified incubator containing 5% CO2 for 24 h. Culture supernatants were then collected for determination of cytokine levels by ELISA and cells were lysed in RLT buffer (QIAGEN, Valencia, CA) and total cellular RNA isolated for Q-PCR analysis.
ELISA
IL-4, IL-5, IL-13 produced by CD4+ cells and serum IgE and IgG1 levels were quantified by OptEIA ELISA kits (BD Biosciences Pharmingen, San Diego, CA, USA). OVA-specific immunoglobulin (Ig) was assayed by coating plates with 10µg/ml OVA, and anti-OVA Ig antibodies detected by ELISA as described previously [41]. Briefly, total OVA-specific IgG was detected using donkey anti-mouse IgG conjugated to horseradish peroxidase (HRP; Chemicon International, Temecula, CA, USA), while OVA-specific IgE was detected using and anti-IgE-biotin and streptavidin-HRP (BD Biosciences Pharmingen) as per manufacturer’s instructions. Titres were considered positive when the optical density was over 0.1, or three standard deviations above background.
Real-time quantitative PCR (Q-PCR)
Mice used for Q-PCR analysis were separate from mice perfused for histology. Following bronchoalveolar lavage, the lower lobe of the right lung and thoracic-draining lymph nodes were collected into TRIzol Reagent. Total cellular RNA from lung, lymph nodes and cultured CD4+ cells was isolated using the RNeasy kit (QIAGEN, Valencia, CA) and first strand cDNA synthesis performed using Superscript II RNASE H− reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Real-time PCR was performed on an ABI Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA). PCR conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 15s, 60°C (SOCS1, SOCS3), 50°C (SOCS5, GAPDH) and 54°C (all remaining primers) for 25s and 72°C for 10s, with a transition rate of 20°C/s and a single fluorescence measurement, melting curve program (60°C-95°C, with a heating rate of 0.1°C/s and continuous fluorescence measurement) and a final cooling step to 40°C. All PCR reactions were performed using the QuantiTect SYBR Green PCR Kit (QIAGEN) in 10µl reactions containing 0.5 pmol of forward and reverse primers, 5 µl of QuantiTect Master Mix and 4µl of cDNA (diluted 1 in 5). Primer sequences are shown in Table 1.
Table 1.
Q-PCR Primers
| Forward | Reverse | |
|---|---|---|
| GAPDH | TTGTCAAGCTCATTTCCTGGT | TTACTCCTTGGAGGCCATGTA |
| SOCS1 | CTCGTCCTCTCTTCGTCCT | GAAGGTGCGGAAGTGAGTGT |
| SOCS4 | GGCGGCAGGCGCTCGGACAG | TCTGGGCACTTTCTGGACGG |
| SOCS5 | GACGGCTTAGTATCGAAGAA | GCTTATACAATGGGTTGACC |
| CIS | TGCTCCACAGCCAGCAAAG | TCCTTTTCTCCTTCCATCCCG |
| IL-4 | GACGCCATGCACGGAGAT | AAGCACCTTGGAAGCCCTACAG |
| IL-5 | ATGAAGTGCTGGAGATGGAACC | GACCCTGATGCAACGAAG |
| IL-13 | CCACGGCCCCTTCTAATGA | GCCTCTCCCCAGCAAAGTCT |
| Muc5ac | TGTCAGGTGGTCAAGAGGGTTAGT | CAGTGACAGGTGGCAGTGGTAGA |
| Eotaxin-1/CCLll | CAGTAACTTCCATCTGTCTCC | AGGTGAAGGAAGTGACCGTGA |
| Arginase I | CACAGCAAAGCAGACAGAACTAAG | AGAAAGGAACTGCTGGGATACATA |
| AMCase | AATGACTTGAAAAACAGGAACAGC | AAACCATGGTAGTGAAAGGAGCAG |
| CLCA3/Gob5 | ACTAAGGTGGCCTACCTCCAA | GGAGGTGACAGTCAAGGTGAGA |
SOCS3 forward and reverse primers used are as described by Wormald and colleagues [42]. The specificity of the SYBR green reaction was assessed by melting point analysis and gel electrophoresis. mRNA levels were quantified from standard curves generated using dilutions of an oligonucleotide corresponding to the amplified fragment and using SDS 2.2 software (Applied Biosytems). For eotaxin-1/CCL11, Muc5ac, Arginase I, AMCase and CLCA3/Gob5, PCR fragments were generated from cDNA derived from OVA-treated lungs and used as a standard amplicon. Relative expression was determined by normalizing the quantity of the gene of interest to the quantity of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Each measurement was carried out in duplicate.
Statistical Analysis
Data were normally distributed and were therefore analysed using the two-tailed student’s t test where p < 0.05 was designated as significant.
Results
To investigate the role of SOCS1 in regulating the asthma response, mice lacking the Socs1 gene on an IFN-γ null background (Socs1−/−Ifnγ−/−), mice lacking IFN-γ (Ifnγ−/−) and C57BL/6 control mice were subjected to OVA sensitization and challenge.
