Summary
Haematological patients with neutropenic fever are frequently evaluated with chest computed tomography (CT) to rule out invasive fungal infections (IFI). We retrospectively analysed data from 100 consecutive patients with neutropenic fever and abnormal chest CT from 1998 to 2005 to evaluate their chest CT findings and the yield of diagnostic approaches employed. For their initial CTs, 79% had nodular opacities, with 24.1% associated with the halo sign. Other common CT abnormalities included pleural effusions (48%), ground glass opacities (37%) and consolidation (31%). The CT findings led to a change in antifungal therapy in 54% of the patients. Fifty-six patients received diagnostic procedures, including 46 bronchoscopies, 25 lung biopsies and seven sinus biopsies, with a diagnostic yield for IFI of 12.8%, 35.0% and 83.3%, respectively. In conclusion, chest CT plays an important role in the evaluation of haematological patients with febrile neutropenia and often leads to a change in antimicrobial therapy. Pulmonary nodules are the most common radiological abnormality. Sinus or lung biopsies have a high-diagnostic yield for IFI as compared to bronchoscopy. Patients with IFI may not have sinus/chest symptoms, and thus, clinicians should have a low threshold for performing sinus/chest imaging, and if indicated and safe, a biopsy of the abnormal areas.
Keywords: Invasive fungal infection, neutropenic fever, pulmonary nodules, biopsy, bronchoscopy, chest CT imaging
Introduction
Despite a vastly expanded armamentarium of antimicrobial agents and diagnostic modalities, infection remains a major cause of morbidity and mortality in patients with haematological malignancies. Such patients frequently develop prolonged neutropenia from their underlying disease or chemotherapy and as a consequence, are predisposed to significant infectious complications. It is well recognised that such patients are at high risk of invasive fungal infection (IFI) of the lungs and early evaluation with chest computed tomography (CT) is prudent for early diagnosis and improved outcome.1,2 Among various radiological abnormalities, nodular opacities, in particular those surrounded by a ‘halo’ of ground-glass attenuation (i.e. the halo sign), or associated with cavitation (i.e. the air-crescent sign) are highly associated with IFI of the lungs.3–5 These radiological findings have been incorporated into criteria that are employed to define probable or possible IFI diagnosis.6 However, it is well known that IFI may present with other radiological findings and other infectious or non-infectious aetiologies can also give similar radiological appearances. Given the concomitant profound thrombocytopenia and/or other co-morbidities in these patients, invasive diagnostic procedures are seldom performed. Rather, these patients are empirically or preemptively treated with antifungal agents. The evaluation and management of these patients remain a major clinical challenge to their treating physicians.
In this study, we retrospectively examined the records of 506 patients with haematological malignancies and neutropenic fever, who were admitted to our institution through a 6-year period. We identified 100 patients with chest CT abnormalities and examined their clinical and radiological characteristics as well as the yield of the various diagnostic procedures performed.
Methods
Patient population
Patients admitted to Stanford University Hospital with a haematological malignancy and neutropenic fever were identified retrospectively through chart review using two searching methodologies. For the first, from August 1998 to November 2004, a total of 424 patients, representing 737 admissions, were discharged from the Hematology Service with the diagnosis of neutropenic fever. Of the 424 patients, 155 (36.6%) had at least one chest CT performed; of these 155 patients, 118 (76.1%) had abnormalities on their chest CT. Forty patients were excluded for the following reasons: prior haematopoietic stem cell transplantation (HSCT; 14), no concurrent episode of neutropenia ≥4 days (12); history of renal transplantation (1); history of lung nodules associated with lymphoma (1); known history of IFI prior to admission (5); incomplete records (4); history of breast cancer (2); and duplicated record under a different medical record number (1). From this database, 78 patients with abnormal chest CT were included in our analysis. Our chart review process revealed that a number of haematology patients with neutropenic fever were not discharged with this diagnosis if a specific infection was identified; thus, a second searching strategy was employed. We further reviewed the medical records of 706 patients, who were discharged with the diagnosis of acute myeloid leukaemia (AML) or acute lymphoblastic leukaemia (ALL) from January 1999 to March 2005. We identified another 82 patients with neutropenic fever that were not captured in the first database. Of these patients, 29 were evaluated with chest CT imaging and 27 had at least one abnormal chest CT. Two patients were excluded due to incomplete records (1) or lack of a concurrent episode of neutropenia ≥4 days (1). As a result, 25 patients were added to the database, which totalled 103 patients. However, original chest CT imaging files were unavailable for three of these patients; thus, only 100 patients were included for final analysis. The study protocol was reviewed and approved by Stanford University’s Institutional Review Board on human subjects research.
Chest CT imaging
The patients included in this study were determined to have abnormal chest CT findings based on the radiology reports from medical records. Original chest CT imaging files were available for detailed analysis in 100 patients. Each CT was re-analysed by consensus by our radiologists Margaret Lin and Ann N. C. Leung in a blinded fashion, without any knowledge of the patients’ clinical data, except that they were known to be haematology patients with neutropenic fever.
