Abstract
The single-celled parasite, Entamoeba histolytica, is an enteric pathogen that ingests bacteria and host cells. Inhibition of phagocytosis renders the parasite avirulent. The ligand/receptor interactions that allow E. histolytica to phagocytose are not well understood. We hypothesized that E. histolytica trophozoites might accomplish ingestion through the utilization of a scavenger receptor for cholesterol. Here we show that acetylated low density lipoprotein cholesterol (AcLDL) was phagocytosed by amebae via receptor-mediated mechanisms. AcLDL competitively inhibited by 31 ± 1.3% (P < 0.005) the ingestion of Escherichia coli, but not erythrocytes and Jurkat T lymphocytes, suggesting a partially redundant phagocytic pathway for E. coli and cholesterol. Inducible expression of a signaling-dead dominant-negative version of E. histolytica transmembrane kinase 39 (TMK39) inhibited ingestion of E. coli by 55 ± 3% (P < 0.005) but not LDL particles. We concluded that ingestion of E. coli was regulated by TMK39 and partially shared the AcLDL uptake pathway.
Keywords: Entamoeba histolytica, Transmembrane kinase, Phagocytosis, Bacteria, LDL Receptor, Cholesterol
1. Introduction
The single-celled protozoan parasite, Entamoeba histolytica, is responsible for the human disease, amebiasis. The distribution of amebiasis is worldwide and afflicts individuals of all ages, but children are particularly susceptible. For example between 40–50% of preschool children enrolled in a 4 year study in Bangladesh experienced at least one E. histolytica infection annually (Haque et al., 2006). Up to 50 million cases of amebiasis are estimated to occur each year; of these, up to 100,000 cases result in death (WHO, 1997). Symptomatic infection by this parasite is characterized by diarrhea, severe dysentery and/or colitis (Haque et al., 2003). Complications include the formation of amebic liver abscess following parasite invasion of the intestine (Ravdin and Weller, 1988; Petri and Singh, 1999; Haque et al., 2003).
Infection occurs following the ingestion of multi-nucleated cysts. After reaching the small intestine, each cyst differentiates into trophozoites, the motile form of the parasite. In the large intestine, trophozoites ingest bacteria, multiply and adhere to the colonic epithelium, causing contact-dependent cytotoxicity (Ravdin and Guerrant, 1981; Christy and Petri, 2011). Host cell death is primarily mediated via an ameba-induced rapid activation of caspase-3 dependent apoptosis (Huston et al., 2000; Huston et al., 2003). Trophozoites encyst in the intestine due to unknown stimuli and are passed in the stool (Petri, 2002).
Trophozoites are phagocytic in the intestine and ingest a wide variety of targets. Phagocytosis is linked to virulence: trophozoites genetically selected for a deficiency in the ingestion of bacteria had reduced virulence in a hamster model of infection (Orozco et al., 1983). Entamoeba histolytica that have phagocytized bacteria have been shown to be more cytotoxic to host cells and have increased invasiveness in animal models (Wittner and Rosenbaum, 1970; Mirelman et al., 1983; Bracha and Mirelman, 1984; Galván-Moroyoqui et al., 2008). Entamoeba dispar, a related non-pathogenic species, also ingests bacteria, but contained fewer bacteria in phagosomes that are structurally dissimilar and mature at a slower rate than those of E. histolytica (Pimenta et al., 2002; Mitra et al., 2005). Furthermore, E. histolytica trophozoites regained virulence after co-culture with bacteria (Padilla-Vaca et al., 1999). High rates of erythrophagocytosis were associated with more virulent strains of E. histolytica (Trissl et al., 1978) and trophozoites defective in erythrophagocytosis were also found to induce fewer cases of amebic colitis in mice (Boettner et al., 2008). Beyond acquisition of nutrients, the ability of E. histolytica to ingest apoptotic corpses of the cells it has killed may contribute to immune evasion by the parasite (Huston et al., 2003).
The receptors, extracellular ligands and signaling mechanisms utilized by E. histolytica to recognize and ingest targets have not been entirely characterized. The Gal/GalNAc specific lectin is both the primary amebic adherence lectin and a receptor for erythrocytes (Petri et al., 1987; Katz et al., 2002). Gram-negative bacteria are at least partially bound by a receptor inhibited by α-methyl-mannose (Bracha et al., 1982). Trophozoites recognize surface-exposed phosphatidylserine, a classical marker of apoptotic cells, on aged human erythrocytes (Boettner et al., 2005). Recent work has also shown that the C1q complement component and members of the collectin family are able to serve as opsonins to promote the uptake of apoptotic Jurkat T lymphocytes by E. histolytica (Teixeira et al., 2008). P21racA, a ras family protein, and phosphoinositide 3-kinase (PI3K) have been shown to inhibit the ingestion of both gram-negative Escherichia coli bacteria and human erythrocytes, suggesting that E. histolytica utilizes similar intracellular signaling pathways during the phagocytosis of these targets (Ghosh and Samuelson, 1997). More recently, phosphatidylinositol 3-phosphate (PI3P) and phosphatidylinositol (3,4,5)-trisphosphate (PIP3), both products of PI3K activity, have been shown to localize specifically to phagosomes associated with erythrocyte ingestion by trophozoites (Powell et al., 2006; Nakada-Tsukui et al., 2009; Byekova et al., 2010).
Several members of a novel family of surface-expressed, single-pass transmembrane kinases (TMK) in E. histolytica have been implicated in phagocytosis regulation. Discovered during analysis of the genome, the rather large TMK family is composed of 90 predicted members divided into nine major sub-families based on the similarity of their ectodomains to the Gal/GalNAc adherence lectin (Cheng et al., 2001; Beck et al., 2005; Loftus et al., 2005). For example PATMK, a B3 sub-family TMK, regulates the ingestion of human erythrocytes (Boettner et al., 2008), and TMK39, a C sub-family TMK, controls apoptotic Jurkat T lymphocyte phagocytosis (Buss et al., 2010). A number of TMKs, including TMK39, bear sequence similarity in their extracellular domains to metazoan scavenger receptors in macrophages such as SR-A1, MARCO, and EATER that recognize lipoproteins and negatively charged ligands on oxidized Low Density Lipoprotein (OxLDL), acetylated LDL (AcLDL), bacteria and apoptotic host cells (Goldstein et al., 1979; Krieger, 1997; Kocks et al., 2005; Murphy et al., 2005; Sateriale and Huston, 2011). TMK family members contain extracellular domains with a varying number of cysteine-rich repeat motifs that may mediate dimerization and/or ligand binding (Beck et al., 2005).
