Abstract
Since the brain's gray matter (GM) and white matter (WM) metabolite concentrations differ, their partial volumes can vary the voxel's 1H MR spectroscopy (1H-MRS) signal, reducing the sensitivity to changes. While single-voxel 1H-MRS cannot differentiate the WM from GM signals, partial volume correction is feasible in MR spectroscopic imaging (MRSI), using segmentation of the MRI that is always acquired for VOI placement. To determine the magnitude of this effect on metabolic quantification, we segmented the 1 mm3 resolution MRI into GM, WM and CSF masks that were co-registered with the MRSI grid to yield their partial volumes in every ~1 cm3 spectroscopic voxel. Each voxel then provided one equation with two unknowns – its i- metabolite's GM and WM concentrations: CiGM, CiWM. With the voxels' GM and WM volumes as independent coefficients, that over-determined system of equations can be solved for the global, averaged CiGM and CiWM. Trading off local concentrations differences offers three advantages: (i) higher sensitivity due to combined data from many voxels; (ii) improved specificity to WM versus GM changes; (iii) reduced susceptibility to partial volume effects. These improvements make no additional demands of the protocol, measurement time or hardware. Applying the approach to 18 volunteers' 3D MRSI sets, 480 voxels each, yielded N-acetylaspartate, creatine, choline and myo-inositol CiGM of 8.5±0.7, 6.9±0.6, 1.2±0.2, 5.3±0.6 mM; and CiWM of 7.7±0.6, 4.9±0.5, 1.4±0.1 and 4.4±0.6 mM. We show that unaccounted voxel WM or GM partial volume can vary absolute quantification by 5–10% (more for ratios) that can often as much as double the sample size required to establish statistical significance.
Keywords: segmentation, gray matter, white matter, spectroscopic quantification, MRS
INTRODUCTION
Proton MR spectroscopy (1H-MRS) adds unique specificity to pathological processes in the brain by quantifying metabolic surrogates: N-acetylaspartate (NAA) for neuronal integrity; creatine (Cr) for glial proliferation; choline (Cho) for membrane turnover; and myo-inositol (mI) for astrogliosis (1,2). Its sensitivity to their concentration changes, however, is low mainly due to: (i) The intrinsic low voxel signal-to-noise ratios (SNR) that reduces its reproducibility (3,4); (ii) the variation in cerebrospinal fluid, white and gray matter (CSF, WM, GM) composition in each voxel. Since metabolites' concentrations in GM differ from WM, with virtually none in the CSF (5), and since pathologies may affect these tissue types differently (6,7), variation in the voxels' compositions will diminish the statistical power to detect changes (8). These partial volume effects are exacerbated by volume of interest (VOI) misregistration in longitudinal and cross-sectional studies.
Unfortunately, the SNR and partial volume effect issues described above, are inextricable. For a given instrumental setup and scan time, addressing the SNR by increasing the voxel size comes at the expense of larger partial volumes and vice versa (9,10). Furthermore, the thin, 1 – 4 mm, tortuous GM ribbon (11) makes it difficult to place the ~2×2×2 cm3 typical single-voxel 1H-MRS volumes in “pure” WM and almost impossible to obtain “pure” GM voxels. In addition, single-voxel 1H-MRS precludes verification of diffuse (or multi-focal) metabolic changes that characterize common neurological disorders (1,2). Surprisingly, even though multi-voxel 1H MR spectroscopic imaging (1H-MRSI) can yield much higher, ~1 cm3, spatial resolution, its analyses are often done on a voxel-by-voxel basis (12), and the consequences of tissue partial volume on its precision (reproducibility) are, to our knowledge, not discussed in the literature.
The misregistration, SNR and partial volume issues can be substentially reduced by combining absolute 1H-MRSI metabolic quantification with the anatomical high-spatial resolution (~1 mm3) MRI that always accompanies it. Using freely available segmentation software, WM/GM/CSF masks can be produced and overlaid on the 1H-MRSI grid to yield their contents in each voxel (13,14). This information can yield the global WM and GM metabolites' concentrations by modeling the 1H-MRSI signal from each voxel as a linear combination of their contributions. Thus, at the cost of averaging out regional metabolic variations (15) - a reasonable tradeoff in diffuse disorders - analysis of all the voxels can dramatically improve the SNR while also accounting for partial volume effects. In this paper we show that this approach improves the sensitivity to diffuse/global differences that predominate in either WM or the GM and estimate the metabolites concentrations' variations that can occur if GM and WM partial volumes are not accounted for.
MATERIALS AND METHODS
Human Subjects
Eighteen (12 women, 6 men) healthy 33 (range 19 – 57) year old individuals were enrolled. Their `healthy' status was determined by self-reported negative answers to disqualifying neurological and MR contraindications before the scan and an unremarkable MRI determined by a neuroradiologist afterwards. All were briefed on the procedure and gave an Institutional Review Board approved written informed consent.
MR Data Acquisition
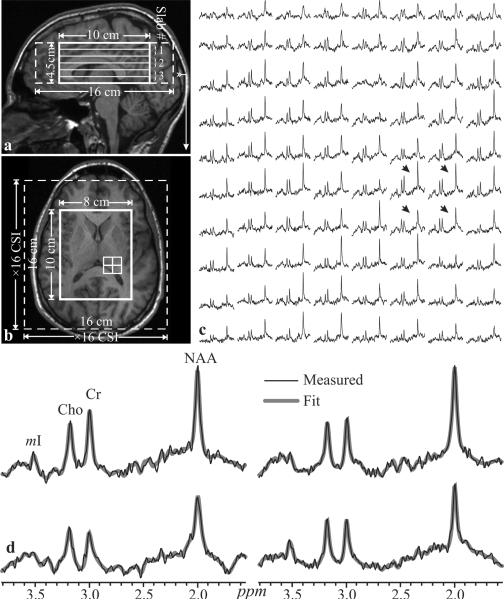
All experiments were carried out on a 3 T scanner (Trio, Siemens AG, Erlangen Germany) with a TEM3000 circularly-polarized, transmit-receive head-coil (MR-Instruments, Minneapolis, MN), capable of delivering a 1 kHz B1 field to the human head with ~1.5 kW of radio-frequency (RF) power. For 1H-MRSI VOI image-guidance and for tissue segmentation, 160 1-mm thick slices were acquired with sagittal Magnetization Prepared RApid Gradient Echo (MP-RAGE): TE/TI/TR=2.6/800/1360 ms, 256×256 mm2 field of view (FOV), 256×256 matrix. They were reconstructed in axial, sagittal and coronal planes at 1 mm3 isotropic resolution, angled to render the genu and splenium of the corpus callosum in the same horizontal plane, as shown in Fig. 1.
