Abstract
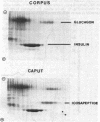
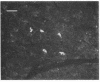
In dogs, the COOH-terminal part of the pancreatic polypeptide precursor gives rise to a stable icosapeptide product against which an antiserum has been raised. By immunohistochemistry, icosapeptide immunoreactivity was localized in human pancrease exclusively to cells that also stored pancreatic polypeptide. Analytical peptide chemistry demonstrated that a peptide corresponding to the canine icosapeptide could be extracted from the pancreatic polypeptide-rich duodenal part of the human pancreas. The human pancreatic icosapeptide was isolated by acid ethanol extraction, gel filtration, anion-exchange chromatography, and reverse-phase high-performance liquid chromatography. The COOH-terminal sequence of the human icosapeptide is very similar to that of the canine icosapeptide, whereas none of the first nine amino acid residues are identical. When the primary structure of peptides from three different species are compared, it is apparent that the pancreatic polypeptide part of the common precursor is a well-conserved sequence as compared to the icosapeptide part, although 8 out of 11 residues in the COOH-terminal sequence of the icosapeptide are identical in all three species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T. E., Greenberg G. R., Barnes A. J., Christofides N. D., Alberti K. G., Bloom S. R. Effects of pancreatic polypeptide on motilin and circulating metabolites in man. Eur J Clin Invest. 1980 Jun;10(3):235–240. doi: 10.1111/j.1365-2362.1980.tb00026.x. [DOI] [PubMed] [Google Scholar]
- COONS A. H., LEDUC E. H., CONNOLLY J. M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J Exp Med. 1955 Jul 1;102(1):49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Floyd J. C., Jr, Fajans S. S., Pek S., Chance R. E. A newly recognized pancreatic polypeptide; plasma levels in health and disease. Recent Prog Horm Res. 1976;33:519–570. doi: 10.1016/b978-0-12-571133-3.50019-2. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., McCloy R. F., Adrian T. E., Chadwick V. S., Baron J. H., Bloom S. R. Inhibition of pancreas and gallbladder by pancreatic polypeptide. Lancet. 1978 Dec 16;2(8103):1280–1282. doi: 10.1016/s0140-6736(78)92042-1. [DOI] [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F. Four basic characteristics of the gastrin-cholecystokinin system. Am J Physiol. 1981 Apr;240(4):G255–G266. doi: 10.1152/ajpgi.1981.240.4.G255. [DOI] [PubMed] [Google Scholar]
- Schwartz S. S., Corkey B., Williamson J. R., Rubenstein A. H. Effect of bovine pancreatic polypeptide on isolated rat liver cells. Endocrinology. 1980 Apr;106(4):1178–1181. doi: 10.1210/endo-106-4-1178. [DOI] [PubMed] [Google Scholar]
- Schwartz T. W., Gingerich R. L., Tager H. S. Biosynthesis of pancreatic polypeptide. Identification of a precursor and a co-synthesized product. J Biol Chem. 1980 Dec 10;255(23):11494–11498. [PubMed] [Google Scholar]
- Schwartz T. W., Tager H. S. Isolation and biogenesis of a new peptide from pancreatic islets. Nature. 1981 Dec 10;294(5841):589–591. doi: 10.1038/294589a0. [DOI] [PubMed] [Google Scholar]
- Sottrup-Jensen L., Petersen T. E., Magnusson S. Analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography using gradient elution with ethanol. Anal Biochem. 1980 Sep 15;107(2):456–460. doi: 10.1016/0003-2697(80)90410-8. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Tatemoto K. Isolation and characterization of peptide YY (PYY), a candidate gut hormone that inhibits pancreatic exocrine secretion. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2514–2518. doi: 10.1073/pnas.79.8.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K. Neuropeptide Y: complete amino acid sequence of the brain peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5485–5489. doi: 10.1073/pnas.79.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramu G., Pillez A., Leonardelli J. An efficient method of antibody elution for the successive or simultaneous localization of two antigens by immunocytochemistry. J Histochem Cytochem. 1978 Apr;26(4):322–324. doi: 10.1177/26.4.207771. [DOI] [PubMed] [Google Scholar]