The overlapping specificity for a range of targets by metazoan scavenger receptors, as well as a large repertoire of such receptors in metazoan professional phagocytes, led us to postulate that E. histolytica may also utilize similar mechanisms for the ingestion of bacteria and modified LDL cholesterol, especially cell-surface proteins, that contribute to its virulence properties compared with non-pathogenic species such as Entamoeba dispar. Here we tested whether ingestion of E. coli and LDL particles utilized common pathways.
2. Materials and methods
2.1. Entamoeba histolytica trophozoite culture
Trophozoites of E. histolytica (strain HM1:IMSS) were cultured axenically in TYI-S-33 medium supplemented with 200 units/ml of penicillin and 200 μg/ml of streptomycin. Trophozoites were harvested during log-phase growth at 37°C by centrifugation at 233 g for 5 min following a brief incubation on ice.
2.2. Escherichia coli and Staphylococcus aureus strains and culture
Escherichia coli strain BM079 expressing GFP with plasmid pFNLTP-6-gro-gfp (Maier et al., 2004) was grown overnight at 37°C in Luria-Bertani (LB) medium under 50 μg/ml kanamycin selection. Staphylococcus aureus strain ALC1435 expressing GFP, a kind gift of Dr. Costi Sifri of the University of Virginia, USA, was grown overnight at 37°C in Tryptic Soy Broth (BD Biosciences, San Jose, CA, USA) supplemented with 5% NaCl and 10 μg/ml chloramphenicol.
2.3. Erythrocyte processing and calcium treatment
Erythrocytes from Rh+ human blood, type O were pelleted by centrifugation (2,000 g, 4°C, 5 min) and counted in a hemocytometer. Cells (2 × 108) were washed once in HEPES buffer (10 mM HEPES, 140 nM sodium chloride, pH 7.2) supplemented with 0.1% heat-inactivated BSA and resuspended to a concentration of 1 × 108 cells/ml in HEPES buffer with or without calcium (2.5 mM calcium chloride) and incubated at 37°C for 48 h. After 48 h erythrocytes were stained with PKH26 Red Fluorescent Cell Linker dye (PKH26-GL; Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol. Briefly, erythrocytes were harvested by centrifugation, washed once in 0.1% BSA-HEPES buffer, resuspended in 1 ml of Diluent C (kit component proprietary from Sigma Aldrich), and added to 1 ml of 4 μM PKH26 dye in Diluent C. Following incubation at 25°C for 5 min, stained erythrocytes were added to 2 ml of 1% BSA-HEPES buffer and washed three times in M199s medium (5.7 mM D-cysteine and 25 mM HEPES, pH 7.2) + 0.5% BSA.
2.4. Jurkat T lymphocyte culture
Jurkat T lymphocytes were grown in RPMI 1640 medium (Gibco, Grand Island, NY, USA) supplemented with 10% FBS, 250 Units/ml of penicillin and 250 μg/ml of streptomycin. Jurkat T lymphocytes were grown to a density of approximately 1 × 106 cells/ml prior to harvesting and stained similarly to erythrocytes as described in Section 2.3.
2.5. LDL particle ingestion
Fluorescent DiI(1,1′-dioctadecyl- 3,3,3′,3′-tetramethylindocarbocyanine perchlorate)-labeled DiI-AcLDL (Biomedical Technologies Inc., Stoughton, MA, USA) were added to trophozoites at 20 μg/ml for 30 min at 37°C in the presence of increasing concentrations of unlabeled AcLDL. In some experiments DiI-AcLDL particles were added to trophozoites at increasing doses. Trophozoites were then washed twice in ice-cold 110 mM D-galactose before fixation in 4% paraformaldehyde for 30 min at 37°C. Trophozoites were washed once in 50 mM NH4CL-PBS to quench paraformaldehyde and reduce auto-fluorescence. Cells were analyzed on a Becton Dickinson FACScalibur (excitation 488 nm) and fluorescence measured in the FL2 channel. Data was acquired using CellQuest Software (BD Biosciences) and analyzed by FlowJo Flow Cytometry Analysis software (Tree Star, Inc., Ashland, OR, USA).
2.6. Ingestion of bacteria, Jurkat T lymphocytes and erythrocytes
Trophozoites (2.5 × 105 per well) were allowed to adhere to the bottom of a 24 well plate under anaerobic conditions (GasPak EZ anaerobe Pouch System; BD Biosciences) in TYI-S-33 medium in the presence of 120 μg/ml AcLDL and washed two times with M199s medium. Equivalent numbers of bacteria (normally 8.75 × 106) were added to each well in the presence or absence of 120 μg/ml AcLDL in M199s medium. The plate was centrifuged at 120 g for 5 min to synchronize ingestion prior to incubation at 37°C for 20 min. Following the incubation, the medium was gently aspirated off the wells of the plate and cells washed three times in M199s medium. Cells were transferred to FACS tubes (BD Biosciences) and washed twice in 110 mM D-galactose in PBS before fixation in 4% para-formaldehyde in PBS for 20 min at 37°C. Finally, cells were washed once in 50 mM ammonium chloride and resuspended in PBS for analysis in the FL1 channel of a Becton Dickinson FACScaliber. Ingestion experiments with Jurkat T lymphocytes and erythrocytes were similarly conducted with the substitution of 5 × 105 Jurkat T lymphocytes or 1.25 × 106 erythrocytes, respectively, for bacteria and analysis in the FL2 channel of the Becton Dickinson FACScaliber. All binding experiments were conducted as described above with co-incubation of trophozoites and extracellular targets performed at 4°C to prevent ingestion.
