Abstract
Naltrexone evokes a cortisol response through its blockade of central opioid receptors on the hypothalamic-pituitary-adrenocortical axis (HPA). The magnitude of this cortisol response may be useful as a probe for central opioid activity in different groups of subjects. Accordingly, the present study examined the effect of opioid blockade on the HPA in 70 women and 58 men with (N = 41) and without (N = 87) a family history of alcoholism, using a randomized, placebo-controlled, double blind administration of oral naltrexone (50 mg). Saliva cortisol was sampled at baseline prior to placebo or naltrexone and again every 30 min over the next 180 min. Women had significantly larger cortisol responses to naltrexone than did the men, F = 6.88, p < .0001. There were no significant differences in cortisol response between groups differing in family history of alcoholism, F = 0.65, p > .69. The present results confirm that women have much greater central opioid restraint on the HPA than men do and that this endogenous restraint is unmasked by opioid blockade. However the results provide no evidence of a differential central opioid tonus in persons with a family history of alcoholism at this dose of naltrexone. The cortisol response to naltrexone may be a useful probe for central opioid activity in women and to a lesser degree in men.
Keywords: sex, family history of alcoholism, cortisol, naltrexone, opioid
The present study examined the salivary cortisol response to naltrexone (50 mg) in men and women with and without a family history of alcoholism (FH+, FH−). Naltrexone is an oral competitive opioid receptor antagonist with a long duration of action at central opioid receptor sites (Lee et al., 1988), and is approved in treatment of alcohol and opioid dependencies (Ling and Wesson, 1990; Moncrieff and Drummond, 1997). Naloxone is an intravenous opioid antagonist that is used in the treatment of acute opiate overdose because of its rapid response and dose titration (Bradberry and Raebel, 1981; Ling and Wesson, 1990).
Opioid agonists and antagonists exert opposing effects on the hypothalamic-pituitary-adrenocortical axis in humans (Kreek, 2001; Vuong et al., 2010). Opioid agonists inhibit cortisol secretion and opioid antagonists increase its secretion (Ambrosi et al., 1989; Grossman and Clement-Jones, 1983; Grossman and Rees, 1983; King et al., 2002). As a result, the cortisol response to opiate receptor blockade may serve as a probe for underlying central opioid activation, and as a way to study differences in endogenous opioid tone in groups such as FH+ and FH− (King et al., 2002).
Studies comparing men and women on cortisol responses to naltrexone and naloxone have reported that women have a large response while men have no significant response (Klein et al., 2000; Roche et al., 2010) or smaller responses (Uhart et al., 2006). Similarly, women were shown to have a significant increase in pain response following naltrexone, while men had no change (Frew and Drummond, 2007). Both sets of results imply greater endogenous opioid tonus in women. Other studies have reported larger cortisol responses to naltrexone or naloxone in FH+ than in FH− (King et al., 2002; Wand et al., 1998). These are of particular interest because central opioid neurons interact with reward pathways (Koob et al., 1994), and modification of these interacting systems through exposure to drugs of abuse is seen as a useful model for the study of addictions (Koob and Le Moal, 2008). However, there are no studies comparing men and women independently within FH+ and FH− groups.
In accord with the preceding, the present study had the immediate goal of using the cortisol response to naltrexone as a probe for central opioid function in FH groups. Use of the cortisol response to naltrexone could then serve as a potential predictor of behavioral variables and risk for alcoholism in later work. Furthermore, prior studies of FH groups have not reported separate results for men vs. women and were generally based on smaller sample sizes. In view of reported sex differences in cortisol response to opioid antagonism, we designed the present study to have sufficient numbers of men and women in each FH category to allow for meaningful comparisons of response magnitudes and to determine if FH effects were equivalent for men and women. We predicted that women would have larger responses than men and that FH+ would have larger responses than FH−, with the largest responses being in FH+ women.
1. MATERIALS AND METHODS
1.1. Overview
The Oklahoma Family Health Patterns Project is a study of healthy young FH+ and FH− adults with the goal of understanding preexisting differences between these groups that might provide insights into risk factors in persons who have not yet developed problem drinking or drug use habits. The parent project currently has 407 participants of whom a subset underwent the naltrexone protocol.
1.2. Subjects
Naltrexone testing included 132 subjects (74 females) recruited through community advertisement, however 4 women experienced nausea and vomiting in response to naltrexone and discontinued the protocol, and so the final sample includes 128 persons. Each subject signed a consent form approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center and the Veterans Affairs Medical Center, Oklahoma City, OK, USA, and received financial compensation for participating. Demographic characteristics are shown in Table 1.
Table 1.
