Abstract
Herpes simplex virus type 1 (HSV-1) is the leading cause of corneal blindness in the developed world due to reactivation of infectious virus and the subsequent immune response. The innate response that facilitates viral control in the cornea is currently unknown. In the present study using a mouse chimera model, we found a bone marrow component is crucial in inhibiting viral replication and identified inflammatory monocytes (F4/80+ GR1+) as the responsible cell. CCL2 was critical for recruiting inflammatory monocytes, and a loss of this chemokine in CCL2−/− mice resulted in a loss of viral containment and inflammatory monocyte recruitment. To confirm these results, clodronate depletion of inflammatory monocytes resulted in elevated viral titers. Furthermore, siRNA targeting the innate sensor p204/IFI-16 resulted in a loss of CCL2 production. In conclusion, CCL2 expression driven by IFI-16 recognition of HSV-1, facilitates the recruitment of inflammatory monocytes into the cornea proper to control viral replication.
Introduction
Herpes simplex virus type I (HSV-1) is a double-stranded DNA virus of which 60–90% of the adult population is seropositive.1 The pathogen is of significant clinical interest due to its role in inducing morbidity in the central nervous system and cornea.1 HSV-1 is spread through mucocutaneous contact in which the virus will first establish a lytic infection in epithelial tissue and progress to invading local sensory fibers. Once the virus has gained access to sensory neurons, it is then transported in a retrograde fashion to the cell body housed in local neural ganglia. In the case of initial oropharyngeal infection, HSV-1 will be transported to the trigeminal ganglia where it can persist as a latent infection indefinitely.2
Following reactivation of HSV-1, infectious virions travel in an anterograde fashion towards sites innervated by divisions of the trigeminal nerve such as the cornea. Recurrent reactivation can then initiate an inflammatory event that if perpetuated can lead to significant corneal scaring known as herpetic keratitis (HSK).3 In individuals that have had an viral reactivation in the cornea, the chance of subsequent reactivations drastically increases, while cornea graft survival drops.4 Thus, identifying mechanisms to inhibit viral replication and establishment of HSV latency could drastically reduce the incidence of HSK.
Unfortunately, highly effective anti-viral compounds such as acyclovir and penciclovir block productive infection but do not reduce the establishment of latency.5 In sites such as the skin, delayed responses of CD8+ T cells reduce the number of infected neurons as well as the amount of latent viral copies but do not thwart viral latency.6 These studies would suggest neither a robust adaptive immune response nor antiviral drugs are effective in reducing initial infection of innervating ganglia to the point of blocking the establishment of latency. Our lab as well as others have shown that type I interferon (IFN) production is critical in preventing viral dissemination and subsequent death of the host.7, 8 Thus, identification of other important innate mechanisms in acute control of viral replication could be tailored to help inhibit HSV replication during the primary infection and prevent establishment of latency in the trigeminal ganglia.
IFI-16/p204 has recently been identified as the innate sensor facilitating the acute anti-viral state of the cornea and other epithelial tissues to HSV by induction of IFN-α production.9 A loss of this critical protein or its downstream adaptor protein, STING, results in a significant rise in the viral titer.9 However, the role of infiltrating innate immune cells (e.g. NK cells, macrophages, and neutrophils) has not been evaluated relative to this sensor during acute infection. Infiltrating leukocytes including granulocytes, monocytes/macrophages, and NK cells are thought to contribute in the resistance to HSV-1 infection by direct or indirect means.10–12 Specifically, NK cells can directly target HSV-1-infected cells and target them for cytolysis.13 In a more indirect fashion, compounds released from immune cells such as nitric oxide (NO) have shown potent antiviral properties in cell culture.14 In the peripheral nervous system, macrophages secreting TNF-α, NO, and IFN-γ control viral replication during the primary infection.15
Thus, we hypothesized that IFI-16/p204 recognition of HSV-1 was required to initiate signals to recruit bone marrow-derived macrophages to the site of infection and contain acute viral replication. Consistent with this hypothesis, we found that IFI-16-driven, IFN-α production initiated inflammatory monocyte recruitment to the site of infection in a CCL2-dependent manner. This response was a critical component of innate immunity in viral surveillance of the cornea.
Materials and Methods
Mice and Virus
C57BL/6J wild type (WT) Trif−/− (Ticam−/−; C57BL/6J background) and CCL2−/− mice were purchased from Jackson Laboratory and housed in the Dean McGee Eye Institute’s and Presbyterian Health Foundation’s animal facilities alongside MyD88−/−, CD118−/− and STING−/− mice.16–18 Animal treatment was consistent with the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals. All experimental procedures were approved by the University of Oklahoma Health Sciences Center and Dean A. McGee Eye Institutes’ Institutional Animal and Care Use Committees. HSV-1 McKrae was passaged as previously described.7
In vivo HSV-1 Infection
Six to ten week old male and female mice were anesthetized by an intraperitoneal (i.p.) injection of xylazine (6.6 mg/kg) and ketamine (100 mg/kg). Corneas were scarified with a 25 gauge 1 ½” needle, and the tear film was blotted. HSV-1 McKrae was resuspended (1,000 PFU / eye) in 3 µLs of sterile 1X PBS and topically applied onto each cornea.
Plaque Assays
The corneas of infected mice were harvested at designated times, resuspended in RPMI 1640 media supplemented with 10% FBS, antibiotic/antimycotic, and gentamicin (normal media, Invitrogen, Gibco), and homogenized with a tissue miser for approximately 20–30 seconds as previously described.7 Supernatant was then clarified with a 10,000 × g spin for 1.5 minutes and serially diluted onto a confluent lawn of HSV-1 susceptible green monkey kidney (Vero) cells. Plaques were allowed to develop for 24–36 hours and then counted with the aid of an inverted Zeiss microscope.
Bone marrow chimeras
Six to ten week old male and female mice were lethally irradiated with two 650-rad doses of γ irradiation separated by 4 hours. Irradiated mice were then given 3 × 106 BM cells by retro-orbital injection isolated from either CD118−/− or WT femurs. The injected BM cells were then given 10 weeks to completely reconstitute the BM as previously described.9, 19 To confirm chimerism, flow analysis was performed on circulating leukocytes and was found to be consistently greater than 95% successful implant. Mice were then infected as previously described and the indicated test performed.
ELISAs and Bioplex Assays
At the indicated time pi, mice were euthanized, and the corneas were harvested. The corneas were then resuspended in normal media containing a 1X protease inhibitor cocktail (Calbiotech) on ice. Following homogenization, homogenates were clarified by a 10,000 × g centrifugation for 1 minute. Supernatants were evaluated for CXCL1, CCL2, and CCL5, by a Bioplex suspension array (BioRad) per manufacturers’ instructions. For rIFN-α experiments, 30,000 Units of purified IFN or PBS was topically applied to corneas.
Flow cytometry
At the appointed time pi, mice were euthanized and corneas harvested. The corneas were then subjected to a 1 mg / ml type I collagenase digestion for 1.5–2 hours at 37°C. Following digestion, single cell suspensions were labeled with monoclonal antibodies and analyzed using a Coulter Epics XL flow cytometer (BD Biosciences, Beckman Coulter). The absolute number of cells residing in the cornea was determined as previously described.7
iNOS inhibition
Mice were anesthetized and topically treated three times daily with 0.05 mg of iNOS inhibitor, aminoguanidine (Sigma Aldrich), resuspended in 1X PBS.20 After two days, mice were infected as previously described. Forty-eight hours pi, mice were euthanized and viral loads in the cornea were evaluated by plaque assay. Mice were treated starting 2 days prior to infection up to 48 hours pi.
In vitro transfection and infection
THCE cells (a gift from Dr. Jerry Shay, UT-Southwestern) were grown in 6-well plates to 50–70% confluency in keratinocyte media containing bovine pituitary extract and recombinant EGF (Invitrogen). The cells were then transfected with 2 or 4 pmols of either nonspecific [5′-UUCUCCGAACGUGUCA CGUTT-3′] or siRNA specific to IFI-16 [5′-GGUGCUGAACGCAAC AGAAUCAUUU-3′] (Invitrogen, medium GC rich content backbone) in serum free keratinocyte media. Following a four-hour incubation, serum free media was removed and replaced with normal media. Twenty-four hours post-transfection, cells were infected at a multiplicity of infection of .001 of HSV-1 McKrae. Media was then replaced after 1 hour at 37°C, and the cells and supernatant harvested 24 hours pi.