2.7. Fluorescence confocal microscopy
Trophozoites were incubated with GFP-expressing E. coli bacteria or DiI-stained AcLDL particles for ingestion and adherence assays (see Sections 2.5 and 2.6) and 20 ul of a sample was placed on a cover-slip with Fluoromount-G mounting medium (Southern Biotech, Birmingham, AL, USA). Amebae were visualized with a 40X objective on a Zeiss LSM510 laser scanning confocal microscope under excitation at 488 nm with an argon laser (GFP visualization) or 543 nm with an HeNe laser (DiI visualization). Image slices of approximately 0.8 μm thickness through the focal plane of the cells were combined to assemble Z-stack images of adherent and ingested E. coli bacteria or AcLDL particles.
2.8. Statistical analyses
Bacterial ingestion experiments were repeated at least three times. Experiments were conducted in triplicate to calculate error bars. Prism 4.0c software by GraphPad was utilized to construct figures and calculate error bars. P values, determined by subjecting the data to unpaired Student’s t-test analysis, less than (<) .05 were considered significant.
3. Results
3.1. Ingestion of LDL particles
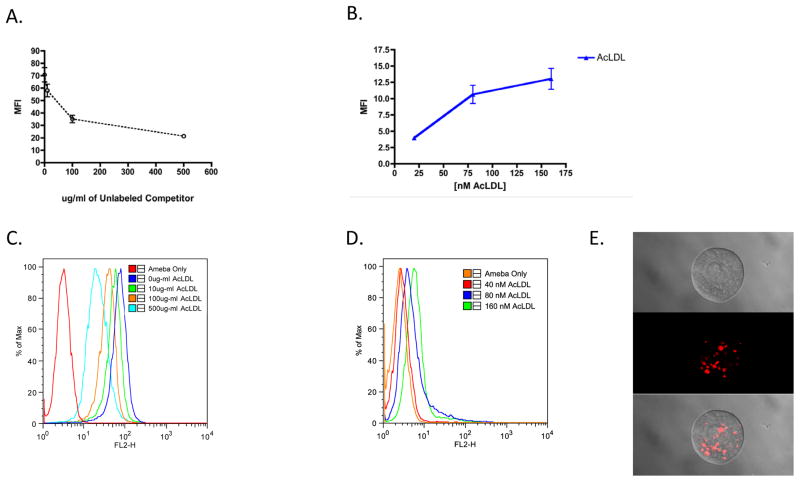
The uptake of modified LDL particles by trophozoites was performed using fluorescent DiI-labeled AcLDL. Following incubation of amebae with AcLDL at 37° C and washing in ice-cold 110 mM D-galactose to remove bound but uninternalized particles, the level of ingestion was measured by flow cytometry. Trophozoites were incubated with DiI-labeled AcLDL particles and increasing concentrations of unlabeled competitor up to 500 μg/ml (Fig. 1C). These concentrations were previously shown to saturate receptor-mediated LDL ingestion in macrophages (Buono et al., 2009). Uptake of AcLDL particles was competitively inhibited in a dose-dependent manner (Fig. 1A). Fluorescence microscopy images showed that AcLDL particles appeared in vesicular-like structures (Fig. 1E). The saturation of AcLDL ingestion was consistent with it being a receptor-mediated process. We also incubated trophozoites with DiI-AcLDL particles at 40, 80 and 160 nM concentrations (Fig. 1D). These concentrations were previously shown to saturate receptor-mediated LDL ingestion in macrophages (Buono et al., 2009). Uptake of both AcLDL and OxLDL particles was essentially saturated beyond 80 nM (Fig. 1B). Modified LDL particles appeared in a small number of membrane-bound structures (Fig. 1E).
Fig. 1.
Competitive and saturable uptake of acetylated low density lipoprotein particles (AcLDL). 1,1′-dioctadecyl- 3,3,3′,3′-tetramethylindocarbocyanine perchlorate labeled AcLDL particles (DiI AcLDL) (20 μg/ml) were incubated with trophozoites for 30 min at 37°C in the presence of increasing concentrations (0, 10, 100, 500 μg/ml) of unlabeled AcLDL and analyzed by flow cytometry (A). DiI AcLDL particles were also incubated with trophozoites at increasing concentrations until saturation was observed (B). Flow cytometry histograms are shown that represent the uptake of DiI AcLDL in the competition dose-response (C) and saturation (D) AcLDL uptake experiments. Confocal fluorescence microscopy of DiI AcLDL uptake is shown (E). MFI, Mean Fluoresent Intensity.
3.2. Ingestion and binding of bacteria, erythrocytes and Jurkat T lymphocytes
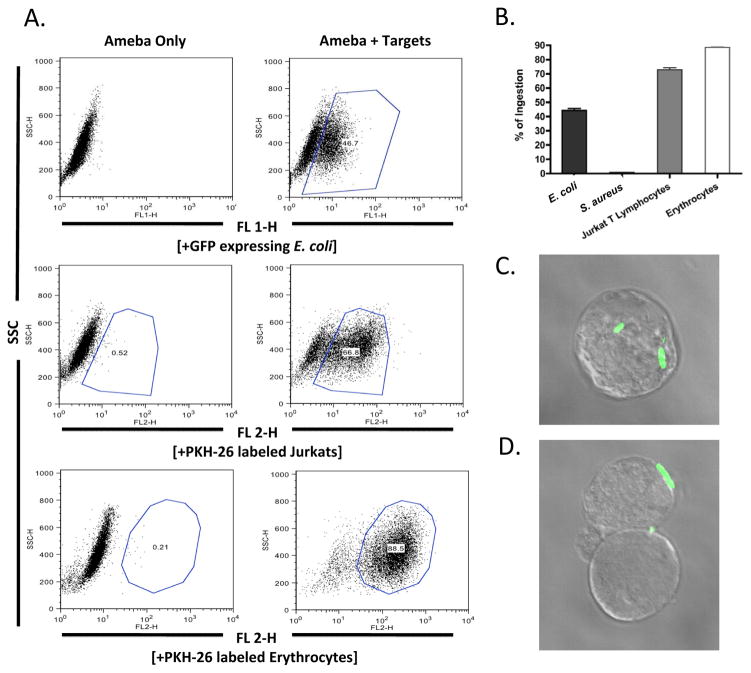
Flow cytometric techniques were used to quantify bacterial phagocytosis by trophozoites (Huston et al., 2003). Trophozoites were incubated with fluorescently-labeled bacteria, erythrocytes or Jurkat T lymphocytes. The percentage of amebae ingesting targets was measured by first gating trophozoite populations based on side scatter (y-axis) (Fig. 2A). Amebae readily phagocytosed GFP-expressing E. coli, PKH-26 membrane labeled human erythrocytes, and PKH-26 labeled apoptotic Jurkat T lymphocytes, but not GFP-expressing S. aureus (Fig. 2B). Confocal fluorescence microscopy confirmed the intracellular location of the bacteria ingested at 37° C (Fig. 2C, Supplementary Movie S1) versus the adherence of bacteria at 4° C (Fig. 2C, Supplementary Movie S2).