Serum IgE levels are elevated in the absence of SOCS1
Elevation of serum IgE is a common trait in animal asthma models as well as in human asthma [3]. OVA-treated Socs1−/−Ifnγ−/−, Ifnγ−/− and C57BL/6 mice all responded with an increase in serum IgE levels, however the increase was most dramatic in the Socs1−/−Ifnγ−/− mice when compared to Ifnγ−/− and C57BL/6 mice (7.8µg/ml c.f. <1.1µg/ml). Saline-treated Socs1−/−Ifnγ−/− mice also presented with elevated serum IgE levels in comparison to controls (0.79µg/ml c.f. <0.1µg/ml). Saline treatment consisted of systemic exposure to alum, which can promote IgE production [43], however, IgE levels were also elevated in untreated Socs1−/−Ifnγ−/− mice, indicating that this effect is independent of Alum (Fig. 1A).
Fig. 1.
IgE levels are elevated in Socs1−/−Ifnγ−/− mice. (A) Serum IgE in C57BL/6, mice lacking IFN-γ (Ifnγ−/−), and mice lacking both SOCS1 and IFN-γ (Socs1−/−Ifnγ−/−). Mice were untreated, subjected to antigen sensitization and challenge (OVA) or treated with Alum alone (Saline) (n ≥ 7). (B) Serum IgG1 levels in C57BL/6, Ifnγ−/− and Socs1−/−Ifnγ−/− mice. Mice were subjected to antigen sensitization and challenge (OVA) or treated with Alum alone (Saline) (n=4). (C) Antigen-specific total IgG was detected by ELISA in OVA-challenged C57BL/6, Ifnγ−/− and Socs1−/−Ifnγ2−/− mice. Bars represent mean ± standard deviation. ND: Not detected. ** p < 0.0001
In contrast, while serum IgG1 levels were elevated in all genotypes following OVA treatment, IgG1 levels were unaltered in Socs1−/−Ifnγ−/− mice relative to controls (Fig. 1B), indicating that SOCS1 may be differentially regulating IgE and IgG1 homeostasis. Consistent with the lack of change in IgG1, antigen-specific immunoglobulin levels were not differentially regulated in Socs1−/−Ifnγ−/− mice, but were modestly reduced in comparison to controls (Fig. 1C). Antigen-specific IgE production was undetectable in OVA-treated C57BL/6 and Ifnγ−/− control mice. By contrast BALB/c, which have a propensity to Th2-type responses and have been reported to produce 10-fold higher OVA-specific IgE levels than C57BL/6 mice [44], produced low but detectable levels of antigen-specific IgE in response to OVA. Socs1−/−Ifnγ−/− mice produced variable responses, where three mice developed low anti-OVA IgE titres comparable to BALB/c titres, whilst two mice did not develop detectable levels of anti-OVA IgE (n=5; data not shown). Given that total serum IgE levels were >7µg/ml, this suggests that the increase in serum IgE observed in OVA-treated Socs1−/−Ifnγ−/− mice is largely antigen-independent.
To determine if the elevation in basal IgE was due to either increased IL-4 production and/or signaling in T cells, we obtained serum from mice with a T cell-specific deletion of SOCS1 (Socs1Lck-Lox) [27]. Socs1Lck-Lox Cre+/+ had similar serum IgE levels to Socs1Lck-Lox Cre−/− littermates and C57BL/6 mice (~0.1µg/ml; data not shown), suggesting that the elevated IgE in untreated Socs1−/−Ifnγ−/− mice is not due to increased Th2 production of IL-4.
Severity of lung infiltrates is exacerbated in Socs1−/−Ifnγ−/− mice
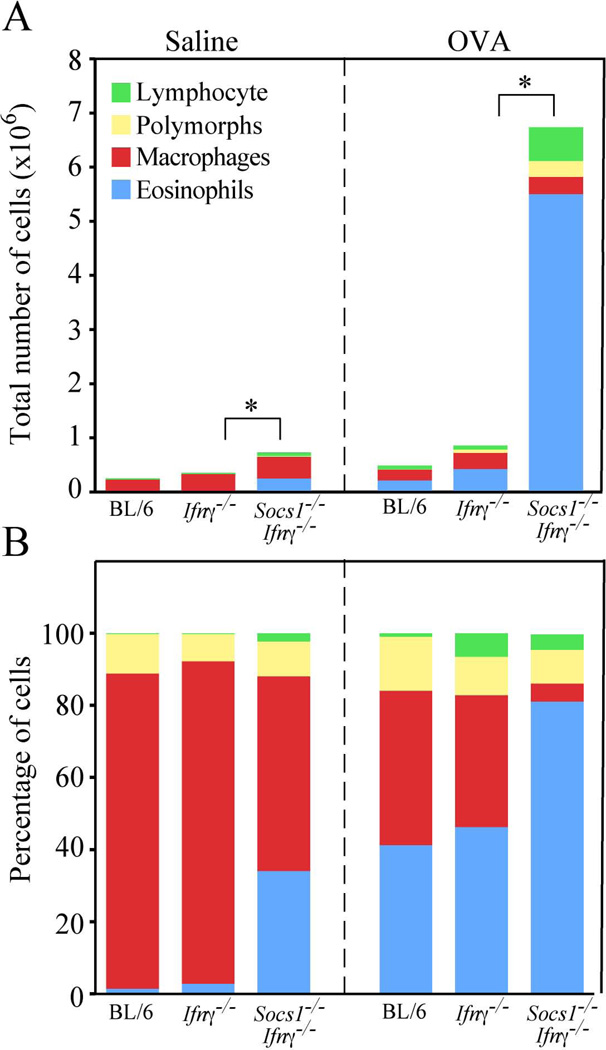
Following OVA or saline treatment a bronchoalveolar lavage was performed to recover infiltrating cells. The number of cells recovered by lavage was increased in saline-treated Socs1−/−Ifnγ−/− mice when compared to Ifnγ−/− or C57BL/6 mice. Following OVA-treatment the number of cells recovered from the lungs was increased in all three genotypes, but was greater in the Socs1−/−Ifnγ−/− mice (6.7 × 106 compared to <106 in Ifnγ−/− or C57BL/6 mice) (Fig. 2A). The majority of cells recovered from saline-treated Ifnγ−/− and C57BL/6 mice were macrophages (80–90%) with 1–2% eosinophils and the remainder lymphocytes and polymorphs. In contrast, saline-treated Socs1−/−Ifnγ−/− mice presented with 30–40% eosinophils (Fig. 2A & B). Following OVA-treatment, there was an increase in the percentage of eosinophils recovered from Ifnγ−/− and C57BL/6 mice (up to 30–40%) and a corresponding reduction in the percentage of macrophages. OVA-treated Socs1−/−Ifnγ−/− mice presented with 80–90% eosinophils and a reduction in the percentage (but not total number) of macrophages (Fig. 2B).