Fig. 1.
Left: Sagittal (a) and axial (b) T1-weighted MP-RAGE MRI of subject #18 in Table 1, superimposed with the 8×10×4.5 cm3 VOI, 16×16×4.5 cm3 FOV (thick and dashed white frames) and 3 second-order 1.5 cm thick Hadamard slabs (six 0.75 cm thick 1H-MRSI slices), every TR. Arrow on a indicates the location of b. Right - c:_Real part of the 8×10 1H spectra matrix from the VOI on b on common 1.7 – 3.7 ppm and intensity scales. Bottom - d: The 4 spectra from the 2×2 white grid on b, indicated by the black arrows on c, are expanded for greater detail, superimposed with the fitted waveform used for quantification in Eq. [1]. Note the SNR and spectral resolution from these 0.75 cm3 voxels in ~30 minutes of acquisition and the fit quality.
Our chemical-shift imaging (CSI) based automatic procedure then adjusted the full second order shims in 3–5 minutes (16). Next, a 10 cm anterior-posterior (AP) ×8 cm left-right (LR) ×4.5 cm inferior-superior (IS) =360 cm3 VOI was image-guided over the corpus callosum, as shown in Fig. 1. It was excited with a TE=35 ms PRESS in three second-order Hadamard encoded 1.5 cm thick slabs (for a total of 6 slices), interleaved along the IS direction every TR=1800 ms (Fig. 1a) for optimal SNR and spatial coverage (17). It also enabled strong, 6 mT/m, slice-select gradient for the 5.12 ms Hadamard PRESS 90° RF pulses, reducing the 1.56 ppm (~200 Hz) chemical shift between NAA and mI to just a ~0.6 mm displacement (18). The slices were partitioned with 16×16 CSI over a 16×16 cm2 FOV to yield 1.0×1.0×0.75 cm3 voxels [1.2×1.2×0.75≈1.1 cm3 given the full-width at half-max (FWHM) of the 2D point spread function (19–21)]. The 8×10 cm VOI was defined with two 11.2 ms numerically-optimized 180° RF pulses (4.5 kHz bandwidth) under 1.34 (LR) and 1.1 mT/m (AP) gradients to yield 8×10×6 =480 voxels. The PRESS 180° RF pulses were calibrated to ensure that their transition bands fell outside the VOI, in voxels which were subsequently discarded during post-processing. The MR signal was acquired for 256 ms at ±1 kHz bandwidth. At two averages, the 1H-MRSI took 34 minutes.
Voxel Volumetry
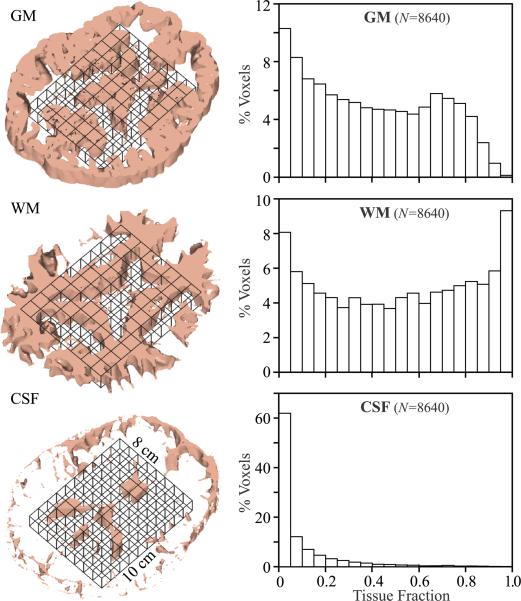
Each subject's MP-RAGE images were segmented using SPM2 [Wellcome Department of Cognitive Neurology, Institute of Neurology, Queen Square, London, UK (22,23)] into CSF, WM and GM masks in ~15 minutes on a Core i7 class workstation. These were co-registered with the 1H-MRSI grid using in-house software that computed their volume in every j-th voxel of the k-th subject (VjkGM, VjkWM, VjkCSF) in ~5 seconds, as shown in Fig. 2. Since the 1H-MRSI and MRI were acquired in the same frame of reference, no deformations or shearing transformations were needed.
Fig. 2.
Left: 3D renderings of a single 7.5 mm thick 1H-MRSI slice (black solid grid, of 6 in subject #18), co-registered with its 7.5 corresponding CSF, WM and GM masks (1 mm thick each) segmented from the T1-weighted MP-RAGE with SPM2. Our in-house software counted the number of pixels of each mask fell into every j-th MRSI voxel in the VOI to estimate its , , for the analysis of Eqs. [3] and [4].
Right: Histograms of the % of the total number of 8,640 voxels (18 subjects × 480 voxels each) containing a given fraction of GM, WM and CSF at 5% bin resolution. Note that even at this relatively high spatial resolution (0.75 cm3) it is hard to get a “pure,” i.e., ❯95% WM voxel, a requirement satisfied by less that 10% of all voxels and nearly impossible (❮1%) to get one that is ❯95% GM.