Fig. 2.
Flow cytometry analysis of bacterial ingestion by Entamoeba histolytica. Trophozoites of E. histolytica were incubated with various targets, washed with D-galactose to remove bound but not ingested targets, fixed in 4% paraformaldehyde and analyzed by flow cytometry (A). Trophozoites readily ingested Escherichia coli bacteria, human erythrocytes, and Jurkat T lymphoycytes but not Staphylococcus aureus bacteria (B). Fluorescence microscopy was utilized to visualize the ingestion (C) and adherence (D) of GFP expressing E. coli bacteria. Data is represented as the Mean Fluorescence Intensity percentage of trophozoites ingesting targets. Error bars shown represent the S.E.M. SSC, Side Scatter.
3.3. Ingestion and binding of E. coli bacteria in the presence of modified LDL cholesterol
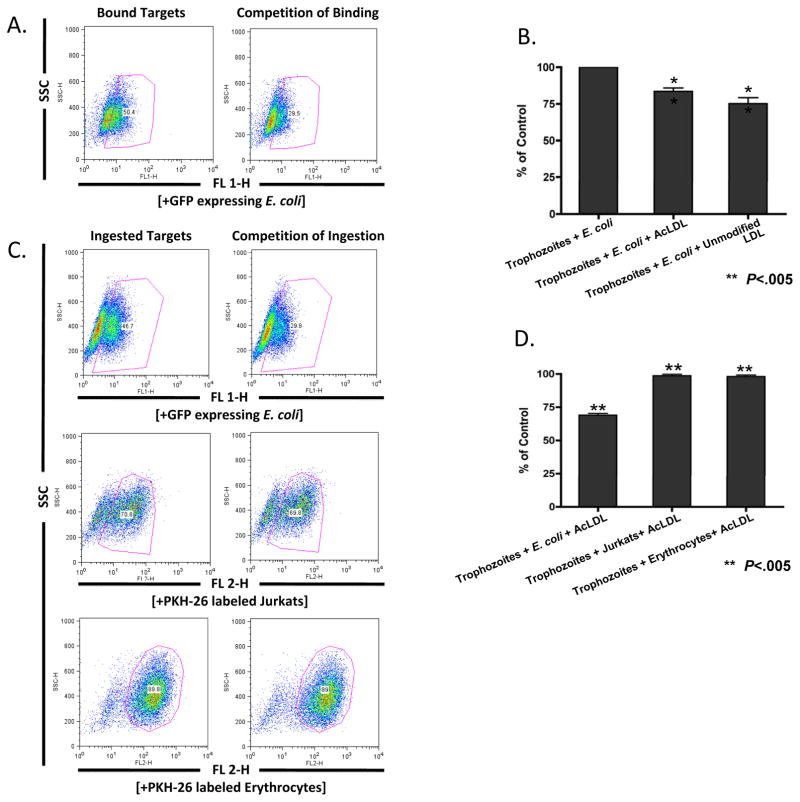
The impact of AcLDL particles on the ingestion of erythrocytes, bacteria and Jurkat T lymphocytes was determined. A modest inhibition of adherence to E. coli bacteria was observed, with 16 ± 2.2%, (P < 0.005) fewer trophozoites binding to E. coli bacteria in the presence of saturating amounts of AcLDL (Fig. 3B). AcLDL particles also inhibited the ingestion of E. coli bacteria (69 ±1.3%; P < 0.005) but not erythrocytes or apoptotic Jurkat T lymphocytes (Fig. 3D), consistent with the ingestion of these targets being via different uptake mechanisms.
Fig. 3.
Partial adherence and ingestion of Escherichia coli bacteria in the presence of acetylated low density lipoprotein particles (AcLDL). In the presence of 120 μg/ml acetylated LDL particles the binding (B) and ingestion (D) of E. coli bacteria but not ingestion of erythrocytes and Jurkat T lymphocytes was inhibited (D). Data is represented as the Mean Fluorescent Intensity percentage of control trophozoites ingesting targets. Error bars shown represent the S.E.M. and ** denotes P values of less than 0.005. (A) and (C) are representative flow cytometry plots of binding and ingestion, respectively. SSC, Side Scatter.