Fig. 2.
Increased eosinophilic lung infiltrates in OVA-challenged Socs1−/−Ifnγ−/− mice. Differential cell counts in BAL recovered from OVA-challenged C57BL/6 (BL/6), Ifnγ−/− and Socs1−/−Ifnγ−/− mice (n≥3). (A) Absolute cell numbers. (B) Percentage of hemopoietic subsets. * p < 0.05, where p refers to eosinophils.
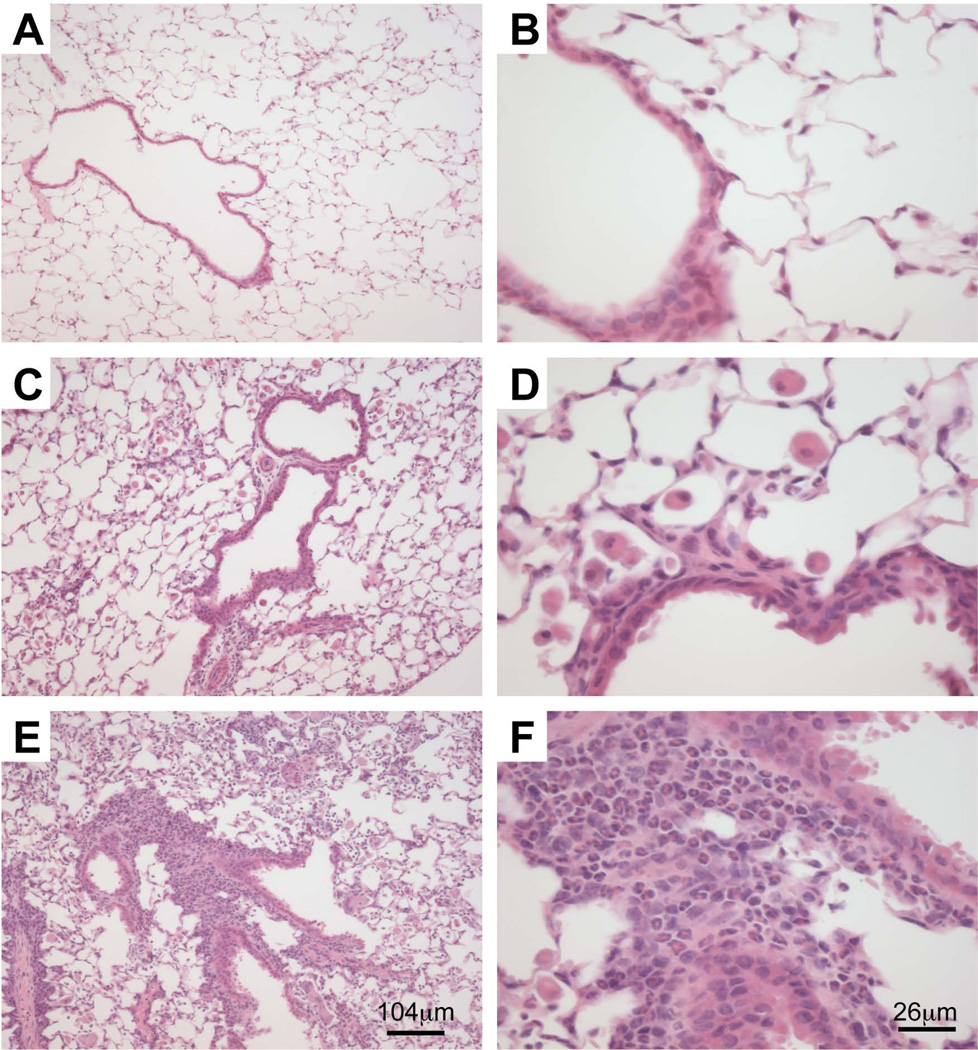
Histological evidence for exacerbated eosinophilic infiltration in OVA-treated Socs1−/−Ifnγ−/− mice
To confirm that BAL results accurately reflected responses in the lungs we obtained transverse pulmonary sections from saline and OVA-treated mice, specifically examining peri-bronchiole and peri-vascular inflammation in airways of comparable size, and proximal infiltration of the alveolar spaces in nearby lung parenchyma. Sections from saline-treated Socs1−/−Ifnγ−/− mice were comparable to Ifnγ−/− and C57BL/6 mice (data not shown). Presumably the slight increase in BAL cell number observed in saline-treated Socs1−/−Ifnγ−/− mice (Fig. 2A) was not sufficient to be observed at a histological level. In contrast, OVA-treated Socs1−/−Ifnγ−/− mice exhibited a greater severity and frequency of peri-bronchiole and peri-vascular inflammation in comparison to Ifnγ−/− and C57BL/6 mice. Higher magnification also revealed increased eosinophil infiltration of the alveolar spaces in OVA-treated Socs1−/−Ifnγ−/− mice (Fig. 3). These data confirmed the BAL results and further suggest enhanced vascular adhesion and diapedesis in OVA-treated Socs1−/−Ifnγ−/− mice.
