Abstract
Signaling through the innate immune system can promote or suppress allergic sensitization. TLR9 has modulatory effects on the mucosal immune system, and we hypothesized that TLR9 would influence susceptibility to allergic sensitization to foods. We observed that TLR9−/− mice were resistant to peanut-induced anaphylaxis. This was associated with a significant impairment in total IgE and peanut-specific IgE and IgA, but not IgG1 or Th2 cytokine production. TLR9−/− mice had reduced development of Peyer’s patches, but resistance to sensitization was not restricted to oral routes. Rag1-deficient mice were reconstituted with TLR9+/+ or −/− B cells plus CD4+ T cells. TLR9−/− B cells regained the ability to produce IgE in the presence of a wild-type environment. Our results demonstrate that TLR9 on an unknown cell type is required for the development of IgE-producing B cells, and we conclude that TLR9 signaling indirectly shapes the immune response for optimal IgE production.
INTRODUCTION
The role of the innate immune system in the development of inappropriate allergic sensitization to innocuous antigens has been of great interest to the field of allergy and immunology. The idea that microbial products promote a regulatory tone in the immune system-and therefore a reduction in exposure to microbial products promotes allergic disease- is the central idea behind the hygiene hypothesis. Alternatively, microbial products such as bacterial toxins (1, 2) or lipopolysaccharide (3) can have adjuvant activity that supports allergic sensitization.
There is interest in harnessing the innate immune system therapeutically for the purpose of reprogramming an immune response from a Th2-biased response to a regulatory or Th1-biased response. Several microbial-based approaches have been tested at the pre-clinical level for the treatment of food allergy. The use of heat-killed listeria as an adjuvant together with peanut allergens, either unmodified or modified to reduce IgE binding, resulted in significant reductions in peanut-induced symptoms in mice and dogs (4, 5). Heat-killed E. coli containing modified peanut allergens was also shown to tolerize mice to peanut when administered by the rectal route (6). TLR9 ligands (CpG oligonucleotides) given at the time of sensitization to peanut could suppress sensitization to peanut (7, 8) in mice. Furthermore, TLR9 agonists coupled to ragweed have been used with some success in human trials for allergic rhinitis. House dust mite has also been conjugated with CpG in virus-like particles for the purpose of immunotherapy, with promising preliminary results (9). TLR9 ligands are potent Th1 adjuvants and can be used at mucosal sites to prime for humoral and cellular immune responses (10, 11). A major source of endogenous TLR9 ligands is the intestinal flora, and a loss of constitutive signaling through the flora in TLR9-deficient mice has been shown to have significant effects on the responsiveness of the mucosal immune system. TLR9−/− mice have a decreased number of effector cells producing IFN-γ and IL-17 in the small intestine, and an increased number of Foxp3+ regulatory T cells (12), suggesting that TLR9 ligands function as endogenous adjuvants. Others have found that TLR9 influences the colonic epithelium leading to a suppressed responsiveness to inflammatory signaling, and they observed that TLR9−/− mice exhibit an enhanced susceptibility to experimental colitis (13). There is a window of responsiveness of the mucosal immune system to CpG oligonucleotides in the neonatal period (14), indicating that early exposure to this class of TLR ligand may profoundly influence the immune tone of the small intestine in adulthood. This idea is supported by the recent finding of a gene-environment interaction between TLR9 polymorphisms and breast-feeding in the development of sensitization to foods (15). We hypothesized that constitutive signaling to the mucosal immune system through TLR9 could influence the susceptibility to allergic sensitization to foods. We tested this hypothesis through the use of experimental models of peanut-induced sensitization and anaphylaxis in mice deficient in TLR9, and found that TLR9 was required to indirectly promote the generation of IgE and IgA from B cells.
RESULTS
TLR9-deficient mice have reduced susceptibility to peanut-induced sensitization and anaphylaxis
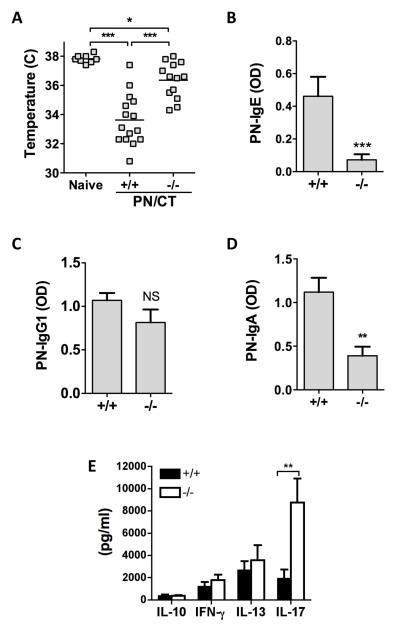
Mice were orally sensitized to peanut by repeated feeding together with the mucosal adjuvant cholera toxin (CT). Mice on the C57BL/6 background strain do not respond to oral peanut challenge with anaphylaxis, but develop a robust peanut-specific IgE response and will respond to intraperitoneal (i.p.) peanut challenge. This model of sensitization to peanut utilizing i.p. re-challenge has been shown to be primarily mast cell and IgE-dependent, with minor but detectable contribution from macrophages and IgG (16, 17). C57BL/6 TLR9+/+ mice sensitized and challenged with peanut extract underwent systemic anaphylaxis, as measured by a significant drop in core body temperature (Figure 1A). The severity of anaphylaxis was significantly less but not absent in TLR9−/− mice (Mean temperature of 36.4 °C in TLR9−/− mice compared to 33.6 °C in TLR9+/+ mice and 37.8 °C in naïve mice). This reduced susceptibility to anaphylaxis in TLR9−/− was associated with significantly lower peanut-specific IgE levels in serum obtained prior to challenge as compared to wild-type controls (Figure 1B). TLR9-deficiency was also associated with a significant reduction in peanut-specific IgA responses in serum (Figure 1D), while peanut-specific IgG1 responses were similar in TLR9+/+ and −/− mice (Figure 1C). Sensitization to peanut was associated with a mixed cytokine response from allergen re-stimulated spleen cells. Similar levels of IL-13, IL-10, and IFN-γ were observed in TLR9−/− mice, while IL-17 levels were significantly elevated in orally sensitized TLR9−/− mice (Figure 1E). IL-4 was routinely below the level of detection.