Macrophage/Neutrophil Depletion
Mice were anesthetized and infected with 1,000 PFU / eye HSV-1. Thirty minutes after infection, mice were given a 10 µl subconjunctival injection of PBS liposomes, clodronate liposomes, IgG control antibody, or anti-Ly6G (eBioscience). For clodronate experiments, mice were given a second injection 24 hours later. Forty-eight hours pi, viral content was evaluated by plaque assay. Macrophage/neutrophil depletion was determined by flow cytometry.
RT-PCR
Corneas were harvested at the indicated time pi, and mRNA transcript was isolated as previously described.9 iNOS primers were purchased from Origene and the other primer sequences can be seen below.
| Primer | Sequence |
|---|---|
| mβ-actinF | 5'-CTTCTACAATGAGCTGCGTGTG-3' |
| mβ-actinR | 5'-TTGAAGGTCTCAAACATGATCTGG-3' |
| hβ-actinF | 5'-AGCCTCGCCTTTGCCGA-3' |
| hβ-actinR | 5'-CATGTCGTCCCAGTTGGTGAC-3' |
| hCCL2F | 5'-ATGAAAGTCTCTGCCGCCCTTCTGT-3' |
| hCCL2R | 5'-CCTCTGCACTGAGATCTTCCTATTGGTG-3' |
| m, mouse; h, human |
Statistical analysis
Statistical analysis was conducted using a GBSTAT one-way analysis of variance followed by the ad hoc Tukey’s t test in experiments with more than two groups. Significance was defined as a p value less than 0.05 throughout the paper.
Disclosure
All authors have reviewed the final manuscript and have no conflicts of interest to declare.
Results
Resident cells and bone marrow leukocytes contribute to HSV-1 surveillance in the cornea
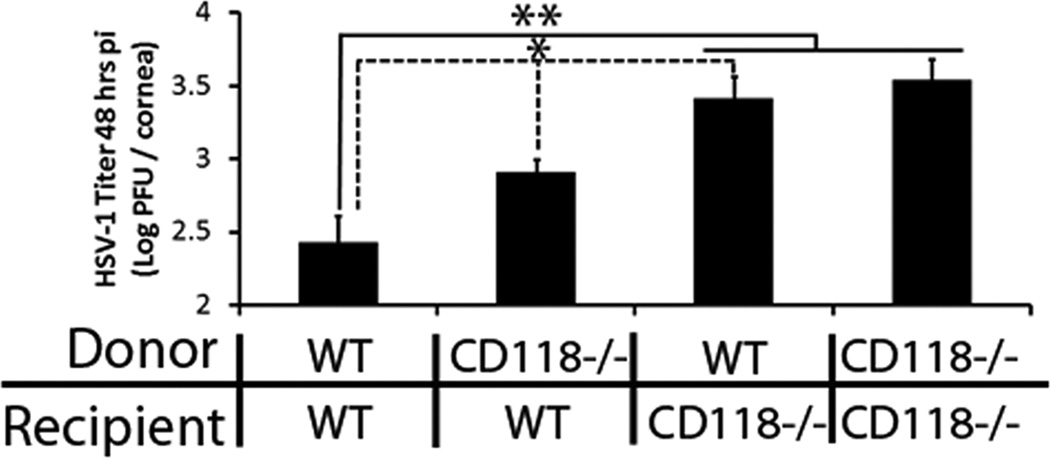
A functional type I IFN pathway is absolutely required for resistance to HSV-1 replication in the cornea as previously shown in IFN receptor A1 deficient mice, CD118−/−, in which these mice no longer respond to IFN signals and rapidly succumb to viral dissimination.7, 9 To dissect the contribution of resident (corneal stromal and epithelial cells) and bone marrow (BM)-derived cells in the innate immune response to HSV-1 in the cornea, a chimera model was chosen to evaluate resistance using WT and highly sensitive CD118−/− mice. The 48 hour post infection (pi) time point was chosen as this period appears to be the critical period in which HSV-1 exploits the cornea in the absence of a functional type I IFN pathway.9 Cornea samples from WTBM>WT and CD118−/−BM>WT mice contained significantly less virus than WTBM>CD118−/− and CD118−/−BM>CD118−/− mice highlighting the importance of IFN-responsive corneal resident cells in HSV-1 containment (Fig. 1). However, WTBM>WT mouse corneas possessed significantly less virus than CD118−/−BM>WT mouse corneas implicating a role for BM-derived leukocytes in viral surveillance of the cornea in concert with IFN-responsive resident cells (Fig. 1).
Figure 1. Corneal defense against HSV-1 requires a bone marrow component.
Chimeric mice were created with bone marrow cells from WT and CD118−/− mice. Ten weeks after irradiation and bone marrow transfer, chimerism was confirmed by flow cytometric analysis of blood samples and was consistently greater than 95%. Following infection with 1,000 PFU HSV-1 / eye, viral loads were measured 48 hr pi by plaque assay. Bars represent 2–4 experiments, n = 2–4 per group per experiment and are expressed as the mean log PFU / cornea ± SEM. **, p < 0.01; *, p < 0.05 when comparing all groups. Solid line, comparing WTBM>WT mice to WTBM>CD118−/− mice and CD118−/−BM>CD118−/− mice; dotted line, comparing WTBM>WT mice to CD118−/−BM>WT mice and CD118−/−BM>WT mice to WTBM>CD118−/−mice.
Resident cell production of CCL2 drives inflammatory monocyte recruitment into the cornea
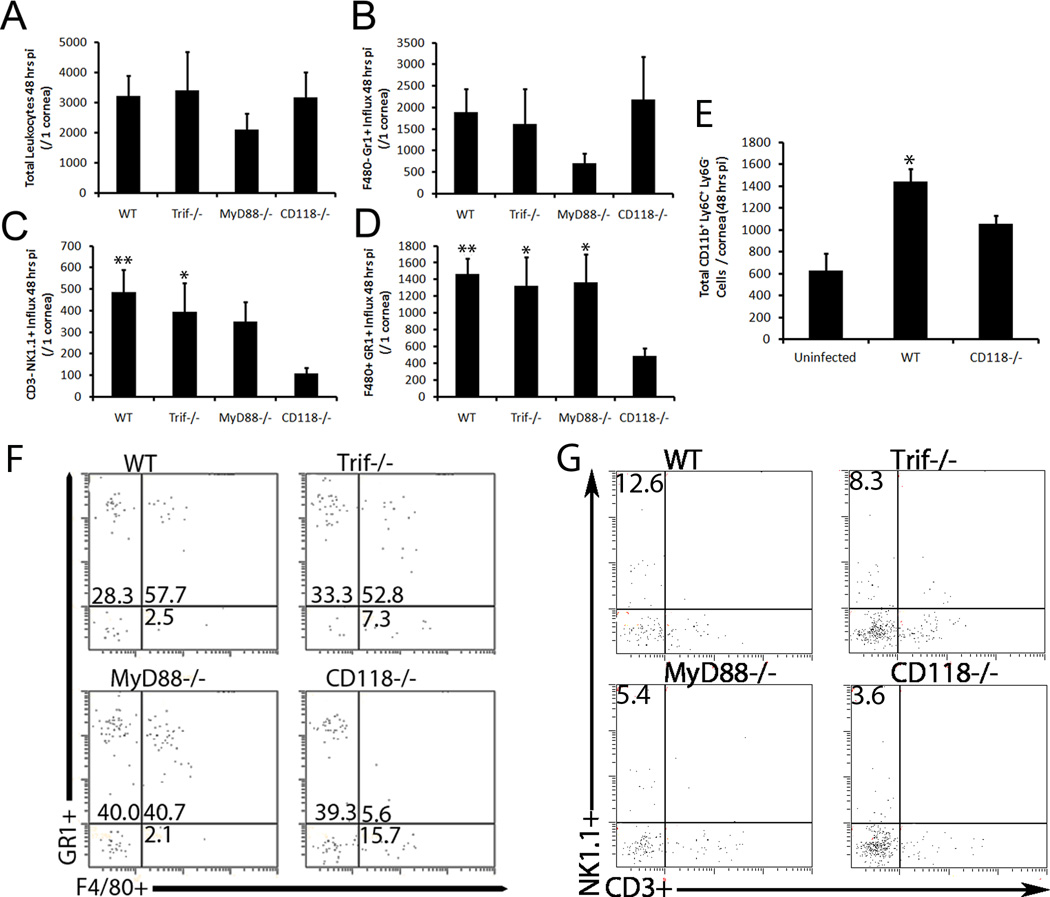
To identify the innate leukocyte population(s) and thus, the BM-derived component of viral surveillance in the cornea of mice that was implicated above, flow cytometric analysis of select infiltrating leukocytes was performed. Previous published work reported viral containment in toll-like receptor (TLR) adaptor protein deficient mice mirrored that of WT controls in the cornea.9 Thus, it was hypothesized the leukocyte population required for HSV containment would be similar in MyD88−/−, Trif−/−, and WT mice; however, recruitment of this specific cell type would be severely reduced in CD118−/− corneas due to their enhanced susceptibility to infection. Forty-eight hours pi, CD118−/− mice exhibited a gross deficiency in NK cells (CD3− NK1.1+) [Fig. 2C, G] and inflammatory monocytes (F4/80+ Gr1+/ CD11b+ Ly6C+ Ly6G−) [Fig. 2D–F] residing in the cornea proper compared to all other groups. In contrast, CD118−/− mice possessed similar numbers of total leukocytes (CD45 hi) [Fig. 2A], macrophages (F4/80+ Gr1−) (data not shown), and neutrophils (F4/80− Gr1+) [Fig. 2B] in the cornea compared to WT, MyD88−/−, and Trif−/− mice. Taken together, these results suggested a potential role for inflammatory monocytes and/or NK cells in viral containment in the cornea 48 hours pi, while further suggesting that the influx of neutrophils was not responsible for HSV surveillance.