Fig. 3.
Increased peri-bronchiole, peri-vascular and alveolar infiltrate in OVA-treated Ifnγ−/−Socs1−/− mice. Transverse sections of lungs taken from OVA-treated C57BL/6 (A & B), Ifnγ−/− (C & D) and Socs1−/−Ifnγ−/− mice (E & F). Representative sections are stained with haematoxylin and eosin.
Th2 cytokine levels are elevated in Socs1−/−Ifnγ−/− mice
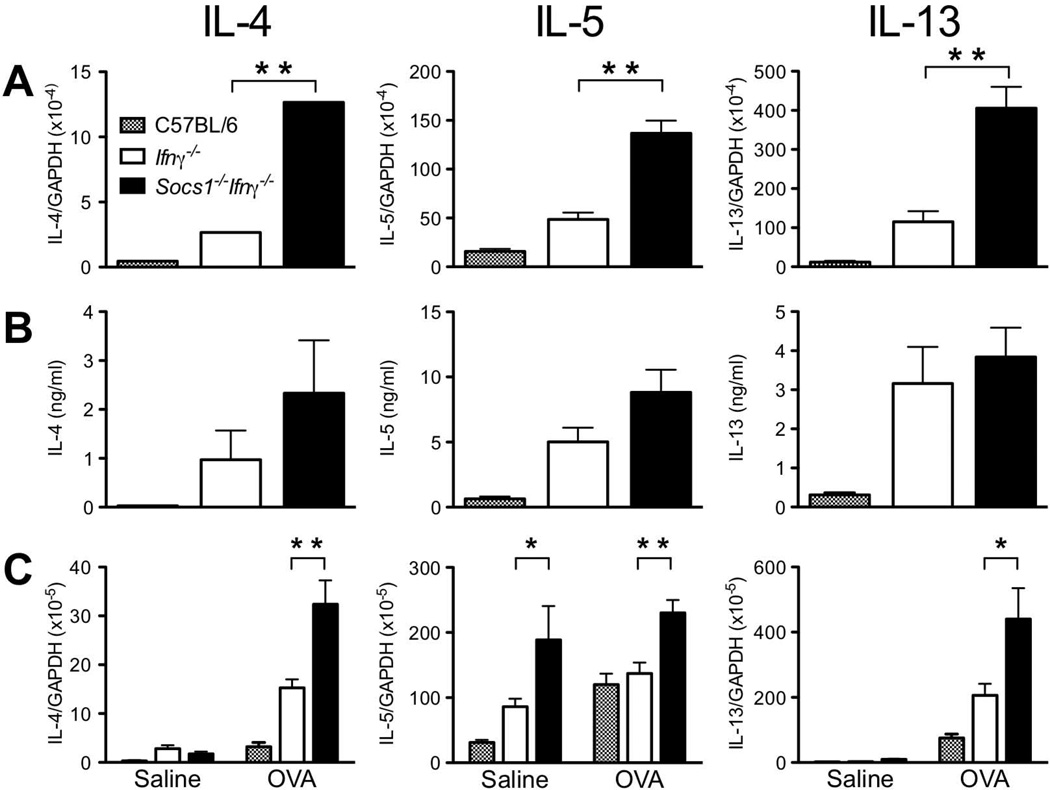
To determine if an increase in Th2 cytokine production was responsible for the enhanced local Th2 response observed in Socs1−/−Ifnγ−/− mice, IL-4, IL-5 and IL-13 levels were measured in OVA-treated Socs1−/−Ifnγ−/− mice. CD4+ T Cells were purified from draining bronchial lymph nodes following OVA-challenge, cultured overnight and IL-4, IL-5 and IL-13 production measured by ELISA and Q-PCR. All three cytokines were significantly elevated (2–3-fold) at both an mRNA and protein level in cultured CD4+ T cells and in lung tissue, in OVA-challenged Socs1−/−Ifnγ−/− mice when compared with C57BL/6 and Ifnγ−/− controls (Fig. 4). Interestingly, IL-5 mRNA levels were elevated in lung tissue from saline-treated Socs1−/−Ifnγ−/− mice, consistent with the increased numbers of eosinophils found in these mice. However, there was no further increase in IL-5 production following OVA treatment (Fig. 4C). Cytokine levels in CD4+ cultures from Ifnγ−/− mice were also elevated compared to wild-type cultures (Fig. 4 A & B).