Figure 1.
Impact of TLR9 deficiency on peanut-induced anaphylaxis and antibody and cytokine responses. TLR9 +/+ or −/− mice were orally sensitized to peanut with cholera toxin (PN/CT), followed by intraperitoneal challenge with crude peanut extract. (A) Body temperature measured 30 min after peanut challenge. Peanut (PN)-specific IgE (B), IgG1 (C) and IgA (D) as measured by ELISA in serum obtained 1-2 days prior to challenge. Cytokine secretion (E) measured after re-stimulation of spleen cells with peanut extract. Spleen cells were harvested immediately after allergen challenge. A is combined data from 2 independent experiments. B-E are from one representative experiment of 2, with n = 10 (+/+) and 8 (−/−). *** p < 0.001, ** p < 0.01, * p < 0.05 comparing the indicated groups.
Impact of TLR9-deficiency on IgE-expressing B cells
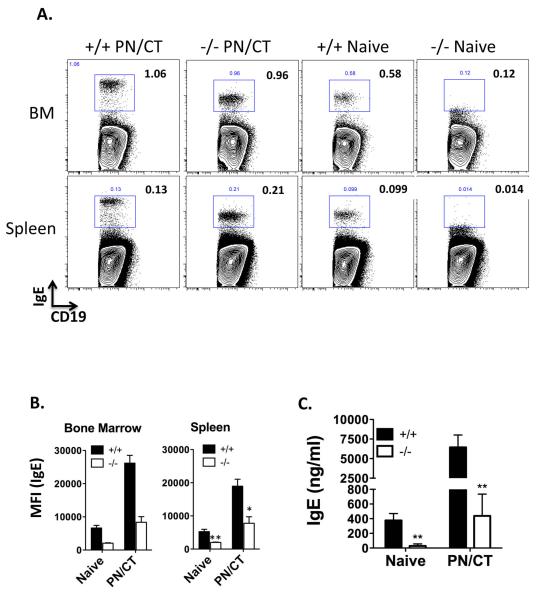
To investigate the source of IgE production in peanut-sensitized mice, we performed flow cytometry to detect IgE-expressing cells. We examined cells in the bone marrow, spleen, mesenteric lymph node (MLN), and Peyer’s Patch (PP). A significant population of IgE+ cells with B cell markers (CD19, B220, but not the plasma cell marker CD138) was found in spleen and bone marrow, but not MLN or PP (Supplementary Figure 1). Mature IgE transcripts in the spleen and bone marrow were confirmed by RT-PCR (data not shown). Permeabilization of the cells was required for detection of these cells, indicating minimal surface IgE expression. The median fluorescence intensity of IgE expression was elevated after sensitization. TLR9−/− mice had a marked reduction in the fluorescence intensity of IgE expressed by the B cells, even in the naïve state (Figure 2). This was confirmed by measuring total serum IgE in naïve and sensitized TLR9+/+ and −/− mice, which demonstrated a significant reduction in total IgE at baseline and after sensitization in TLR9−/− mice. In contrast, total IgA as measured in either serum or feces at baseline was not affected by TLR9-deficiency.
Figure 2.
Detection of IgE+ B cells. Mice were left naïve as control, or sensitized with PN/CT for 6 weeks. Sensitization was verified by measurement of peanut-specific IgE in serum. Mice were not allergen challenged prior to collection of tissues. (A) Representative staining in bone marrow (BM) and spleen cells. Live CD19+ cells were gated, and plots show IgE versus CD19 in TLR9+/+ and TLR9−/− mice orally sensitized to peanut (PN/CT) or naive. (B) Median fluorescence intensity (MFI) of IgE in IgE+ B cells gated as above. N = 3/group (spleen) and 2/group (BM). *p < 0.05 (C) Total IgE measured by ELISA in serum of naïve or peanut-sensitized (PN/CT) TLR9+/+ or −/− mice. ** p < 0.01, n = 8-15 mice/group.