Each exam was analysed for the presence or absence of ground glass opacity (GGO), consolidation, nodules, masses and pleural effusions. Standard terminology was used as per the Fleischner Society Glossary of Terms for Thoracic Imaging.7 GGO is seen on CT as hazy increased opacity of lung, with preservation of bronchial and vascular margins. Consolidation is a homogeneous increase in pulmonary parenchymal attenuation that obscures the margins of vessels and airway walls. A nodule is a rounded or irregular opacity, measuring up to 3 cm in diameter. A mass is any pulmonary, pleural, or mediastinal lesion greater than 3 cm in diameter that is a solid or partly solid opacity.
The presence or absence of cavitation, or the development of a gas-filled space, seen as a lucency or low-attenuation area, was noted in consolidation, nodules and masses. The presence or absence of a halo of GGO surrounding a nodule or mass was also noted.
The total number of nodules (1, 2–5, 6–10 or >10), the average diameter of the largest nodule (≤10 or 11–30 mm), and their distribution (centrilobular, perilymphatic or random) were all determined. The two-dimensional size of the five largest nodules and/or masses was measured on axial images, and the sum of the longest diameter of all target lesions was calculated. 8 Pleural effusions in each hemithorax were rated as none, small, moderate or large in size.
Sinus CT or magnetic resonance imaging (MRI)
Findings of the sinus imaging studies were obtained from the original radiology reports, without further re-analysis.
Bronchoscopy and biopsy procedures
Bronchoscopy and biopsy procedures were performed by various physicians, radiologists and surgeons through a span of 6 years. For each procedure, the general approach employed is described, but variation of the techniques or methodologies is expected.
Patients undergoing bronchoscopy received conscious sedation with intravenous fentanyl and midazolam. Nebulised lidocaine at 4% and topical liquid lidocaine at 2% were used to anaesthetise the posterior oropharynx. A small size, adult, flexible fibreoptic bronchoscope (Olympus, Center Valley, PA, USA) was then advanced through a bite block in the mouth into the traceho-bronchial tree through the vocal cords. Bronchoalveolar lavage (BAL) was performed by instillation of normal saline. The volume of saline instilled and the amount of fluid returned varied in different cases. A typical lavage included instillation of 60 ml of sterile normal saline with an approximate return of 25–35 ml (considered a satisfactory return). Specimens were then sent for cytopathology and culture. In some cases, transbronchial forceps biopsies were taken under fluoroscopic guidance. Throughout the procedure, heart rate, rhythm, blood pressure, respiratory rate and pulse oximetry are continually monitored. Supplemental oxygen is also administered to maintain adequate oxygen saturation over 92%.
Percutaneous (transthoracic) lung biopsies were performed by Interventional Radiology in the CT suite. The patient undergoing the procedure was positioned to optimise access to the targeted lesion. A limited scout view of the chest was obtained followed by localising axial images through the area of abnormality. The skin overlying the targeted area was prepped and draped in the usual sterile fashion. Moderate sedation was administered and local anaesthesia was achieved using 1% lidocaine. A 19-gauge thin-walled coaxial introducer needle was advanced under CT fluoroscopic guidance to the targeted area. Once correctly positioned, fine needle aspiration (FNA) was performed using a 20- to 22- gauge needle through the introducer needle. A cytotechnologist was present to determine adequacy of specimens. In some cases, multiple FNA and/or core samples were obtained. Axial CT images through the targeted area were obtained after the biopsy was completed. The specimens were then sent to cytopathology and to microbiology.
Sinus biopsies were performed by Otolaryngology-Head and Neck Surgery. The specimens were obtained transnasally, endoscopically, or via an open approach, such as an antrotromy, depending on the site of the lesion in individual patients. Video-assisted thoracoscopic surgery (VATS) and open lung biopsies were performed by cardiothoracic surgery. Description of these surgical procedures is beyond the scope of this article.
Mycology cultures
Fungal species were isolated in the Stanford Microbiology Laboratory and were identified using standard methodologies.9,10 The filamentous fungi were identified by their phenotypic characteristics.9,10 The Candida species were identified by the Dalmau method as well as the API 20C Yeast Identification System (bioMerieux, Marcy-l’Etoile, France) according to the manufacturer’s protocols.
Aspergillus galactomannan test
Detection of Aspergillus galactomannan antigen from patient sera was performed in 24 patients using the Bio-Rad Platelia® Aspergillus EIA method per protocol of the manufacturer (Bio-Rad Laboratories, Hercules, CA, USA). A total of 70 tests were performed, ranging from 1 to 10 tests per patient. An optical index of ≥0.5 was considered as positive.