Fig. 4.
IL-4, IL-5 and IL-13 levels following OVA-challenge. (A) Cytokine mRNA levels in pooled cultures of CD4+ T cells from OVA-treated C57BL/6, Ifnγ−/− and Socs1−/−Ifnγ−/− mice were normalised against GAPDH mRNA (n≥3). (B) Cytokine production by cultured CD4+ T cells as measured by ELISA (n≥3). (C) Cytokine mRNA levels in lungs from saline and OVA-treated mice were normalised against GAPDH mRNA (n≥3). Bars represent mean ± S.E.M. * p < 0.05, ** p < 0.01
Infiltrating hematopoietic cells express SOCS1
CIS, SOCS1, SOCS3, SOCS4 and SOCS5 mRNAs were detected by Q-PCR in lungs, bronchoalveolar lymph nodes, purified CD4+ T cells and cells recovered from BAL in C57BL/6 mice. Surprisingly, with the exception of CIS in CD4+ cells, which increased modestly (p<0.05), SOCS mRNA levels did not increase in lungs, lymph nodes or CD4+ cells following OVA-treatment. In fact, SOCS4 and CIS decreased slightly in OVA-treated lung (p<0.05) (Fig. 5A, B & D). It is likely that changes in SOCS expression in these tissues are either transient or restricted to small sub-populations of cells. In contrast, SOCS1 mRNA was highly expressed in infiltrating hematopoietic cells in OVA-treated lungs and was undetectable in saline-treated BAL cells, whilst CIS was modestly increased following OVA-treatment (p=0.059) (Fig. 5C). As the composition of the BAL changes following OVA-treatment with an influx of 30–40% eosinophils, it is impossible to know whether existing cells such as macrophages up-regulate SOCS1 or whether infiltrating eosinophils are expressing SOCS1.
Fig. 5.
SOCS expression in OVA-treated C57BL/6 mice. SOCS mRNA levels in lung (A), draining bronchial lymph nodes (B), infiltrating lung hematopoietic cells (C) and cultured CD4+ T cells (D) from saline and OVA-treated C57BL/6 mice were normalised against GAPDH mRNA. (n≥3) Bars represent mean ± S.E.M. ND: Not detectable
Although SOCS levels were unchanged in lung tissue, mRNA levels for a number of key IL-4 and IL-13 responsive genes were up-regulated in response to OVA-challenge. Expression of the eosinophil chemokine eotaxin-1/CCL11, the acidic mammalian chitinase AMCase and two genes associated with goblet cells, Muc5ac and CLCA3/Gob5, although elevated in response to OVA, were not differentially regulated in Socs1−/−Ifnγ−/− mice when compared to Ifnγ−/− mice. The exception was Arginase I, which was increased in lungs from OVA-challenged Socs1−/−Ifnγ−/− mice (10-fold compared to Ifnγ−/− mice) (Fig. 6). Together this data suggests that, in general, the IL-4/IL-13 transcriptional response in airway epithelial cells of the lung is not enhanced in mice lacking SOCS1.
Fig. 6.
Gene expression in OVA-treated Socs1−/−Ifnγ−/− mice. Muc5ac (A), Arginase I (B), Gob5 (C), eotaxin-1/CCL11 (D), and AMCase (E) mRNA levels in lungs from saline and OVA-treated C57BL/6, Ifnγ−/− and Socs1−/−Ifnγ−/− mice were normalised against GAPDH. (n≥3) Bars represent mean ± S.E.M. * p < 0.05
Discussion
SOCS1 is not only an important negative regulator of IFN-γ and IL-12 signaling in Th1 and dendritic cells, but is also a negative regulator of IL-4 in macrophages and Th2 cells. Allergy and allergic asthma result from an imbalance in Th1/Th2 polarization. The analysis of SOCS1-deficient mice on the IFN-γ null background has allowed us to examine in isolation, the role of SOCS1 in the Th2-mediated asthma response. We have shown that following OVA-sensitization and challenge, mice lacking SOCS1 respond with an exaggerated Th2 response characterized by an increased eosinophil infiltration in the lungs and elevated serum IgE (but not IgG1) levels. While our results clearly indicate that in the context of this genetic background, SOCS1 is an important regulator of Th2-type responses, in the presence of IFN-γ, SOCS1 will be involved in regulating both Th1 and Th2 cytokines.
Socs1−/−Ifnγ−/− mice appear to be already skewed towards a Th2-type response, as treatment with alum alone resulted in an increased number of infiltrating eosinophils compared to controls, elevated Th2 cytokine levels and resting mice displayed elevated IgE levels. The mechanism for the latter is not clear, but may relate to altered B cell signalling thresholds, consistent with findings in mice deficient for the Src family kinase Lyn, where abnormally high basal polyclonal IgE is consequent to a B cell signalling defect [45]. Given that eosinophil differentiation and recruitment to the lungs is coordinated by IL-4, IL-5 and IL-13 [15, 16, 46], the over-production of these cytokines in the absence of SOCS1 is likely to be responsible for the increased eosinophil infiltrate in the lungs and is in accordance with a previous report that Socs1−/− CD4+ T cells produce more IL-4 than wild-type cells [29].