Subject Demographic and Biometric Characteristics
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Negative | Positive | p | Negative | Positive | p | |
| N | 42 | 16 | 45 | 25 | ||
| Age (yr) | 23.8 (0.4) | 24.4 (0.7) | 0.4 | 24.1 (0.4) | 23.6 (0.6) | 0.5 |
| Education (yr) | 16.3 (0.3) | 16.2 (0.5) | 0.8 | 16.1 (0.3) | 15.5 (0.4) | 0.2 |
| SES | 49 (2.0) | 46 (3.0) | 0.4 | 49 (1.9) | 42 (2.5) | 0.03 |
| Race (% White) | 93 | 81 | 0.3 | 91 | 96 | 0.6 |
| Smokers (%) (n) | 5 (2) | 6 (1) | 1.0 | 4 (2) | 12 (3) | 0.3 |
| AUDIT | 4.93 (0.5) | 4.44 (0.6) | 0.6 | 4.0 (0.3) | 3.7 (0.5) | 0.7 |
| QFI | 60 (6.9) | 69 (11.2) | 0.5 | 47 (3.6) | 47 (8.6) | 0.9 |
| BMI | 23.9 (0.5) | 25.9 (1.0) | 0.06 | 23.0 (0.5) | 22.5 (0.7) | 0.6 |
1.3. Inclusion and exclusion criteria
Prospective volunteers were excluded if they had: a history of alcohol or drug dependence; met criteria for any substance abuse within the past 2 mo; provided a positive urine drug screen (iCup, Instant Technologies, Norfolk VA; including d-amphetamine, secobarbital, benzodiazepines, buprenorphine, cocaine, marijuana, methadone, methamphetamine, methylenedioxymetamphetamine, morphine, oxycodone, phencyclidine, propoxyphene, tricyclic antidepressants) or a positive breath-alcohol test on days of testing; had a history of any Axis I disorder, other than past depression (> 60 days prior), as defined by the Diagnostic and Statistical Manual of Mental disorders, 4th ed. (American_Psychiatric_Association, 1994). Women were required to have a negative urine pregnancy test on each day of testing. All participants were in good physical health, between the ages of 18 and 30 yr, had a body mass index 18.5–29 kg/m2, were not taking prescription medications other than hormonal contraceptives, and had no reported history of serious medical disorder. Smoking and smokeless tobacco use were not exclusionary. Eight subjects (6.3%) reported using tobacco (Table 1). Smokers were allowed a cigarette immediately prior to the start of the protocol to minimize cravings during testing.
1.4. Assessment of family history
Family history classification was established using the Family History Research Diagnostic Criteria (FH-RDC) (Andreasen et al., 1977). The FH-RDC has a high degree of interrater reliability (0.95) for reports of substance use disorders (Andreasen et al., 1977; Zimmerman et al., 1988). Participants were excluded if either they or the parent reported possible fetal exposure to alcohol or other drugs. All family history positive participants reported that at least 1 biological parent had a problem with drinking not limited to isolated incidents in at least one of four areas of functioning in order to meet criteria for alcohol or substance abuse. Eleven subjects had a parent who used alcohol and other drugs and only 2 subjects were included who reported a family history of substance abuse without a history of alcohol abuse. Family history negative participants reported no alcohol or substance use disorders in their biological parents and grandparents. In the larger study sample, the participant’s FH-RDC was confirmed by parent interview in all possible cases (79% of the sample), and parents confirmed the reported FH status in 89% of these cases. Based on these interviews, FH status could confidently be reassigned in 3% of the cases and 6% were dropped for inconsistent or insufficient information. For the participants with no parent interview, we assume that 89% are also correctly classified, and by extrapolation, that 97% of the sample retained for analysis is correctly classified.
1.5. Study design and procedure
The study was a randomized, placebo-controlled, double-blind administration of placebo vs. oral naltrexone (50 mg, Malinkrodt, St. Louis, MO, USA) in identical appearing capsules prepared by Innovative Pharmacy Solutions (Edmond, OK, USA). Test days were separated by a minimum of 72 hrs.
The subject entered the General Clinical Research Center at the University of Oklahoma Health Sciences Center at 0800h, provided a urine sample to check for the presence of opiates or other drugs, and was served a light breakfast. At 0900h he or she provided a baseline saliva sample and immediately consumed the naltrexone or placebo capsule. Saliva was then collected every 30 min for the next 180 min. Every 60 min, the subject filled out a naltrexone-specific side effects questionnaire (King et al., 2002), and also rated their moods using visual-analogue scales (Lundberg and Frankenhaeuser, 1980). The subject remained seated in a recliner chair through the entire protocol and read general interest magazines or watched videos of nature or history programs. A research nurse monitored subjects during the protocol.
