Abstract
Objectives
To assess fat distribution, prevalence of obesity and the metabolic syndrome (MetS) among diverse 6–13 year old Colorado youth to better understand racial/ethnic influences on adiposity and metabolic syndrome.
Study design
We measured body mass index (BMI), subscapular-triceps skinfold ratio (STR), waist circumference (WC), dietary fat, and physical activity in 422 youth, 47% non-Hispanic White, 44% Hispanic, 9% African American. Visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and intramyocellular lipid (IMCL) were measured with magnetic resonance techniques. Multiple-linear regression was used to assess associations between race/ethnicity and adiposity patterns.
Results
Hispanic and African American youth had a higher prevalence of obesity and metabolic syndrome compared with non-Hispanic White youth. Both groups displayed a more centralized fat distribution and larger volumes of SAT, compared with non-Hispanic White youth. After controlling for BMI, these differences were attenuated and for a given body size, African American youth showed significantly lower VAT than non-Hispanic White youth. However, both Hispanic and African American youth showed higher IMCL in skeletal muscle compared with non-Hispanic Whites, independent of body size.
Conclusions
Racial/ethnic minorities experience higher overall adiposity, and may also have an increased risk for early development of metabolic syndrome relative to non-Hispanic White youth, beyond their increased obesity risk.
Keywords: IMCL, race, ethnicity, Hispanic, African American, childhood obesity, subcutaneous fat, visceral fat, metabolic syndrome
The prevalence of overweight and obesity in children has increased markedly over the past 30 years in the United States; ~32% of children and teens are currently overweight or obese(1). Moreover, African-American (AA) and Hispanic children in the United States are disproportionately affected by obesity in preschool and beyond(1). According to data from the 2007–2008 National Health and Nutrition Examination Survey (NHANES), the prevalence of obesity among 6–19 year olds was 23.2% in Hispanic, 22.3% in African American and 17% in non-Hispanic White (NHW) children(1). Obesity in childhood and adolescence is associated with overweight and obesity in adulthood as well as increased risk of future adverse health outcomes such as diabetes mellitus, cardiovascular disease, and consequences to psychosocial development(2).
Previous studies have suggested that childhood obesity is also associated with an imbalance in fat distribution in the abdominal fat depot and skeletal muscle tissues, leading to increased insulin resistance (3, 4). A high proportion of abdominal visceral (VAT) relative to subcutaneous adipose tissue (SAT) was shown to be associated with intramyocellular lipid (IMCL) accumulation(5), hepatic steatosis, insulin resistance, as well as an increased risk of the metabolic syndrome (MetS) among obese adolescents(6).
Differences in fat patterning, prevalence of metabolic syndrome, and the relationship between various fat depots and insulin resistance have been observed among various racial/ethnic groups. African-American adults have more subcutaneous (SAT) but less visceral adipose tissue (VAT) than non-Hispanic Whites, despite the former having higher overall body mass index (BMI)(7). It also appears that African-Americans exhibit impaired insulin resistance at a lower threshold of visceral fat accumulation when compared with non-Hispanic White and Hispanics(8). Moreover, evidence exists that some of these differences may start to manifest early in life, even among pre-pubertal youth(9). However, limited epidemiologic data exists on patterns of fat distribution and their relationship to the prevalence of metabolic syndrome among healthy youth of diverse racial and ethnic backgrounds. Our study aims to address this limitation by exploring racial/ethnic differences in fat distribution patterns and the prevalence of obesity and metabolic syndrome among 6–13 year old healthy youth from Colorado of non-Hispanic White, Hispanic and African-American origin.
Methods
This report includes data from a retrospective cohort study conducted in Colorado: Exploring Perinatal Outcomes among Children (EPOCH). Participants were healthy 6–13 year old children whose mothers were members of Kaiser Permanente of Colorado Health plan and who attended an in-person fasting study visit. All participants received informed consent and youths provided written assent. The study was approved by the Colorado Multiple Institutional Review Board.