Metabolic quantification
The 1H-MRS data was processed offline using in-house software. The data was voxel-shifted to align the NAA grid with the VOI, then Fourier transformed in the time, AP and LR direction and Hadamard-transformed along the IS dimension. Each spectrum was frequency-aligned and zero-order phased in reference to the NAA peak. Relative levels of the i-th (i=NAA, Cr, Cho, mI) metabolite in the j-th (j-1…480) voxel in the k-th (k-1…18) subject, Sijk, were estimated from their peak area using the freely available SITools-FITT spectral modeling software of Soher et al. (24). It used the full lineshapes of aspartate, glutamate, glutamine, Cho, Cr, mI, NAA and taurine as model functions obtained with the GAVA simulation program for our pulse sequence (25). This process, which takes about 30 minutes, uses a priori spectral information and includes non-parametric baseline signal components characterization and Lorenz-Gauss lineshape assumption. Analysis of this baseline modeling showed that for spectra with 5 Hz linewidth, the mean errors of the fit are 3.4%, 2.3% and 2.8% for NAA, Cr and Cho (26). The Sijk-s were scaled into absolute amounts, Qijk, against a 2 L sphere of Civitro=12.5, 10.0, 3.0 and 7.5 mM NAA, Cr, Cho and mI in water at physiological ionic strength to load the coil and VOI size and position similar to the in vivo studies to approximate a similar B1 profile up to the intrinsic differences between the phantom and the head due to tissue – RF field interactions at 3 T:
| [1] |
where V is the voxel volume (0.75 cm3), SijR the sphere voxels' metabolites' signal, Pk180° and PR180° are the RF power for a non-selective 1 ms 180° inversion pulse on the k-th subject and reference. To account for different relaxation times in vivo (T1vivo, T2vivo) and in the phantom (T1vitro, T2vitro), the Qijk in were corrected for each metabolite i with (27):
| [2] |
Global VOI concentrations
Individual, age-adjusted, T2vivo WM and GM values for NAA, Cr and Cho were calculated using published formulae (28). For the whole VOI we used their WM and GM values weighted by each tissue volume fraction in that individual's VOI (sum of their fractions in all the voxels - see Results below). Their mean T2vivo values over all 18 subjects were 350, 174 and 251 ms. T2vivo=200 ms was used for mI with no GM/WM or age difference (29). Since no significant GM/WM or age differences are reported for T1vivo at 3 T, we used 1360, 1300, 1145 and 1170 ms (29,30). The corresponding values measured in the phantom were T2vitro=483, 288, 200, 233 ms and T1vitro=605, 336, 235, 280 ms. The average whole-VOI tissue concentration for each metabolite, Cik was obtained as:
| [3] |
This sum has the advantage of (number of voxels)½ ≈22 fold less variance than individual elements and consequently, expected to yield better precision, as described by Kreis (4).
Global WM and GM concentrations
Since CSF does not contribute to the 1H-MRS signal, the i-th metabolite amount in the j-th voxel of the k-th subject can be modeled as a sum of two (GM, WM) compartments:
| [4] |
where CikWM, CikGM are the (unknown) global WM and GM concentrations of the i-th metabolite in the k-th subject, and fiGM, fiWM are calculated by Eq. [2] using GM T2vivos of 275, 157, 241 and 200 ms for NAA, Cr, Cho and mI, and 400, 185, 258 and 200 ms for WM (28,29). The corresponding T1vivos, T2vitros and T1vitros, are the same as for Eq. [3].
For each volunteer, Eq. [4] comprises a set of 480 over determined equations for CikWM, and CikGM that can be solved with least-squares optimization that minimizes the total error:
| [5] |
for each metabolite, i, and patient, k. Since the brain's GM and WM spatial heterogeneity is on a scale smaller than the 1 cm3 of the voxels, the j=1,…,480 VjkGM and VjkWM coefficients (480 equations), are independent, guaranteeing a non-degenerate solution.
RESULTS
Our shim produced a consistent 22±3 Hz FWHM VOI water linewidth. An example of the VOI size, position and 1H-MRSI, is shown in Fig. 1. The SNRs in 8,640 voxels (18 subjects × 480 voxels each) were: NAA=30±6, Cr=15±3, Cho=13±2 and mI=8±1 (mean ± standard deviation), estimated as peak height divided by twice the root-mean-square of the noise. The metabolites' voxel FWHM linewidth was 6.6±1.1 Hz.
The average VOI composition was 9±2% CSF, 39±2% GM and 52±3% WM. Its tissue fraction was 43±2% GM and 57±2% WM. Analysis of the individual voxels' GM , WM and CSF composition, shown in Fig. 2, reveals that, even at this relatively high spatial resolution, less than 1% of the voxels can be considered “pure” (95% or greater) GM and under 10% “pure” WM, as shown in Fig. 2. Of the 8640 voxels in the 18 VOIs, about 86% contained less than 10% CSF (see Fig. 2).