Figure 2. CD118−/− mice exhibit gross deficiencies in inflammatory monocyte and NK cell recruitment into the cornea.
Forty-eight hr pi, corneas from HSV-1-infected mice (n = 6–10 corneas / group) were harvested and subsequently digested in type I collagenase. Single cell suspensions were then stained with specific monoclonal antibodies to phenotypically identify infiltrating CD45hi total leukocytes (A), F4/80− Gr1+ neutrophils (B), CD3− NK1.1+ NK cells (C), and F4/80+ Gr1+/CD11b+ Ly6C+ Ly6G− inflammatory monocytes (D–E) by flow cytometry. Results summarize the mean phenotypic leukocyte count / cornea ± SEM of 3 independent experiments. **, p < 0.01; *, p < 0.05 comparing all groups to CD118−/−. (F–G) Representative histograms for F4/80+ Gr1+ (F) and CD3+ NK1.1+ (G) are depicted.
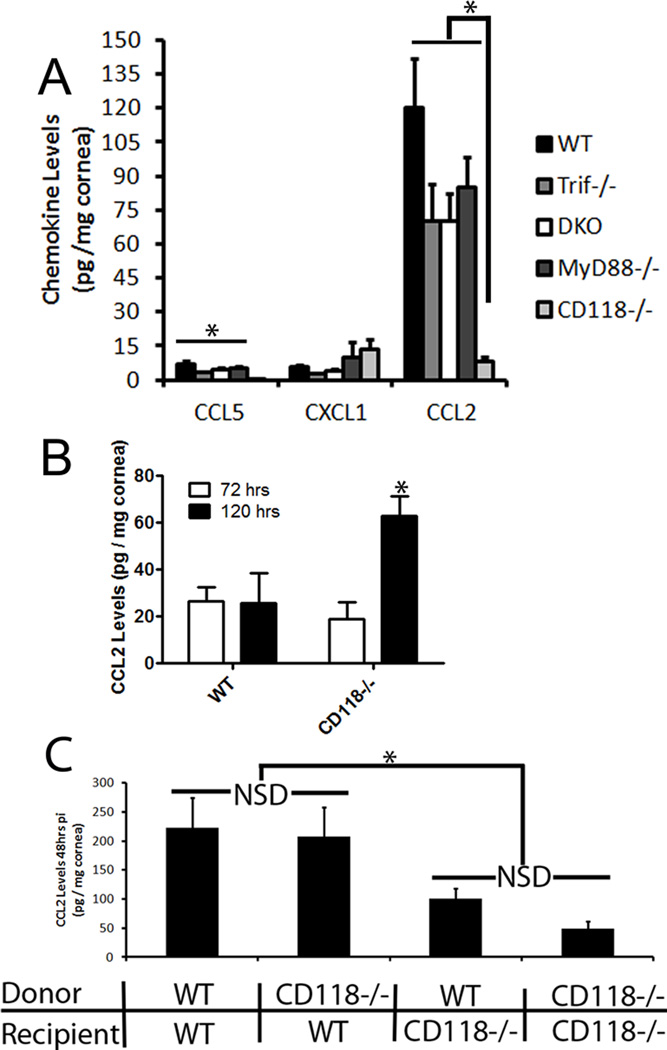
In order to identify the predominant signal for inflammatory monocyte and NK cell recruitment into the infected cornea, chemokine levels of CXCL1, CCL2, and CCL5, known chemoattractants for early-responding leukocyte populations,21 were evaluated in TLR adaptor deficient mice. In the absence of infection, CXCL1, CCL2, and CCL5 were below the level of detection in the cornea (data not shown). Forty-eight hours pi, there were no appreciable difference in CXCL1 production amongst all groups (Fig. 3A). Conversely, CCL5 expression levels were diminished, while CCL2 production was severely abated in CD118−/− mouse corneas compared to WT, MyD88−/−, and Trif−/− mice (Fig. 3A). Furthermore, to negate TLR redundancy, DKO mouse chemokine production resembled that of Trif−/− and MyD88−/− mice (Fig. 3A). Even though CCL2 production was significantly elevated in TLR adaptor-deficient mice compared to CD118−/− mice, HSV-1 infected MyD88−/−, DKO, and Trif−/− mice displayed lower levels of CCL2 compared to those of WT mice (Fig. 3A). We interpret these results to suggest TLR activation contributes to CCL2 production but additional pathways play the predominant role in response to HSV-1. By 72 hours pi, high CCL2 levels began to subside in WT mice and by 120 hours pi, CD118−/− mice produced significantly more CCL2 in the cornea than WT controls (Fig. 3B).
Figure 3. Early resident cell production of CCL2 is minimized in CD118−/− mice.
Corneas (4–10 corneas / group) were harvested from infected TLR adaptor protein deficient 48 hrs pi (A), WT and CD118−/− mice 72 and 120 hrs pi (B) and chimeric mice (C) 48 hrs pi and evaluated for specific chemokine production by suspension arrays. Results represent 2–3 independent experiments and are summarized as the mean pg / mg ± SEM. **, p < .01; *, p < 0.05 when compared to CD118−/−, WTBM>CD118−/− or CD118−/−BM>CD118−/−. NSD, no significant difference.
To localize the source of CCL2 production in the cornea, the previously described mouse model chimera was utilized. At 48 hours pi, CCL2 levels were significantly elevated in WTBM>WT and CD118−/−BM>WT mice compared to WTBM>CD118−/− and CD118−/−BM>CD118−/− mice establishing resident, IFN-responsive corneal cells as the primary source of CCL2 (Fig. 3C). There was no significant difference between CD118−/− mouse recipients of WT or CD118−/−BM cells or between WT mouse recipients of WT or CD118−/−BM cells further emphasizing the importance of the resident population in CCL2 production (Fig. 3C).