1.6. Saliva collection times and cortisol assay
Saliva samples were collected using the Salivette device (Sarstedt, Newton, NC, USA). Salivettes were centrifuged at 4200 RPM for 20 min. The saliva was transferred to cryogenic storage tubes and placed into a − 20° C freezer until shipping. Saliva free cortisol assays were conducted by Salimetrics (State College, PA, USA) using a competitive enzymatic immunoassay (Salimetrics, 2011) with a sensitivity of < .083 µg/dL and an interassay coefficient of variation of < 6.42%.
Estrogen levels may affect cortisol binding, and so preliminary analyses on cortisol data were done in the women. These showed no differences in cortisol response to naltrexone between women tested in the self-reported luteal and follicular phases of their menstrual cycles, t = 0.55, p > 0.58. Similarly, women using hormonal birth control (35 %) did not differ from those not doing so, t = 0.02, p > .98. Menstrual cycle and hormonal contraceptive effects accordingly were not considered in the subsequent analyses. Prior work has shown that cortisol responses to the opiate receptor agonist fentanyl was not affected by menstrual cycle phase (Hoehe, 1988).
1.7. Subjective responses and side effects
Subjects rated their moods at four time points (0, 60, 120, and 180 min) using 12 10-point visual-analogue scales containing a Distress subscale (impatience, irritability, distress, pleasantness, and control), and an Activation subscale (effort, tension, concentration, interest, and stimulation) adapted from Foresman (Lundberg and Frankenhaeuser, 1980).
Adverse effects were assessed at two time points (0 and 180 min) using an opioid-specific questionnaire (King et al., 2002) covering nausea, vomiting, headache, distress, warm or flushed feelings, anxiety, libido, hives or rash, insomnia, diarrhea, pain, sleepiness and agitation on a 3-point scale, “none,” “mild,” or “severe.” Other than the 4 subjects who vomited, only 6 (4.7%) of the subjects reported severe nausea. As a result, adverse effects were analyzed as 0 (none) or 1 (mild or severe).
1.9. Data analyses
Demographic and personality characteristics were compared between FH− and FH+ individuals using a Student’s t-test for continuous variables and an exact chi-square for categorical variables. A multivariable model with repeated measures was used to estimate the association between response to naltrexone and FH and sex, with differences from placebo to naltrexone day over time periods as a repeated measure. A similar model with repeated measures was used to estimate the association between self-reported distress and activation scores and sex and family history with treatment day and time period as repeated measures. Naltrexone adverse effects were analyzed using exact chi-square tests for men and women for each symptom. Two-tailed tests were used for all analyses and significance level was set at α <0.05. Data were analyzed using SAS software, Ver. 9.2 for Windows. Copyright© 2008 SAS Institute Inc., Cary, NC, USA.