Data analysis
Comparisons between two groups were analysed by Student’s t-test and Fisher’s exact test for interval and categorical data, respectively. Comparisons across three groups were analysed by ANOVA and Chi-square test for interval and categorical data, respectively. For nonparametric data, Kruskal–Wallis test was used. The findings are considered significant if the two-tailed probability of type I error was ≤0.05.
Definitions of invasive fungal infection and neutropenia
IFIs were classified as proven, probable or possible according to the revised definitions put forth by the IFIs Cooperative Group of the European Organization for Research and Treatment of Cancer and Mycoses Study Group of the National Institute of Allergy and Infectious Diseases (EORTC/MSG).6
Neutropenia is defined as an absolute neutrophil count (ANC) of ≤500 μl−1. For patient who deceased before the resolution of neutropenia, the duration of neutropenia is defined as the first day of ANC ≤500 μl−1 to the day of their demise.
Results
Patient characteristics
The demographics of the 100 patients are described in Table 1. For their underlying haematological malignancies (Table 1), the majority was diagnosed with either AML (n = 70) or ALL (n = 18). On average, the patients had received 2.4 cycles (median = 1; range = 1–8) of chemotherapy prior to the current episode of neutropenic fever. Fifty-five patients were status post their first cycle of induction chemotherapy. The duration of documented neutropenia ranged from 4 to 241 days (mean = 32 days; median = 22 days). All five patients who were neutropenic for ≥90 days suffered from either aplastic anaemia or AML transformed from myelodysplastic syndrome. Ninety-five patients and 88 patients were neutropenic for ≥7 days and for ≥10 days, respectively.
Table 1.
Demographics and underlying hematologic malignancies of 100 neutropenic febrile patients with chest CT abnormalities.
| Total no. of patients | 100 (%) |
|---|---|
| Age (years) | |
| Mean ± SD1 | 48.4 ± 14.5 |
| Median (range) | 49.5 (20–80) |
| Female | 41 |
| Race | |
| Caucasian | 61 |
| Asian/Pacific | 18 |
| Others | 21 |
| Hematological malignancy2 | |
| AML3 | 70 |
| ALL | 18 |
| Aplastic anaemia | 4 |
| CLL | 4 |
| Others | 4 |
Data are no. of patients (= percentage of total no. evaluated), unless otherwise indicated.
SD, standard deviation.
AML, acute myeloid leukaemia; ALL, acute lymphoblastoid leukaemia; CLL, chronic lymphocytic leukaemia. ‘Others’ include biphenotypic leukaemia (one), T cell lymphoblastic lymphoma (one) and Burkitt leukaemia/lymphoma (two).
AML subtypes: M0, n = 3; M1, n = 11; M2, n = 7, M3, n = 4; M4, n = 13; M5, n = 10; M7, n = 1; NOS/multilineage/unknown, n = 21.
Imaging studies
Chest CT results
Chest CTs were performed in these 100 patients due to persistent fever on broad-spectrum antibiotics or the development of pulmonary symptoms, at a mean of 25.6 days from the onset of neutropenia (median = 19 days; range = 1–154 days). Sixty-two patients reported pulmonary symptoms, including cough (n = 46), dyspnoea (n = 27), chest pain (n = 14), haemoptysis (n = 11) and sputum production (n = 10). Another nine patients developed pulmonary symptoms subsequent to the first chest CT, including two that developed haemoptysis. Fifty-eight patients were receiving antifungal drug(s) when the first chest CT was performed. The CT findings led to a change of the antifungal management within 48 h in 54 patients. Antifungal treatment was initiated in 28, discontinued in seven, or changed from one regimen to another in 19. Among the patients continued on antifungal therapy, 31 received monotherapy and 16 received combination therapies. The combinations included voriconazole plus caspofungin (n = 12), an amphotericin product plus voriconazole or itraconazole (n = 3), and an amphotericin product plus caspofungin (n = 1).
The radiological findings of the first chest CTs and the number of days elapsed from the onset of neutropenia to the CT imaging are detailed in Table 2. Nodular opacities accounted for the majority of abnormalities (79%), but only a small proportion of them had associated GGO (i.e. the ‘halo sign’) or cavitation (24.1% and 8.9%, respectively). For those patients with nodules ≤10 mm in size, a majority (55%) had at least six or more nodules. Others major CT abnormalities included GGO (37%) and consolidations (31%). Pleural effusion was a common finding (48%), but when present, the effusions were usually small. Examples of nodules, GGO and consolidation are depicted in Fig. 1.
Table 2.