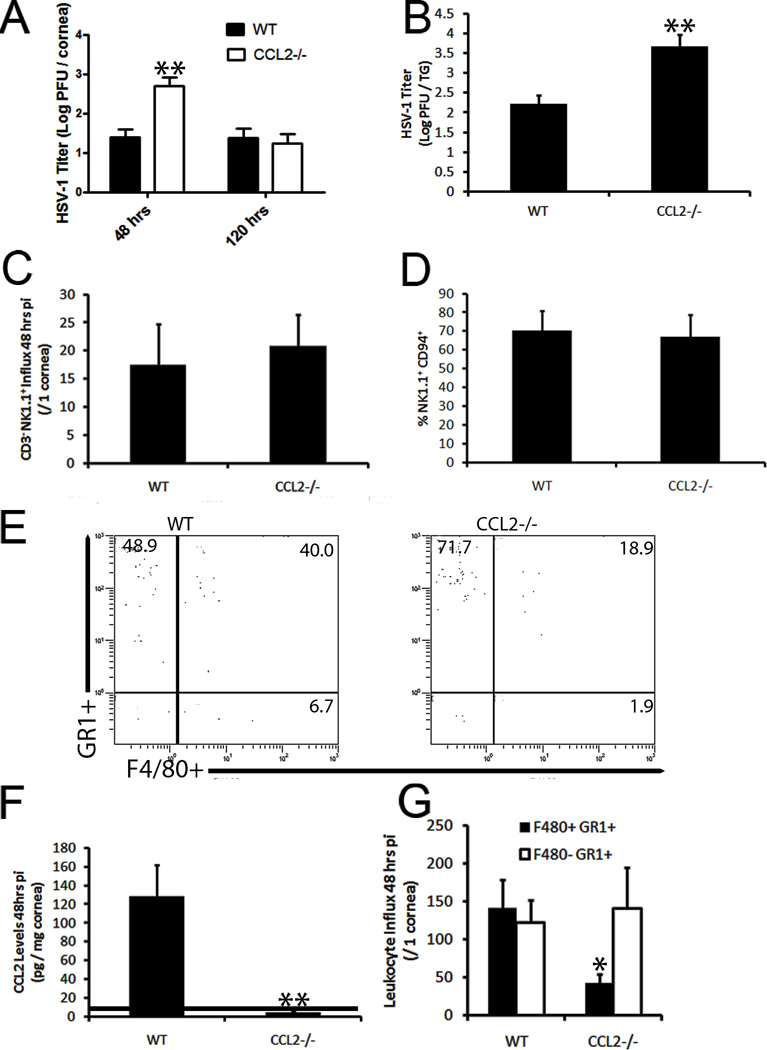
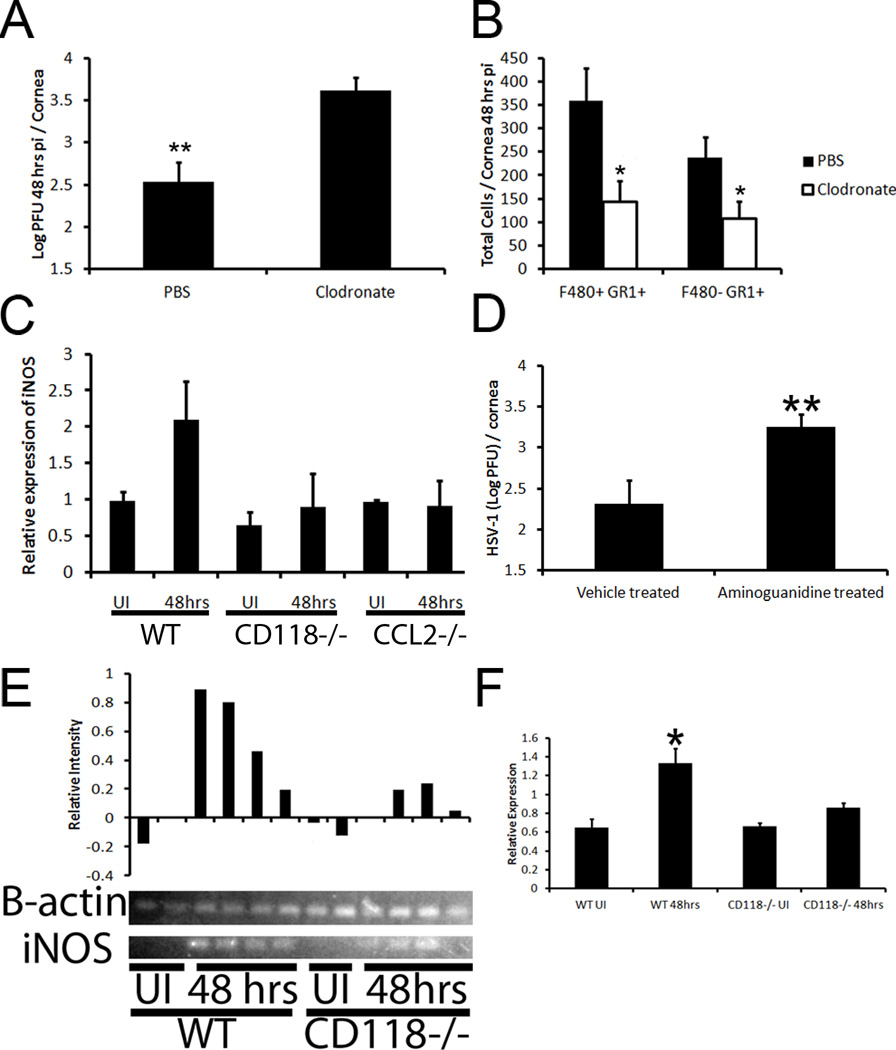
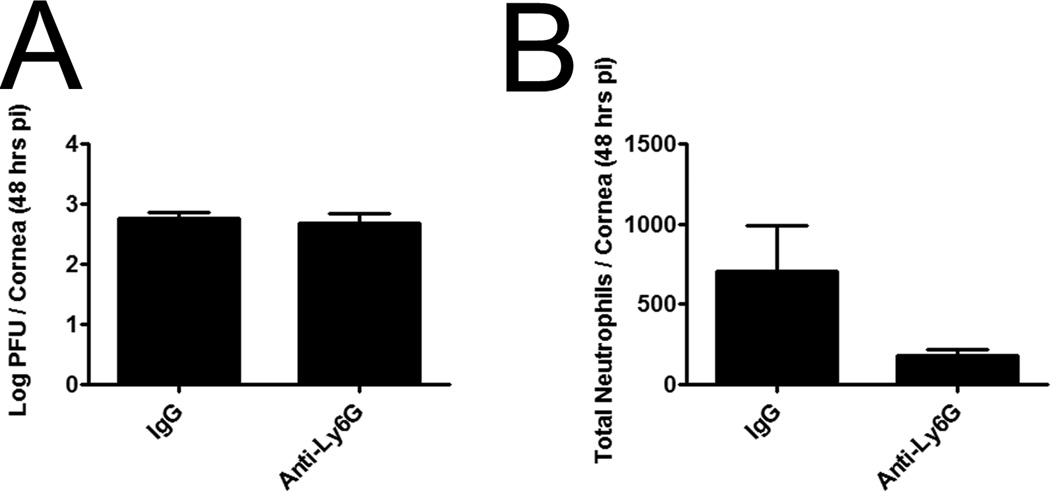
Previously, CCL2 had been shown to mediate NK cell trafficking and activity as well as macrophage recruitment to sites of infection and inflammation.22–24 However, its role in the cornea is poorly understood in relation to these cell types. Thus, we sought to elucidate the contribution of CCL2 production in viral surveillance of the cornea utilizing WT and CCL2 deficient (CCL2−/−) mice. Consistent with our previous results, CCL2−/− mice suffered from a significant rise in infectious virus in the cornea compared to WT animals at 48 hr pi but viral levels returned to those of WT controls 120 hrs pi whereas more virus was recovered in the TG 5 days pi (Fig. 4A, B). Changes in replicating virus levels occurred despite similar numbers of NK cells (Fig. 4C), neutrophils (Fig. 4E), and total leukocytes in the cornea proper (data not shown). CCL2 levels were readily detectable in the cornea of WT mice compared to CCL2−/− mice at this time point (Fig. 4F). Furthermore, the activation status of NK cells (expression of NK cell activation marker, CD9425, 26) was similar in CCL2−/− and WT mice as well as mean fluorescent intensity (Fig. 4D, data not shown). We interpreted these results to suggest NK cells were not the cell type responsible for immune surveillance of the cornea 48 hrs pi. Thus, the sensitivity of CCL2−/− mice to HSV-1 infection of the cornea was assigned exclusively to a gross deficiency in the recruitment of inflammatory monocytes (F4/80+ Gr1+) to the cornea (Fig. 4E, G). To solidify the critical role of inflammatory monocytes, subconjunctival injections of clodronate liposomes were used to show a significant drop in viral containment associated with a loss of inflammatory monocytes in the cornea proper compared to those of PBS-liposome controls (Fig. 5A–B). While the clodronate liposome treatment resulted in a decrease in total neutrophils as well, previous data shown herein negated their contribution to ocular HSV immunity (Fig. 2). Additionally, depletion of neutrophils did not result in an enhanced corneal susceptibility to HSV-1 infection consistent with previous findings in an HSV-1 flank infection model (Fig. 6).27
Figure 4. CCL2 production drives inflammatory monocyte recruitment.