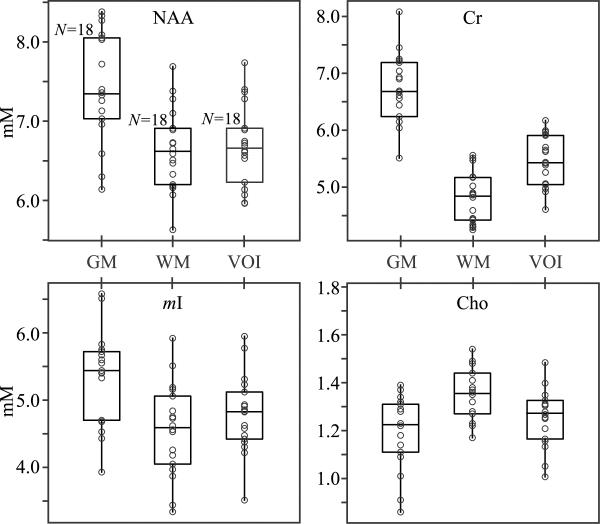
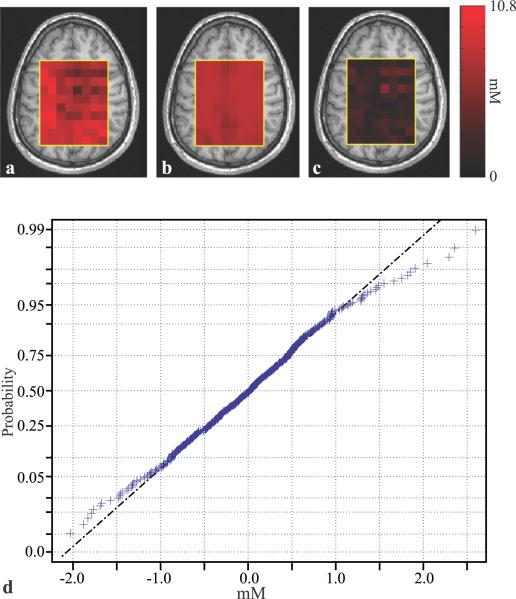
Global VOI, WM and GM metabolites' concentrations from our 18 subjects, are compiled in Table 1 with their least-squares errors (Eik in Eq. [5]) as quantitative “goodness of the fit” metric. To demonstrate their different GM and WM distributions and sensitivity gain from the segmentation process, the concentrations, are also plotted in Fig. 3. The absolute-valued NAA residual error map, obtained by subtracting the synthesized NAA map (Qijk calculated from Eq. [4] for each voxel using its VjkGM and VjkWM and the global least-squares CikWM, CikGM) from the experimental one, is shown in Fig. 4. Also shown is the normal probability plot for the, Eik,s for the whole VOI for that metabolite and patient, for visual assessment of the statistical normalcy of the errors.
TABLE 1.
Absolute mM whole-VOI tissue concentrations (VOI), global WM and GM concentrations (CWM, CGM) of the NAA, Cho, Cr, mI for the 18 subjects, using Eqs. [3], Eq. [4] and least-squares fitting. The global fitting error (Eik in Eq. [5]) per voxel (divided by the number of terms in the sum) were calculated for each patient and metabolite, and averaged over all patients.
| Concentrations [mM] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NAA | Cr | Cho | mI | |||||||||
| subject | VOI | CWM | CGM | VOI | CWM | CGM | VOI | CWM | CGM | VOI | CWM | CGM |
| 1 | 7.2 | 7.1 | 8.0 | 5.4 | 4.9 | 6.4 | 1.4 | 1.5 | 1.1 | 4.6 | 4.2 | 5.4 |
| 2 | 8.3 | 8.3 | 9.3 | 6.7 | 5.0 | 7.3 | 1.4 | 1.5 | 1.3 | 4.3 | 3.9 | 5.3 |
| 3 | 7.9 | 7.9 | 9.1 | 5.9 | 4.9 | 8.1 | 1.3 | 1.4 | 1.3 | 4.8 | 4.6 | 5.7 |
| 4 | 7.5 | 7.5 | 8.4 | 5.1 | 4.3 | 6.7 | 1.2 | 1.3 | 1.2 | 4.6 | 4.1 | 5.8 |
| 5 | 7.6 | 7.2 | 9.0 | 5.0 | 4.5 | 6.2 | 1.3 | 1.4 | 1.3 | 4.9 | 4.8 | 5.4 |
| 6 | 7.7 | 7.7 | 8.3 | 5.4 | 4.9 | 6.6 | 1.3 | 1.4 | 1.3 | 5.8 | 5.5 | 6.5 |
| 7 | 7.0 | 6.6 | 8.0 | 5.0 | 4.3 | 6.0 | 1.0 | 1.0 | 1.1 | 4.2 | 4.0 | 4.7 |
| 8 | 7.1 | 7.7 | 7.3 | 4.6 | 4.4 | 5.5 | 1.2 | 1.3 | 1.1 | 6.0 | 5.9 | 6.6 |
| 9 | 8.7 | 8.7 | 9.4 | 6.0 | 5.5 | 7.0 | 1.2 | 1.2 | 1.1 | 5.3 | 5.2 | 5.8 |
| 10 | 7.6 | 7.7 | 8.0 | 5.6 | 4.8 | 6.9 | 1.3 | 1.4 | 1.3 | 4.4 | 4.3 | 4.7 |
| 11 | 7.0 | 7.2 | 7.6 | 5.0 | 4.6 | 6.2 | 1.1 | 1.2 | 1.0 | 4.5 | 4.7 | 4.5 |
| 12 | 7.9 | 8.1 | 8.3 | 5.7 | 5.2 | 6.9 | 1.3 | 1.4 | 1.3 | 4.8 | 4.6 | 5.4 |
| 13 | 8.4 | 7.9 | 9.3 | 5.3 | 4.3 | 6.7 | 1.3 | 1.3 | 1.2 | 4.4 | 3.4 | 5.7 |
| 14 | 7.6 | 7.6 | 8.1 | 5.4 | 4.4 | 7.2 | 1.3 | 1.2 | 1.4 | 4.9 | 4.5 | 5.6 |
| 15 | 7.1 | 7.3 | 7.1 | 4.9 | 4.3 | 6.2 | 1.1 | 1.3 | 0.9 | 3.5 | 3.3 | 3.9 |
| 16 | 7.8 | 7.2 | 9.1 | 5.9 | 5.2 | 7.2 | 1.4 | 1.4 | 1.4 | 5.1 | 4.8 | 5.6 |
| 17 | 8.4 | 8.4 | 8.4 | 6.0 | 5.6 | 6.6 | 1.3 | 1.5 | 0.9 | 4.9 | 5.1 | 4.4 |
| 18 | 7.7 | 7.5 | 8.7 | 6.2 | 5.5 | 7.5 | 1.5 | 1.8 | 1.2 | 5.2 | 5.2 | 5.4 |
| Mean | 7.7 | 7.6 | 8.4 | 5.5 | 4.8 | 6.7 | 1.3 | 1.4 | 1.2 | 4.8 | 4.6 | 5.4 |
| SD | 0.5 | 0.5 | 0.7 | 0.5 | 0.4 | 0.6 | 0.1 | 0.2 | 0.2 | 0.6 | 0.7 | 0.7 |
| CV | 7% | 7% | 8% | 8% | 9% | 9% | 10% | 13% | 13% | 12% | 15% | 13% |
The results are (mean ± SD): NAA=0.11±0.1, Cho=0.03±0.01, Cr=0.08±0.01, mI=0.08±0.08.