Radiologic findings of first chest CTs and days elapsed from the onset of neutropenia to CT imaging.
| Total no. of CTs | 100 (%) | Days from onset of neutropenia to CT Mean (median; range) |
|---|---|---|
| Nodules (% of all first CTs) | 79 (79.0) | 26.9 (19; 1–154) |
| with halo (% of CTs with nodules) | 19 (24.1) | 20.8 (21; 2–43) |
| with cavitation (% of CTs with nodules) | 7 (8.9) | 37.9 (30; 12–78) |
| Masses1 (% of all first CTs) | 6 (6.0) | 13.8 (10; 3–29) |
| with halo (% of CTs with masses) | 4 (66.7) | 11.0 (9; 3–23) |
| with cavitation | 0 | / |
| GGO (% of all first CTs) | 37 (37.0) | 23.7 (22; 1–103) |
| Consolidation (% of all first CTs) | 31 (31.0) | 25.1 (20; 1–154) |
| with cavitation (% of CTs with consolidation) | 4 (12.9) | 66.0 (49; 12–154) |
| Pleural effusion (% of all first CTs) | 48 (48.0) | 23.1 (18.5; 1–154) |
| Large (% of CTs with pleural effusion) | 4 (8.3) | 16.8 (19; 10–19) |
| Moderate (% of CTs with pleural effusion) | 8 (16.7) | 38.5 (30.5; 13–103) |
| Small (% of CTs with pleural effusion) | 36 (75.0) | 20.3 (15; 1–154) |
| Number of nodules (% of CTs with nodules) | ||
| 1 | 12 (15.2) | 25.8 (19; 4–103) |
| 2–5 | 22 (27.8) | 26.2 (22.5; 1–58) |
| 6–10 | 16 (20.3) | 41.6 (27.5; 5–154) |
| >10 | 29 (36.7) | 19.7 (14; 2–78) |
| Size of nodules (% of CTs with nodules) | ||
| ≤10 mm | 31 (39.2) | 22.9 (18; 4–89) |
| 11–30 mm | 41 (51.9) | 32.0 (23; 1–154) |
| >30 mm | 6 (7.6) | 13.8 (10; 3–29) |
CT, chest computed tomography; GGO, ground glass opacity.
A mass is defined as a nodular opacity of >30 mm. All patients with mass(es) also had nodules of ≤30 mm.
Figure 1.
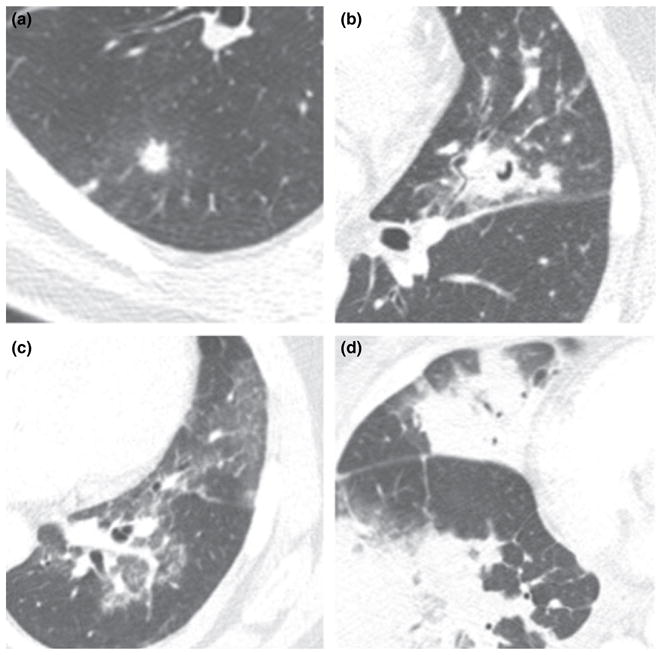
Axial CT images demonstrating (a) nodule with a halo of surrounding GGO, (b) nodule with ‘air crescent’ sign of cavitation, (c) geographic areas of GGO and (d) multifocal parenchymal opacification ranging from ground-glass to consolidation. CT, chest computed tomography; GGO, ground glass opacity.
Nodules associated with cavitation appeared later in the course of neutropenia than those associated with the halo sign (at a mean of 37.9 days vs. 20.8 days from the onset of neutropenia). However, this trend does not reach statistical significance.
Sinus imaging studies
Sinus imaging was performed on 19 patients (18 CTs and 1 MRI), with 14 studies (73.7%) reported as abnormal. Abnormalities included mucosal thickening of the maxillary, ethmoid, frontal and/or sphenoid sinuses, with or without fluid levels and/or opacification. Only four of these 19 patients had documented sinus symptoms; for the rest, sinus imaging was performed in evaluation of their protracted fever. All four patients with sinus symptoms had abnormal sinus CT findings.
Diagnostic procedures and tests
Data on diagnostic procedures and tests are presented in Table 3.
Table 3.