3.4. Ingestion and binding of bacteria by TMK39 dominant-negative transfected trophozoites
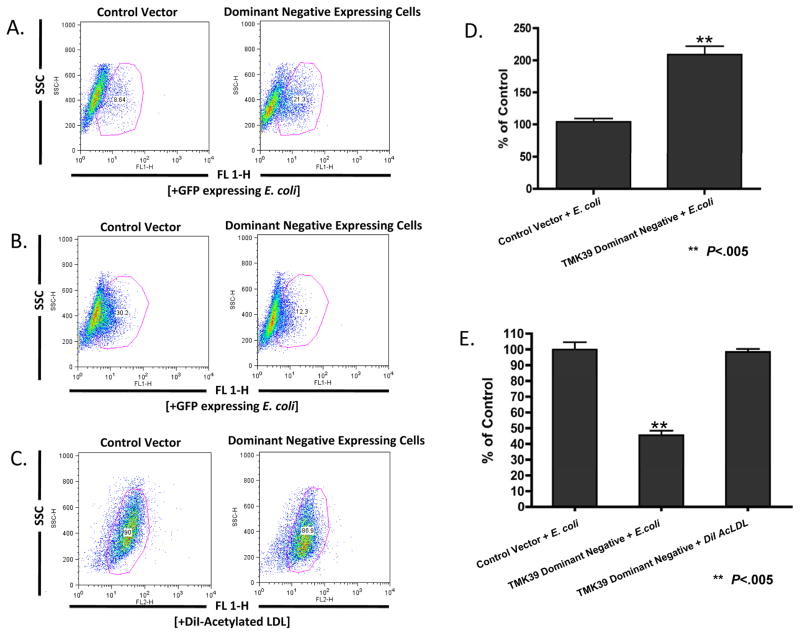
The role of TMK39, a member of the transmembrane kinase family, in the ingestion of bacteria was determined via the inducible expression of a TMK39 dominant-negative mutant. TMK39 has been implicated in the phagocytosis of apoptotic Jurkat T lymphocytes by studies with trophozoites expressing this truncated version of the protein lacking the putative intracellular kinase domain (Buss et al., 2010). This dominant-negative strain did not show differences in growth (Buss et al., 2010). We induced expression of this construct in amebic trophozoites using a tetO - tetR repressor system. Expression of the dominant-negative TMK39 increased E. coli bacteria adherence to the amebae (209% ± 13.1, P < 0.005) compared with that of empty-vector transfected control cells also under tetracycline selection (Fig. 4B). However, amebae expressing the dominant-negative version of TMK39 ingested E. coli bacteria at a rate of 45 ± 2% of control compared with empty-vector transfected trophozoites (Fig. 4D) (P < 0.005). Amebae expressing the dominant-negative version of TMK39 did not ingest DiI-AcLDL particles at a rate different from that of empty-vector control cells (Fig. 4D).
Fig. 4.
Ingestion and binding of extracellular targets by Entamoeba histolytica trophozoites expressing a dominant negative version of the cell surface protein TMK39. Trophozoites were incubated with GFP expressing Escherichia coli bacteria or 1,1′-dioctadecyl- 3,3,3′,3′-tetramethylindocarbocyanine perchlorate labeled AcLDL particles (DiI AcLDL) for 20 min at 4°C (D) or 37°C (E), washed with D-galactose (ingestion experiments only) and analyzed by flow cytometry. Control vector cells represent trophozoites transfected with an empty vector control. Data is represented as the Mean Fluorescent Intensity percentage of control cells. Error bars shown represent the S.E.M. and ** denotes P values of less than 0.005. (A), (B) and (C) are representative flow cytometry plots of bacterial binding, bacterial ingestion and AcLDL particle ingestion, respectively.
4. Discussion
The most important conclusion of this work is that E. coli ingestion by E. histolytica is regulated by TMK39 in a common pathway with LDL. This discovery is of importance due to the role of cholesterol and bacterial uptake in the pathogenesis of amebiasis (Laughlin et al., 2004; Mittal et al., 2008).
The inhibition of both the adherence and ingestion of E. coli bacteria by AcLDL implied that these targets partially occupied a common binding site on an amebic surface receptor. Given the redundancy seen by scavenger receptors in metazoan cells such as macrophages, it was not surprising that a novel receptor pathway for LDL particle recognition by E. histolytica also appeared to function in bacterial cell recognition. Many LDL receptors in metazoan cells can also recognize bacterial and/or apoptotic or necrotic host cells, allowing singular receptor pathways to perform multiple functions by the recognition of various targets (Murphy et al., 2005).
Surprisingly, the presence of AcLDL particles did not affect the ingestion of Jurkat T lymphocytes or erythrocytes as it did for E. coli bacteria, indicating the lack of complete redundancy in phagocytic pathways. We concluded that the previously documented receptor pathways for the ingestion of Jurkat T lymphocytes and erythrocytes are distinct from that demonstrated for AcLDL, or at least that ligand recognition differs significantly between these targets. Also, the level of inhibition of bacterial uptake by AcLDL in our experiments was not complete, which suggested that there are multiple pathways for the recognition of ligands on E. coli bacteria. It was possible that saturating concentrations of LDL particles affected the biophysical properties of the parasite membrane. However, the lack of an effect on Jurkat and erythrocyte uptake made this less likely.
Previously the Gal/GalNAc adherence lectin was shown to bind to the O55 E. coli strain (Padilla-Vaca et al., 1999). Our identification of a separate pathway for E. coli ingestion that also controls LDL particle uptake is indicative of the redundancy of ligand recognition on gram-negative bacteria by various receptor pathways. It was of interest that the gram-positive bacterium S. aureus was not ingested under the experimental conditions used, as previously described (Bracha et al., 1982).
The discovery of a novel family of putative transmembrane kinases related to the Igl subunit of the Gal/GalNAc lectin in the genome of E. histolytica has promised a more complete understanding of the mechanisms of ingestion by this parasite. An important role for PATMK and TMK39 in the ingestion of erythrocytes (Boettner et al., 2008) and apoptotic Jurkat T lymphocytes (Buss et al., 2010), respectively, has previously been demonstrated. The current work demonstrated that TMK39 occupied a key role in the specific recognition of bacteria. Two experiments supported this conclusion: first, the increased binding of bacteria to trophozoites over-expressing the ectodomain segment of TMK39; and second, that trophozoites transfected with this construct were defective in the internalization of bacteria, implying that the truncated intracellular domain was important for signaling during the ingestion of E. coli bacteria. Aside from PATMK and TMK39, the SREHP surface protein of E. histolytica has previously been shown to modulate the ingestion of apoptotic Jurkat T lymphocytes and may serve as a receptor or co-receptor for the phagocytosis of dying host cells (Teixeira and Huston, 2008).