Fig. 3.
Box plots showing the first, second (median) and third quartiles (box) and ±95% (whiskers) of the non-tissue specific, WM and GM NAA, Cho, Cr and mI whole-VOI concentrations' distributions, superimposed with their respective dot plots showing the concentrations in each for the 18 healthy volunteers. Note the differences between WM, and GM tissue-specific concentrations differences that can modulate the non-specific VOI concentration depending on their partial-volume, as described by Eqs. [8] and [9].
Fig. 4.
(a) Top, left - a: the distribution of Qijk (i=NAA) in spectroscopic slice #4 (of 6) in subject #18 in Table 1. Center - b: the modeled NAA distribution, in the same slice, reconstructed a posteriori using the global least-squares WM and GM concentrations , Right - c: the absolute value of the difference maps on |a – b|, showing the residual errors. Note the small residual values on c, demonstrating the quality of the global concentration assumption. Bottom - d: A normal probability plot for the error residuals from the entire VOI of that subject. Note that most voxels lie on the (dashed - dot) line indicating a normal distribution, providing visual indication of the normal distribution of the residuals.
DISCUSSION
The problem of inferring WM and GM-specific metabolites' concentrations has been addressed in many 1H-MRS studies. Although single voxel 1H-MRS studies often attempt to circumvent this issue by placing the VOI in “mostly” WM or GM (31), partial volume effects, quantified in Fig. 2, SNR limitations and misregistration, all introduce quantification errors. A remedy in 1H-MRSI is to apply linear regression to the metabolites' concentrations and either the GM or WM voxel volume fractions (13,32–37). In such linear model, the voxels' signals are assumed to be of the form given by Eq. [4], while the constraint VjkGM+VjkWM+VjkCSF=V is addressed by dividing Qijk by V− VjkCSF=VjkGM+VjkWM; the voxels' signals are then fitted to a straight line of the form:
| [6] |
In contrast, minimizing the error given by Eq. [5] seeks to fit the data to a (2D) plane, constrained to pass through the origin: Qijk=0 for VjkWM=VjkGM=0. While both approaches are equivalent for a noiseless signal, once a random, normally distributed error term εijk is added to the model (Eq. [4]), its division by (V−VjkCSF) raises the variance of the statistical estimators for CGM, CWM (38) by the ratio of the variances of the undivided and the divided errors:
| [7] |
This effect can be mitigated by increasing the number of voxels and excluding all those above a certain threshold for VjkCSF. This, however, may become prohibitive for smaller structures or 2D MRSI data. It can be avoided altogether by employing a two-dimensional constrained fitting procedure (Eq. [5]), where division by V−VjkCSF is unnecessary.
Two other studies applied linear regression in sub-structures, e.g. the right temporal lobe (33,34). While concentrations variation among structures is well documented (13,15), arguably in diffuse (or multi-focal) diseases affecting the whole brain, changes are of interest not absolute magnitudes. Applying Eq. [4] to the entire, heterogeneous VOI, therefore, should not detract from the method's power to detect this change and only serves to increase the sensitivity (3,4).
While the improved SNR of this approach is reflected in small, 7–15%, coefficients of variations (CV=standard deviation/mean) of the CiGMs and CiWMs in Table 1, the partial volume confounds are more subtle and will be treated separately. First, random GM and WM voxel composition due to either: (i) contamination of an intended “pure” tissue; or (ii) misregistration in different exams, each may lead to variations in the MRS signal, reducing statistical power to detect pathology-induced changes. Second, these variations also decrease the ability to detect changes specific to one tissue type, but not the other.
Quantification errors from partial volume variation
To estimate the error introduced by WM and GM voxel composition variations due to misregistration [scenario (i) above], let VWM, VGM and V be the WM, GM and total voxel (assume for simplicity no CSF: V=VWM+VGM), and CWM, CGM the tissue metabolite concentration. This metabolite's 1H-MRS signal from that voxel will be proportional to its amount: Q =VWM˙CWM + VGM˙CGM. Ideally, for a pure WM, one would have VGM=0. However, if the voxel contained unintentional VGM≠0, then its signal would differ by a multiplicative “fractional error” factor, δ:
| [8] |
Using the median CGM=6.7 mM and CWM=4.7 mM for Cr (often used as an internal standard in 1H-MRS) from Fig. 3 and assuming a commonly encountered VGM/V=0.2 – difficult to avoid for large(er) single-voxel studies (see Fig. 2) - yields δ ≈1.1. Thus, the apparent Cr CWM would be biased ~10% upwards. Note that Eq. [8] holds true for any change from an initial VGM/V on subsequent measurements (scenario (ii) above). Specifically, an easily incurred 20% WM and GM voxel composition difference due to misregistration can lead to quantification variations of the order of 10% for absolute quantification and even more for metabolite ratios (39).
WM and GM specificity loss due to partial volume variation
The MRSI signal of each metabolite in every voxel represents the sum of its GM and WM contributions. However, if a diffuse disease affects the concentration of a metabolite predominantly in GM, e.g. in cognitive disorders, or WM, e.g. in leukodystrophies, these tissue-specific changes will be “diluted” and modulated by the signal from the other unaffected tissue fractions, reducing the contrast between patients and controls and, consequently, the sensitivity.
