Abstract
The incidence of breast cancer is increasing worldwide, and this seems to be related to an increase in lifestyle risk factors, including physical inactivity, and overweight/obesity. We previously reported that exercise induced a circulating angiostatic phenotype characterized by increased sFlt-1 and endostatin and decreased unbound-VEGF in men. However, there is no data on women. The present study determines the following: 1) whether moderate exercise increased sFlt-1 and endostatin and decreased unbound-VEGF in the circulation of adult female volunteers; 2) whether overweight/obese women have a higher plasma level of unbound-VEGF than lean women. 72 African American and Caucasian adult women volunteers aged from 18–44 were enrolled into the exercise study. All the participants walked on a treadmill for 30 minutes at a moderate intensity (55–59% heart rate reserve), and oxygen consumption (VO2) was quantified by utilizing a metabolic cart. We had the blood samples before and immediately after exercise from 63 participants. ELISA assays (R&D Systems) showed that plasma levels of sFlt-1 were 67.8±3.7 pg/ml immediately after exercise (30 minutes), significantly higher than basal levels, 54.5±3.3 pg/ml, before exercise (P < 0.01; n=63). There was no significant difference in the % increase of sFlt-1 levels after exercise between African American and Caucasian (P=0.533) or between lean and overweight/obese women (P=0.892). There was no significant difference in plasma levels of unbound VEGF (35.28±5.47 vs. 35.23±4.96 pg/ml; P=0.99) or endostatin (111.12±5.48 vs. 115.45±7.15 ng/ml; P=0.63) before and after exercise. Basal plasma levels of unbound-VEGF in overweight/obese women were 52.26±9.6 pg/ml, significantly higher than basal levels of unbound-VEGF in lean women, 27.34±4.99 pg/ml (P < 0.05). The results support our hypothesis that exercise-induced plasma levels of sFlt-1 could be an important clinical biomarker to explore the mechanisms of exercise training in reducing breast cancer progression and that VEGF is an important biomarker in obesity and obesity-related cancer progression.
Keywords: Exercise, Young adult women, Overweight/obese, sFlt-1, Endostatin, VEGF
Introduction
Breast cancer is the most common malignancy among women in the US (Jemal et al. 2006). The incidence of breast cancer is increasing worldwide, and this seems to be related to an increase in lifestyle risk factors, including physical inactivity, and overweight/obesity (Edwards et al. 2005; Huang et al. 1997; Bardia et al. 2006). Physically active individuals have lower rates of many cancers including breast cancer. The probable protective effect of physical activity on breast cancer is associated with a risk reduction ranging from 20 to 30% (Friedenreich et al. 2008; Friedenreich et al. 2010; Eliassen et al. 2010; Monninkhof et al. 2007). A prospective study of 64,777 premenopausal women indicates that leisure-time activity is associated with a reduced risk for premenopausal breast cancer (Maruti et al. 2008). Obese postmenopausal women have a 50% higher risk of breast cancer than non-obese women (Trentham-Dietz et al. 2000). Current physical activity guidelines developed by The American College of Sports Medicine recommend healthy adults and cancer survivors perform a minimum of 30-min moderate-intensity exercise for 5 days/week, to promote health (Schmitz et al. 2010). While numerous observational studies have described an association between physical activity, adiposity, and cancer, the evidence linking biomarkers with exercise, cancer etiology and progression has not been clearly defined.
The growth of a tumor and its biological behavior are mainly dependent on angiogenesis, whereas avascular tumors cannot grow over a microscopic size and can be eliminated by the immune system. Angiogenesis is regulated by the net balance between the positive (angiogenic or proangiogeneic) and negative (angiostatic or anti-angiogenic) regulators of blood vessel growth (O'Reilly et al. 1994; Hanahan et al. 1996). Vascular endothelial growth factor (VEGF) is a key angiogenic factor that stimulates growth of tumors including breast cancer (Ferrara et al. 2003; Young et al. 2010), in which VEGF exerts paracrine (especially angiogenesis) and autocrine (proliferation and migration) effects to promote breast cancer progression. Many VEGF receptor tyrosine kinase inhibitors have been used in clinical trials as anti-tumor angiogenesis agents for breast cancer (Gasparini et al. 2005). Administration of angiostatic factors such as endostatin or soluble vascular endothelial growth factor receptor 1 (sFlt-1) inhibits progression of cancers including breast cancer (Folkman. 2002; Cho et al. 2005; Mahasreshti et al. 2001). We have reported that exercise induces a circulating angiostatic phenotype, characterized by decreased free-VEGF and increased endostatin and sFlt-1 plasma levels in men (Gu et al. 2004; Bailey et al. 2006) and in a rodent model (Gu et al. 2006). However, there is no current data reported on whether exercise can induce a circulating angiostatic phenotype in young adult women. We hypothesize that in women, exercise induces a circulating angiostatic phenotype characterized by increased endostatin and sFlt-1, and decreased free-VEGF.