All measurements were performed by a trained technician. Children were weighed and measured in light clothing and without shoes. Weight was measured to the nearest 0.1 kilogram using a portable electronic SECA scale (Hanover, MD, USA), and height was measured to the nearest 0.1 centimeter, using a portable SECA stadiometer. BMI was calculated as kg/m2. BMI percentiles and BMI Z-scores were determined according to the World Health Organization and Centers for Disease Control standard growth charts(10). Overweight was defined as BMI value between the 85th and 95th percentile for age and sex, and obesity as BMI ≥95th percentile. Waist circumference was measured to the nearest 1 mm at the midpoint between the lower ribs and the pelvic bone with a fiberglass non-springloaded tape measure. Skinfolds were measured in triplicate using Holtain calipers (average: subscapular, 20 mm below the tip of the scapula; triceps, halfway between the acromion process and the olecranon process). The subscapular-to-triceps skinfold ratio (STR) was calculated to assess regional differences in subcutaneous fat distribution. Magnetic resonance imaging (MRI) of the abdominal region was used to quantify VAT and SAT with a 3T HDx imager (General Electric, Waukasha, WI, USA) by a trained technician. Each study participant was placed supine and a series of T1-weighted coronal images were taken to locate the L4/L5 plane. One axial, 10-mm, T1-weighted image at the L4/L5 disc space was analyzed to determine SAT and VAT content. The ratio of VAT to SAT (VSR) was calculated to assess higher abdominal visceral fat patterning. IMCL accumulation was assessed by magnetic resonance spectroscopy (MRS). Each subject was positioned to the mid-calf area, using a T1 weighted image as a localizer and homogenous muscle regions were selected for measurement. The spectroscopy acquisition was performed using the PRESS pulse sequence (TR/TE = 2000msec/100msec) with the Cr peak at 3.0 ppm used for an internal reference (11). Water suppression was not used so that the IMCL concentration could be scaled to the basis set water peak and therefore normalized. IMCL, although not given in absolute units, can therefore be compared across the subjects studied. Spectral images were analyzed using the SAGE spectroscopy analysis package. The areas of the intramyocellular and extramyocellular triglyceride peaks were obtained from the fit results and were corrected for the effects of T1 and T2 relaxation. The triglyceride concentrations were expressed as a percentage of the water content.
Race/ethnicity was self-reported using 2000 U.S. Census-based questions and categorized as Hispanic (any race), non-Hispanic White (NHW), or non-Hispanic African American. Pubertal development was self-reported using a diagrammatic representation of Tanner staging adapted from Marshall and Tanner(12). Pubertal self-assessment was shown to correlate well with physician assessment of sexual maturation in children and adolescents(13). Youth were categorized as Tanner <2 (pre-pubertal) and ≥2 (pubertal). Percent daily calories from fat was assessed using Kid’s Block Food Frequency Questionnaire, a semi-quantitative food frequency questionnaire(14). Usual participation in both sedentary and non-sedentary activities was queried using the Block Kid’s Food Questionnaire questions based on the CDC Youth Risk Behavior Surveillance(15) (YRBS). Physical activity results were reported as average number of 30 minute blocks of moderate-to-vigorous activity per day. Three resting blood pressure measurements were obtained by a trained technician and averaged. Blood samples were drawn after an overnight fast and laboratory analyses were performed at the University of Colorado Hospital laboratory and the Children’s Hospital laboratory (Aurora, CO, USA). Glucose, HDL-cholesterol and triglyceride levels were measured using the Olympus AU400 advanced chemistry analyzer system (Center Valley, PA, USA). Presence of metabolic syndrome was defined according to the criteria proposed by Cook as presence of any 3 or more of the following abnormalities(16): elevated blood pressure (systolic or diastolic blood pressure ≥90th percentile for age, sex, and height), large waist circumference (≥90th percentile for age and sex)(17), low HDL cholesterol (≤40 mg/dl), high triglycerides (≥110 mg/dl), high blood glucose levels (≥110 mg/dl).