Mast cells from TLR9−/− mice become functional upon passive sensitization with IgE
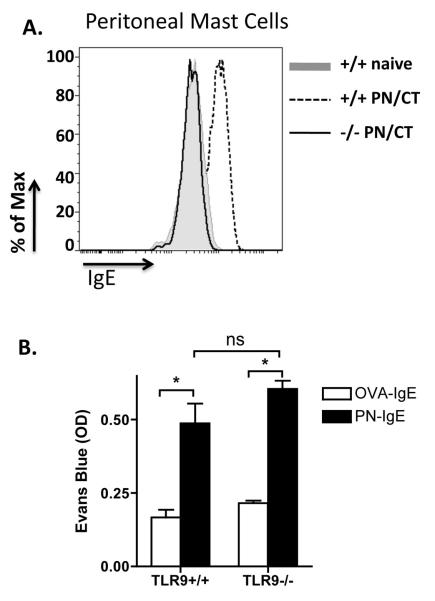
Peanut-induced anaphylaxis is dependent primarily on activation of mast cells by cross-linking IgE, although minor contributions from macrophages and IgG have also been demonstrated (16, 17). To assess the function of mast cells in TLR9−/− mice, we first examined peritoneal mast cells by flow cytometry. The number of c-kit+ IgE+ cells in the peritoneal cavity was not significantly different in TLR9+/+ and −/− mice (data not shown), however as shown in Figure 3A, there was a marked reduction in the level of IgE on the surface of peritoneal mast cells from sensitized TLR9−/− mice as compared with TLR9+/+ mice. To test the function of mast cells in TLR9+/+ and −/− mice, mice were passively sensitized with serum containing high levels of peanut-specific IgE by injection into the ear pinna. As control, the other ear was injected with OVA-IgE-positive serum. The next day, mice were injected intravenously with evans blue and peanut extract, and the extent of extravasation in the ear was measured (Figure 3B). TLR9+/+ and −/− mice responded to peanut challenge with a similar level of extravasation in the ear passively sensitized with peanut-IgE+ serum, indicating that mast cells of TLR9−/− mice are functional and the major defect is at the level of IgE production.
Figure 3.
Impact of TLR9 deficiency on mast cells. (A) Peritoneal cells were harvested from naïve or orally peanut-sensitized (PN/CT) TLR9+/+ or −/− mice. Peritoneal cells were harvested from sensitized un-challenged mice. Mast cells were gated based on c-kit and IgE staining, and the level of IgE positivity was compared in naïve and sensitized +/+ and −/− mice. (B) Naïve TLR9+/+ or −/− mice were passively sensitized in the ear with serum from peanut-sensitized mice or OVA-sensitized mice as controls. The next day, mice were intravenously injected with Evans Blue and peanut extract, and extravasation in each ear was quantified by spectrophotometry. * p < 0.05, ns = not significant. (n = 5/group).
TLR9 influences Peyer’s patch development
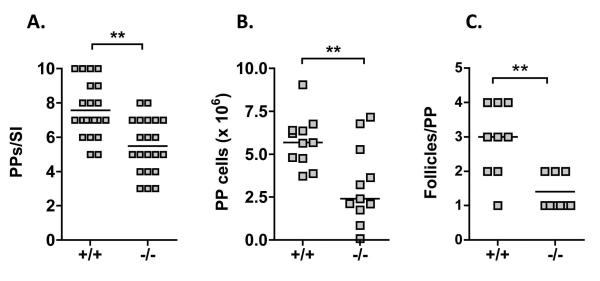
It has been described that TLR9−/− mice have elevated numbers of regulatory T cells in their small intestinal lamina propria and within Peyer’s patches (PPs) (12) that could potentially explain the suppressed response to oral sensitization. In addition, the defect in IgA suggested a mucosal immunoglobulin production defect. In our colony of co-housed TLR9+/+ and −/− mice, we did not observe any difference in the number of intestinal CD4+ Foxp3+ cells (in lamina propria, MLN, or PP) (Figure S2), nor did we observe any quantitative difference in CD4 or CD8 T cells, B cells, or dendritic cells (Figure S2). However, we did observe that the PPs were reduced in size in TLR9−/− mice. Quantification of the number of grossly visible PPs per small intestine and the total yield of cells from the PPs per small intestine showed that there was a significant reduction in number of visible PPs as well as the size of PPs in TLR9−/− mice. In addition, histological examination showed that the number of follicles per patch was also reduced in TLR9−/− mice (Figure 4).
Figure 4.
Impact of TLR9 deficiency on Peyer’s patches. The entire small intestine from naïve TLR9+/+ or −/− mice was removed and the total number of visible Peyer’s patches per mouse was counted (A). Cells were isolated by collagenase digestion and the yield of cells per mouse was determined (B). Segments of intestine containing a Peyer’s patch were fixed, embedded, and cross-sections stained with hematoxylin and eosin. The number of follicles per Peyer’s patch was counted (C). ** p < 0.01.
Resistance to sensitization to peanut in TLR9−/− mice is not restricted to the gastrointestinal tract
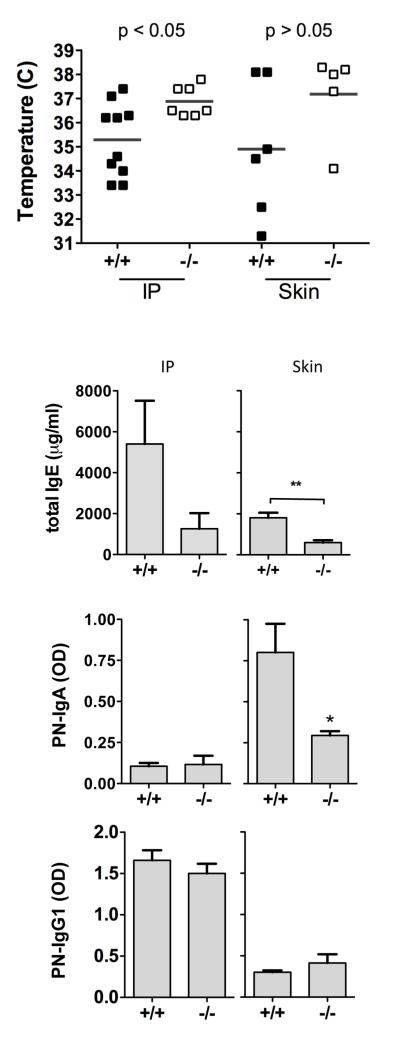
It has been described that crude peanut contains insoluble particles that are readily taken up by M cells overlying the Peyer’s patch (18), and we have previously observed that site of uptake in the gastrointestinal tract plays a significant role in the allergic response to food allergens (19). To determine if resistance to sensitization was due to the reduced Peyer’s patch development we sensitized mice to peanut by non-oral routes, either intraperitoneal injection with alum adjuvant, or by the epicutaneous route by applying peanut and cholera toxin topically to the skin (20). As shown in Figure 5, TLR9−/− mice were resistant to peanut-induced anaphylaxis whether sensitization occurred through the intraperitoneal route or the epicutaneous route. There was no significant difference between body temperatures in epicutaneously-sensitized TLR9+/+ and −/− mice after challenge, but +/+ mice had a significant drop in body temperature compared to pre-challenge temperatures, while TLR9−/− mice did not. By both routes of sensitization, there was a significant suppression of IgE in TLR9−/− mice. Peanut-specific IgA was elevated in response to cutaneous, but not intraperitoneal sensitization, and this was also significantly suppressed in TLR9−/− mice. These data indicate that the resistance of TLR9−/− mice to peanut sensitization is systemic, and not restricted to the induction of responses within the intestinal mucosa.