The existence of a saturable and likely receptor-mediated process for the uptake of acetylated forms of LDL particles is an additional observation from this work. The saturability of uptake of AcLDL particles was as expected, in direct contrast to the linear rate of dextran pinocytosis (data not shown) (Welter et al., 2006). The presence in amebic trophozoites of a saturable LDL particle receptor that allows for lipoprotein and cholesterol scavenging from the host has precedence in the evidence of such receptors in Schistosoma and Trichomonas spp. as well as the appearance of receptor-mediated uptake of human LDL by Giardia spp. (Peterson and Alderete, 1984; Rumjanek et al., 1988; Rogers et al., 1989; Lujan et al., 1996). Entamoeba histolytica has a limited capacity for the de novo synthesis of cholesterol and its virulence is increased following incubation in cholesterol (Snyder and Meleney, 1943; Sharma, 1959; Das et al., 2002). Importantly, the parasite’s requirement for cholesterol has been shown to be satisfied by that packaged in lipoprotein form (Mata-Cárdenas et al., 2000). Such a scavenging mechanism may allow it to survive in various environments within the host. Interestingly, TMK39 dominant-negative trophozoites did not show a difference in the uptake of AcLDL particles, suggesting that TMK39 may function in a signaling pathway for bacterial ingestion that is distinct from that recognizing LDL particles.
In conclusion, this work advances the understanding of bacterial and lipoprotein-particle phagocytosis by E. histolytica. A novel pathway for bacterial ingestion as well as a likely role for receptor-mediated cholesterol uptake by the parasite was identified. Finally, TMK39 was demonstrated to occupy a key regulatory role in the regulation of E. coli adherence and ingestion. Future insights into the pathogenesis of amebiasis may be garnered by the identification of amebic receptors for ingestion and their connection to the signaling pathways governing endocytosis.
Supplementary Material
Supplementary Movie S1. Z-stack movie of the ingestion of Escherichia coli bacteria by Entamoeba histolytica trophozoites. Trophozoites were incubated with GFP-expressing bacteria for 20 min at 37°C before washing in 110 mM D-Galactose, fixation in 4% paraformaldehyde and mounting on slides in Fluoromount-G, then imaged on a Zeiss LSM510 Confocal Microscope. Images were analyzed with Zeiss Zen 2009 LE software.
Supplementary Movie S2. Z-stack movie of the adherence of Escherichia coli bacteria to Entamoeba histolytica trophozoites. Trophozoites were incubated with GFP-expressing bacteria for 20 min at 4°C before fixation in 4% paraformaldehyde, and mounting on slides in Fluoromount-G, then imaged on a Zeiss LSM510 Confocal Microscope. Images were analyzed with Zeiss Zen 2009 LE software.
Highlights.
Entamoeba histolytica ingests low density lipoprotein(LDL) particles in a saturable manner.
LDL particles are a competitive ligand for the ingestion of Escherichia coli bacteria.
The adherence and ingestion of E. coli bacteria is regulated by transmembrane kinase 39.
Acknowledgments
Supported by National Institutes of Health (NIH), USA, grant AI 26649 to WAP.
Footnotes
Note: Supplementary data associated with this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck DL, Boettner DR, Dragulev B, Ready K, Nozaki T, Petri WA. Identification and gene expression analysis of a large family of transmembrane kinases related to the Gal/GalNAc lectin in Entamoeba histolytica. Eukaryotic Cell. 2005;4:722–732. doi: 10.1128/EC.4.4.722-732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner DR, Huston CD, Linford AS, Buss SN, Houpt E, Sherman NE, Petri WA. Entamoeba histolytica phagocytosis of human erythrocytes involves PATMK, a member of the transmembrane kinase family. PLoS Pathog. 2008;4:e8. doi: 10.1371/journal.ppat.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettner DR, Huston CD, Sullivan JA, Petri WA. Entamoeba histolytica and Entamoeba dispar utilize externalized phosphatidylserine for recognition and phagocytosis of erythrocytes. Infect Immun. 2005;73:3422–3430. doi: 10.1128/IAI.73.6.3422-3430.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha R, Kobiler D, Mirelman D. Attachment and ingestion of bacteria by trophozoites of Entamoeba histolytica. Infect Immun. 1982;36:396–406. doi: 10.1128/iai.36.1.396-406.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha R, Mirelman D. Virulence of Entamoeba histolytica trophozoites. Effects of bacteria, microaerobic conditions, and metronidazole. J Exp Med. 1984;160:353–368. doi: 10.1084/jem.160.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono C, Anzinger JJ, Amar M, Kruth HS. Fluorescent pegylated nanoparticles demonstrate fluid-phase pinocytosis by macrophages in mouse atherosclerotic lesions. J Clin Invest. 2009;119:1373–1381. doi: 10.1172/JCI35548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss SN, Hamano S, Vidrich A, Evans C, Zhang Y, Crasta OR, Sobral BW, Gilchrist CA, Petri WA. Members of the Entamoeba histolytica transmembrane kinase family play non-redundant roles in growth and phagocytosis. Int J Parasitol. 2010;40:833–43. doi: 10.1016/j.ijpara.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byekova YA, Powell RR, Welter BH, Temesvari LA. Localization of phosphatidylinositol (3,4,5)-trisphosphate to phagosomes in Entamoeba histolytica achieved using glutathione S-transferase- and green fluorescent protein-tagged lipid biosensors. Infect Immun. 2010;78:125–137. doi: 10.1128/IAI.00719-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng XJ, Hughes MA, Huston CD, Loftus B, Gilchrist CA, Lockhart LA, Ghosh S, Miller-Sims V, Mann BJ, Petri WA, Tachibana H. Intermediate subunit of the Gal/GalNAc lectin of Entamoeba histolytica is a member of a gene family containing multiple CXXC sequence motifs. Infect Immun. 2001;69:5892–5898. doi: 10.1128/IAI.69.9.5892-5898.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy NC, Petri WA. Mechanisms of adherence, cytotoxicity and phagocytosis modulate the pathogenesis of Entamoeba histolytica. Future Microbiol. 2011;6:1501–1519. doi: 10.2217/fmb.11.120. [DOI] [PubMed] [Google Scholar]