Epidemiological studies suggest that overweight/obesity is associated with increased adipokines such as VEGF and leptin (Vona-Davis et al. 2009), and has been causally associated with breast cancer in postmenopausal populations and a poor prognosis regardless of menopausal status. Several studies have reported increased plasma levels of VEGF in human obesity (Gomez-Ambrosi et al. 2010; Miyazawa-Hoshimoto et al. 2003; Seida et al. 2003), although others have failed to find the same results (Rehman et al. 2003). Another study has described higher plasma level of VEGF only in postmenopausal obese women (Silha et al. 2005). There is no data on whether overweight/obese young adult women have higher plasma levels of unbound VEGF than lean young adult women.
The present study determines the following: 1) whether moderate exercise induces a circulating angiostatic phenotype characterized by increased sFlt-1 and endostatin, and decreased unbound-VEGF in young adult female volunteers; 2) whether overweight/obese young adult women have a higher plasma level of unbound-VEGF than lean young adult women; and 3) whether there is a positive correlation between basal levels of plasma sFlt-1 and age.
Methods
Selection of subjects
72 African American and Caucasian adult women volunteers aged from 18–44 were enrolled into the exercise study. The study excluded any subjects who were male, menopausal, pregnant, or had any medical condition that would inhibit them from walking on a treadmill. Informed consent was obtained from all subjects. The study was approved by the University of Mississippi’s Institutional Review Board (IRB).
Exercise study protocol
All the participants performed an exercise test by walking on a treadmill for 30 minutes at a moderate intensity. During exercise, submaximal oxygen consumption (VO2) was determined by utilizing a ParvoMedics metabolic cart that analyzed pulmonary and metabolic variables from the subjects (Hodeges et al. 2005). The subjects walked at the speed and grade corresponding to 55–59% of their heart rate reserve while VO2 was measured (Steele et al. 2008). This test provided VO2 data on an actual 30 minute bout of moderate intensity endurance exercise. Six ml of blood were collected by venipuncture tube with EDTA solution immediately before and after 30 minutes of exercise. The blood samples were immediately centrifuged at 700×g in a micro-centrifuge for 5 minutes at 4°C; and the plasma was stored at −80°C. We had access to the blood samples from 63 of the participants. The exercise test was conducted between the first and seventh day of the participant’s menstrual cycle.
Measurement of plasma levels of sFlt-1, unbound VEGF, and Endostatin
Plasma levels of sFlt-1 and endostatin were measured using sFlt-1 and Endostatin ELISA kits (R&D Systems, Minneapolis, MN) according to manufacturer protocol. Plasma levels of unbound VEGF were determined using a human ELISA kit with monoclonal anti-VEGF antibodies (R&D Systems, Minneapolis, MN) because Sandwich ELISA kits with monoclonal antibodies fail to detect the antigen if the epitopes are masked by soluble receptors, as is the case with VEGF bound to sFlt-1.
Statistical analysis
Where indicated, data is presented as mean ± SE. Statistical significance was defined as a two-tail Student’s t-test value of P < 0.05. Paired Student’s t-test was used to compare variables between two groups (before and after exercise, lean and overweight/obese, African American and Caucasian). The comparisons of the differences by obesity status and race were performed by using ANOVA and Dunnett’s t test. Linear regression was performed for the correlation analysis between two continuous variables. All statistical calculations were performed using SPSS software (SPSS Inc., Chicago, IL).
Results
General characteristics and response to exercise
Of the 72 volunteers, 35 were African American and 37 were Caucasian. Average age, BMI, and VO2max determined during 30 minutes of exercise were summarized in table 1. BMI less than 25 kg/m2 was considered lean. Overweight was defined as a BMI over 25 kg/m2 and obese over 30 kg/m2. We found that in our population of women, 65% were overweight/obese (BMI: 29.1±0.82 kg/m2). Average BMI and average age in years for the African American group and Caucasian group were: (28.5±1.21 kg/m2, 26.1±1.25) and (29.7±1.11 kg/m2, 22.1±0.84) respectively. There was no significant difference in average BMI between the two groups (P < 0.05). Submaximal VO2 (ml/kg/min) during 30 minutes of exercise for the African American group averaged 31.4±1.20 ml/min/kg and 27.1±1.18 ml/min/kg for the Caucasian group.