Statistical analyses
Univariate analyses (Chi-square and t tests) were used to compare demographic, behavioral, adiposity and metabolic syndrome -related characteristics between African-American and Hispanic youth and non-Hispanic White (reference category). Multiple linear regression was used to explore the associations between race/ethnicity (with non-Hispanic White as reference group) and parameters of fat distribution (waist circumference, STR, VAT, SAT, VSR, IMCL), adjusted for potential confounders in sequential models. Model 1 included adjustment for age, sex and pubertal development. Model 2 adjusted for behavioral risk factors (percent calories from fat and physical activity), in addition to Model 1 variables. Model 3 added current BMI to Model 2 variables. A significant interaction between age and Tanner stage was noted in previous studies based on this dataset (18, 19), suggesting that the effect of age on child adiposity deposition patterns depends on pubertal development; therefore, all 3 models also included adjustment for age*Tanner interaction. All statistical analyses were performed using SAS version 9.2 (Cary, NC, USA).
Results
A total of 197 non-Hispanic White, 185 Hispanic, and 40 African American study participants had complete data on all variables of interest. Table I shows the demographic, behavioral, adiposity, and metabolic syndrome -related characteristics of the study population, by race/ethnicity. Compared with non-Hispanic White youth, Hispanic youth were more likely to be males and less likely to have begun puberty. Hispanic youth reported higher average daily percent of calories from fat, and both African American and Hispanic youth reported a lower physical activity level relative to non-Hispanic White. In univariate analyses, both Hispanic and African-American youth had higher BMI and BMI Z-scores, higher waist circumference, SAT, STR, and IMCL than non-Hispanic White youth. Moreover, both Hispanic and African-American youth had a higher prevalence of elevated blood pressure, and African-Americans also had a higher prevalence of large waist circumference, compared with non-Hispanic Whites. There were no significant differences by race in the prevalence of hypertriglyceridemia, although Hispanic youth had a significantly reduced prevalence of low HDL-cholesterol, and no participants had fasting blood glucose levels at or above 110 mg/dL.