- Das S, Stevens T, Castillo C, Villasenõr A, Arredondo H, Reddy K. Lipid metabolism in mucous-dwelling amitochondriate protozoa. Int J Parasitol. 2002;32:655–675. doi: 10.1016/s0020-7519(02)00006-1. [DOI] [PubMed] [Google Scholar]
- Galván-Moroyoqui JM, Del Carmen Domínguez-Robles M, Franco E, Meza I. The interplay between Entamoeba and enteropathogenic bacteria modulates epithelial cell damage. PLoS Negl Trop Dis. 2008;2:e266. doi: 10.1371/journal.pntd.0000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SK, Samuelson J. Involvement of p21racA, phosphoinositide 3-kinase, and vacuolar ATPase in phagocytosis of bacteria and erythrocytes by Entamoeba histolytica: suggestive evidence for coincidental evolution of amebic invasiveness. Infect Immun. 1997;65:4243–4249. doi: 10.1128/iai.65.10.4243-4249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Nat Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA. Amebiasis. N Engl J Med. 2003;348:1565–1573. doi: 10.1056/NEJMra022710. [DOI] [PubMed] [Google Scholar]
- Haque R, Mondal D, Duggal P, Kabir M, Roy S, Farr BM, Sack RB, Petri WA. Entamoeba histolytica Infection in Children and Protection from Subsequent Amebiasis. Infect Immun. 2006;74:904–909. doi: 10.1128/IAI.74.2.904-909.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston CD, Houpt ER, Mann BJ, Hahn CS, Petri WA. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell Microbiol. 2000;2:617–625. doi: 10.1046/j.1462-5822.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- Huston CD, Boettner DR, Miller-Sims V, Petri WA. Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica. Infect Immun. 2003;71:964–972. doi: 10.1128/IAI.71.2.964-972.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz U, Ankri S, Stolarsky T, Nuchamowitz Y, Mirelman D. Entamoeba histolytica expressing a dominant negative N-truncated light subunit of its gal-lectin are less virulent. Mol Biol Cell. 2002;13:4256–4265. doi: 10.1091/mbc.E02-06-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C, Cho JH, Nehme N, Ulvila J, Pearson AM, Meister M, Strom C, Conto SL, Hetru C, Stuart LM. Eater, a Transmembrane Protein Mediating Phagocytosis of Bacterial Pathogens in Drosophila. Cell. 2005;123:335–346. doi: 10.1016/j.cell.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Krieger M. The other side of scavenger receptors: pattern recognition for host defense. Curr Opin Lipidol. 1997;8:275–280. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Laughlin RC, McGugan GC, Powell RR, Welter BH, Temesvari LA. Involvement of raft-like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion. Infect Immun. 2004;72:5349–5357. doi: 10.1128/IAI.72.9.5349-5357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus B, Anderson I, Davies R, Alsmark UCM, Samuelson J. The genome of the protist parasite Entamoeba histolytica. Nature. 2005;433:865–868. doi: 10.1038/nature03291. [DOI] [PubMed] [Google Scholar]
- Lujan HD, Mowatt MR, Nash TE. Lipid requirements and lipid uptake by Giardia lamblia trophozoites in culture. J Eukaryot Microbiol. 1996;43:237–242. doi: 10.1111/j.1550-7408.1996.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Maier TM, Havig A, Casey M, Nano FE, Frank DW, Zahrt TC. Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl Environ Microbiol. 2004;70:7511–7519. doi: 10.1128/AEM.70.12.7511-7519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Cárdenas BD, Vargas-Villarreal J, Martínez-Rodríguez HG, Castro-Garza J, González-Garza MT, Said-Fernández S. Auxotrophy to lipoproteins of Entamoeba histolytica cultivated under axenic conditions. Parasitol Res. 2000;86:1018–1021. doi: 10.1007/pl00008522. [DOI] [PubMed] [Google Scholar]
- Mirelman D, Feingold C, Wexler A, Bracha R. Interactions between Entamoeba histolytica, bacteria and intestinal cells. Ciba Found Symp. 1983;99:2–30. doi: 10.1002/9780470720806.ch2. [DOI] [PubMed] [Google Scholar]
- Mitra BN, Yasuda T, Kobayashi S, Saito-Nakano Y, Nozaki T. Differences in morphology of phagosomes and kinetics of acidification and degradation in phagosomes between the pathogenic Entamoeba histolytica and the non-pathogenic Entamoeba dispar. Cell Motil Cytoskeleton. 2005;62:84–99. doi: 10.1002/cm.20087. [DOI] [PubMed] [Google Scholar]
- Mittal K, Welter BH, Temesvari LA. Entamoeba histolytica: lipid rafts are involved in adhesion of trophozoites to host extracellular matrix components. Exp Parasitol. 2008;120:127–134. doi: 10.1016/j.exppara.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S. Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis. 2005;182:1–15. doi: 10.1016/j.atherosclerosis.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Nakada-Tsukui K, Okada H, Mitra BN, Nozaki T. Phosphatidylinositol-phosphates mediate cytoskeletal reorganization during phagocytosis via a unique modular protein consisting of RhoGEF/DH and FYVE domains in the parasitic protozoon Entamoeba histolytica. Cell Microbiol. 2009;11:1471–1491. doi: 10.1111/j.1462-5822.2009.01341.x. [DOI] [PubMed] [Google Scholar]