In contrast to the elevated Th2 cytokine levels, mRNA levels of the IL-4/IL-13-responsive genes, Muc5ac, CCL11, Gob5 and AMCase were not differentially regulated in lung tissue from OVA-treated Socs1−/−Ifnγ−/− mice. This suggests that the exacerbated local Th2 response observed in the absence of SOCS1 does not result from enhanced signalling in lung epithelium in response to IL-4 or IL-13, but more likely results from increased cytokine production by hematopoietic cells infiltrating the lung. Together, IL-5 and CCL11 orchestrate the recruitment of eosinophils to the site of allergic inflammation [47]. IL-5 and CCL11 mRNA levels were increased in saline-treated Socs1−/−Ifnγ−/− mice, but did not change significantly following OVA-treatment. While this is consistent with the increased eosinophil number found in the lungs of saline-treated Socs1−/−Ifnγ−/− mice, the lack of further elevation suggests that these regulators are unlikely to be responsible for the dramatic eosinophilia observed in OVA-treated Socs1−/−Ifnγ−/− mice, and other mechanisms such as changes in eosinophil lifespan or proliferation may be altered in SOCS1-deficient mice.
Altered L-arginine metabolism is a candidate asthma severity determinant [48, 49] and while further work is required to substantiate the SOCS1-specific regulation of L-arginine metabolism via Arginase 1, it is appealing to speculate that augmentation of SOCS1 function may prove useful in severe asthma.
It remains possible that the phenotype in the SOCS null mice results, at least in part, from enhanced intracellular signalling in hematopoietic cells in response to IL-4 (as has been shown by others [30]), or in response to other Th2 cytokines such as IL-5 or IL-9. Infiltrating hematopoietic cells in the lungs of wild-type C57BL/6 mice expressed high levels of SOCS1 following OVA treatment and it will be of interest to further define this population.
An interesting aspect of this study is the apparent dissociation of IgE and IgG1 regulation. Isotype switching to IgE and IgG1 is dependent on IL-4 [50, 51] although IL-13 may also play a role in vivo [52], whilst STAT6 is critical for IgE production [9–11]. In most instances when IgE is elevated in response to parasitic infection, IgG1 is also elevated. While at this stage we are unable to explain why IgE, but not IgG1 is elevated in SOCS1-deficient mice, it is possible that IgE clearance and/or class switching is selectively regulated by a SOCS1-dependent pathway. Given the low levels of OVA-specific IgE in the serum of SOCS1-deficient mice it is likely that the increase in serum IgE is largely antigen-independent.
Little is known regarding the role of SOCS proteins in human asthma. Harada and colleagues [39] have identified a polymorphism in the SOCS1 promoter, which correlates with asthma severity and, in vitro with enhanced SOCS1 expression. Others have found an association between increased SOCS1 expression, allergic sensitization and Th2 polarization [40]. These results can be interpreted in several ways. Enhanced SOCS1 expression may result in inhibition of IFN-γ signaling, skewing towards Th2 polarisation and exacerbating asthma pathology. Conversely, the elevated levels of Th2 cytokines found in asthmatic individuals, may result in cytokine-induced elevation of SOCS1 as part of the normal transcriptional response. In the latter, SOCS1 is clearly unable to control the extent of Th2-cytokine signaling, perhaps due to deregulation at other points in the cascade. There is great interest in SOCS proteins as therapeutic candidates for the treatment of allergy and asthma. It is important that we fully understand the underlying biology and the consequences of SOCS intervention. Our results would predict that treatment with exogenous SOCS1 or a SOCS1 mimetic would alleviate the Th2-driven symptoms of asthma.
While the sub-chronic mouse model of asthma recapitulates some of the classic symptoms of human asthma, it will also be of interest to determine in the future, if SOCS1 regulates airway remodelling, pulmonary fibrosis or airway smooth muscle function [53]. Recent and meticulous lung physiology data has confirmed the long held suspicion that airway hyperresponsiveness is not a consequence of airway inflammation, but rather a separately regulated trait [54]. It can therefore not be assumed that regulation of inflammation by SOCS1 will also alter lung function.
Despite these caveats, it is clear that at least in mice, SOCS1 is an important negative regulator of asthma-like allergic inflammation.
Acknowledgements
This work was supported in part by the National Health and Medical Research Council (NHMRC), Australia (Program grant #461219, Project grant #461232) and in part by the National Institutes of Health (RO1 CA22556-26). C.L. was supported by an Alan Harris Student Scholarship, S.E.N. by an NHMRC Biomedical Career Development Award and Fellowship (#516705) and W.S.A. by an NHMRC Fellowship (#305503). We are grateful to D. Metcalf for instruction in differential cell counts, and T. Kay and R. Starr for provision of Socs1Lck-Cre blood samples. We thank Michele Grimbaldeston (IMVS, SA) for the kind gift of OVA-specific IgE antibodies. We thank G. Siciliano and J. McSween for excellent animal husbandry.