Table 1.
Characteristics of study participants, by race/ethnicity.
| NHW (ref) | Hispanic | AA | |
|---|---|---|---|
| (n=197) | (n=185) | (n=40) | |
| Demographic Characteristics | |||
| Age (years) | 10.2 (1.1) | 10.2 (1.5) | 10.2 (1.6) |
| Males: N (%) | 86 (43.7%) | 99 (53.5%)* | 19 (47.5%) |
| Pre-Pubertal: N (%) | 77 (39.1%) | 87 (47%) | 13 (32.5%) |
| Behavioral Characteristics | |||
| Total calories (kcal/day) | 1803 (532) | 1831 (594) | 1915 (654) |
| Calories from fat (% of total) | 35.1 | 36.1* | 36.8 |
| Physical Activity (blocks/day) | 5.2 (3.1) | 3.8 (2.4)* | 2.6 (1.9)* |
| Adiposity and Fat Patterning | |||
| BMI (kg/m2) | 17.8 (3.2) | 19.6 (4.9)* | 22.5 (6.6)* |
| BMI z-score | −0.06 (1.2) | 0.4 (0.4)* | 0.9 (1.3)* |
| Waist (cm) | 62.3 (8.1) | 67.8 (13.1)* | 73.4 (18.1)* |
| VAT (cm2) | 2.1 (1.6) | 2.4 (1.7) | 2.3 (1.5) |
| SAT (cm2) | 9.3 (7) | 13.6 (11.6)* | 19.5 (17.7)* |
| VSR | 0.27 | 0.23* | 0.18* |
| STR | 0.7 | 0.8* | 0.8* |
| IMCL (institutional units) | 2 | 2.6* | 3* |
| Components of the Metabolic Syndrome | |||
| Waist ≥ 90th percentile: N (%) | 18 (9.1%) | 27 (14.6%) | 14 (35%)* |
| Triglycerides ≥ 110 mg/dl: N (%) | 49 (24.9%) | 46 (24.9%) | 8 (20%) |
| Glucose ≥ 110 mg/dl: N (%) | 0 | 0 | 0 |
| HDLc mg/dl < 40 mg/dl: N (%) | 42 (21.3%) | 26 (14.1%)* | 7 (17.5%) |
| BP ≥ 90th percentile: N (%) | 53 (26.9%) | 67 (36.2%) | 21 (52.5%)* |
α=0.05, p≤0.05, as compared with NHW (reference group)
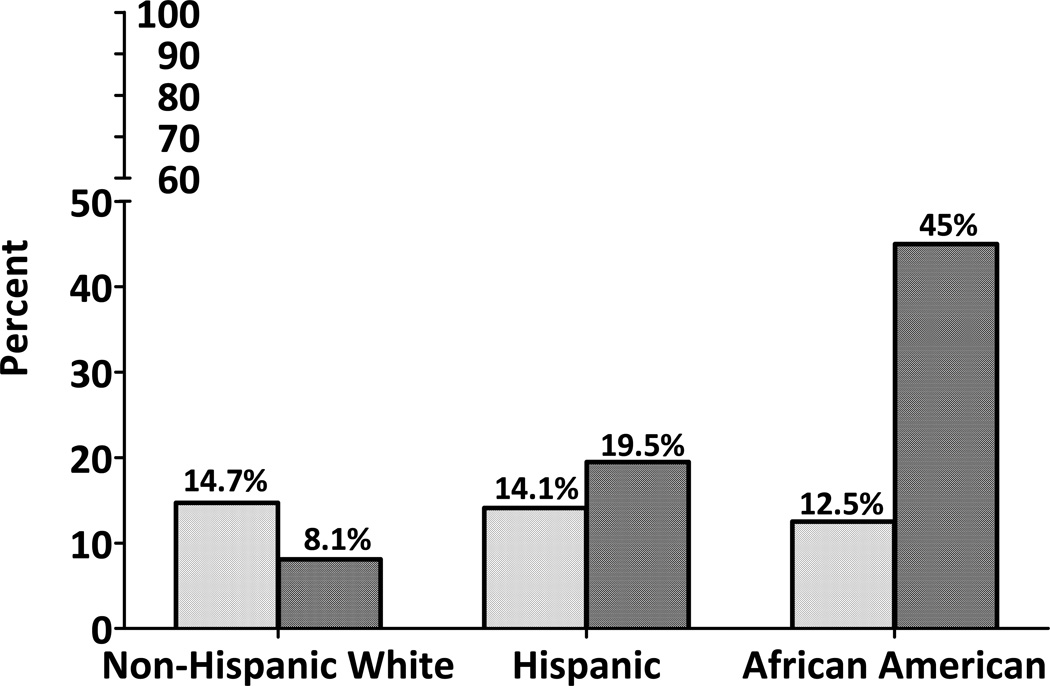
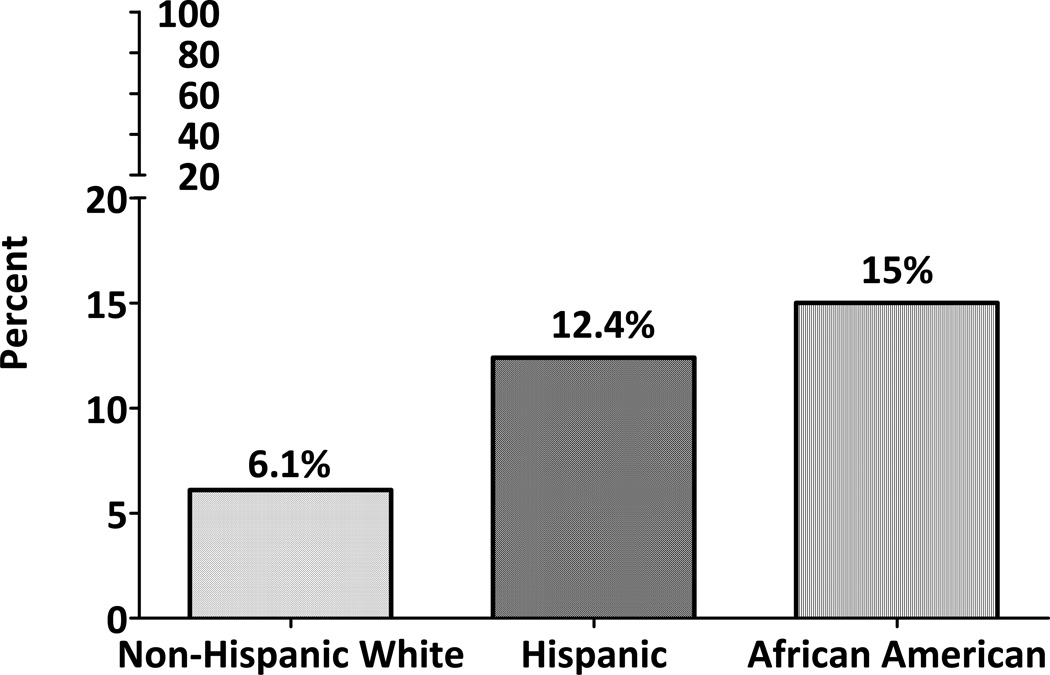
Figure 1 shows the prevalence of overweight and obesity among youth of non- Hispanic White, Hispanic and African-American backgrounds. Although the prevalence of overweight was similar among all ethnic groups, the prevalence of obesity was significantly higher in Hispanics and African-Americans versus non-Hispanic White youth. A total of 14.7% of non-Hispanic White, 14.1% of Hispanic and 12.5% of African-American youth were overweight, and 8.1%, 19.5% and 45% were obese, respectively. Similar patterns were noted regarding the prevalence of metabolic syndrome (Figure 2) with 6.1% of non-Hispanic White, 12.4% of Hispanics and 15% of African-American youth being classified as having the metabolic syndrome.