Table 1.
Characteristics of Female Participants
| Participant # | Age | BMI(kg/m2) | VO2max (ml/min/kg) | |
|---|---|---|---|---|
| African American | 35 | 26.1 ± 1.25 | 28.5 ± 1.21 | 31.4 ± 1.20 |
| Caucasian | 37 | 22.1 ± 0.84 | 29.7 ± 1.11 | 27.1 ± 1.18 |
| Total | 72 | 24.0 ± 0.78 | 29.1 ± 0.82 | 29.2 ± 0.87 |
Effect of exercise on plasma levels of sFlt-1
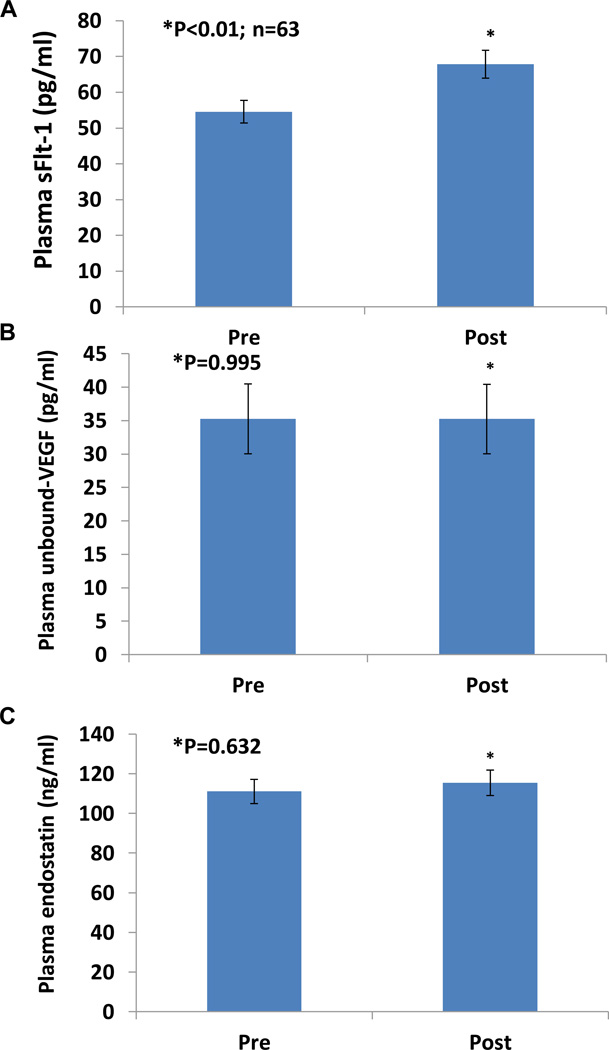
Figure 1A shows significantly increased plasma sFlt-1 in adult women after a 30 minute bout of moderate intensity exercise walking on a treadmill. Plasma levels of sFlt-1 were 67.8±3.7 pg/ml immediately after 30 minutes of exercise, significantly higher than basal levels, 54.5±3.3 pg/ml, before exercise (P < 0.01; n=63). Based on the percent change of sFlt-1 in each individual before and after exercise, we calculated the average percent change to be 49%. There was no significant difference in the percent increase of sFlt-1 levels after exercise between African American and Caucasian (P=0.563) or between lean and overweight/obese women (P=0.841).
Figure 1.
Effect of exercise on plasma sFlt-1, unbound VEGF, and endostatin concentrations in young adult women. Panel A shows plasma sFlt-1 levels were significantly increased by 49% (P < 0.01, n=63) immediately after 0.5 h exercise, compared to pre-exercise levels (54.5±3.3 pg/ml, mean ± SE). Panel B shows similar levels of unbound VEGF before (35.28±5.47 pg/ml) and immediately after (35.23±4.96 pg/ml; P=0.995) exercise. Panel C shows no significant difference in plasma levels of endostatin before (111.12±5.48 ng/ml) and immediately after (115.45±7.15 ng/ml; P=0.632) exercise.
Effect of exercise on plasma levels of unbound-VEGF and Endostatin
We also investigated the effect of exercise on plasma levels of free VEGF and endostatin. There was no change in Plasma unbound-VEGF (Figure 1B) or endostatin (Figure 1C) in young adult women after a 30 minute bout of moderate intensity exercise walking on treadmill. The basal plasma levels of unbound VEGF (35.28±5.47 pg/ml) were similar to the plasma levels of unbound VEGF immediately after (35.23±4.96 pg/ml; P=0.995) exercise. There was no significant difference in plasma levels of endostatin before (111.12±5.48 ng/ml) and immediately after (115.45±7.15 ng/ml; P=0.632) exercise.