Diagnostic procedures and yields for fungal infections.
| Procedures | Total Number | Culture + Fungus1 | Staining + Fungus2 | Culture and staining + Fungus3 | Diagnostic yield for fungal infection in %4 |
|---|---|---|---|---|---|
| Bronchoscopy | |||||
| Number of patients (pts) | 39 | 3 | 5 | 3 | 12.8 |
| Number of bronchoscopies | 46 | 3 | 5 | 3 | 10.9 |
| All biopsy (bx) | |||||
| Number of pts | 28 | 8 | 11 | 4 | 53.6 |
| Number of bx | 38 | 8 | 14 | 4 | 47.4 |
| All lung bx5 | |||||
| Number of pts | 20 | 2 | 6 | 1 | 35.0 |
| Number of bx | 25 | 2 | 8 | 1 | 36.0 |
| Transthoracic FNA | |||||
| Number of pts | 17 | 1 | 4 | 0 | 29.4 |
| Number of bx | 18 | 1 | 4 | 0 | 27.8 |
| VATS | |||||
| Number of pts = no. of bx | 2 | 0 | 0 | 0 | 0 |
| Open lung bx | |||||
| Number of pts = number of bx | 4 | 1 | 4 | 1 | 100 |
| Sinus bx | |||||
| Number of pts | 6 | 4 | 3 | 2 | 83.3 |
| Number of bx | 7 | 4 | 3 | 2 | 71.4 |
| Liver bx | |||||
| Number of pts | 4 | 2 | 2 | 1 | 75 |
| Number of bx | 6 | 2 | 3 | 1 | 66.7 |
| Brain bx | |||||
| Number of pts = no. of bx | 1 | 0 | 0 | 0 | 0 |
| Blood culture | |||||
| Number of pts | 100 | 4 | – | – | 4.0 |
| Autopsy | |||||
| Number of pts | 2 | 1 | 2 | 1 | 100 |
Cultures of BAL/biopsy/autopsy specimens or blood that yielded the growth of a fungal species. Isolation of Candida species from BAL or non-sterile body sites was excluded.
Cytopathological or histopathological staining of BAL/biopsy/autopsy specimens that demonstrated the presence of hyphal elements consistent with a fungus. Yeast-like organisms from BAL or non-sterile body sites were excluded.
Both cultures and cytopathological/histopathological examination of the specimens were positive for the presence of a fungus. Findings of Candida species or yeast-like organisms from BAL or non-sterile body sites were excluded.
Diagnostic yield for fungal infection per total number of patients or per total number of procedures. Samples positive for P. jiroveci, or other non-fungal organisms (such as viral or bacterial pathogens) are excluded from the analysis.
Including transthoracic fine needle aspiration (FNA), video-assisted thoracoscopic surgery (VATS) and open lung biopsies. Transbronchial biopsies are excluded. Some patients underwent more than one type of lung biopsies.
Bronchoscopy
Thirty-nine patients underwent bronchoscopy, including five patients who underwent the procedure twice and one patient thrice. Fungal elements were observed in five BAL samples and three of them were identified as Aspergillus spp. by culture. One sample with Aspergillus fumigatus was also positive for cytomegalovirus by viral culture. Three additional samples yielded Pneumocystis jiroveci by histopathological staining and one sample grew out parainfluenza virus by viral culture. Two patients were diagnosed with bacterial pneumonia with BAL positive for Serratia marcescens and Escherichia coli, respectively. Transbronchial biopsy was performed in only four patients who underwent bronchoscopy and did not increase the yield of the procedure. For fungal infection, the diagnostic yield of bronchoscopy was 12.8%/10.9% per total number of patients/procedures. Overall diagnostic yields for any infections were 28.2%/24.1% per total number of patients/procedures. Diffuse alveolar haemorrhage was also noted in two patients by BAL. One patient was diagnosed with Influenza A by Rapid Flu test and one patient was diagnosed with atypical pneumonia with a positive IgM for Mycoplasma pneumoniae; these two patients did not undergo bronchoscopy.
Biopsies
Twenty-eight patients received one or more biopsies (excluding transbronchial biopsies) for diagnostic purpose, with a diagnostic yield of 53.6% for fungal infection. A total of 38 biopsies were performed, including 25 lung biopsies among 20 patients. Eleven of these patients had first undergone bronchoscopy but without any positive findings. Among the 20 patients with lung biopsies, a diagnosis of IFI was established in seven by either positive culture and/or histopathological findings, resulting in a total diagnostic yield of 35.0%. The diagnostic yield of transthoracic FNA biopsy was 29.4%, while that for open lung biopsy was 100%. Whether the patients were receiving antifungal treatment or not at the time of their chest CT imaging did not affect the diagnostic yield of the bronchoscopy or of lung biopsy (data not shown). The presence or absence of pulmonary symptoms also did not affect the yield of these procedures (data not shown). While 12 of 15 patients (80%) diagnosed with IFI of the lungs had pulmonary symptoms, all three patients diagnosed with Pneumocystis pneumonia (PcP) by bronchoscopy did not have any documented pulmonary symptoms. For those biopsy procedures that did not yield any fungal or other microbiological diagnoses, histopathological findings of the biopsy specimens include chloroma (1), reactive changes (1) and organising pneumonitis (1) among the transthoracic FNAs, as well as mixed inflammatory cells (1) and necrotising abscesses with granulomatous formation (1) with VATS. All the other ‘non-diagnostic’ biopsy specimens revealed benign lung tissues. Stainings for microbiological organisms were negative in these specimens. One patient that underwent transthoracic FNA developed a small pneumothorax, but placement of a chest tube was not required. None of the other patients who underwent transthoracic biopsy, video-assisted thoracoscopic surgery or open lung biopsy experienced any complications from the procedures.