Figure 1.
Prevalence of overweight (BMI ≥85th and <95th percentile for age and sex) and obesity (BMI ≥95th percentile for age and sex) by race/ethnicity, in children aged 6–13 years
Figure 2.
Prevalence of Metabolic Syndrome by Race/Ethnicity in children aged 6–13 years
Table II shows mean adjusted levels of each adiposity measure for Hispanic and African-American compared with non-Hispanic Shite. Model 1 shows that Hispanics and African-Americans have increased waist circumference (67.9 cm and 73.1 cm vs. 62.2 cm, p<0.0001 for both), increased SAT (140.8 cm2 and 194.4 cm2 vs 92.5 cm2, p< 0.0001 for both), higher STR (0.8 and 0.8 vs 0.7, p=0.002 and p=0.0002, respectively) and more IMCL (2.6 and 2.9 vs 2.0, p< 0.0001) compared with non-Hispanic White youth and independent of age, sex, and Tanner stage. VAT was not significantly different in Hispanic or African-American youth, compared with non-Hispanic White (24.9 cm2 and 22.9 cm2 vs 21.2 cm2, p=0.06 and 0.8, respectively). As a result, both Hispanics and African-Americans had a lower ratio of visceral to subcutaneous adipose tissue (VSR), relative to their non-Hispanic White peers (0.23 and 0.18 vs. 0.27, p=0.0009 and p<0.0001, respectively). This pattern of differences remained virtually unchanged upon adjustment for behavioral characteristics (Model 2). After additionally adjusting for BMI (Model 3), most of the differences described above were substantially attenuated and became non-significant, with two notable exceptions: VAT became significantly lower in African-American versus non-Hispanic White youth (13.7 cm2 vs 24.9 cm2, p<0.0001), and IMCL remained higher in both Hispanic and African-American youth, compared with non-Hispanic Whites (2.5 and 2.6 vs 2.1), although this difference only reached statistical significance in Hispanics.
Table 2.