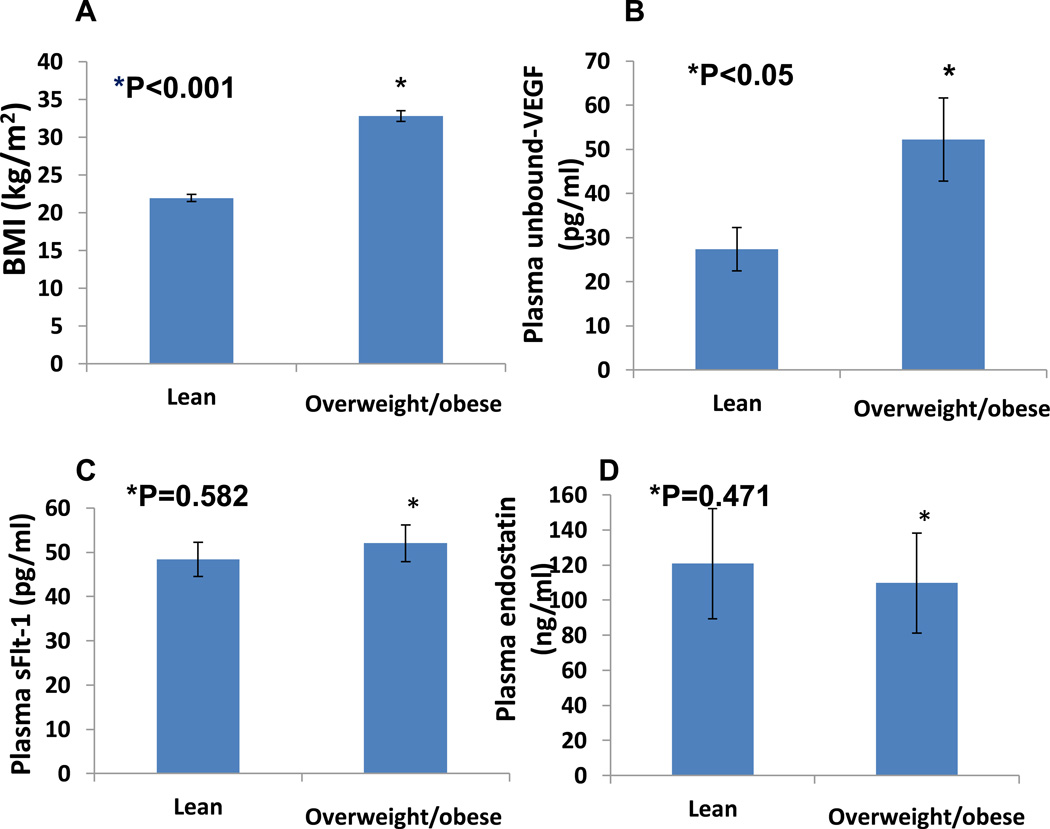
Comparison of basal plasma levels of unbound-VEGF, sFlt-1, and Endostatin in lean and overweight/obese young adult women
VEGF has been recognized as an adipokines (18). It is necessary to analyze whether increased plasma levels of VEGF is associated with increased BMI. Figure 2A shows that in our population of women aged 18–44, the average lean BMI was 21.95±0.47 kg/m2 and the average overweight/obese BMI was 32.83±0.79 kg/m2 (P < 0.001). Figure 2B shows that overweight/obese women have a higher plasma level of unbound-VEGF than lean women. Basal plasma levels of unbound-VEGF in overweight/obese women were 52.26±9.6 pg/ml, significantly higher than basal levels of unbound-VEGF in lean women, 27.34±4.99 pg/ml (P < 0.05).
Figure 2.
Comparison of basal plasma levels of unbound-VEGF (Panel B), sFlt-1 (Panel C), and Endostatin (Panel D) in lean and overweight/obese women aged 18–44. Panel A shows the average lean BMI was 21.95±0.47 kg/m2 (n=22) and the average overweight/obese BMI was 32.83±0.79 kg/m2 (P < 0.001; n=41). Basal plasma levels of unbound-VEGF in overweight/obese young adult women were 52.26±9.6 pg/ml, significantly higher than basal levels of unbound-VEGF in lean young adult women, 27.34±4.99 pg/ml (P < 0.05). No significant changes in basal plasma levels of sFlt-1 (48.37±4.66 vs. 52.06±3.75 pg/ml; P=0.582) or endostatin (120.81±9.66 vs. 109.77±9.04 ng/ml; P=0.471) between lean and overweight/obese young adult women.