CCL2−/− and WT mice were infected with 1,000 PFU HSV-1 / eye and 48 or 120 hrs pi corneas (6–8 corneas / group) were harvested and evaluated for viral content in the cornea (A) and TG (B), leukocyte influx (C–E), and chemokine production (F). Representative histograms depicted in (G). Values summarize 2 independent experiments and are represented as the mean ± SEM. **, p < 0.01; *, p < 0.05 when WT and CCL2−/− groups were compared. Dotted line, limit of detection.
Figure 5. Clodronate depletion of inflammatory monocytes significantly reduces immune control of HSV infection due to loss of the antiviral agent nitric oxide.
WT mice were infected with HSV-1 and then treated with a subconjunctival injection of clodronate or PBS containing liposomes. Twenty-four hours later, the liposome treatment was repeated. Forty-eight hours pi, WT mice were euthanized and corneal viral content was quantified by plaque assay (A) or immune cell infiltration was assessed by flow cytometry (B). Graphs are represented as the mean ± SEM (n = 6–8 corneas / group). **, p <0.01; *, p < 0.05. (C–D) RT-PCR products were analyzed by gel electrophoresis and data and image are representative of 2 independent experiments. Normalized (to β-actin) intensity of each sample can be seen above gel (C) and mean normalized intensity ± SEM of the entire experiment is expressed in (D). (E) Forty-eight hours pi (1,000 PFU HSV-1 / eye), mRNA was isolated from whole corneas of WT, CD118−/−, and CCL2−/− mice (n = 3–10) and RT-PCR was performed to determine the presence of iNOS. The results were normalized to uninfected (UI) controls and the housekeeping gene β-actin. (D) WT mice were topically treated with aminoguanidine (0.05 mg) or vehicle three times daily starting 48 hr prior to infection with HSV-1 (1,000 PFU / eye). Viral titers in the cornea were evaluated 48 hr pi and compared to vehicle-treated WT mice (n = 6–8 corneas / group). Data is presented as the mean ± SEM. **, p < 0.01.
Figure 6. Loss of neutrophils does not enhance corneal susceptibility to HSV infection.
WT mice were infected with HSV-1 (1,000 PFU/eye) and then given a subconjunctival injection of either IgG control or anti-Ly6G antibody. Forty-eight hours pi, viral content (A) was evaluated by plaque assay and presented as the Log PFU / cornea ± SEM (n = 7 / group, 2 experiments). To confirm depletion, flow cytometry was used to evaluate total neutrophils (B) residing within the cornea and is representative of 2 independent experiments. Values are presented as the total neutrophils ± SEM.
Inflammatory monocytes secrete nitric oxide to inhibit HSV-1 replication
Inflammatory monocytes have been shown to restrict HSV-1 replication in vitro28 likely due to the secretion of antiviral compounds driven by IFN and NF-κB signaling such as nitric oxide (NO).20, 29 In order to test this hypothesis and to identify one likely key antiviral compound secreted by infiltrating activated monocytes, we had to first establish the presence or absence of inducible nitric oxide synthase (iNOS). Within 48 hr pi, iNOS transcript levels in the cornea of both infected CD118−/− and CCL2−/− mice were indistinguishable from uninfected controls (Fig. 5C). In stark contrast, iNOS expression was elevated two-fold in the cornea of WT animals. Furthermore, gel electrophoresis of RT-PCR products confirmed a reduction or absence of iNOS in uninfected CD118−/− and mouse corneas (Fig. 5C–D). To determine the significance of NO production in viral containment, HSV-1 levels were determined following iNOS inhibition with aminoguanidine.20 Viral titers were significantly elevated in aminoguanidine-treated mice compared to vehicle-treated WT controls 48 hr pi (Fig. 5E). Taken together, these results suggested a reduction of inflammatory monocyte migration into the corneas of CD118−/− and CCL2−/− mice results in the loss of the primary source of NO production and consequential rise in virus output.
CCL2 production driven by IFN-α
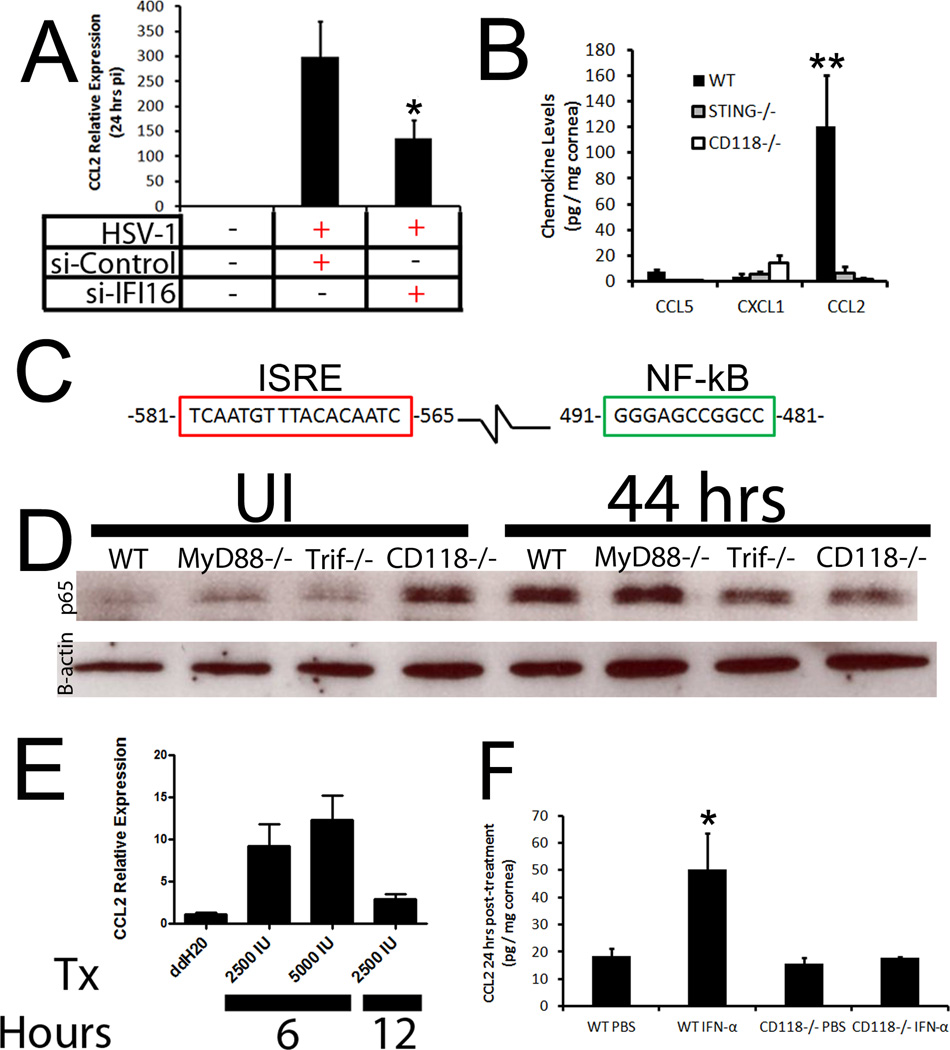
We previously identified the innate sensor, p204/IFI-16, as the crucial regulator of IFN-α in response to HSV infection of the cornea and thus, hypothesized that signals such as CCL2 were induced by the sensor to recruit the BM arm of innate immunity. To first identify a potential role of IFI-16/p204 in CCL2 production, CCL2 transcript expression was evaluated 24 hours pi in human corneal epithelial cells (THCE) transfected with nonspecific siRNA or that directed to IFI-16. A loss of IFI-16 (~70% reduction)9 resulted in a significant reduction in CCL2 mRNA transcript solidifying a role of the innate sensor in CCL2 production (Fig. 7A). This was confirmed in vivo with mice deficient in the adaptor protein for IFI-16, STING, in which CCL2 levels mirrored those of CD118−/− mice and were severely diminished compared to those of WT controls (Fig. 7B). We interpret these results suggested that signals emanating from p204/IFI-16 were responsible for regulating CCL2 production.