Association of race/ethnicity with fat patterning measures in children aged 6–13 years from multivariate linear regression models
| NHW (ref) | Hispanic | p-value1 | AA | p-value2 | |
|---|---|---|---|---|---|
|
Waist (cm) Model 1 Model 2 Model 3 |
62.2 (0.8) 62.4 (0.9) 64.9 (0.7) |
67.9 (0.9) 67.9 (0.9) 66.4 (0.3) |
<0.0001 <0.0001 0.07 |
73.1 (1.8) 73 (1.8) 65.4 (0.3) |
<0.0001 <0.0001 0.8 |
|
VAT (cm2) Model 1 Model 2 Model 3 |
21.2 (1.2) 21.5 (1.3) 24.9 (0.9) |
24.9 (1.3) 24.9 (1.3) 23.3 (1) |
0.06 0.1 0.4 |
22.9 (2.6) 22.6 (2.7) 13.7 (2) |
0.8 0.9 <0.0001 |
|
SAT (cm2) Model 1 Model 2 Model 3 |
92.5 (7.7) 93.1 (8) 121.5 (3.3) |
140.8 (8.4) 140.7 (8.4) 127.3 (3.4) |
<0.0001 <0.0001 0.9 |
194.4 (16.5) 193.7 (16.8) 121.5 (3.2) |
<0.0001 <0.0001 0.4 |
|
VSR Model 1 Model 2 Model 3 |
0.27 (0.009) 0.27 (0.009) 0.26 (0.008) |
0.23 (0.009) 0.23 (0.009) 0.24 (0.008) |
0.0009 0.0013 0.2 |
0.18 (0.02) 0.18 (0.02) 0.22 (0.02) |
<0.0001 <0.0001 0.2 |
|
STR Model 1 Model 2 Model 3 |
0.7 (0.02) 0.7 (0.02) 0.7 (0.02) |
0.8 (0.02) 0.8 (0.02) 0.8 (0.02) |
0.002 0.002 0.2 |
0.8 (0.04) 0.8 (0.04) 0.8 (0.03) |
0.0002 0.02 0.9 |
|
IMCL Model 1 Model 2 Model 3 |
2 (0.09) 2 (0.1) 2.1 (0.1) |
2.6 (0.1) 2.6 (0.1) 2.5 (0.1) |
<0.0001 <0.0001 0.006 |
2.9 (0.2) 2.9 (0.2) 2.6 (0.2) |
<0.0001 <0.0001 0.1 |
Model 1: Adjusting for age, sex, Tanner stage, Tanner × age interaction
Model 2: Model 1 + dietary fat, physical activity
Model 3: Model 2 + BMI
Hispanic vs. NHW α=0.05
AA vs. NHW α=0.05
Discussion
We found that both Hispanic and African-American youth age 6–13 years from Colorado have a higher prevalence obesity and metabolic syndrome, compared with their non-Hispanic White counterparts. Both groups display a tendency for more centralized fat distribution (higher STR) and larger abdominal subcutaneous fat deposition, compared with non-Hispanic White youth, but at this age, these differences are primarily due to their larger overall body size. However, for a given body size, African-American youth showed significantly lower visceral fat deposition than non-Hispanic White youth. Both Hispanic and African-American youth showed a tendency for higher lipid accumulation in skeletal muscle compared with non-Hispanic Whites, independent of their overall body size, suggesting an increased risk for metabolic syndrome, which appears to not be completely mediated through overall obesity or increased VAT and/or SAT. Our results are consistent with previous studies in adults and add to the limited information in children.
Though the prevalence of overweight was similar among all racial/ethnic groups in our study (non-Hispanic White: 14.7%, Hispanics: 14.1%, and African-Americans: 12.1%), Hispanics (19.5%) and African-Americans (45%) showed significantly higher prevalence of obesity relative to non-Hispanic Whites (8.1%). Our cohort was very similar in terms of prevalence of overweight and obesity to the NHANES 2001–2004 estimates for Hispanic youth (18.8% and 18.3%, respectively), and our non-Hispanic White participants were similarly overweight (15.9%) though somewhat less obese than NHANES non-Hispanic White children (15.8%). However, the small sample of African-American children participating in EPOCH tended to be more obese than NHANES African-American youth (45% vs 20.2%), which is probably why they also had a higher prevalence of metabolic syndrome than expected based on population-based studies in adults and youth(20, 21). The NHANES IV (1999–2000) reported a prevalence of metabolic syndrome of 7.2% among non-Hispanic White, 8.5% among Hispanics and 5.1% among African-American youth age 12–19 years, using the Cook definition(20). They also reported a significant increase in the prevalence of metabolic syndrome across all three racial/ethnic groups, in parallel with an increasing prevalence of overweight and obesity(20). Our estimates of metabolic syndrome are similar for non-Hispanic Whites (6.1%) but higher for Hispanic (12.4%) and especially for African-American youth (15%), likely for the reasons mentioned above. Nevertheless, similar to NHANES IV participants(20), our African-American youth showed a lower prevalence of dyslipidemia (elevated triglycerides and decreased HDLc) and a higher prevalence of elevated blood pressure relative to non-Hispanic White youth.