2. RESULTS
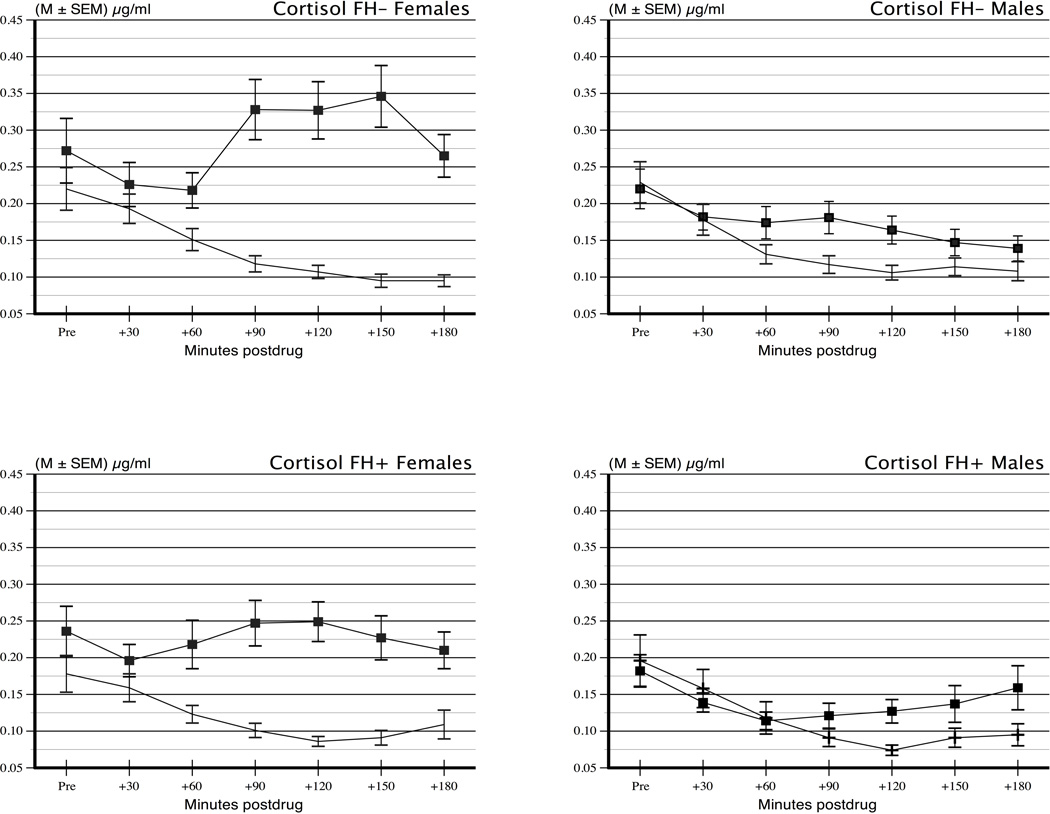
There were no differences between men and women in the FH groups on major demographic and background characteristics (Table 1). Cortisol values on naltrexone and placebo days are shown in Figure 1. In order to establish that the groups had equivalent levels of cortisol secretion under basal conditions, we carried out a preliminary analysis of cortisol values at each time point on placebo days on sex and FH groups using a repeated measure ANOVA. There was a significant effect of period reflecting the expected diurnal decline in cortisol values during the waking hours, F (6, 744) = 40.1, p < .0001. There were no significant effects of sex or FH nor any interactions with period, indicating that men and women and FH+ and FH− had equivalent diurnal secretion levels and patterns, all F’s ≤ 2.09, p’s ≥ .15.
Figure 1.
Cortisol values on naltrexone and placebo days for males (N = 58) and females (N = 70) with and without a family history of alcoholism (Ns = 41 and 87, respectively).
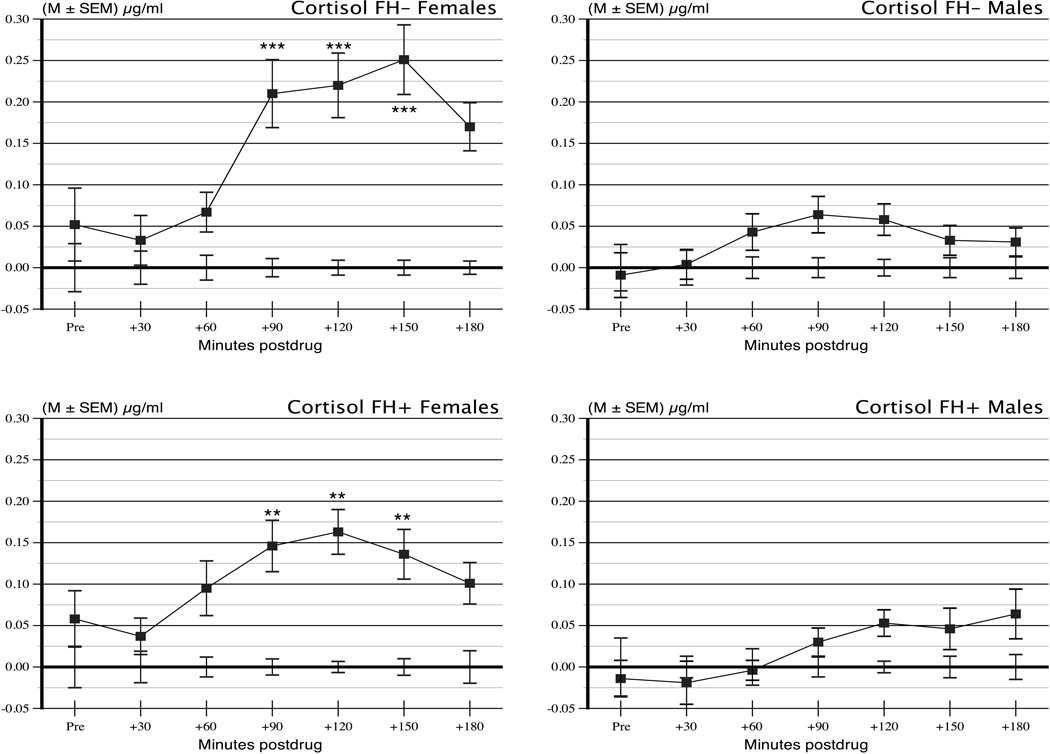
Given the equivalence of the placebo values, and for visual clarity, the placebo values were plotted in Figure 2 as zero at all time points, and the naltrexone values are plotted as differences from placebo in the original measurement units. A Sex × FH × Period repeated measure analysis of variance ANOVA on the difference scores in Figure 2 showed a significant Sex × Period interaction, F (6,744) = 6.88, p < .0001, reflecting larger changes over time among the women. In addition, there were main effects of Sex, F (1,124) = 27.2, p < .0001, and Period, F (6,744) = 15.41, p < .0001. There were no effects of FH or any other interactions, Fs ≤ 1.55, ps ≥ .22. As an additional test of the effects in men and women separately, we carried out planned comparisons of naltrexone difference from placebo in each group at the peak response periods 90, 120, and 150 min. Women had large, highly significant responses at each of these times (ps < .0001). In contrast men had smaller responses that were significant only at 90 and 120 min (ps = .028 and .023, respectively) and not at 180 min (p = .140). Results were the same after removing smokers from the analysis.