Figure 5.
Sensitization to peanut by non-oral routes. TLR9+/+ and −/− mice were sensitized to peanut by intraperitoneal injection with alum (IP), or by repeated epicutaneous exposure (skin). A) Body temperature measured 30 min after intraperitoneal challenge with 500 g of peanut extract. B) IgE, C) IgA, and D) IgG1 antibodies were measured in serum obtained 1-2 days prior to challenge. * p < 0.05, ** P < 0.01.
TLR9-dependent promotion of IgE production is through indirect activity on B cells
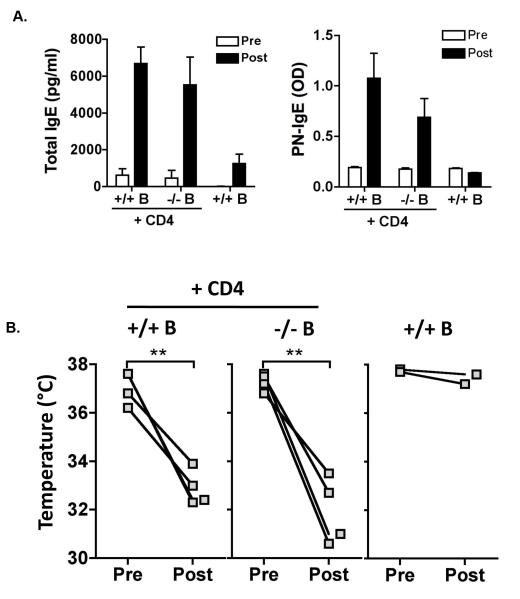
TLR9 ligands have been shown to have direct activity on IgE production from B cells in vitro (21) (22), although the results were conflicting with one report showing suppression while the other showed enhancement of IgE. We wanted to test if the effect of TLR9 deficiency on IgE production was through direct or indirect actions on B cells. To test this, we reconstituted Rag1-deficient mice with B cells from naïve TLR9+/+ or −/− mice, together with CD4+ T cells from TLR9+/+ mice primed with peanut/alum to increase the precursor frequency of peanut-specific T cells. Total and peanut-specific IgE was measured before (4 weeks post-transfer) and 6 weeks after sensitization of the recipient mice. Transfer of B cells without peanut-primed T cells did not support peanut-specific IgE production in recipient mice, demonstrating the T cell-dependence of the generation of peanut-specific IgE. Sensitization of the recipient mice was necessary to generate peanut-specific IgE responses. Rag1-deficient mice receiving wild-type or TLR9−/− B cells plus wild-type peanut-primed T cells generated similar levels of total and peanut-specific IgE after sensitization, and were similarly susceptible to peanut-induced anaphylaxis (Figure 6), demonstrating that TLR9−/− B cells acquire the capacity to generate robust IgE responses in a wild-type milieu.
Figure 6.
Reconstitution of Rag1-deficient mice with TLR9+/+ or TLR9−/− B cells. Rag1−/− mice were reconstituted with B cells from naïve TLR9 +/+ or −/− mice together with CD4+ T cells from TLR9+/+ mice primed with peanut. (n = 4/group, and 2 controls receiving no CD4+ T cells) (A) Total (left) and peanut-specific (right) IgE was measured 4 weeks after cell transfer (pre-sensitization) and after 6 weeks of oral sensitization with peanut plus CT. (B) Mice were challenged by the intraperitoneal route with peanut extract, and rectal temperature measured before and 30 min after challenge to assess anaphylaxis severity. ** p < 0.01 using a paired T test.
DISCUSSION
Exposure to microbial factors has been hypothesized to play a critical role in the development of allergic sensitization. Epidemiologic evidence indicates that lifestyle factors associated with higher rates of microbial exposure or infection are protective against atopy. Studies have also implicated changes in the gastrointestinal flora as a risk factor for eczema in children. Evidence does not indicate that microbial factors are solely protective: bacterial toxins such as cholera toxin (2) and staphylococcal enterotoxin B (1), lipopolysaccharide (3), and NOD2 (23) have adjuvant activity and promote allergic sensitization and inflammation using experimental models of allergy or asthma. Endogenous signals via TLR9 have been shown to modulate the adaptive immune response in the small intestine (12), and we hypothesized that signals via TLR9 would alter susceptibility to allergic sensitization. We found that TLR9−/− mice had reduced susceptibility to allergic sensitization to peanut. This was not due to an altered Th2 cytokine response to peanut but was associated with a selective defect in IgE and IgA production that could be reversed by transferring TLR9−/− B cells to TLR9+/+ mice.