We did not observe any significant changes in basal plasma levels of sFlt-1 or endostatin between lean and overweight/obese young adult women. The basal plasma levels of sFlt-1 in lean women (48.37±4.66 pg/ml) compared to overweight/obese women (52.06±3.75 pg/ml) are shown in Figure 2C (P=0.582). As indicated in Figure 2D, there was no significant difference in the basal plasma levels of endostatin in lean young adult women (120.81±9.66 ng/ml) compared to overweight/obese young adult women (109.77±9.04 ng/ml; P=0.471).
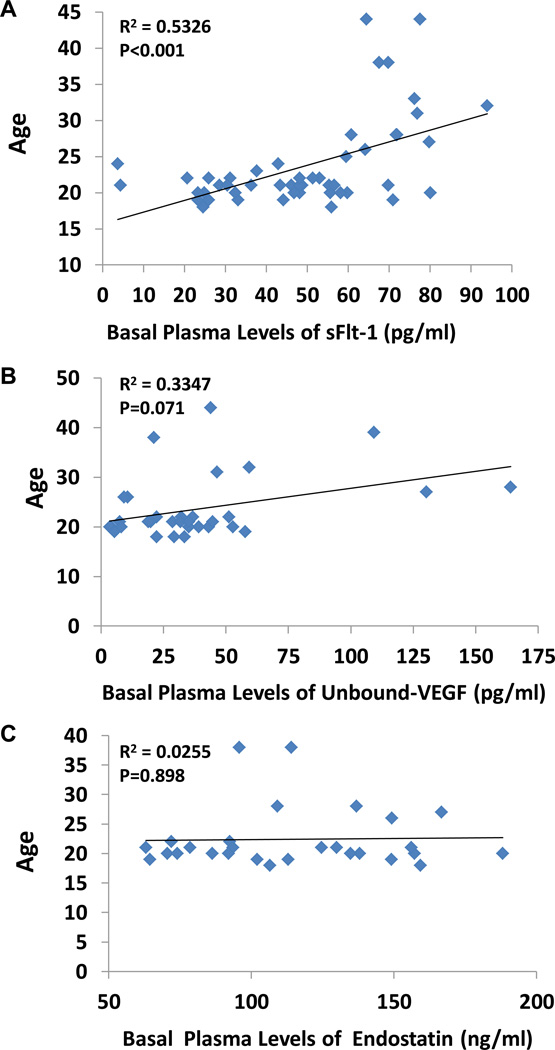
Correlation between basal levels of plasma sFlt-1, unbound-VEGF, and Endostatin vs. age
Figure 3A shows a significant positive linear correlation between basal levels of plasma sFlt-1 and age (R2=0.5326; P < 0.001). We found no significant correlation between basal levels of plasma unbound VEGF (R2=0.335; P < 0.071) or endostatin (R2=0.025; P < 0.898) with age, shown in Figures 3B and 3C respectively.
Figure 3.
Correlation between basal levels of plasma sFlt-1 (Panel A), unbound-VEGF (Panel B), and Endostatin (Panel C) vs. age. There was a significant positive linear correlation between basal levels of plasma sFlt-1 and age (R2=0.5326; P < 0.001, n=63). No significant correlation between basal levels of plasma unbound VEGF (R2=0.335; P < 0.071) or endostatin (R2=0.025; P < 0.898) with age.
Discussion
The present study has clearly demonstrated that moderate exercise significantly increases plasma sFlt-1 levels in young adult women. Based on the percent change of sFlt-1 in each individual before and after exercise, the percent increase in sFlt-1 levels is 49% (n=63; P < 0.01). This data supports our hypothesis that exercise induces a circulating angiostatic factor, sFlt-1 in young adult women.
The VEGF family (including its ligands and receptors) are the prime molecular targets of pro-angiogenic and anti-angiogenic therapies. Soluble fms-like tyrosine kinase-1 (sFlt-1) is an extra-cellular Ig-like domain of the VEGF receptor-1 that is released into the extracellular space and circulation where it inhibits the activities of VEGF (Wu et al. 2010). The natural occurrence of sFlt-1 is predominantly derived from alternative splicing (Kendall et al. 1993), but is also derived from proteolytic cleavage of full-length VEGFR1 (Cai et al. 2006). There is growing interest in biomedical research to explore sFlt-1 as a disease marker (Woolcok et al. 2008; Bando et al. 2005; Jaroszewicz et al. 2008; Shapiro et al. 2008) and therapeutic vector for angiogenesis inhibition (Kommareddy et al. 2007; Mahendra et al. 2005; Gehlbach et al. 2003). Some animal studies have demonstrated that sFlt-1 gene therapy suppresses growth of human ovarian carcinoma (Mabasreshti et al. 2001) and human breast adeno-carcinoma (Kommareddy et al. 2007). Evidence exists for a probable protective effect of physical activity on breast cancer, with a risk reduction ranging from 20 to 30% (Friedenreich et al. 2010; Eliassen et al. 2010; Friedenreich et al. 2008; Monninkhof et al. 2007). Based on the information mentioned above, we assume that increased plasma sFlt-1 is an important biomarker linking the protective effect of exercise on breast cancers. Currently, we are working on a clinical study in breast cancer patients to test this hypothesis.