We also showed that minority youth tended to have a more centralized fat patterning (higher STR) and increased subcutaneous adipose tissue distribution (higher SAT) relative to non-Hispanic White, independent of dietary fat intake and levels of physical activity, suggesting an increased risk for obesity and central fat patterning, which may not be entirely explained by lifestyle differences. However, upon adjustment for current body size (BMI), these associations were substantially attenuated and became non-significant. This suggests that, at this young age, some differences in fat patterning across racial/ethnic groups are likely due to differences in overall adiposity. Additionally, after adjustment for current BMI, VAT became significantly lower in African-American as compared with non-Hispanic White youth (p<0.0001), which is consistent with prior studies in adults (22, 23) and children (24, 25). Though not statistically significant, Hispanic youth also tended to have lower levels of VAT after adjustment for BMI. One of the most novel findings of our study is that, although minority youth had less VAT for their body size compared with non-Hispanic Whites, they had higher levels of IMCL, both in absolute terms as well as adjusted for BMI differences. Upon adjustment for differences in VAT, this association became even more pronounced: Hispanics and African-American youth had on average 19% and 23% higher IMCL relative to non- Hispanic Whites (data not shown, p=0.0003 and 0.02, respectively). Because IMCL is a marker of insulin resistance at the level of skeletal muscle(5), our findings suggest that, relative to non-Hispanic White counterparts, youth of African-American and Hispanic origin may have an increased risk for metabolic syndrome, possibly genetically determined, which is above and beyond the risk conferred by their overall higher adiposity and independent of differences in fat patterning.
There are several limitations to our study. Because this is a relatively large cohort we were not able to conduct direct measurements of insulin sensitivity using euglycemic-hyperinsulinemic clamps. However, we were able to assess IMCL, which has been associated with skeletal muscle insulin resistance in adults and youth(26). The age range of the cohort was large: about half our participants had initiated puberty, a time when overall adiposity and VAT levels begin to increase at differential rates with age and sex(9). We adjusted for differences in age, sex, puberty and an interaction between age and puberty; however, follow-up of this cohort is necessary to assess the effect of pubertal development on VAT and other fat parameters. Finally, although our cohort over-sampled minority youth, relative to the proportional distribution of Hispanic (19.2%) and African-American youth (4.1%) in the entire state of Colorado(27), our sample of African-American youth was relatively small (N=40), perhaps limiting our ability to detect some significant differences for this group, and tended to over-represent obese individuals.
Our study also has several important strengths. This study investigated fat patterning and adiposity in diverse healthy children using state-of-the-art abdominal fat imaging technology (MRI). The study population included children from three racial/ethnic subgroups, allowing us to explore differences in adiposity-related parameters, while controlling for relevant potential confounders.
In conclusion, Hispanic and African American youth from Colorado have a significantly higher prevalence of obesity and a higher prevalence of metabolic syndrome relative to their non-Hispanic White peers. Both Hispanics and African Americans also show a more centralized fat distribution pattern, but at this age differences are mostly due to their overall higher body size. Importantly, Hispanic and African American youth have higher IMCL deposition relative to non-Hispanic White youth, independent of differences in body size and fat patterning. These findings add to the body of evidence suggesting that racial/ethnic minorities not only experience higher overall adiposity, but may also have an increased risk for early development of metabolic syndrome relative to non-Hispanic White youth, above and beyond their increased obesity risk. This may contribute to several health disparities present early in life, such as an increased risk of early onset type 2 diabetes and related cardio-metabolic outcomes.
Acknowledgments
Supported by General Clinical Research Centers Program (RO1 DK068001 to D.D.) and (M01 RR00069).