To estimate the magnitude of this contrast loss, consider a diffuse disease that alters only a metabolite's GM concentration, CGM, by an amount ΔCGM. Denoting that metabolite's overall (unsegmented) concentration in controls by Cctrl, its concentration in patients, Cpts, will be:
| [9] |
where the metabolite (i) and patient (k) indices were omitted for brevity, , , are the total GM, WM and VOI tissue volumes. The observed global change in that metabolite's concentration will be VGM/V, smaller than the true change, ΔCGM, since VGM/V (or for that matter VWM/V) is always <1, reflecting a loss of both tissue specificity and sensitivity.
The consequences of such a loss can be estimated by assuming the GM and WM metabolites' concentrations are homogeneous within each patient and normally distributed in both controls and patients: , and , , where and since the pathology does not alter the WM concentrations. Here N(μ, σ) symbolizes a normal distribution having mean μ and standard deviation σ. We also assume, to first order, that the pathology only alters the means, not the standard deviations of the GM distributions. The number of patients needed to observe a change ΔCGM in a GM only VOI with 80% power and a two-sided significance level of 0.05, is given by (38):
| [10] |
.
The global non tissue-specific concentration of a metabolite in question, Cctrl, is given by the weighted average of its tissue concentrations,, where and are the VOI GM and WM fractions. Thus, Cctrl, Cpts are also normally distributed:
| [11] |
The number of patients needed to observe this difference, with the same statistical power (80%) and significance level (0.05) as before, has now increased:
| [12] |
For example, in the VOIs studied herein, the WM:GM≈3:2. Given whole-brain WM:GM of ~0.7 (40,41), fGMVOI ≈fGMVOI ≈ 0.5 is a reasonable first order approximation in any VOI large enough to observe diffuse metabolic changes. Furthermore, Table 1 reveals that for most metabolites . Substituting into Eq. [12], yields:
| [13] |
Eq. [13] represents a general rule of thumb for any sufficiently large, heterogeneous VOI: A linear regression like the one described can increase a study's statistical power two-fold.
Applications
The method is applicable in the following clinical scenarios: (i) In diseases with a known diffuse component, such as traumatic brain injury, multiple sclerosis, encephalopathies, late stage dementias and HIV-associated neurological disorder, tumors, leukodystrophies (in those diseases with focal centers, the method can be used to observe the diffuse component in the adjacent normal appearing tissue); (ii) In focal disease, where a hypothesis tests for diffuse involvement of normal-appearing tissue; (iii) In focal disease, known to advance to diffuse (e.g. dementias) in order to time the progression; (iv) in both diffuse and focal disease to assess global drug effects.
Caveats
Global WM and GM quantification is subject to several limitations arising from an approach geared to maximize sensitivity at the expense of localization: (i) It is insensitive to focal changes that may occur in specific small brain regions. (ii) Since metabolites' concentration are assumed to be the same in a given tissue type (GM or WM), only uniform diffuse changes (all increases or all decreases) are detectable. Large changes in concentrations between brain structures within a particular tissue - e.g., between the cerebrum and cerebellum (42) - will be averaged out, which will diminish the approach's statistical power. This limitation, however, can be relaxed since, although we used 480 equations (Eq. [4]) to deduce CWM, CGM, only two equations are actually necessary. The other 480-2=478 merely increase the robustness. Therefore, if the VOI is divided into subregions of at least 2 voxels each, their local CWM and CGM could be determined on spatial scale only slightly coarser than the acquisition grid. (iii) Long post-processing (~45 Role of tissue segmentation in brain 1H-MRSI min./subject) may impede clinical application. Finally, (iv) although our VOI covered substantially more brain than most single voxel or 2D 1H-MRSI studies, it excluded most of the cortex and infratentorial brain. Given the average brain GM and WM volumes of ~700 cm3 and 500 cm3 (41) our VOI contained 20% of the total GM (including cortical and most of the deep structures) and 40% of the WM. The cortical periphery has been excluded due to technical challenges of cortical spectroscopy, including lipid contamination and shimming artifacts. However, given a (multivoxel) sequence capable of overcoming these limitations, the cortex would make an excellent candidate for investigation using this technique: the tortuous, thin (1–4 mm) GM cortical strip is often impossible to observe directly with multivoxel spectroscopy due to the large spectroscopic voxels and the partial volume effects from the adjacent white matter. A linear-regression based method, such as the one described herein, in combination with the excellent performance of most segmentation algorithms for the other regions of the brain, can be used to obtain cortexspecific spectra. Given the functional and cognitive importance of the cortical GM, we anticipate the outlined approach to become a useful tool for probing cortical GM metabolite concentrations.
CONCLUSION
Although 1H-MRSI can yield spatial distribution maps of brain metabolites, these often do not come at sufficient SNR (sensitivity) to identify small changes incurred in diffuse pathologies. Amongst the (controllable) factors that lower the sensitivity is voxel tissue partial volume variations. Readily available segmentation software in conjunction with the anatomical MRI that is always available, allows us now to leverage the myriad 1H-MRSI voxels to obtain global tissue-specific metabolite concentrations. The process also dramatically, ∝(number of voxels)½, increases the precision and by its nature addresses the WM, GM and CSF partial volume metabolic quantification variations issue encountered in 1H-MRSI. Together, these may allow the detection of smaller metabolic changes with greater statistical power, and to better assign them to pathologies that may preferentially target the GM, WM or both, leading to higher sensitivity and consequently, fewer patients(or measurements) needed to determined a change. Finally note that this improvement is “free,” i.e., achieved entirely in post-processing, with no additional demands of the measurement time, protocol or hardware.