The major limitation for the present study of exercise in women is that only one blood sample was taken immediately after exercise. In our previous exercise study in men we found: 1) significantly increased plasma sFlt-1 after 30 minutes of exercise; 2) significantly increased plasma endostatin after 2 hours of exercise; and 3) significantly decreased plasma unbound-VEGF after 2 hours of exercise (Gu et al. 2004; Bailey et al. 2006). However, the present study shows that a 30 minute bout of moderate exercise significantly increases plasma sFlt-1 levels in adult women and there is no change in plasma unbound-VEGF or endostatin in adult women. In the future exercise study in women, we will take multiple blood samples after exercise, for example at 30 minutes and 2 hours, to measure sFlt-1, free VEGF, and endostatin. We predict that similar to our findings in men, exercise in women can induce a circulating angiostatic phenotype characterized by increased endostatin and sFlt-1, and decreased free VEGF.
The sFtl-1 is produced in the microvascular and macrovascular endothelial cells (Kendall et al. 1993; Karumanchi et al. 2004), vascular smooth muscle cells (Sela et al. 2008), and activated peripheral blood mononuclear monocytes (Karumanchi et al. 2004), that exist throughout skeletal muscle tissues. It is therefore plausible that release of sFlt-1 from skeletal muscle into the circulation might be due to exercise-dependent reductions in oxygen tension in the skeletal muscle. In future studies, we will determine whether sFlt-1 can be released directly from the exercised muscle.
The present study has also demonstrated that basal plasma levels of unbound-VEGF in overweight/obese young adult women were 52.26±9.6 pg/ml, significantly higher than basal levels of unbound-VEGF in lean young adult women, 27.34±4.99 pg/ml (P < 0.05). These results suggest that obesity has a pro-angiogenic phenotype characterized by increased plasma levels of VEGF. This data is consistent with our previous reports on increased plasma VEGF in MC4R −/− mice (Brandon et al. 2009) and postmenopausal obese mice (Gu et al. 2011), and some clinical studies of increased plasma levels of VEGF in obese men (Gomez-Ambrosi et al. 2010; Miyazawa-Hoshimoto et al. 2003; Seida et al. 2003). It is a significant finding that overweight/obese young adult women have a higher plasma level of unbound-VEGF than lean young adult women because adipose tissue-derived VEGF promotes breast cancer progression in animal model (Gu et al. 2011). Further studies are needed.
Ageing is associated with a decreased capacity for angiogenesis by impairing the functions of some molecules such as VEGF, hypoxia inducible factor-1α, and endothelial nitric oxide synthase (Revard et al. 1999; Hoenig et al. 2008). Decreased metabolic activity and sex hormones probably contribute to impaired angiogenesis in the elderly. In analyzing the correlation between basal levels of plasma sFlt-1 and age in women from 18 to 44 years, we have found that there is a significant positive linear correlation (R2=0.5326; P < 0.001). The data may suggest that younger people show a more angiogenic phenotype. However, further studies are needed to determine whether individuals over 50 years of age have higher plasma levels of sFlt-1.
In conclusion, the measurement of plasma sFlt-1 levels before and after exercise in 63 African American and Caucasian adult women volunteers aged from 18–44 reveals that moderate exercise significantly increases plasma sFlt-1 levels. The data supports the hypothesis that exercise-induced plasma levels of sFlt-1 could be an important clinical biomarker to explore the mechanisms of exercise training in reducing breast cancer progression. The present study also shows that in overweight/obese women the increased levels of plasma unbound VEGF are linking to the relationship between obesity and breast cancer progression. However, the conclusions are based on 62 participates which is a small number of samples. Further extended studies are necessary. Identifying the biomarkers associated with the VEGF pathway could be an important strategy for treatment and prevention of breast cancer, especially in obesity.