Figure 2.
Cortisol responses to 50 mg naltrexone shown as differences from placebo across time periods in the Sex and Family History groups. As noted in the text and as indicated by asterisks in the figure, women had significant cortisol elevations relative to placebo at 90, 120, and 150 min following ingestion. Men had no significant rises at any time based on simple effects tests corrected for multiple comparisons.
Subjective reports were examined in a Sex × FH × Day × Period (0, 60, 120, 180 min) ANOVA. Feelings of distress did not vary as a function of any of these variables or any of their interactions (all Fs < 1.50, ps > .22), indicating that naltrexone did not increase feelings of dysphoria. Activation reports declined across periods, F (1, 124) = 13.39, p < .0004 on both days. There were no other main effects or interactions, all Fs ≤ 1.25, ps ≥ .127.
Side effects analyses showed only that women were more likely than men to report mild nausea on the naltrexone day, chi-square = 10.18, p < .003. Since nausea may have affected cortisol secretion, the Sex × FH × Period ANOVA on cortisol values was rerun with nausea as a covariate, and the results did not change.
3. DISCUSSION
The present study confirms that women have larger and longer lasting cortisol responses to 50 mg of naltrexone than do men. Perhaps more interestingly, the results provide no confirmation that FH+ are more reactive than FH− to that dose of naltrexone. These findings provide several points for consideration in relation to naltrexone’s utility as a probe for central opioid activity in relation to risk for alcoholism.
In light of our present findings we reexamined studies of cortisol responses to opioid blockade. In general these studies show that men have minimal cortisol responses to opioid blockade when sex comparisons are available. Fourteen studies reported significant cortisol responses to opioid blockade in combined groups of men and women (al'Absi et al., 2004; Ceballos et al., 2007; Cohen et al., 1985; Cohen et al., 1983; Farren et al., 1999; Hernandez-Avila et al., 2002; Kiefer et al., 2006; King et al., 2002; O'Malley et al., 2002; Ooteman et al., 2007; Ray et al., 2009; Wand et al., 2001; Wand et al., 1999; Wand et al., 1998). However, in the three prior studies that made sex comparisons, men failed to respond to 50 or 100 mg of naltrexone, while women responded at both doses (Klein et al., 2000; Roche et al., 2010), and men had smaller responses to doses of naloxone up to 400 µg/kg (Uhart et al., 2006). Taken together with the present data, it appears that men are less responsive than women to the cortisol effects of opioid blockade.
Similarly, there is only equivocal evidence of that FH groups have different cortisol responses to opioid blockade at most doses. Five studies have reported cortisol comparisons between FH groups (Hernandez-Avila et al., 2002; King et al., 2002; Wand et al., 2001; Wand et al., 1999; Wand et al., 1998). One study reported larger responses to 50 mg naltrexone in FH+ than FH− although the sample included only 15 subjects, indicating a low level of statistical power (King et al., 2002). The remaining four studies provide only equivocal support that FH+ are differentially reactive to naloxone at doses at or below 125 µg/kg IV (Hernandez-Avila et al., 2002; Wand et al., 2001; Wand et al., 1999; Wand et al., 1998). In three of these studies the FH+ group had higher placebo values than the FH− subjects, but these placebo differences were not accounted for in the statistical analysis. However, the graphed data show that the naltrexone group differences are only minimally larger when the placebo difference is accounted for (Hernandez-Avila et al., 2002; Wand et al., 1999; Wand et al., 1998). In a fourth study FH+ subjects appear to have larger responses than FH− to 125 and 375 µg/kg of naloxone but not to 50 µg/kg or less (Wand et al., 2001). However, the graphs do not have error bars to allow a clear comparison of the FH groups. In light of the present data, it appears unlikely that FH+ have larger cortisol responses to opioid blockade than FH−, with the possible exception of responses to naloxone doses ≥ 125 µg/kg.