TLR9 polymorphisms have been found to be associated with wheeze, but not allergen-specific IgE (24), and with atopic eczema (25). In the latter study, a gain-of-function allele was associated with increased risk of atopic eczema. Hong et al recently reported gene-environment interactions between TLR9 and breast-feeding in IgE sensitization to foods (15). The finding that the intestine is highly responsive to TLR9 in the neonatal period (14) supports the hypothesis that early exposure to microbial antigens may be decisive in programming the IgE response to food allergens. Our results show that a deficiency in TLR9 signaling can result in protection or resistance in generation of IgE, we speculate that a gain of function in TLR9 signaling could have the opposite effect.
TLR9 is a cytoplasmic pattern recognition molecule that is widely expressed on hematopoietic cells and has also been described as a surface receptor on intestinal epithelial cells (13, 26). Ligands for TLR9 include unmethylated DNA containing CpG motifs (common in bacterial DNA) and viral DNA. TLR9 ligands are potent stimulators of type-I interferon from plasmacytoid dendritic cells (pDCs), and promote the activation and proliferation of B cells. Stimulation via TLR9 suppresses class-switching of naïve B cells to IgE (21, 27), and in vivo treatment with synthetic oligonucleotides that activate TLR9 have been shown to suppress IgE production (8, 28). Furthermore, in models of fungal asthma induced by Aspergillus, it was found that TLR9−/− mice had enhanced IgE responses after fungal infection (29). However, in contrast with those findings, it was recently reported that TLR9 ligands could synergize with CD40 and TACI to promote B cell activation and IgE class-switching (22). The authors hypothesized that the difference in responses could be due to sub-optimal concentrations of CpG used in their study compared to the doses used in the previous study showing direct inhibitory effects of CpG on IgE production in vitro. Our studies with reconstituted Rag1-deficient mice suggest that the modulatory effect of TLR9 deficiency on IgE production is not due to direct effects on the B cell. Our finding of the impact of TLR9 deficiency on IgE production is quite different than the impact of the gut flora as a whole. It was recently shown by Hill et al that eradication of the gut flora with antibiotics induced a significant increase in IgE (30). Total IgE was also elevated in germ-free mice, and in mice lacking the TLR signaling adapter MyD88. This effect of the flora was found to be dependent on MyD88 expression in the B cell compartment. Treatment with exogenous CpG led to a modest reduction in total IgE levels, consistent with previous reports. This is similar to an earlier finding in a model of peanut allergy which demonstrated that antibiotic treatment enhanced susceptibility to sensitization to peanut, and exogenous CpG suppressed sensitization to peanut (7). Therefore, we think that signaling through TLR9 is important for supportive mechanisms of IgE and IgA production during development, rather than directly influencing B cell production of IgE. In addition, peanut may be unique in its dependence on TLR9 for IgE production.
IgE class-switching in mice is supported by IL-4 and IL-13. IL-4 was routinely below the level of detection in our assays, but IL-13 was secreted in an antigen-specific manner and was not different between TLR9+/+ and −/− mice. We observed a significant increase in peanut-induced IL-17 production in TLR9−/− mice in response to oral sensitization (shown in Figure 1). IL-17 and IgE have been shown to negatively correlate in some conditions, such as hyper-IgE syndrome (31). However, in data not shown, sensitization by the epicutaneous route was not associated with enhanced production of IL-17 in TLR9−/− mice yet IgE and IgA suppression was observed. Therefore we did not find a consistent negative correlation between IgE levels and IL-17 production to support a T cell basis for the altered immunoglobulin production. Our data do not suggest that the reduced production of IgA and IgE is due to suppressed cytokine responses.
The finding that both IgE and IgA were suppressed suggested a possible deficiency in mucosal isotype switching. We observed that there were developmental changes in the GALT of TLR9-deficient mice. TLR9−/− mice had a significant decrease in the cellularity of their Peyer’s patches. Although Peyer’s patches are programmed lymphoid organs whose development begins prenatally, environmental cues can modulate the enlargement of these tissues. An impairment in Peyer’s patch development has been noted in a number of knockout mice, including those lacking the chemokine receptor CCR6 (32) and the chemokine CXCL13 (33). It was shown that neonatal feeding of CpG induced an upregulation of chemokine expression in the small intestine (14), that we hypothesize would play an important role in the chemoattraction of B cells to the Peyer’s patch leading to enlargement of the Peyer’s patch after microbial colonization. The Peyer’s patch is a major site of IgA production, and has also been described to be a significant induction site of IgE production, even in response to distal systemic immunization (34). However, we could find no evidence for a local mucosal production of IgE. We did not address the impact of TLR9 deficiency on the formation of isolated lymphoid follicles that are highly sensitive to regulation from the gut flora and are also a significant source of IgA production (35). The contribution of isolated lymphoid follicles to IgE production has not yet been addressed. The selective effect of TLR9 deficiency on IgE and IgA in the absence of effects on IgG1 or Th2 cytokines suggests that TLR9 is altering factors that are common to the support of IgE and IgA production in vivo. Our data, and the recent finding that TLR9 polymorphisms are associated with increased risk of food sensitization in breast-fed infants (15) highlights the potential importance of constitutive signaling through the TLR9 pathway in development of sensitization and tolerance to foods early in life. More studies are needed to identify the mechanism of this TLR9-dependent source of support for IgE production from B cells. A greater understanding of the regulation of IgE production is needed to address the increasing health problem of atopic diseases.