Six patients underwent sinus biopsies, with four samples yielded a fungus by culture and three samples revealed the presence of fungal elements by histopathological examination. The diagnostic yield of sinus biopsy was 83.3%/71.4% per total number of patients/procedures. Among the five patients diagnosed with fungal sinusitis, only two had reported sinus symptoms.
Four patients underwent liver biopsies because of the findings of hepatic microabscesses by abdominal CT. The diagnostic yield of liver biopsies was 75%/66.7% per total number of patients/procedures. Two of the liver biopsy samples grew out Candida albicans and one yielded Scedosporium apiospermum. Brain biopsy was performed in one patient with a mass lesion in the right temporo-occipital lobe, concerning for infectious aetiology. Histopathological examination showed necrotic tissues; the biopsy was otherwise non-diagnostic. Patients who underwent biopsies but did not have any abnormal chest CTs were not included in our analysis.
Blood cultures
All 100 patients had multiple sets of blood cultures performed during their neutropenic febrile episodes; four of them had candidemia. Findings of bacteraemia are beyond the scope of this paper and will not be discussed.
Aspergillus galactomannan assay
During the latter part of 2003, the aspergillus galactomannan assay became available and was utilised in 24 patients. The test was performed from 1 to 11 times per patient (mean = 2.9; median = 1.5). Only two patients had galactomannan levels >0.5, but one of them had received piperacillin-tazobactam and the result was interpreted as false positive. The one patient with a positive galactomannan test underwent a lung biopsy by transthoracic FNA, which was non-diagnostic.
Autopsy data
Two patients underwent autopsy and IFI of the lungs was demonstrated in both cases. One patient was known to have IFI antemortem per open lung biopsy. The other patient developed candidemia and initial chest CT performed 28 days prior to her death showed GGO. Subsequent chest radiographs showed the development of bibasilar and right upper lobe parenchymal opacities, but no followup chest CT was performed prior to her demise. Aspergillus-like species was identified in her lung tissues at autopsy.
Diagnoses of IFI and other pulmonary infections
IFI was categorised as proven, probable and possible in 15, 9 and 47 patients, respectively, per revised EORTC/MSG criteria (Table 4).6 Patients who did not meet any criteria for IFI are categorised as ‘other’. Those in the ‘Possible’ category were re-classified as ‘other’ if a specific non-fungal infection of the lungs was diagnosed.
Table 4.
Invasive fungal infection (IFI) diagnosis in 100 neutropenic febrile patients.
| IFI diagnosis | No. of patients (%) |
|---|---|
| Proven | 15 |
| Probable | 9 |
| Possible | 47 |
| Other1 | 29 |
| PcP | 3 |
| Viral | 2 |
| Bacterial | 2 |
| Without specific diagnosis | 22 |
| Proven/probable IFI | 24 (100) |
| Organ system involved: | |
| Blood (total) | 4 (16.6) |
| Liver (total) | 3 (12.5) |
| Lung (total) | 15 (62.5) |
| Sinus (total) | 5 (20.8) |
| Liver and blood | 1 (4.2) |
| Lung and blood2 | 1 (4.2) |
| Lung and sinus | 1 (4.2) |
| Positive cultures | 16 (100) |
| Aspergillus spp. | 9 (52.9) |
| A. fumigatus | 7 (43.8) |
| Non-fumigatus | 2 (12.5) |
| Scedoporium apiospermum | 2 (11.8) |
| Candida spp. | 5 (29.4) |
| C. albicans | 2 (12.5) |
| C. glabrata | 1 (6.3) |
| C. krusei | 1 (6.3) |
| C. tropicalis | 1 (6.3) |
Patients who did not meet revised EORTC/MSG criteria for ‘Proven’, ‘Probable’ or ‘Possible’ fungal infections are grouped into ‘Other’. Seven patients in ‘Other’ received specific diagnoses based on positive BAL cultures/cytology, serology or histopathology findings.
Candidemia and invasive mould infection in lungs.
Among the 24 patients with proven/probable IFI, the organ systems involved are listed in Table 4. Major abnormal chest CT findings among these patients include nodules in 20 patients (83.3%), GGO in three (12.5%) and consolidation in one (4.2%) The three patients with GGO had candidemia (and one of them also grew S. marcescens in BAL), while the one patient with consolidation had biopsy-proven fungal sinusitis. For the 15 patients with pulmonary IFI, all but one had pulmonary nodules; however, only five had the halo sign and three had cavitation associated with the nodules. For the one patient without lung nodules, GGO was the predominant chest CT finding and IFI of the lungs was diagnosed at autopsy as described above. Sixteen patients had cultures positive for fungus, including 11 moulds and five Candida spp. (Table 4).