ACKNOWLEDGMENTS
This work was supported by NIH Grants EB01015, NS050520, NS39135 and NS29029. Assaf Tal acknowledges the support of the Human Frontiers Science Project.
This work was supported by NIH Grants EB01015, NS39135, NS29029 and NS0050520, and the Human Frontiers Science Project's Cross-Disciplinary Fellowship (HFSP's CDF).
Abbreviations Used
- GM
gray matter
- WM
white matter
- CSF
cerebrospinal fluid
- MRSI
magnetic resonance spectroscopic imaging
- SPM
statistical parametric mapping
- VOI
volume of interest
- FWHM
full-width at half-maximum
- CSI
chemical shift imaging
- NAA
N-Acetyl-Aspartate
- Cho
choline
- Cr
creatine
- mI
myo-inositol
REFERENCES
- 1.Mountford CE, Stanwell P, Lin A, Ramadan S, Ross B. Neurospectroscopy: the past, present and future. Chemical Reviews. 2010;110(5):3060–3086. doi: 10.1021/cr900250y. [DOI] [PubMed] [Google Scholar]
- 2.Tran T, Ross B, Lin A. Magnetic resonance spectroscopy in neurological diagnosis. Neurologic Clinics. 2009;27(1):21–60. doi: 10.1016/j.ncl.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Kirov II, George IC, Jayawickrama N, Babb JS, Perry NN, Gonen O. Longitudinal inter- and intra-individual human brain metabolic quantification over 3 years with proton MR spectroscopy at 3 T. Magnetic Resonance in Medicine. 2011 doi: 10.1002/mrm.23001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreis R, Slotboom J, Hofmann L, Boesch C. Integrated data acquisition and processing to determine metabolite contents, relaxation times, and macromolecule baseline in single examinations of individual subjects. Magn Reson Med. 2005;54(4):761–768. doi: 10.1002/mrm.20673. [DOI] [PubMed] [Google Scholar]
- 5.Kreis R. Quantitative localized 1H MR spectroscopy for clinical use. Progress in NMR Spectroscopy. 1997;31(2–3):155–195. [Google Scholar]
- 6.Barker PB, Bizzi A, Stefano ND, Gullapalli R, Lin DDM. Clinical MR Spectroscopy: Techniques and Applications. Cambridge University Press; 2009. [Google Scholar]
- 7.Caramanos Z, Narayanan S, Arnold DL. 1H-MRS quantification of tNA and tCr in patients with multiple sclerosis: a meta-analytic review. Brain. 2005;128(11):2483–2506. doi: 10.1093/brain/awh640. [DOI] [PubMed] [Google Scholar]
- 8.Jansen JF, Backes WH, Nicolay K, Kooi ME. 1H MR spectroscopy of the brain: absolute quantification of metabolites. Radiology. 2006;240(2):318–332. doi: 10.1148/radiol.2402050314. [DOI] [PubMed] [Google Scholar]
- 9.Li BS, Babb JS, Soher BJ, Maudsley AA, Gonen O. Reproducibility of 3D proton spectroscopy in the human brain. Magn Reson Med. 2002;47(3):439–446. doi: 10.1002/mrm.10081. [DOI] [PubMed] [Google Scholar]
- 10.Macovski A. Noise in MRI. Magn Reson Med. 1996;36(3):494–497. doi: 10.1002/mrm.1910360327. [DOI] [PubMed] [Google Scholar]
- 11.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maudsley AA, Domenig C, Sheriff S. Reproducibility of serial whole-brain MR Spectroscopic Imaging. NMR in Biomedicine. 2010;23:251–256. doi: 10.1002/nbm.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonekamp D, Horska A, Jacobs MA, Arslanoglu A, Barker PB. Fast Method for Brain Image Segmentation: Application to Proton Magnetic Resonance Spectroscopic Imaging. Magn Reson Med. 2005;54:1268–1272. doi: 10.1002/mrm.20657. [DOI] [PubMed] [Google Scholar]
- 14.Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, Nichols TE. Statistical Parametric Mapping: The Analysis of Functional Brain Images. Academic Press; 2006. [Google Scholar]
- 15.Degaonkar MN, Pomper MG, Barker PB. Quantitative proton magnetic resonance spectroscopic imaging: regional variations in the corpus callosum and cortical gray matter. Journal of Magnetic Resonance Imaging. 2005;22(2):175–179. doi: 10.1002/jmri.20353. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Javaid T, Arias-Mendoza F, Liu Z, McNamara R, Brown TR. A fast, reliable, automatic shimming procedure using 1H chemical-shift-imaging spectroscopy. Journal of Magnetic Resonance B. 1995;108:213–219. doi: 10.1006/jmrb.1995.1126. [DOI] [PubMed] [Google Scholar]
- 17.Goelman G, Liu S, Hess D, Gonen O. Optimizing the Efficiency of High-Field Multivoxel Spectroscopic Imaging by Multiplexing in Space and Time. Magn Reson Med. 2006;56:34–40. doi: 10.1002/mrm.20942. [DOI] [PubMed] [Google Scholar]
- 18.Goelman G, Liu S, Fleysher R, Fleysher L, Grossman RI, Gonen O. Chemical-Shift Artifact Reduction in Hadamard-Encoded MR Spectroscopic Imaging at High (3T and 7T) Magnetic Fields. Magn Reson Med. 2007;58:167–173. doi: 10.1002/mrm.21251. [DOI] [PubMed] [Google Scholar]
- 19.Brooker HR, Mareci TH, Mao JT. Selective Fourier transform localization. Magn Reson Med. 1987;5(5):417–433. doi: 10.1002/mrm.1910050503. [DOI] [PubMed] [Google Scholar]
- 20.Mareci TH, Brooker HR. Essential Considerations for Spectral Localization Using Indirect Gradient Encoding of Spatial Information. Journal of Magnetic Resonance. 1991;92:229–246. [Google Scholar]