METHODS
Mice
TLR9−/− mice were originally generated by Hemmi et al (36) and were maintained as a breeding colony at Mount Sinai School of Medicine. Wild-type C57BL/6 mice were purchased from NCI (Frederick, MD). TLR9 +/+ and −/− mice were co-caged in specific pathogen-free conditions for at least two weeks prior to the start of experiments in order to normalize the gut flora. Rag1-deficient mice were obtained from Jackson Laboratories and maintained as a breeding colony at the Mount Sinai School of Medicine. The Institutional Animal Care and Use Committee approved all experimental procedures utilizing mice.
Sensitization
Mice were orally sensitized to peanut by intragastric administration of 10 mg ground peanut (prepared from whole shelled peanuts) in 0.2M sodium bicarbonate with 10 μg of cholera toxin (CT, List Biologicals, Campbell, CA) once a week for 6 weeks. Alternatively mice were injected with 100 μg of ground peanut mixed 1:1 with Alum Imject (Pierce, Rockford, IL) every two weeks for six weeks. Mice were sensitized epicutaneously by first removing the abdominal hair with depilatory cream (Veet, Reckitt Benckiser, Parsippany, NJ) under anesthesia. A total of 50 μl of a solution containing 10 mg of crude ground peanut and 10 μg of cholera toxin was spread topically on the abdominal skin. Sensitization was repeated weekly for 6 weeks as previously described for milk allergens (20). Mice were challenged or used for other experiments one week after the last sensitization dose.
Challenge of mice
Mice were challenged with 500 μg of a defatted crude peanut extract by intraperitoneal injection. Body temperature was measured 30 min after challenge by intrarectal temperature probe (World Precision Instruments, Sarasota, FL). Immediately after assessment of anaphylaxis severity, mice were euthanized and spleens harvested for in vitro re-stimulation.
Antigen-specific cytokine production
Spleen cells were isolated, red blood cells lysed, and cells plated in complete RPMI in the presence or absence of 100 μg/ml of a sterile extract of defatted peanut. Cells were cultured for 72 h prior to harvesting of supernatants for measurement of cytokines by ELISA (all from eBioscience, San Diego, CA).
Immunoglobulins
Serum was obtained 1-2 days prior to allergen challenge. Peanut-specific IgE in serum was measured by a capture ELISA using digoxygenin-labeled peanut extract for detection as described previously (37). Peanut-specific IgG1 and IgA were detected by applying serum dilutions to peanut-coated plates and detecting with biotinylated anti-IgG1 and IgA, respectively (antibodies from BD Pharmingen, San Diego, CA). Total IgE was measured in serum by capture ELISA using antibodies from BD Pharmingen. Total IgA was measured in serum and fecal extracts. Fecal pellets were collected and homogenized in PBS. After centrifugation, total IgA was measured in supernatants by ELISA (eBioscience, San Diego, CA) and adjusted to total protein measured by Bradford assay (Pierce).
Isolation of cells
Cells were isolated from the intestine using published protocols (38). The entire small intestine was excised and Peyer’s patches removed for separate isolation. After treatment with dithiothreitol and ethylenediaminetetraacetic acid to remove epithelium, the tissue was incubated with collagenase D (Roche, Indianapolis, IN) and DNase (Roche) prior to passing through a 70 μm filter and separation on a discontinuous Percoll gradient (GE Healthcare, Pittsburgh, PA).
Peritoneal cells were harvested by injection of RPMI into the peritoneum prior to aspiration. Bone marrow cells were harvested by flushing the femurs and tibias with 5 ml of PBS.
Flow Cytometry
All reagents were from eBioscience (La Jolla, CA) unless otherwise stated. Cells were blocked with FcBlock prior to staining with surface antibodies and live/dead violet viability reagent (Invitrogen, Carlsbad, CA). Cells were fixed and permeabilized (using Foxp3-specific buffers from eBioscience for Foxp3 staining, or general fixative and permeabilization buffer for IgE). Intracellular staining for Foxp3 or IgE was performed, and cells were acquired on a LSR II flow cytometer (BD Biosciences, San Diego, CA). Analysis was performed using FlowJo software (Tree Star, Ashland, OR).
Histology
Rings of small intestine containing a Peyer’s patch were fixed in 10% formalin, and embedded in paraffin. Tissue sections (4 μm) were cut as rings and stained with hematoxylin and eosin. The number of visible follicles per Peyer’s patch were quantified and averaged per mouse.
Passive Cutaneous Anaphylaxis
Mice were injected in the ear pinna with 25 μl of pooled serum from peanut-sensitized mice in one ear or from OVA-sensitized mice in the other ear as control. The next day, mice were intravenously injected with 0.2 ml of 1% Evans Blue containing 500 μg of peanut extract. After 60 min, at the time when there was obvious blueing of the positive ear, the mice were euthanized and entire ears were harvested, minced, and extracted with dimethylformamide at 56 °C for 18 h. The supernatant was collected, and the absorbance of the extract was measured at 655 nm (39). Anaphylaxis was localized to the ear injected with serum from peanut-sensitized mice.
Reconstitution of Rag-deficient mice
Resting B cells were purified from spleen and mesenteric lymph nodes of naive TLR9+/+ and −/− mice by negative selection (Miltenyi Biotec, Auburn, CA). CD4+ T cells were purified by negative selection (StemCell, Vancouver, BC) from the spleen of TLR9+/+ mice primed twice with peanut and alum on days 0 and 14, and harvested on day 28. 5 × 106 +/+ or −/− B cells were transferred together with 5 × 106 CD4+ T cells. Mice were allowed to recover for 4 weeks, and then orally sensitized to peanut with CT adjuvant for 6 weeks as described above. Peanut-specific and total IgE levels in serum were measured before and after sensitization to peanut.