References
- 1.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 3.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Ann Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 4.Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 5.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 6.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Resp Cell Mol Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. New Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 8.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 9.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 10.Akimoto T, Numata F, Tamura M, Takata Y, Higashida N, Takashi T, Takeda K, Akira S. Abrogation of bronchial eosinophilic inflammation and airway hyperreactivity in signal transducers and activators of transcription (STAT)6-deficient mice. J Exp Med. 1998;187:1537–1542. doi: 10.1084/jem.187.9.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyle AJ, Le Gros G, Bertrand C, Tsuyuki S, Heusser CH, Kopf M, Anderson GP. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am J Resp Cell Mol Biol. 1995;13:54–59. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- 13.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 14.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nature Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 15.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. quiz 691. [DOI] [PubMed] [Google Scholar]
- 18.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Ann Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 19.Tabata Y, Khurana Hershey GK. IL-13 receptor isoforms: breaking through the complexity. Curr Allergy Asthma Rep. 2007;7:338–345. doi: 10.1007/s11882-007-0051-x. [DOI] [PubMed] [Google Scholar]
- 20.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nature Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 21.Coyle AJ, Tsuyuki S, Bertrand C, Huang S, Aguet M, Alkan SS, Anderson GP. Mice lacking the IFN-gamma receptor have impaired ability to resolve a lung eosinophilic inflammatory response associated with a prolonged capacity of T cells to exhibit a Th2 cytokine profile. J Immunol. 1996;156:2680–2685. [PubMed] [Google Scholar]
- 22.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 23.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 24.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 25.Cornish AL, Chong MM, Davey GM, Darwiche R, Nicola NA, Hilton DJ, Kay TW, Starr R, Alexander WS. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003;278:22755–22761. doi: 10.1074/jbc.M303021200. [DOI] [PubMed] [Google Scholar]
- 26.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TW, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 27.Chong MM, Cornish AL, Darwiche R, Stanley EG, Purton JF, Godfrey DI, Hilton DJ, Starr R, Alexander WS, Kay TW. Suppressor of cytokine signaling-1 is a critical regulator of interleukin-7-dependent CD8+ T cell differentiation. Immunity. 2003;18:475–487. doi: 10.1016/s1074-7613(03)00078-5. [DOI] [PubMed] [Google Scholar]
- 28.Naka T, Tsutsui H, Fujimoto M, Kawazoe Y, Kohzaki H, Morita Y, Nakagawa R, Narazaki M, Adachi K, Yoshimoto T, Nakanishi K, Kishimoto T. SOCS-1/SSI-1-deficient NKT cells participate in severe hepatitis through dysregulated cross-talk inhibition of IFN-gamma and IL-4 signaling in vivo. Immunity. 2001;14:535–545. doi: 10.1016/s1074-7613(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 29.Fujimoto M, Tsutsui H, Yumikura-Futatsugi S, Ueda H, Xingshou O, Abe T, Kawase I, Nakanishi K, Kishimoto T, Naka T. A regulatory role for suppressor of cytokine signaling-1 in T(h) polarization in vivo. Int Immunol. 2002;14:1343–1350. doi: 10.1093/intimm/dxf094. [DOI] [PubMed] [Google Scholar]
- 30.Dickensheets H, Vazquez N, Sheikh F, Gingras S, Murray PJ, Ryan JJ, Donnelly RP. Suppressor of cytokine signaling-1 is an IL-4-inducible gene in macrophages and feedback inhibits IL-4 signaling. Genes Immun. 2007;8:21–27. doi: 10.1038/sj.gene.6364352. [DOI] [PubMed] [Google Scholar]
- 31.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–3187. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 32.Seki Y, Inoue H, Nagata N, Hayashi K, Fukuyama S, Matsumoto K, Komine O, Hamano S, Himeno K, Inagaki-Ohara K, Cacalano N, O'Garra A, Oshida T, Saito H, Johnston JA, Yoshimura A, Kubo M. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nature Med. 2003;9:1047–1054. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 33.Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T, Yoshimura A. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J Exp Med. 2006;203:1021–1031. doi: 10.1084/jem.20052333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seki Y, Hayashi K, Matsumoto A, Seki N, Tsukada J, Ransom J, Naka T, Kishimoto T, Yoshimura A, Kubo M. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc Natl Acad Sci USA. 2002;99:13003–13008. doi: 10.1073/pnas.202477099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozaki A, Seki Y, Fukushima A, Kubo M. The control of allergic conjunctivitis by suppressor of cytokine signaling (SOCS)3 and SOCS5 in a murine model. J Immunol. 2005;175:5489–5497. doi: 10.4049/jimmunol.175.8.5489. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe H, Kubo M, Numata K, Takagi K, Mizuta H, Okada S, Ito T, Matsukawa A. Overexpression of suppressor of cytokine signaling-5 in T cells augments innate immunity during septic peritonitis. J Immunol. 2006;177:8650–8657. doi: 10.4049/jimmunol.177.12.8650. [DOI] [PubMed] [Google Scholar]