Figure 7. IFI-16 drives CCL2 production by way of type I IFN.
(A) THCE cells were transfected with control or siRNA specific to IFI-16 and infected with HSV-1. Twenty-four hours pi, mRNA transcript expression was evaluated for CCL2 and normalized to uninfected controls and the housekeeping gene β-actin. Results are expressed as the mean ± SEM. (B) STING−/−, CD118−/−, and WT mice (n = 4–8) were infected with 1,000 PFU / eye. Forty-eight hours pi chemokine content was evaluated by suspension array and is presented as the mean pg / mg ± SEM. (C) Evaluation of the upstream promoter of CCL2 identified IFN (red) and NF-κB (green) responsive elements. (D) WT, MyD88−/−, Trif−/−, and CD118−/− mice were infected and NF-κB nuclear translocation was assessed 44 hours pi by Western blot and compared to uninfected (UI) controls. Image is representative of 2 independent experiments. (E) THCE cells (n = 9) were treated with rIFN-α and CCL2 mRNA transcript assessed at 6 or 12 hours post-treatment. Results were normalized to ddH20 controls and the housekeeping gene β-actin. Tx, treatment; IU, units; Values are expressed as the mean ± SEM of 2–3 independent experiments. (F) To confirm IFN was responsible for driving CCL2, WT and CD118−/− mice were treated with PBS or 30,0000 U rIFN-α. Twenty-four hrs later, chemokine levels were assessed and are presented as the mean ± SEM of 3 independent experiments of 2–3 corneas / group. **, p < 0.01; *, p < 0.05
IFI-16 has been shown to activate both NF-κB and IRF-3/IFN cascades, and CCL2 is a known NF-κB-driven chemokine through a phospholipase C-dependent mechanism.30, 31 Both an IFN-stimulated regulatory element (ISRE) and NF-κB binding site are found upstream of the start site at position −580 to −566 and −490 to −480 respectively (Fig. 7C). To evaluate the role of NF-κB on CCL2 production, western blot analysis was performed on nuclear extracts on TLR adaptor protein-deficient mice to explain the differences seen in CCL2 production (Fig. 3A). However, there were no appreciable differences between any of the groups tested suggesting that NF-κB activity was not responsible for CCL2 production 48 hours pi (Fig. 7D). To then evaluate the role of IFN, THCE cells were treated with recombinant IFN-α and CCL2 mRNA was evaluated. CCL2 transcript levels rose 10-fold 6 hours post treatment but returned to near baseline 6 hours later suggesting that IFN alone could drive CCL2 production (Fig. 7E). To further solidify this point, WT mice topically treated with rIFN-α had significantly higher levels of CCL2 24 hrs post-treatement than PBS-treated controls and CD118−/− mice treated with PBS or IFN (Fig. 7F).
Discussion
We have previously shown IFI-16 was responsible for the recognition of HSV in epithelial tissue and once activated, the sensor drove IRF-3-dependent IFN production critical in containing viral replication.9 However, we did not explore the contribution of infiltrating leukocytes.9 In the study described herein, we have identified inflammatory monocytes (F4/80+ Gr1+) as the BM component of innate immunity within the cornea proper that contributes to HSV-1 surveillance. To substantiate the BM arm of innate immunity, a loss of any part of the CCL2-inflammatory monocyte response results in a significant reduction in the host’s ability to contain viral replication.
The chemokine CCL2 is widely studied due to its up-regulation in autoimmune diseases, tumors, and infections.22, 32, 33 CCL2 attributes can be favorable or unfavorable to the host. From a negative aspect, the chemokine facilitates tumor metastasis and HIV entry into the CNS.34, 35 However, CCL2 is also needed in muscle repair36 and as we have found, is required in the cornea to recruit inflammatory monocytes to contain HSV-1 replication. Additionally, CCL2 production has been shown to be regulated by both TNF-α and STAT-2 signals.37, 38 However, we show the CCL2 upstream promoter contains an IFN-responsive element, and cells treated with recombinant IFN alone up-regulate CCL2 mRNA. Such results imply multiple host signals elicit CCL2 expression dependent upon the initiating stimulus. We hypothesize the timing of CCL2 production is facilitated by different signals and is likely tissue-specific. This notion is best observed in CD118−/− mice that lack downstream IFN signals. Forty-eight hours pi, CD118−/− mice produce very low levels of CCL2 in the cornea; however, by 5 days pi, CCL2 concentrations are significantly higher in CD118−/− mice than WT controls. Furthermore, we have previously shown in peripheral nervous system tissue such as the trigeminal ganglia that there were no identified differences in CCL2 production comparing HSV-1 infected WT to CD118−/− mice.7 This was in stark contrast to the brain stem where the only difference in levels of the chemokine was detected 5 days pi when CD118−/− mouse brain stem possessed significantly more CCL2.7 Thus, the regulation of CCL2 is complex and warrants further inspection. Furthermore, while we found no precedent for enhanced p65 nuclear translocation in uninfected CD118−/− mice, an immediate early gene of HSV-1, ICP0, has been shown to inhibit NF-κB signaling by reducing specific TLR adaptor protein levels possibly explaining the loss of nuclear p65 in infected CD118−/− corneas in which viral levels are much higher than WT controls.39
Although the role of inflammatory monocytes in controlling parasitic and bacterial infections is well described,40, 41 the contribution of these cells in HSV infection was ambiguous. Most studies would suggest neutrophils and NK cells are the predominant immune cell required to contain HSV replication during the innate immune response.42–44 However, we show in mice that recruit inflammatory monocytes with far less efficiency (CD118−/− and CCL2−/− mice) than WT animals, such mice suffer from significantly more virus in the cornea which correlates with a reduction in NO levels. Although NO is likely only one of several viral replication inhibitors secreted by infiltrating monocytes (e.g. TNF-α),45 its ability to antagonize viral replication has been previously noted.14, 20, 46 To further substantiate the role of inflammatory monocytes in innate immunity of the cornea, localized clodronate depletion was performed and resulted in significantly higher HSV-1 titers. The one caveat to these results is that the treatment resulted in a loss of neutrophils (F4/80− Gr1+) as well. However, CD118−/− and CCL2−/− mice have similar numbers of neutrophils despite significantly more infectious virus in the cornea than those of WT controls. Additionally, specific depletion of neutrophils in WT mice did not result in an increase in viral titers recovered from the cornea following HSV-1 infection. This would suggest and be supported by previous work with herpetic lesions in the skin showing that depletion of Ly6G+ cells (neutrophils) did not compromise the anti-viral response.27 However, this study did suggest the loss of Gr1+ cells (includes both neutrophils and inflammatory monocytes) resulted in a deficiency in viral containment.27 Thus, the role of neutrophils in HSV surveillance of the cornea is likely only minimal at best with a notable contribution to ocular pathology.47 Additionally, viral resistance mediated by NK cells during acute HSV infection of the cornea is likely minimal compared to that of inflammatory monocytes and a later response than that of monocytes. This is evident in CCL2−/− mice that are compromised in viral surveillance 48 hours pi but activity (CD94 expression25) and total numbers of NK cells does not differ from those of WT controls. Studies implicating NK cells in ocular surveillance have used depletion techniques to confirm their role within the first ninety-six hours;44 however, the antibody targeting NK cells (anti-ASGM-1) has also been reported to reduce inflammatory monocyte recruitment into the cornea proper.44, 48 Furthermore, we identified that the major contribution of inflammatory monocytes occurs within the first forty-eight hours after infection. Thus, it is unclear what role, if any, NK cells contribute to the earliest phases of the inflammatory monocyte-driven innate immunity within the cornea and how these responses are orchestrated.