Seven patients without any IFI were diagnosed with other pulmonary infections, including PcP (three patients), Influenza A, Parainfluenza, M. pneumoniae and E. coli. One patient with PcP and the patient with M. pneumoniae had both pulmonary nodules and GGO. Another patient with PcP had only nodules, but no GGO. For the other four patients, GGO or consolidation was the predominant radiological abnormalities.
The characteristics of patients categorised into proven/probable IFI, possible IFI, or ‘other’ were compared (Table 5). Patients with proven/probable or possible IFI had a longer episode of neutropenia as compared to ‘other’ (52.7 days or 30.5 days versus 18.7 days; P = 0.01 and 0.005, respectively). Chi-square analysis also showed significant differences among the three groups in the number of patients that had pulmonary nodules in their chest CTs (P < 0.001) or had nodules >10 mm in size (P = 0.023). Further statistical analysis showed that patients with proven/probable and possible IFI were more likely to have pulmonary nodules than patients categorised as ‘other’ (RR = 2.5, P < 0.001). Patients with proven/probable IFI were also more likely to have pulmonary nodules >10 mm as compared to the other two groups (RR = 2.5, P = 0.019).
Table 5.
Comparison of patients with proven/probable IFI, possible IFI or ‘Other’
| Variables | Proven/probable IFI n (%) | Possible IFI n (%) | ‘Other’ n (%) | P-value1 |
|---|---|---|---|---|
| Total | 24 (100) | 47 (100) | 29 (100) | |
| Age | ||||
| Mean ± SD | 51.7 ± 14.2 | 48.9 ± 14.3 | 47.5 ± 13.8 | 0.55 |
| Female | 13 (54.2) | 16 (34.0) | 12 (41.4) | 0.26 |
| Diagnosed with AML | 16 (66.7) | 34 (72.3) | 20 (69.0) | 0.88 |
| Chemotherapy2 | ||||
| One cycle | 16 (66.7) | 28 (59.6) | 11 (37.9) | 0.08 |
| ≥3 cycles | 4 (16.7) | 17 (36.2) | 13 (44.8) | 0.09 |
| Prior relapse3 | 9 (37.5) | 22 (46.8) | 8 (27.6) | 0.24 |
| Total days of neutropenia | ||||
| Mean ± SD | 52.7 ± 65.7 | 30.5 ± 16.2 | 18.7 ± 17.3 | <0.001 |
| Median | 25.5 | 26 | 11 | |
| First CT with nodules | 20 (80.0) | 45 (93.8) | 14 (46.7) | <0.001 |
| >10 nodules4 | 7 (35.0) | 17 (37.8) | 5 (35.7) | 0.97 |
| >10 mm4 | 17 (85.0) | 22 (48.9) | 8 (57.1) | 0.023 |
CT, chest computed tomography; IFI, invasive fungal infection; AML, acute myeloid leukaemia.
P-values are calculated by ANOVA and Chi-square test for interval and categorical data, respectively, except that for ‘Total days of neutropenia ‘, Kruskal--Wallis analysis is used.
Number of patients that had 1 or ≥3 chemotherapy cycles performed prior to the first chest CT.
Number of patients that had a history of relapse of their hematologic malignancy prior to the first chest CT.
Percentages calculated out of patients who had first CT with nodules.
Discussion
IFI causes significant mortality and morbidity in immunocompromised hosts and remains one of the greatest diagnostic challenges to clinicians. Chest CT imaging is frequently employed to evaluate haematological patients with neutropenic fever and the chest CT characteristics of IFI (or specifically, of invasive pulmonary aspergillosis, IPA) have been extensively described. CT findings may include nodules (with or without associated halo sign or crescent sign), patchy or segmental consolidation, peribronchial consolidation or centrilobular nodules, or a combination of these.11–14 Among all these radiological findings, the halo sign has been hailed as the one highly suggestive of IPA.15 Pathophysiologically, the halo sign represents a discrete nodule of an angioinvasive mould with infarction and coagulative necrosis surrounded by alveolar haemorrhage. Although a number of pathologic processes, both infectious and non-infectious, can lead to pulmonary nodules (infarcts) with surrounding hemorrhage,16–20 in the right clinical setting, such as patients with haematological malignancies and neutropenic fever, this radiological finding is highly suggestive of IPA,21 with sensitivity and specificity up to 75% and 100%, respectively.12 Among our 15 patients with proven or probable pulmonary IFI, over 93% had pulmonary nodules, but only five had nodules associated with a halo sign. However, it is now recognised the halo sign is a transient phenomenon, primarily associated with the early phase of the infection.5,22 It is conceivable that some of the initial CTs in our cohort might be obtained during the later phase of infection, leading to a low sensitivity of the halo sign for IFI diagnosis. Consistent with the literature, we also noted the appearance of cavitation later than that of the halo sign during the course of neutropenia.5,22 There were also patients with pulmonary nodules (with or without associated halo) that received non-fungal diagnoses for their pulmonary diseases, but certainly, concomitant IFI cannot be ruled out in those cases. Nevertheless, it is important to emphasise that the CT manifestations of IFI can be variable and clinicians treating neutropenic febrile patients cannot lower their suspicion for IFI based on the lack of a halo sign.