The present results, taken in light of these mixed earlier findings, suggest that naltrexone (50 mg PO) and lower doses of naloxone (< 125 µg/kg IV) are not likely to be useful probes for differential central opioid function in FH+ persons, although the sex difference seen here indicates that naltrexone may be of interest in probing individual differences in opioid function among women. The peak cortisol response to naltrexone in women at 90–150 min corresponds to the interval of peak naltrexone biotransformation and peak blood levels of naltrexone and its primary metabolite (Meyer et al., 1984; Verebey et al., 1976). The greater cortisol responses in women suggest possible clinical implications for treatment of addictive disorders. As noted, naltrexone is approved for treatment of alcohol and opioid dependencies and also shows some promise as an adjunct treatment for nicotine dependence (King et al., 2012; King et al., 2006; O'Malley et al., 2006). There is evidence that lowered HPA function accompanies addiction and risk for addiction; persons at high risk for alcoholism have reduced cortisol responses to alcohol challenge (King et al., 2011; Schuckit et al., 1987) and alcoholics and persons at risk have blunted cortisol responses to laboratory stressors (Errico et al., 1993; Lovallo, 2006; Sinha et al., 2011; Sorocco et al., 2006). Most importantly, blunted cortisol responses to stress and drug challenge predict higher cravings and relapse risk (Adinoff et al., 2005; al'Absi et al., 2005; O'Malley et al., 2002). Accordingly naltrexone may normalize HPA function in such persons, and this may aid in its clinical efficacy (Adinoff et al., 2005; Kiefer et al., 2006). Women’s greater sensitivity to the HPA effects of naltrexone may play a role in the differential sex-specific effects observed with naltrexone in treatment of nicotine dependence (Epperson et al., 2010; King et al., 2012; King et al., 2006) and/or alcohol dependence (Garbutt et al., 2005), although at least one study found no sex differences in alcoholism outcomes with naltrexone (Greenfield et al., 2010). While prior studies have lacked sufficient numbers of women for meaningful sex comparisons, there is evidence that compared to men, women given naltrexone report more antinociception (al'Absi et al., 2004) and sedation (Epstein and King, 2004), and we found greater experience of nausea and vomiting in women. Increased focus on the use of naltrexone in women is warranted, particularly in regard to its normalization of HPA function in relation to treatment effectiveness.
The present study has three limitations. Several studies have examined the effect of opioid blockade on ACTH, which was not measured here. We focused on cortisol as the target hormone in the present study in accord with work showing a degree of independence between ACTH and cortisol responses to naloxone (Leslie et al., 1985). In addition, this study relied on a single dose of naltrexone, which was chosen as the standard daily dose in abstinence studies. It is possible that responses may have been seen in men with higher doses, and accordingly a dose-ranging study using naltrexone would be called for.
The primary strength of the present study is the relatively large sample size, which lends confidence that the lack of differential cortisol response between FH groups is a true negative finding. Similarly, the results agree with prior studies that reported larger cortisol responses to opioid blockade in women than in men (Klein et al., 2000; Roche et al., 2010). We conclude that women have a higher level of central opioid restraint on the HPA than men do. Future work should examine whether the cortisol response to opioid blockade may be useful in understanding individual differences across cognitive functions, behavioral tendencies, and stress reactivity. Increased attention to naltrexone’s effectiveness in treatment of addictions in women also appears warranted.
Acknowledgements
This work was supported by the Department of Veterans Affairs, the National Institutes of Health, NIAAA (R01 AA12207), NIRR (M01 RR14467, UL1 RR025767, KL2 RR025766, and RR025766), and NHLBI (F32 HL083689).
Role of the funding source
The funding sources had no role in the design of the study or in the analysis and interpretation of the results. The content is solely the view of the authors and does not necessarily represent the official view of the National Institutes of Health or the VA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no financial interest in any product associated with this research.