We surmise inflammatory monocytes and IFI-16-driven IFN production act together to contain viral replication in the cornea and likely other epithelial tissues.9 These two arms of the innate immune response are required during acute HSV infection and as such would be potential targets for vaccine development. Current attempts of incorporating HSV glycoproteins in vaccine designs have failed49, 50 suggesting additional factors are likely required to provide protective immunity. Thus, attenuated strains of HSV51 that provide host epithelial cells with viral DNA to activate IFI-16-driven cascades would likely facilitate a more robust immune response strengthening the overall vaccine while reducing the establishment of latency.52 Furthermore, the widely used adjuvant, alum, is known to excite IRF-3-dependent cascades due to the release of host DNA from dying cells.53 We hypothesize that this could be due at least in part to IFI-16 activity as a result of the ability of the protein to respond to both host and viral DNA54 and thus, boost the overall immune response to the vaccine. Consequently, activating the IFI-16 pathway may be more fruitful in preventing early viral replication and establishment of latency due to its significant role in initiating the innate immune response to HSV-1 and HSV-2 in epithelial tissue.9
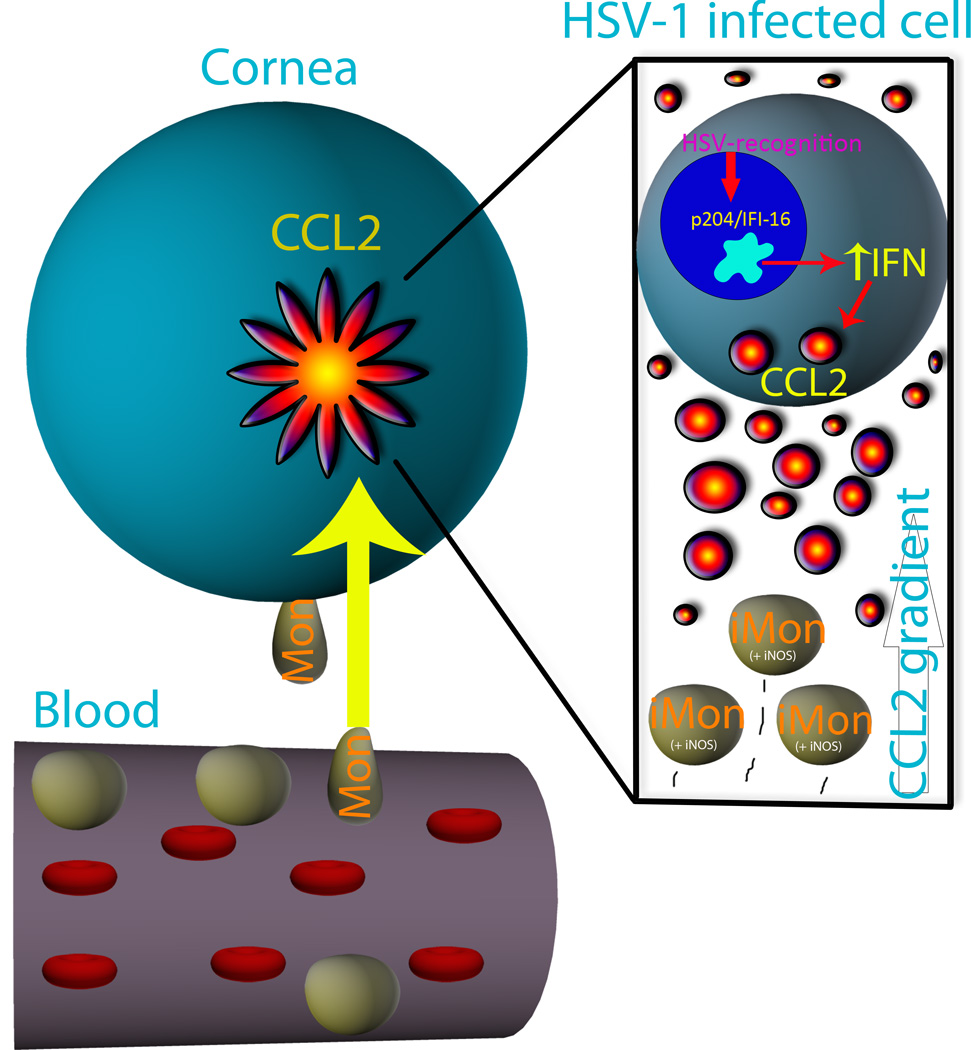
In conclusion, we propose that IFI-16 activates IFN production and NF-κB signaling through a STING-dependent manner in response to HSV-1 within resident cells of the cornea and likely other epithelial tissues.9 Up-regulated products of IFN signaling such as CCL2, PKR, and RNase L facilitate an antiviral state in the cornea. The CCL2 chemokine gradient recruits inflammatory monocytes to the infectious lesion, where the leukocytes secrete compounds such as NO to antagonize HSV-1 replication. IFN-responsive proteins such as PKR and RNase L initiate an antiviral state within infected and uninfected cells of the cornea to further inhibit viral spread (Fig. 8). These two orchestrated processes act synergistically to contain HSV-1 replication in the cornea even in the absence of TLR signaling.
Figure 8. Schematic representation of innate immunity in the cornea in response to HSV-1.
IFI-16/p204 binds HSV-1 and subsequently activates IFN production through an IRF-3-STING-dependent mechanism and NF-κB signaling to induce antiviral proteins such as protein kinase R and RNAse L as well as CCL2. The localized CCL2 gradient chemoattracts circulating inflammatory monocytes (iMon) from the blood to the site of infection in the cornea, where the monocytes secrete NO to further block HSV-1 spread. These finely-tuned responses (BM and resident) work in concert to orchestrate acute resistance to HSV-1 in the cornea and are first initiated by p204. iMon, inflammatory monocyte; iNOS+, iNOS expressing cell; IFN, interferon; Mon, monocyte.
Acknowledgements
We would like to thank Julie Tran, Sara Moore, Linh Sramek, and Gabby Nguyen for technical support and Drs. Helen Rosenberg, Russell Vance, and Shizuro Akira for the CD118−/−, STING−/−, and MyD88−/− mice respectively. The authors would also like to thank Roche Diagnostics GmbH for clodronate liposomes. Principal support for the study was from NIH AI053108 to DJJC. Additional support includes an OUHSC Presbyterian Health Foundation Presidential Professorship award to DJJC, NIH/NCRR P20 RR017703, and a senior investigator award (to DJJC) from Research to Prevent Blindness.
Footnotes
Disclosure: All authors have reviewed the final manuscript and have no conflicts of interest to declare.