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism Grant AA-013821 and the National Heart, Lung and Blood Institute Grant HL-51971.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bailey AP, Shparago M, Gu JW. Exercise Increases Soluble Vascular Endothelial Growth Factor Receptor-1 (sFlt-1) in Circulation of Healthy Volunteers. Medical Science Monitor. 2006;12:CR45–CR50. [PubMed] [Google Scholar]
- 2.Bando H, Weich HA, Brokelmann M, Horiguchi S, Funata N, Ogawa T, Toi M. Association between intratumoral free and total VEGF, soluble VEGFR-1, VEGFR-2 and prognosis in breast cancer. Br J Cancer. 2005;92:553–561. doi: 10.1038/sj.bjc.6602374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bardia A, Hartmann LC, Vachon CM, Vierkant RA, Wang AH, Olson JE, et al. Recreational physical activity and risk of postmenopausal breast cancer based on hormone receptor status. Arch Intern Med. 2006;166:2478–2483. doi: 10.1001/archinte.166.22.2478. [DOI] [PubMed] [Google Scholar]
- 4.Brandon EL, Gu JW, Cantwell L, He Z, Wallace G, Hall JE. Obesity promotes melanoma tumor growth: Role of leptin. Cancer Biol Ther. 2009;8:1871–1879. doi: 10.4161/cbt.8.19.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai J, Jiang WG, Grant MS, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem. 2006;281:3604–3613. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 6.Cho HM, Rosenblatt JD, Kang YS, Iruela-Arispe ML, Morrison SL, Penichet ML, et al. Enhanced inhibition of murine tumor and human breast tumor xenografts using targeted delivery of an antibody-endostatin fusion protein. Mol Cancer Ther. 2005;4:956–967. doi: 10.1158/1535-7163.MCT-04-0321. [DOI] [PubMed] [Google Scholar]
- 7.Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005;97:1407–1427. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 8.Eliassen AH, Hankinson SE, Rosner B, Holmes MD, Willett WC. Physical activity and risk of breast cancer among postmenopausal women. Arch Intern Med. 2010;170:1758–1764. doi: 10.1001/archinternmed.2010.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara N, Gerber HP, LeCouter J. The biological properties of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;6:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 11.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42:636–647. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 12.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46:2593–2604. doi: 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Gasparini G, Longo R, Torino F, Morabito A. Therapy of breast cancer with molecular targeting agents. Ann Oncol. 2005;16 (suppl 4):26–28. doi: 10.1093/annonc/mdi905. [DOI] [PubMed] [Google Scholar]
- 14.Gehlbach P, Demetriades AM, Yamamoto S, Deering T, Xiao WH, Duh EJ, et al. Periocular gene transfer of sFlt-1 suppresses ocular neovascularization and vascular endothelial growth factor-induced breakdown of the blood-retinal barrier. Hum Gene Ther. 2003;14:129–141. doi: 10.1089/104303403321070829. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Ambrosi J, Catalan V, Rodriguez A, Ramirez B, Silva C, Gil MJ, et al. Involvement of serum vascular endothelial growth factor family members in the development of obesity in mice and humans. J Nutritional Biochemistry. 2010;21:774–780. doi: 10.1016/j.jnutbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Gu JW, Gadonski G, Wang J, Makey I, Adair TH. Exercise increases endostatin in circulation of healthy volunteers. BMC Physiology. 2004;4:2. doi: 10.1186/1472-6793-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu JW, Shparago M, Tan W, Bailey AP. Tissue endostatin correlates inversely with capillary network in rat heart and skeletal muscles. Angiogenesis. 2006;9:93–99. doi: 10.1007/s10456-006-9035-z. [DOI] [PubMed] [Google Scholar]
- 18.Gu JW, Young E, Patterson SG, Makey KL, Wells J, Miele L. Postmenopausal obesity promotes tumor angiogenesis and breast cancer progression in mice. Cancer Biol Ther. 2011;11:910–917. doi: 10.4161/cbt.11.10.15473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 20.Hodges LD, Brodie DA, Bromley PD. Validity and reliability of selected commercially available metabolic analyzer systems. Scand J Med Sci Sports. 2005;15:271–279. doi: 10.1111/j.1600-0838.2005.00477.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoenig MR, Bianchi C, Rosenzweig A, Sellke FW. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia-inducible factor-1. Current Molecular Medicine. 2008;8:754–767. doi: 10.2174/156652408786733685. [DOI] [PubMed] [Google Scholar]
- 22.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. [PubMed] [Google Scholar]