Statistics
Difference between TLR9+/+ and −/− mice were measured by two-tailed T test, unless indicated otherwise.
Supplementary Material
Figure S1: Representative flow cytometry showing IgE versus CD19 in bone marrow (BM), spleen, Peyer’s patch (PP), or mesenteric lymph node (MLN). IgE+ cells were negative for the plasma cell marker CD138 at all sites.
Figure S2: Impact of TLR9 deficiency on gastrointestinal lymphoid tissue. Cells from naïve mice were isolated from the Peyer’s patch (A) and mesenteric lymph node (B) of TLR9+/+ and −/− mice, and live cell populations identified by flow cytometry. (C) Regulatory T cells in the mesenteric lymph node (MLN), lamina propria (LP) or Peyer’s patches (PP) of the small intestine were identified by CD4+ Foxp3+ co-staining. Representative flow plots are shown, and the bar graph depicts the mean + SEM of 5 mice per group.
ACKNOWLEDGMENTS
The work was funded by U19AI66738 and U19AI044236 (from NIAID) and R834064 (from EPA). We would like to thank the CoFAR basic science group (Kim Bottomly, Michael Caplan, Pierre Pochard and Brian Vickery) as well as Hugh Sampson and Lloyd Mayer for helpful discussions.
Footnotes
Conflicts of Interest: None
REFERENCES
- 1.Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009 Jan;123(1):231–8. e4. doi: 10.1016/j.jaci.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 1994 Jul 15;153(2):647–57. [PubMed] [Google Scholar]
- 3.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002 Dec 16;196(12):1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frick OL, Teuber SS, Buchanan BB, Morigasaki S, Umetsu DT. Allergen immunotherapy with heat-killed Listeria monocytogenes alleviates peanut and food-induced anaphylaxis in dogs. Allergy. 2005 Feb;60(2):243–50. doi: 10.1111/j.1398-9995.2004.00711.x. [DOI] [PubMed] [Google Scholar]
- 5.Li XM, Srivastava K, Huleatt JW, Bottomly K, Burks AW, Sampson HA. Engineered recombinant peanut protein and heat-killed Listeria monocytogenes coadministration protects against peanut-induced anaphylaxis in a murine model. J Immunol. 2003 Mar 15;170(6):3289–95. doi: 10.4049/jimmunol.170.6.3289. [DOI] [PubMed] [Google Scholar]
- 6.Li XM, Srivastava K, Grishin A, Huang CK, Schofield B, Burks W, et al. Persistent protective effect of heat-killed Escherichia coli producing “engineered,” recombinant peanut proteins in a murine model of peanut allergy. J Allergy Clin Immunol. 2003 Jul;112(1):159–67. doi: 10.1067/mai.2003.1622. [DOI] [PubMed] [Google Scholar]
- 7.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004 Jun 1;172(11):6978–87. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 8.Adel-Patient K, Ah-Leung S, Bernard H, Durieux-Alexandrenne C, Creminon C, Wal JM. Oral sensitization to peanut is highly enhanced by application of peanut extracts to intact skin, but is prevented when CpG and cholera toxin are added. Int Arch Allergy Immunol. 2007;143(1):10–20. doi: 10.1159/000098221. [DOI] [PubMed] [Google Scholar]
- 9.Senti G, Johansen P, Haug S, Bull C, Gottschaller C, Muller P, et al. Use of A-type CpG oligodeoxynucleotides as an adjuvant in allergen-specific immunotherapy in humans: a phase I/IIa clinical trial. Clin Exp Allergy. 2009 Apr;39(4):562–70. doi: 10.1111/j.1365-2222.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 10.McCluskie MJ, Davis HL. Oral, intrarectal and intranasal immunizations using CpG and non-CpG oligodeoxynucleotides as adjuvants. Vaccine. 2000 Oct 15;19(4-5):413–22. doi: 10.1016/s0264-410x(00)00208-5. [DOI] [PubMed] [Google Scholar]
- 11.Demento SL, Bonafe N, Cui W, Kaech SM, Caplan MJ, Fikrig E, et al. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J Immunol. 2010 Sep 1;185(5):2989–97. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008 Oct;29(4):637–49. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006 Dec;8(12):1327–36. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix-Lamande S, Rochereau N, Mancassola R, Barrier M, Clauzon A, Laurent F. Neonate intestinal immune response to CpG oligodeoxynucleotide stimulation. PLoS One. 2009;4(12):e8291. doi: 10.1371/journal.pone.0008291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong X, Wang G, Liu X, Kumar R, Tsai HJ, Arguelles L, et al. Gene polymorphisms, breast-feeding, and development of food sensitization in early childhood. J Allergy Clin Immunol. 2011 Aug;128(2):374–81. e2. doi: 10.1016/j.jaci.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol. 2007 Nov 15;179(10):6696–703. doi: 10.4049/jimmunol.179.10.6696. [DOI] [PubMed] [Google Scholar]
- 17.Arias K, Chu DK, Flader K, Botelho F, Walker T, Arias N, et al. Distinct immune effector pathways contribute to the full expression of peanut-induced anaphylactic reactions in mice. J Allergy Clin Immunol. 2011 Jun;127(6):1552–61. e1. doi: 10.1016/j.jaci.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Chambers SJ, Wickham MS, Regoli M, Bertelli E, Gunning PA, Nicoletti C. Rapid in vivo transport of proteins from digested allergen across pre-sensitized gut. Biochem Biophys Res Commun. 2004 Dec 24;325(4):1258–63. doi: 10.1016/j.bbrc.2004.10.161. [DOI] [PubMed] [Google Scholar]