Contributors
William R. Lovallo designed the study, interpreted the results and contributed to writing the paper
Andrea C. King consulted on interpreting the results and contributed to writing the paper
Noha H. Farag contributed to analysis and interpretation of the results
Kristen H. Sorocco carried out side effects assessments and contributed to writing the paper
Andrew S. Cohoon carried out the primary analysis of the results
Andrea S. Vincent contributed to the study design and the analysis of the results
REFERENCES
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Wittmers LE, Ellestad D, Nordehn G, Kim SW, Kirschbaum C, Grant JE. Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med. 2004;66:198–206. doi: 10.1097/01.psy.0000116250.81254.5d. [DOI] [PubMed] [Google Scholar]
- Ambrosi B, Bochicchio D, Ferrario R, Colombo P, Faglia G. Effects of the opiate agonist loperamide on pituitary-adrenal function in patients with suspected hypercortisolism. J Endocrinol Invest. 1989;12:31–35. doi: 10.1007/BF03349913. [DOI] [PubMed] [Google Scholar]
- American_Psychiatric_Association. Diagnostic and Statistical Manual of Mental Disorders. 4th (text rev.) ed. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria. Reliability and validity. Arch Gen Psychiatry. 1977;34:1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Bradberry JC, Raebel MA. Continuous infusion of naloxone in the treatment of narcotic overdose. Drug Intell Clin Pharm. 1981;15:945–950. doi: 10.1177/106002808101501205. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, France CR, al'Absi M. Influence of naltrexone administration on dehydroepiandrosterone sulfate levels in male and female participants. Biol Psychol. 2007;74:414–416. doi: 10.1016/j.biopsycho.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Cohen RM, Pickar D, Kreger D, McLellan C, Murphy DL. Hormonal effects of high dose naloxone in humans. Neuropeptides. 1985;6:373–380. doi: 10.1016/0143-4179(85)90010-1. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Cohen RM, Pickar D, Weingartner H, Murphy DL. High-dose naloxone infusions in normals. Dose-dependent behavioral, hormonal, and physiological responses. Arch Gen Psychiatry. 1983;40:613–619. doi: 10.1001/archpsyc.1983.04390010023003. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Toll B, Wu R, Amin Z, Czarkowski KA, Jatlow P, Mazure CM, O'Malley SS. Exploring the impact of gender and reproductive status on outcomes in a randomized clinical trial of naltrexone augmentation of nicotine patch. Drug Alcohol Depend. 2010;112:1–8. doi: 10.1016/j.drugalcdep.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AM, King AC. Naltrexone attenuates acute cigarette smoking behavior. Pharmacol Biochem Behav. 2004;77:29–37. doi: 10.1016/j.pbb.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Errico AL, Parsons OA, King AC, Lovallo WR. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J Stud Alcohol. 1993;54:393–398. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- Farren CK, O'Malley S, Grebski G, Maniar S, Porter M, Kreek MJ. Variable dose naltrexone-induced hypothalamic-pituitary-adrenal stimulation in abstinent alcoholics: A preliminary study. Alcohol Clin Exp Res. 1999;23:502–508. [PubMed] [Google Scholar]
- Frew AK, Drummond PD. Negative affect, pain and sex: the role of endogenous opioids. Pain. 2007;132(Suppl 1):S77–S85. doi: 10.1016/j.pain.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Pettinati HM, S OM, Randall PK, Randall CL. Gender differences in alcohol treatment: An analysis of outcome from the COMBINE study. Alcohol Clin Exp Res. 2010;34:1803–1812. doi: 10.1111/j.1530-0277.2010.01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A, Clement-Jones V. Opiate receptors: enkephalins and endorphins. Clin Endocrinol Metab. 1983;12:31–56. doi: 10.1016/s0300-595x(83)80028-0. [DOI] [PubMed] [Google Scholar]
- Grossman A, Rees LH. The neuroendocrinology of opioid peptides. Br Med Bull. 1983;39:83–88. doi: 10.1093/oxfordjournals.bmb.a071796. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Oncken C, Van Kirk J, Wand G, Kranzler HR. Adrenocorticotropin and cortisol responses to a naloxone challenge and risk of alcoholism. Biol Psychiatry. 2002;51:652–658. doi: 10.1016/s0006-3223(01)01334-8. [DOI] [PubMed] [Google Scholar]
- Hoehe M. Influence of the menstrual cycle on neuroendocrine and behavioral responses to an opiate agonist in humans: preliminary results. Psychoneuroendocrinology. 1988;13:339–344. doi: 10.1016/0306-4530(88)90059-5. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Otte C, Naber D, Wiedemann K. Hypothalamic-pituitary-adrenocortical axis activity: a target of pharmacological anticraving treatment? Biol Psychiatry. 2006;60:74–76. doi: 10.1016/j.biopsych.2005.11.023. [DOI] [PubMed] [Google Scholar]
- King AC, Cao D, O'Malley SS, Kranzler HR, Cai X, deWit H, Matthews AK, Stakoviak RJ. Effects of naltrexone on smoking cessation outcomes in nicotine dependent men and women. J Clin Psychopharmacol. 2012 doi: 10.1097/JCP.0b013e3182676956. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, Riley RC, Cao D, Niaura R, Hatsukami D. Efficacy of naltrexone in smoking cessation: a preliminary study and an examination of sex differences. Nicotine Tob Res. 2006;8:671–682. doi: 10.1080/14622200600789767. [DOI] [PubMed] [Google Scholar]