Abbreviations
- NHW
non-Hispanic White
- AA
African American
- BMI
body mass index
- WC
waist circumference
- VAT
visceral adipose tissue
- SAT
subcutaneous tissue
- VSR
visceral to subcutaneous fat ratio
- STR
subscapular/triceps skinfold thickness ratio
- IMCL
intermyocellular lipid
- BP
blood pressure
- HDLc
high density lipoprotein cholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 2.Daniels SR. Complications of obesity in children and adolescents. Int J Obes (Lond) 2009;33(Suppl 1):S60–S65. doi: 10.1038/ijo.2009.20. [DOI] [PubMed] [Google Scholar]
- 3.Weiss R, Dufour S, Taksali SE, Tamborlane WV, Petersen KF, Bonadonna RC, et al. Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet. 2003;362:951–957. doi: 10.1016/S0140-6736(03)14364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, et al. The "obese insulin-sensitive" adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab. 2005;90:3731–3737. doi: 10.1210/jc.2004-2305. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, et al. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, central adiposity. Diabetes. 2002;51:1022–1027. doi: 10.2337/diabetes.51.4.1022. [DOI] [PubMed] [Google Scholar]
- 6.Taksali SE, Caprio S, Dziura J, Dufour S, Cali AM, Goodman TR, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 7.Sisson SB, Katzmarzyk PT, Srinivasan SR, Chen W, Freedman DS, Bouchard C, et al. Ethnic differences in subcutaneous adiposity and waist girth in children and adolescents. Obesity (Silver Spring) 2009;17:2075–2081. doi: 10.1038/oby.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lara-Castro C, Garvey WT. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol Metab Clin North Am. 2008;37:841–856. doi: 10.1016/j.ecl.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 10.CDC.gov [homepage on the Internet] Atlanta: National Center for Health Statistics; 2000. [updated 10 September 2010; cited 17 January 2012]. Available from: http://www.cdc.gov/growthcharts/cdc_charts.htm/. [Google Scholar]
- 11.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 12.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 13.Lamb MM, Beers L, Reed-Gillette D, McDowell MA. Feasibility of an Audio Computer-Assisted Self-Interview method to self-assess sexual maturation. J Adolesc Health. 48:325–330. doi: 10.1016/j.jadohealth.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Block G, Murphy M, Roullet JB, Wakimoto P, Crawford PB, Block T. Pilot validation of a FFQ for children 8–10 years. The Fourth International Conference on Dietary Assessment Methods.2000. [Google Scholar]
- 15.Eaton DK, Kann L, Kinchen S, Shanklin S, Ross J, Hawkins J, et al. Youth risk behavior surveillance - United States, 2009. MMWR Surveill Summ. 2010;59:1–142. [PubMed] [Google Scholar]
- 16.Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 18.Crume TL, Ogden L, Maligie M, Sheffield S, Bischoff KJ, McDuffie R, et al. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care. 2011;34:641–645. doi: 10.2337/dc10-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crume TL, Ogden L, West NA, Vehik KS, Scherzinger A, Daniels S, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87–92. doi: 10.1007/s00125-010-1925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among u.s. Adolescents, 1999–2000. Diabetes Care. 2004;27:2438–2443. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 21.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009;155(S7):e-11. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, et al. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91:7–15. doi: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le KA, Ventura EE, Fisher JQ, Davis JN, Weigensberg MJ, Punyanitya M, et al. Ethnic differences in pancreatic fat accumulation and its relationship with other fat depots and inflammatory markers. Diabetes Care. 2011;34:485–490. doi: 10.2337/dc10-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goran MI, Nagy TR, Treuth MS, Trowbridge C, Dezenberg C, McGloin A, et al. Visceral fat in white and African American prepubertal children. Am J Clin Nutr. 1997;65:1703–1708. doi: 10.1093/ajcn/65.6.1703. [DOI] [PubMed] [Google Scholar]
- 25.Huang TT, Johnson MS, Figueroa-Colon R, Dwyer JH, Goran MI. Growth of visceral fat, subcutaneous abdominal fat, and total body fat in children. Obes Res. 2001;9:283–289. doi: 10.1038/oby.2001.35. [DOI] [PubMed] [Google Scholar]
- 26.Ravussin E, Smith SR. Increased fat intake, impaired fat oxidation, and failure of fat cell proliferation result in ectopic fat storage, insulin resistance, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2002;967:363–378. doi: 10.1111/j.1749-6632.2002.tb04292.x. [DOI] [PubMed] [Google Scholar]
- 27.Dola.colorado.gov [homepage on the Internet] Denver: Colorado State Demography Office; 2010. [updated 10 March 2011; cited 30 May 2011]. Available from: http://dola.colorado.gov/dlg/demog/race_colo.html. [Google Scholar]