- 21.Goelman G, Liu S, Gonen O. Reducing Voxel Bleed in Hadamard Encoded MRI and MRS. Magn Reson Med. 2006;55:1460–1465. doi: 10.1002/mrm.20903. [DOI] [PubMed] [Google Scholar]
- 22.Ashburner JT, Friston KJ. Multimodal image coregistration and partitioning--a unified framework. Neuroimage. 1997;6(3):209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- 23.Ashburner JT, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 24.Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: application to in vivo proton MR spectroscopy and spectroscopic imaging. Magn Reson Med. 1998;40(6):822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- 25.Soher BJ, Young K, Bernstein A, Aygula Z, Maudsley AA. GAVA: Spectral simulation for in vivo MRS applications. J Magn Reson. 2007 doi: 10.1016/j.jmr.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soher BJ, Young K, Maudsley AA. Representation of strong baseline contributions in 1H MR spectra. Magn Reson Med. 2001;45(6):966–972. doi: 10.1002/mrm.1129. [DOI] [PubMed] [Google Scholar]
- 27.Inglese M, Li BS, Rusinek H, Babb JS, Grossman RI, Gonen O. Diffusely elevated cerebral choline and creatine in relapsing-remitting multiple sclerosis. Magn Reson Med. 2003;50(1):190–195. doi: 10.1002/mrm.10481. [DOI] [PubMed] [Google Scholar]
- 28.Kirov II, Fleysher L, Fleysher R, Patil V, Liu S, Gonen O. Age dependence of regional proton metabolites T2 relaxation times in the human brain at 3T. Magn Reson Med. 2008;60(4):790–795. doi: 10.1002/mrm.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG, Marjanska M, Gasparovic C, Zuo C, Magnotta V, Mueller B, Mullins P, Renshaw P, Ugurbil K, Lim KO, Alger JR. Proton echo-planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magn Reson Med. 2007;58(2):236–244. doi: 10.1002/mrm.21287. [DOI] [PubMed] [Google Scholar]
- 30.Traber F, Block W, Lamerichs R, Gieseke J, Schild HH. 1H metabolite relaxation times at 3.0 tesla: Measurements of T1 and T2 values in normal brain and determination of regional differences in transverse relaxation. Journal of Magnetic Resonance Imaging. 2004;19(5):537–545. doi: 10.1002/jmri.20053. [DOI] [PubMed] [Google Scholar]
- 31.Malucelli E, Manners DN, Testa C, Tonon C, Lodi R, Barbiroli B, Lotti S. Pitfalls and advantages of different strategies for the absolute quantification of N-acetyl aspartate, creatine and choline in white and grey matter by 1H-MRS. NMR in Biomedicine. 2009;22:1003–1013. doi: 10.1002/nbm.1402. [DOI] [PubMed] [Google Scholar]
- 32.Doyle TJ, Bedell BJ, Narayana PA. Relative Concentrations of Proton MR Visible Neurochemicals in Gray and White Matter in Human Brain. Magn Reson Med. 1995;33:755–759. doi: 10.1002/mrm.1910330603. [DOI] [PubMed] [Google Scholar]
- 33.Govind V, Gold S, Kaliannan K, Saigal G, Falcone S, Arheart KL, Harris L, Jagid J, Maudsley AA. Whole-Brain Proton MR Spectroscopic Imaging of Mild-to-Moderate Traumatic Brain Injury and Correlation with Neuropsychological Deficits. Journal of Neurotrauma. 2010;27:483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeo RA, Gasparovic C, Merideth F, Ruhl D, Doezema D, Mayer AR. A Longitudinal Proton Magnetic Resonance Spectroscopy Study of Mild Traumatic Brain Injury. Journal of Neurotrauma. 2011;28:1–11. doi: 10.1089/neu.2010.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hetherington HP, Pan JW, Mason GF, Adams D, Vaughn MJ, Twieg DB, Pohost GM. Quantitative 1H Spectroscopic Imaging of Human Brain at 4.1 T Using Image Segmentation. Magn Reson Med. 1996;36:21–29. doi: 10.1002/mrm.1910360106. [DOI] [PubMed] [Google Scholar]
- 36.McLean MA, Barker GJ. Concentrations and Magnetization Transfer Ratios of Metabolites in Gray and White Matter. Magn Reson Med. 2006;56:1365–1370. doi: 10.1002/mrm.21070. [DOI] [PubMed] [Google Scholar]
- 37.Pan JW, Twieg DB, Hetherington HP. Quantitative Spectroscopic Imaging of the Human Brain. Magn Reson Med. 1998;40:363–369. doi: 10.1002/mrm.1910400305. [DOI] [PubMed] [Google Scholar]
- 38.Wasserman L. All of Statistics: A Concise Course in Statistical Inference. Springer; 2010. [Google Scholar]
- 39.Li BS, Wang H, Gonen O. Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magnetic Resonance Imaging. 2003;21(8):923–928. doi: 10.1016/s0730-725x(03)00181-4. [DOI] [PubMed] [Google Scholar]
- 40.Herndon RC, Lancaster JL, Giedd JN, Fox PT. Quantification of White Matter and Gray Matter Volumes from Three Dimensional Magnetic Resonance Volume Studies Using Fuzzy Classifiers. Journal of Magnetic Resonance Imaging. 1998;8(5):1097–1105. doi: 10.1002/jmri.1880080515. [DOI] [PubMed] [Google Scholar]
- 41.Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. Part II: quantitative magnetization transfer ratio histogram analysis. American Journal of Neuroradiology. 2002;23(8):1334–1341. [PMC free article] [PubMed] [Google Scholar]
- 42.Pouwels PJW, Frahm J. Regional metabolite concentrations in human brain as determined by quantitative localized proton MRS. Magn Reson Med. 1998;39(1):53–60. doi: 10.1002/mrm.1910390110. [DOI] [PubMed] [Google Scholar]