References
- 1.Conrady CD, Drevets DA, Carr DJ. Herpes simplex type I (HSV-1) infection of the nervous system: is an immune response a good thing? J Neuroimmunol. 2010;220(1–2):1–9. doi: 10.1016/j.jneuroim.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khanna KM, Lepisto AJ, Hendricks RL. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 2004;25(5):230–234. doi: 10.1016/j.it.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198(5):659–663. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- 4.Larkin DF. Corneal transplantation for herpes simplex keratitis. Br J Ophthalmol. 1998;82(2):107–108. doi: 10.1136/bjo.82.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith RL, Morroni J, Wilcox CL. Lack of effect of treatment with penciclovir or acyclovir on the establishment of latent HSV-1 in primary sensory neurons in culture. Antiviral Res. 2001;52(1):19–24. doi: 10.1016/s0166-3542(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 6.Wakim LM, Jones CM, Gebhardt T, Preston CM, Carbone FR. CD8(+) T-cell attenuation of cutaneous herpes simplex virus infection reduces the average viral copy number of the ensuing latent infection. Immunol Cell Biol. 2008;86(8):666–675. doi: 10.1038/icb.2008.47. [DOI] [PubMed] [Google Scholar]
- 7.Conrady CD, Thapa M, Wuest T, Carr DJ. Loss of mandibular lymph node integrity is associated with an increase in sensitivity to HSV-1 infection in CD118-deficient mice. J Immunol. 2009;182(6):3678–3687. doi: 10.4049/jimmunol.0803878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leib DA, Harrison TE, Laslo KM, Machalek MA, Moorman NJ, Virgin HW. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189(4):663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrady CD, Zheng M, Fitzgerald KA, Liu C, Carr DJ. Resistance to HSV-1 infection in the epithelium resides with the novel innate sensor, IFI-16. Mucosal Immunol. 2012 doi: 10.1038/mi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reading PC, Whitney PG, Barr DP, Smyth MJ, Brooks AG. NK cells contribute to the early clearance of HSV-1 from the lung but cannot control replication in the central nervous system following intranasal infection. Eur J Immunol. 2006;36(4):897–905. doi: 10.1002/eji.200535710. [DOI] [PubMed] [Google Scholar]
- 11.van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, Carbone FR. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J Immunol. 2004;172(1):392–397. doi: 10.4049/jimmunol.172.1.392. [DOI] [PubMed] [Google Scholar]
- 12.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322(5899):268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chisholm SE, Howard K, Gomez MV, Reyburn HT. Expression of ICP0 is sufficient to trigger natural killer cell recognition of herpes simplex virus-infected cells by natural cytotoxicity receptors. J Infect Dis. 2007;195(8):1160–1168. doi: 10.1086/512862. [DOI] [PubMed] [Google Scholar]
- 14.Croen KD. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993;91(6):2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodukula P, Liu T, Rooijen NV, Jager MJ, Hendricks RL. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol. 1999;162(5):2895–2905. [PubMed] [Google Scholar]
- 16.Garvey TL, Dyer KD, Ellis JA, Bonville CA, Foster B, Prussin C, et al. Inflammatory responses to pneumovirus infection in IFN-alpha beta R gene-deleted mice. J Immunol. 2005;175(7):4735–4744. doi: 10.4049/jimmunol.175.7.4735. [DOI] [PubMed] [Google Scholar]
- 17.Sauer JD, Sotelo-Troha K, von Moltke J, Monroe KM, Rae CS, Brubaker SW, et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect Immun. 2011;79(2):688–694. doi: 10.1128/IAI.00999-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 19.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci U S A. 2009;106(14):5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benencia F, Courreges MC, Gamba G, Cavalieri H, Massouh EJ. Effect of aminoguanidine, a nitric oxide synthase inhibitor, on ocular infection with herpes simplex virus in Balb/c mice. Invest Ophthalmol Vis Sci. 2001;42(6):1277–1284. [PubMed] [Google Scholar]
- 21.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469–499. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 22.Morrison BE, Park SJ, Mooney JM, Mehrad B. Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis. J Clin Invest. 2003;112(12):1862–1870. doi: 10.1172/JCI18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169(4):1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taub DD, Sayers TJ, Carter CR, Ortaldo JR. Alpha and beta chemokines induce NK cell migration and enhance NK-mediated cytolysis. J Immunol. 1995;155(8):3877–3888. [PubMed] [Google Scholar]
- 25.Ho EL, Heusel JW, Brown MG, Matsumoto K, Scalzo AA, Yokoyama WM. Murine Nkg2d and Cd94 are clustered within the natural killer complex and are expressed independently in natural killer cells. Proc Natl Acad Sci U S A. 1998;95(11):6320–6325. doi: 10.1073/pnas.95.11.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115(2):274–281. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wojtasiak M, Pickett DL, Tate MD, Londrigan SL, Bedoui S, Brooks AG, et al. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J Gen Virol. 2010;91(Pt 9):2158–2166. doi: 10.1099/vir.0.021915-0. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H, Tumpey TM, Staats HF, van Rooijen N, Oakes JE, Lausch RN. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Invest Ophthalmol Vis Sci. 2000;41(6):1402–1409. [PubMed] [Google Scholar]
- 29.Farlik M, Reutterer B, Schindler C, Greten F, Vogl C, Muller M, et al. Nonconventional initiation complex assembly by STAT and NF-kappaB transcription factors regulates nitric oxide synthase expression. Immunity. 2010;33(1):25–34. doi: 10.1016/j.immuni.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fantuzzi L, Spadaro F, Purificato C, Cecchetti S, Podo F, Belardelli F, et al. Phosphatidylcholine-specific phospholipase C activation is required for CCR5-dependent, NF-kB-driven CCL2 secretion elicited in response to HIV-1 gp120 in human primary macrophages. Blood. 2008;111(7):3355–3363. doi: 10.1182/blood-2007-08-104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199(9):1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193(6):713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26(4):1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H, Huang D, Ransohoff RM, Zhou L. Acute skeletal muscle injury: CCL2 expression by both monocytes and injured muscle is required for repair. FASEB J. 2011;25(10):3344–3355. doi: 10.1096/fj.10-178939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khorooshi R, Babcock AA, Owens T. NF-kappaB-driven STAT2 and CCL2 expression in astrocytes in response to brain injury. J Immunol. 2008;181(10):7284–7291. doi: 10.4049/jimmunol.181.10.7284. [DOI] [PubMed] [Google Scholar]
- 38.Bonello GB, Pham MH, Begum K, Sigala J, Sataranatarajan K, Mummidi S. An evolutionarily conserved TNF-alpha-responsive enhancer in the far upstream region of human CCL2 locus influences its gene expression. J Immunol. 2011;186(12):7025–7038. doi: 10.4049/jimmunol.0900643. [DOI] [PubMed] [Google Scholar]
- 39.van Lint AL, Murawski MR, Goodbody RE, Severa M, Fitzgerald KA, Finberg RW, et al. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J Virol. 2010;84(20):10802–10811. doi: 10.1128/JVI.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, et al. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29(2):306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, et al. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34(5):769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tumpey TM, Chen SH, Oakes JE, Lausch RN. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70(2):898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siebens H, Tevethia SS, Babior BM. Neutrophil-mediated antibody-dependent killing of herpes-simplex-virus-infected cells. Blood. 1979;54(1):88–94. [PubMed] [Google Scholar]
- 44.Frank GM, Buela KA, Maker DM, Harvey SA, Hendricks RL. Early Responding Dendritic Cells Direct the Local NK Response To Control Herpes Simplex Virus 1 Infection within the Cornea. J Immunol. 2012;188(3):1350–1359. doi: 10.4049/jimmunol.1101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossol-Voth R, Rossol S, Schutt KH, Corridori S, de Cian W, Falke D. In vivo protective effect of tumour necrosis factor alpha against experimental infection with herpes simplex virus type 1. J Gen Virol. 1991;72(Pt 1):143–147. doi: 10.1099/0022-1317-72-1-143. [DOI] [PubMed] [Google Scholar]
- 46.Karupiah G, Xie QW, Buller RM, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261(5127):1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 47.Yan XT, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci. 1998;39(10):1854–1862. [PubMed] [Google Scholar]
- 48.Nishikado H, Mukai K, Kawano Y, Minegishi Y, Karasuyama H. NK cell-depleting anti-asialo GM1 antibody exhibits a lethal off-target effect on basophils in vivo. J Immunol. 2011;186(10):5766–5771. doi: 10.4049/jimmunol.1100370. [DOI] [PubMed] [Google Scholar]
- 49.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366(1):34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr, et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA. 1999;282(4):331–340. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 51.Halford WP, Puschel R, Gershburg E, Wilber A, Gershburg S, Rakowski B. A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS One. 2011;6(3):e17748. doi: 10.1371/journal.pone.0017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin WR, Jennings R, Smith TL, Wozniak MA, Itzhaki RF. Vaccination prevents latent HSV1 infection of mouse brain. Neurobiol Aging. 2001;22(5):699–703. doi: 10.1016/s0197-4580(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 53.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 54.Liu CJ, Wang H, Lengyel P. The interferon-inducible nucleolar p204 protein binds the ribosomal RNA-specific UBF1 transcription factor and inhibits ribosomal RNA transcription. EMBO J. 1999;18(10):2845–2854. doi: 10.1093/emboj/18.10.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]