- Orozco E, Guarneros G, Martinez-Palomo A, Sánchez T. Entamoeba histolytica Phagocytosis as a virulence factor. J Exp Med. 1983;158:1511–1521. doi: 10.1084/jem.158.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Vaca F, Ankri S, Bracha R, Koole LA, Mirelman D. Down regulation of Entamoeba histolytica virulence by monoxenic cultivation with Escherichia coli O55 is related to a decrease in expression of the light (35-kilodalton) subunit of the Gal/GalNAc lectin. Infect Immun. 1999;67:2096–2102. doi: 10.1128/iai.67.5.2096-2102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson KM, Alderete JF. Selective acquisition of plasma proteins by Trichomonas vaginalis and human lipoproteins as a growth requirement for this species. Mol Biochem Parasitol. 1984;12:37–48. doi: 10.1016/0166-6851(84)90042-2. [DOI] [PubMed] [Google Scholar]
- Petri WA, Singh U. Diagnosis and management of amebiasis. Clin Infect Dis. 1999;29:1117–1125. doi: 10.1086/313493. [DOI] [PubMed] [Google Scholar]
- Petri WA, Smith RD, Schlesinger PH, Murphy CF, Ravdin JI. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Invest. 1987;80:1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petri WA. Pathogenesis of amebiasis. Curr Opin Microbiol. 2002;5:443–447. doi: 10.1016/s1369-5274(02)00335-1. [DOI] [PubMed] [Google Scholar]
- Pimenta PFP, Diamond LS, Mirelman D. Entamoeba histolytica Schaudinn, 1903 and Entamoeba dispar Brumpt, 1925: differences in their cell surfaces and in the bacteria-containing vacuoles. J Eukaryot Microbiol. 2002;49:209–219. doi: 10.1111/j.1550-7408.2002.tb00525.x. [DOI] [PubMed] [Google Scholar]
- Powell RR, Welter BH, Hwu R, Bowersox B, Attaway C, Temesvari LA. Entamoeba histolytica: FYVE-finger domains, phosphatidylinositol 3-phosphate biosensors, associate with phagosomes but not fluid filled endosomes. Exp Parasitol. 2006;112:221–231. doi: 10.1016/j.exppara.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Ravdin JI, Guerrant RL. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica Study with mammalian tissue culture cells and human erythrocytes. J Clin Invest. 1981;68:1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin JI, editor. Amebiasis: Human Infection by Entamoeba histolytica. John Wiley and Sons, Inc; New York: 1988. [Google Scholar]
- Rogers MV, Henkle KJ, Fidge NH, Mitchell GF. Identification of a multispecific lipoprotein receptor in adult Schistosoma japonicum by ligand blotting analyses. Mol Biochem Parasitol. 1989;35:79–88. doi: 10.1016/0166-6851(89)90145-x. [DOI] [PubMed] [Google Scholar]
- Rumjanek FD, Campos EG, Afonso LC. Evidence for the occurrence of LDL receptors in extracts of schistosomula of Schistosoma mansoni. Mol Biochem Parasitol. 1988;28:145–152. doi: 10.1016/0166-6851(88)90062-x. [DOI] [PubMed] [Google Scholar]
- Sateriale A, Huston CD. A Sequential Model of Host Cell Killing and Phagocytosis by Entamoeba histolytica. J Parasitol Res. 2011;2011:926706. doi: 10.1155/2011/926706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. Effect of cholesterol on the growth and virulence of Entamoeba histolytica. Trans Royal Soc Trop Med Hyg. 1959;53:278–281. doi: 10.1016/0035-9203(59)90008-2. [DOI] [PubMed] [Google Scholar]
- Snyder T, Meleney H. Anaerobiosis and Cholesterol as Growth Requirements of Entamoeba histolytica. J Parasitol. 1943;29:278–284. [Google Scholar]
- Teixeira JE, Huston CD. Participation of the serine-rich Entamoeba histolytica protein in amebic phagocytosis of apoptotic host cells. Infect Immun. 2008;76:959–966. doi: 10.1128/IAI.01455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira JE, Heron BT, Huston CD. C1q- and collectin-dependent phagocytosis of apoptotic host cells by the intestinal protozoan Entamoeba histolytica. J Infect Dis. 2008;198:1062–1070. doi: 10.1086/591628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trissl D, Martínez-Palomo A, de la Torre M, de la Hoz R, Pérez de Suárez E. Surface properties of Entamoeba: increased rates of human erythrocyte phagocytosis in pathogenic strains. J Exp Med. 1978;148:1137–1143. doi: 10.1084/jem.148.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welter BH, Powell RR, Laughlin RC, McGugan GC, Bonner M, King A, Temesvari LA. Entamoeba histolytica: comparison of the role of receptors and filamentous actin among various endocytic processes. Exp Parasitol. 2006;113:91–99. doi: 10.1016/j.exppara.2005.12.012. [DOI] [PubMed] [Google Scholar]
- WHO. WHO/PAHO/UNESCO report. A consultation with experts on amoebiasis Mexico City, Mexico 28–29 January, 1997. Epidemiol Bull. 1997;18:13–14. [PubMed] [Google Scholar]
- Wittner M, Rosenbaum RM. Role of bacteria in modifying virulence of Entamoeba histolytica Studies of amebae from axenic cultures. Am J Trop Med Hyg. 1970;19:755–761. doi: 10.4269/ajtmh.1970.19.755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie S1. Z-stack movie of the ingestion of Escherichia coli bacteria by Entamoeba histolytica trophozoites. Trophozoites were incubated with GFP-expressing bacteria for 20 min at 37°C before washing in 110 mM D-Galactose, fixation in 4% paraformaldehyde and mounting on slides in Fluoromount-G, then imaged on a Zeiss LSM510 Confocal Microscope. Images were analyzed with Zeiss Zen 2009 LE software.
Supplementary Movie S2. Z-stack movie of the adherence of Escherichia coli bacteria to Entamoeba histolytica trophozoites. Trophozoites were incubated with GFP-expressing bacteria for 20 min at 4°C before fixation in 4% paraformaldehyde, and mounting on slides in Fluoromount-G, then imaged on a Zeiss LSM510 Confocal Microscope. Images were analyzed with Zeiss Zen 2009 LE software.