- 37.Ohshima M, Yokoyama A, Ohnishi H, Hamada H, Kohno N, Higaki J, Naka T. Overexpression of suppressor of cytokine signalling-5 augments eosinophilic airway inflammation in mice. Clin Exp Allergy. 2007;37:735–742. doi: 10.1111/j.1365-2222.2007.02707.x. [DOI] [PubMed] [Google Scholar]
- 38.Brender C, Columbus R, Metcalf D, Handman E, Starr R, Huntington N, Tarlinton D, Odum N, Nicholson SE, Nicola NA, Hilton DJ, Alexander WS. SOCS5 is expressed in primary B and T lymphoid cells but is dispensable for lymphocyte production and function. Mol Cell Biol. 2004;24:6094–6103. doi: 10.1128/MCB.24.13.6094-6103.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada M, Nakashima K, Hirota T, Shimizu M, Doi S, Fujita K, Shirakawa T, Enomoto T, Yoshikawa M, Moriyama H, Matsumoto K, Saito H, Suzuki Y, Nakamura Y, Tamari M. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am J Resp Cell Mol Biol. 2007;36:491–496. doi: 10.1165/rcmb.2006-0090OC. [DOI] [PubMed] [Google Scholar]
- 40.Daegelmann C, Herberth G, Roder S, Herbarth O, Giese T, Kramer U, Behrendt H, Borte M, Heinrich J, Emmrich F, Lehmann I. Association between suppressors of cytokine signalling, T-helper type 1/ T-helper type 2 balance and allergic sensitization in children. Clin Exp Allergy. 2007 doi: 10.1111/j.1365-2222.2007.02913.x. [DOI] [PubMed] [Google Scholar]
- 41.Caminschi I, Proietto AI, Ahmet F, Kitsoulis S, Teh JS, Lo JC, Rizzitelli A, Wu L, Vremec D, van Dommelen SL, Campbell IK, Maraskovsky E, Braley H, Davey GM, Mottram P, van de Velde N, Jensen K, Lew AM, Wright MD, Heath WR, Shortman K, Lahoud MH. The dendritic cell subtype restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood. 2008;112:3264–3273. doi: 10.1182/blood-2008-05-155176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wormald S, Zhang JG, Krebs DL, Mielke LA, Silver J, Alexander WS, Speed TP, Nicola NA, Hilton DJ. The comparative roles of suppressor of cytokine signaling-1 and-3 in the inhibition and desensitization of cytokine signaling. J Biol Chem. 2006;281:11135–11143. doi: 10.1074/jbc.M509595200. [DOI] [PubMed] [Google Scholar]
- 43.Brewer JM, Conacher M, Satoskar A, Bluethmann H, Alexander J. In interleukin-4-deficient mice, alum not only generates T helper 1 responses equivalent to freund's complete adjuvant, but continues to induce T helper 2 cytokine production. Eur J Immunol. 1996;26:2062–2066. doi: 10.1002/eji.1830260915. [DOI] [PubMed] [Google Scholar]
- 44.Brewer JP, Kisselgof AB, Martin TR. Genetic variability in pulmonary physiological, cellular, and antibody responses to antigen in mice. Am J Respir Crit Care Med. 1999;160:1150–1156. doi: 10.1164/ajrccm.160.4.9806034. [DOI] [PubMed] [Google Scholar]
- 45.Beavitt SJ, Harder KW, Kemp JM, Jones J, Quilici C, Casagranda F, Lam E, Turner D, Brennan S, Sly PD, Tarlinton DM, Anderson GP, Hibbs ML. Lyn-deficient mice develop severe, persistent asthma: Lyn is a critical negative regulator of Th2 immunity. J Immunol. 2005;175:1867–1875. doi: 10.4049/jimmunol.175.3.1867. [DOI] [PubMed] [Google Scholar]
- 46.Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24:73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 47.Mould AW, Matthaei KI, Young IG, Foster PS. Relationship between interleukin-5 and eotaxin in regulating blood and tissue eosinophilia in mice. J Clin Invest. 1997;99:1064–1071. doi: 10.1172/JCI119234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lara A, Khatri SB, Wang Z, Comhair SA, Xu W, Dweik RA, Bodine M, Levison BS, Hammel J, Bleecker E, Busse W, Calhoun WJ, Castro M, Chung KF, Curran-Everett D, Gaston B, Israel E, Jarjour N, Moore W, Peters SP, Teague WG, Wenzel S, Hazen SL, Erzurum SC. Alterations of the arginine metabolome in asthma. Am J Respir Crit Care Med. 2008;178:673–681. doi: 10.1164/rccm.200710-1542OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Litonjua AA, Lasky-Su J, Schneiter K, Tantisira KG, Lazarus R, Klanderman B, Lima JJ, Irvin CG, Peters SP, Hanrahan JP, Liggett SB, Hawkins GA, Meyers DA, Bleecker ER, Lange C, Weiss ST. ARG1 is a novel bronchodilator response gene: screening and replication in four asthma cohorts. Am J Respir Crit Care Med. 2008;178:688–694. doi: 10.1164/rccm.200709-1363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 51.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 52.Emson CL, Bell SE, Jones A, Wisden W, McKenzie AN. Interleukin (IL)-4-independent induction of immunoglobulin (Ig)E, and perturbation of T cell development in transgenic mice expressing IL-13. J Exp Med. 1998;188:399–404. doi: 10.1084/jem.188.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar RK, Foster PS. Modeling allergic asthma in mice: pitfalls and opportunities. Am J Resp Cell Mol Biol. 2002;27:267–272. doi: 10.1165/rcmb.F248. [DOI] [PubMed] [Google Scholar]
- 54.Wagers SS, Haverkamp HC, Bates JH, Norton RJ, Thompson-Figueroa JA, Sullivan MJ, Irvin CG. Intrinsic and antigen-induced airway hyperresponsiveness are the result of diverse physiological mechanisms. J Appl Physiol. 2007;102:221–230. doi: 10.1152/japplphysiol.01385.2005. [DOI] [PubMed] [Google Scholar]