- 23.Jaroszewicz J, Januszkiewicz M, Flisiak R, Rogalska M, Kalinowska A, Wierzbicka I. Circulating vascular endothelial growth factor and its soluble receptors in patients with liver cirrhosis: possible association with hepatic function impairment. Cytokine. 2008;44:14–17. doi: 10.1016/j.cyto.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer Statistics. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 25.Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chickenand-egg” question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 26.Kendall RL, Thomas KA. Inhibition of vascular endothelial growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kommareddy S, Amiji M. Antiangiogenic gene therapy with systemically administered sFlt-1 plasmid DNA in engineered gelatin-based nanovectors. Cancer Gene Ther. 2007;14:488–498. doi: 10.1038/sj.cgt.7701041. [DOI] [PubMed] [Google Scholar]
- 28.Mahasreshti PJ, Navarro JG, Kataram M, Wang MH, Carey D, Siegal GP, et al. Adenovirus-mediated soluble FLT-1 gene therapy for ovarian carcinoma. Clin Cancer Res. 2001;7:2057–2066. [PubMed] [Google Scholar]
- 29.Mahendra G, Kumar S, Isayeva T, Mahasreshti PJ, Curiel DT, Stockardt CR, et al. Antiangiogenic cancer gene therapy by adeno-associated virus 2-mediated stable expression of the soluble FMS-like tyrosine kinase-1 receptor. Cancer Gene Ther. 2005;12:26–34. doi: 10.1038/sj.cgt.7700754. [DOI] [PubMed] [Google Scholar]
- 30.Maruti SS, Willett WC, Feskanich D, Rosner B, Colditz GA. A prospective study of age specific physical activity and premenopausal breast cancer. J Natl Cancer Inst. 2008;100:728–737. doi: 10.1093/jnci/djn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyazawa-Hoshimoto S, Takahashi K, Bojo H, Hashimoto N, Salto Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483–1488. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 32.Monninkhof E, Elias S, Vlems F, van der Tweel I, Schuit A, Voskuil D, et al. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 33.O'Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, et al. Angiogenesis: a novel angiogenesis inhibitor that mediates the suppression of metastases by Lowis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 34.Rehman J, Considine RV, Bovenkerk JE, Li J, Slavens CA, Jones RM, et al. Obesity is associated with increased levels of circulating hepatocyte growth factor. J Am Coll Cardiol. 2003;41:1408–1413. doi: 10.1016/s0735-1097(03)00231-6. [DOI] [PubMed] [Google Scholar]
- 35.Revard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, et al. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 36.Sela S, Itin A, Natanson-Yaron S, Greenfield C, Goldman-Wohl D, Yagel S, et al. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res. 2008;102:1566–1574. doi: 10.1161/CIRCRESAHA.108.171504. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sport Exerc. 2010;42:1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 38.Seida A, Wada J, Kunitomi M, Tsuchiyama Y, Miyataka N, Fujii M, et al. Serum bFGF levels are reduced in Japanese overweight man and restored by a 6-month exercise education. Int J Obese Relat Meta b Disord. 2003;27:1325–1331. doi: 10.1038/sj.ijo.0802408. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro NI, Yano K, Okada H, Fischer C, Howell M, Spokes KC, et al. A prospective, observational study of soluble Flt-1 and vascular endothelial growth factor in sepsis. Shock. 2008;29:452–457. doi: 10.1097/shk.0b013e31815072c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes. 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 41.Steele RM, Finucane FM, Griffin SJ, Wareham NJ, Ekelund U. Obesity is associated with altered lung function independently of physical activity and fitness. Obesity. 2008;17:578–584. doi: 10.1038/oby.2008.584. [DOI] [PubMed] [Google Scholar]
- 42.Trentham-Dietz A, Nowcomb PA, Egan KM, Titus-Ernstoff L, Baron JA, Storer BE, et al. Weight change and risk of postmenopausal breast cancer (United States) Cancer Causes and Control. 2000;11:533–542. doi: 10.1023/a:1008961931534. [DOI] [PubMed] [Google Scholar]
- 43.Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009;20:193–201. doi: 10.1016/j.cytogfr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Woolcok J, Hennessy A, Xu B, Thornton C, Tooher J, Makris A, et al. Soluble flt-1 as a diagnostic marker of preeclampsia. Aust N Z J Obstet Gynaecol. 2008;48:64–70. doi: 10.1111/j.1479-828X.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 45.Wu FTH, Stefanini MO, Gabhann FM, Kontos CD, Annex BH, Popel AS. A systems biology perspective on sVEGFR1: its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14:528–552. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young E, Miele L, Tucker KB, Min Huang M, Wells J, Gu JW. SU11248, a selective tyrosine kinases inhibitor suppresses breast tumor angiogenesis and growth via targeting both tumor vasculature and breast cancer cells. Cancer Biology & Therapy. 2010;10:703–711. doi: 10.4161/cbt.10.7.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]