- King AC, Schluger J, Gunduz M, Borg L, Perret G, Ho A, Kreek MJ. Hypothalamic-pituitary-adrenocortical (HPA) axis response and biotransformation of oral naltrexone: preliminary examination of relationship to family history of alcoholism. Neuropsychopharmacology. 2002;26:778–788. doi: 10.1016/S0893-133X(01)00416-X. [DOI] [PubMed] [Google Scholar]
- Klein LC, Jamner LD, Alberts J, Orenstein MD, Levine L, Leigh H. Sex differences in salivary cortisol levels following naltrexone administration. J Appl Biobehav Res. 2000;5:144–153. [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Rassnick S, Heinrichs S, Weiss F. Alcohol, the reward system and dependence. EXS. 1994;71:103–114. doi: 10.1007/978-3-0348-7330-7_11. [DOI] [PubMed] [Google Scholar]
- Kreek MJ. Drug addictions: Molecular and cellular endpoints. Ann N Y Acad Sci. 2001;937:1749–6632. [PubMed] [Google Scholar]
- Lee MC, Wagner HN, Jr, Tanada S, Frost JJ, Bice AN, Dannals RF. Duration of occupancy of opiate receptors by naltrexone. J Nucl Med. 1988;29:1207–1211. [PubMed] [Google Scholar]
- Leslie RD, Prescott RW, Kendall-Taylor P, Cook D, Weightman D, Ratcliffe J, Ingram MC. Opiate receptor blockade and diurnal pituitary and adrenal hormone levels. Horm Metab Res. 1985;17:86–89. doi: 10.1055/s-2007-1013458. [DOI] [PubMed] [Google Scholar]
- Ling W, Wesson DR. Drugs of abuse--opiates. West J Med. 1990;152:565–572. [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR. Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol. 2006;59:195–202. doi: 10.1016/j.ijpsycho.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg U, Frankenhaeuser M. Pituitary-adrenal and sympathetic-adrenal correlates of distress and effort. J Psychosom Res. 1980;24:125–130. doi: 10.1016/0022-3999(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Meyer MC, Straughn AB, Lo MW, Schary WL, Whitney CC. Bioequivalence, dose-proportionality, and pharmacokinetics of naltrexone after oral administration. J Clin Psychiatry. 1984;45:15–19. [PubMed] [Google Scholar]
- Moncrieff J, Drummond DC. New drug treatments for alcohol problems: a critical appraisal. Addiction. 1997;92:939–947. discussion 49–64. [PubMed] [Google Scholar]
- O'Malley SS, Cooney JL, Krishnan-Sarin S, Dubin JA, McKee SA, Cooney NL, Blakeslee A, Meandzija B, Romano-Dahlgard D, Wu R, Makuch R, Jatlow P. A controlled trial of naltrexone augmentation of nicotine replacement therapy for smoking cessation. Arch Intern Med. 2006;166:667–674. doi: 10.1001/archinte.166.6.667. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Verheul R, Schippers GM, van den Brink W. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system and neuroendocrine reactions to alcohol-related cues in alcoholics. Eur Neuropsychopharmacol. 2007;17:558–566. doi: 10.1016/j.euroneuro.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Leggio L, Morgan M, Hutchison KE. Effects of naltrexone on cortisol levels in heavy drinkers. Pharmacol Biochem Behav. 2009;91:489–494. doi: 10.1016/j.pbb.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Childs E, Epstein AM, King AC. Acute HPA axis response to naltrexone differs in female vs. male smokers. Psychoneuroendocrinology. 2010;35:596–606. doi: 10.1016/j.psyneuen.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimetrics High sensitivity salivary cortisol enzyme immunoassay kit. 2011 [Google Scholar]
- Schuckit MA, Gold E, Risch C. Plasma cortisol levels following ethanol in sons of alcoholics and controls. Arch Gen Psychiatry. 1987;44:942–945. doi: 10.1001/archpsyc.1987.01800230022005. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorocco KH, Lovallo WR, Vincent AS, Collins FL. Blunted hypothalamic-pituitary-adrenocortical axis responsivity to stress in persons with a family history of alcoholism. Int J Psychophysiol. 2006;59:210–217. doi: 10.1016/j.ijpsycho.2005.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Verebey K, Volavka J, Mule SJ, Resnick RB. Naltrexone: disposition, metabolism, and effects after acute and chronic dosing. Clin Pharmacol Ther. 1976;20:315–328. doi: 10.1002/cpt1976203315. [DOI] [PubMed] [Google Scholar]
- Vuong C, Van Uum SH, O'Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand G, McCaul ME, Gotjen D, Reynolds J, Lee S. Confirmation that offspring from families with alcohol-dependent individuals have greater hypothalamic-pituitary-adrenal axis activation induced by naloxone compared with offspring without a family history of alcohol dependence. Alcohol Clin Exp Res. 2001;25:1134–1139. [PubMed] [Google Scholar]
- Wand GS, Mangold D, Ali M, Giggey P. Adrenocortical responses and family history of alcoholism. Alcohol Clin Exp Res. 1999;23:1185–1190. [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55:1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W, Pfohl B, Stangl D. The reliability of the family history method for psychiatric diagnoses. Arch Gen Psychiatry. 1988;45:320–322. doi: 10.1001/archpsyc.1988.01800280030004. [DOI] [PubMed] [Google Scholar]