- 19.Roth-Walter F, Berin MC, Arnaboldi P, Escalante CR, Dahan S, Rauch J, et al. Pasteurization of milk proteins promotes allergic sensitization by enhancing uptake through Peyer’s patches. Allergy. 2008 Jul;63(7):882–90. doi: 10.1111/j.1398-9995.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 20.Dunkin D, Berin MC, Mayer L. Allergic sensitization can be induced via multiple. doi: 10.1016/j.jaci.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003 Jul;4(7):687–93. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan E, Rauter I, Garibyan L, Dillon SR, Geha RS. Toll-like receptor 9, transmembrane activator and calcium-modulating cyclophilin ligand interactor, and CD40 synergize in causing B-cell activation. J Allergy Clin Immunol. 2011 Sep;128(3):601–9. e1–4. doi: 10.1016/j.jaci.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan W, Mehta AK, Magalhaes JG, Ziegler SF, Dong C, Philpott DJ, et al. Innate signals from Nod2 block respiratory tolerance and program T(H)2-driven allergic inflammation. J Allergy Clin Immunol. 2010 Nov 2; doi: 10.1016/j.jaci.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genuneit J, Cantelmo JL, Weinmayr G, Wong GW, Cooper PJ, Riikjarv MA, et al. A multi-centre study of candidate genes for wheeze and allergy: the International Study of Asthma and Allergies in Childhood Phase 2. Clin Exp Allergy. 2009 Dec;39(12):1875–88. doi: 10.1111/j.1365-2222.2009.03364.x. [DOI] [PubMed] [Google Scholar]
- 25.Novak N, Yu CF, Bussmann C, Maintz L, Peng WM, Hart J, et al. Putative association of a TLR9 promoter polymorphism with atopic eczema. Allergy. 2007 Jul;62(7):766–72. doi: 10.1111/j.1398-9995.2007.01358.x. [DOI] [PubMed] [Google Scholar]
- 26.Ewaschuk JB, Backer JL, Churchill TA, Obermeier F, Krause DO, Madsen KL. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun. 2007 May;75(5):2572–9. doi: 10.1128/IAI.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, Gerth AJ, Peng SL. CpG DNA redirects class-switching towards “Th1-like” Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004 May;34(5):1483–7. doi: 10.1002/eji.200324736. [DOI] [PubMed] [Google Scholar]
- 28.Spiegelberg HL, Tighe H, Roman M, Broide D, Raz E. Inhibition of IgE formation and allergic inflammation by allergen gene immunization and by CpG motif immunostimulatory oligodeoxynucleotides. Allergy. 1998;53(45 Suppl):93–7. doi: 10.1111/j.1398-9995.1998.tb04948.x. [DOI] [PubMed] [Google Scholar]
- 29.Ramaprakash H, Ito T, Standiford TJ, Kunkel SL, Hogaboam CM. Toll-like receptor 9 modulates immune responses to Aspergillus fumigatus conidia in immunodeficient and allergic mice. Infect Immun. 2009 Jan;77(1):108–19. doi: 10.1128/IAI.00998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012;18(4):538–46. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008 Apr 10;452(7188):773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutierrez J, et al. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. 2001 Mar;107(6):R37–45. doi: 10.1172/JCI11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003 May 5;197(9):1191–8. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auci DL, Chice SM, Heusser C, Athanassiades TJ, Durkin HG. Origin and fate of IgE-bearing lymphocytes. II. Gut-associated lymphoid tissue as sites of first appearance of IgE-bearing B lymphocytes and hapten-specific IgE antibody-forming cells in mice immunized with benzylpenicilloyl-keyhole limpet hemocyanin by various routes: relation to asialo GM1 ganglioside+ cells and IgE/CD23 immune complexes. J Immunol. 1992 Oct 1;149(7):2241–8. [PubMed] [Google Scholar]
- 35.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008 Nov 27;456(7221):507–10. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 36.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000 Dec 7;408(6813):740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 37.Berin MC, Zheng Y, Domaradzki M, Li XM, Sampson HA. Role of TLR4 in allergic sensitization to food proteins in mice. Allergy. 2006 Jan;61(1):64–71. doi: 10.1111/j.1398-9995.2006.01012.x. [DOI] [PubMed] [Google Scholar]
- 38.Lefrancois L, Lycke N. Current Protocols of Immunology. John Wiley & Sons; 1996. Isolation of Mouse Small Intestinal Intraepithelial Lymphocytes, Peyer’s Patch, and Lamina Propria Cells. [DOI] [PubMed] [Google Scholar]
- 39.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999 Dec 24;286(5449):2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Representative flow cytometry showing IgE versus CD19 in bone marrow (BM), spleen, Peyer’s patch (PP), or mesenteric lymph node (MLN). IgE+ cells were negative for the plasma cell marker CD138 at all sites.
Figure S2: Impact of TLR9 deficiency on gastrointestinal lymphoid tissue. Cells from naïve mice were isolated from the Peyer’s patch (A) and mesenteric lymph node (B) of TLR9+/+ and −/− mice, and live cell populations identified by flow cytometry. (C) Regulatory T cells in the mesenteric lymph node (MLN), lamina propria (LP) or Peyer’s patches (PP) of the small intestine were identified by CD4+ Foxp3+ co-staining. Representative flow plots are shown, and the bar graph depicts the mean + SEM of 5 mice per group.