The chest CT findings led to the performance of diagnostic procedures in over half of our patients, including 50 who received flexible bronchoscopy and/or lung biopsy. The diagnostic yield of bronchoscopy was low in our patient population, leading to an infectious disease diagnosis in only 28.2% of the patients (with 12.8% diagnosed with a fungal infection). Other studies have shown that the diagnostic yield of flexible bronchoscopy for histologically proven IPA ranges from 0% to 67%, with an average of 43% (summarised in [23]). Such wide variation may reflect the heterogeneity of the patient populations. Our study includes only neutropenic febrile patients with haematological malignancies and without a history of HSCT. In contrast, many of these prior studies included patients with other immunocompromised states, such as solid organ transplantation or HSCT, chronic steroid use or HIV infection.
Reichenberger et al. [23] evaluated 23 patients with neutropenia and proven IPA who underwent diagnostic bronchoscopy with BAL. The diagnostic yield for pulmonary fungal infection was only 30%. Similar to our experience, BAL was often negative despite extensive fungal infection confirmed by lung resection or autopsy. These authors noted that patients with a positive bronchoscopic result had received a lower cumulative dose of amphotericin B prior to bronchoscopy (not significant) or had more extensive disease radiologically as compared to patients with a negative bronchoscopy (P < 0.05). Therefore, the authors proposed that BAL should be performed before antifungal treatment is started. Although our study identified only five patients with bronchoscopy diagnostic for fungal infection, comparison of this group with those with non-diagnostic bronchoscopy did not yield any significant differences in the total days of neutropenia or the total days of anti-fungal therapy administered prior to bronchoscopy (data not shown). In fact, nine patients with non-diagnostic bronchoscopy (including two with biopsy proven pulmonary IFI) did not receive any anti-fungal therapy prior to the procedure. Therefore, from our experience, withholding antifungal therapy until bronchoscopy did not increase the yield of the procedure. Another group has also observed that the sensitivity of BAL depended on the size of the focal lesions, at 50–80% for lesions >2 cm in size and about 15% for smaller lesions.24 For our five patients with bronchoscopy yielding evidence of IFI, all had nodules of ≤1 cm. Their chest CT findings (e.g. the size or number of nodules, the presence or absence of halo sign/cavitation, or the extent of radiological abnormalities) also did not significantly differ from those with non-diagnostic bronchoscopy (data not shown). We did observe that four of these five patients (80%) had haemoptysis, while only 9 out of 36 patients (25%) with non-diagnostic bronchoscopy haemoptysised (P = 0.05). It is conceivable that patients with haemoptysis likely had more extensive invasive disease of the airways, which then led to a higher diagnostic yield.
Given the low diagnostic yield of bronchoscopy/BAL at our institution, 20 patients proceeded to lung biopsy. Our overall diagnostic yield of all lung biopsies was 35% and that of CT-guided transthoracic FNA was 29.4%. Recent studies showed that the yield of transthoracic FNA in patients with haematological malignancies is about 56–80%.25,26 In one study, complications occurred in up to 25% of the patients.26 Besides pneumothorax and hemothorax, other complications such as fatal pulmonary haemorrhage and cutaneous dissemination of the fungal infection have also been reported.27,28 In our study, only one patient suffered from a small pneumothorax. Overall, CT-guided transthoracic FNA is relatively safe, especially if the patients can be transfused to achieve a platelet count of >50 × 109 l−1.
For open lung biopsy, two previous studies in patients with haematological malignancies or HSCT29,30 showed that the procedure provided a specific diagnosis in about two-third of the cases, which then led to a change in therapy in 40–57% of the patients. It was, therefore, concluded that open lung biopsy has a significant yield and impact on the management of these patients29 and may lead to a better outcome.30 For our study, four patients underwent open lung biopsy with a 100% diagnostic yield for fungal infection. However, all four patients were already receiving appropriate antifungal therapy prior to the procedure and there was no significant change of management based on the biopsy findings. None of our patients developed any significant complications from the procedures despite their profound neutropenia and thrombocytopenia, but certainly, the possibility of selection bias must be considered. Despite the encouraging diagnostic yield of open lung biopsy, given its highly invasive nature, it is hardly considered routine.
While the diagnostic yield of lung biopsies for IFI is quite low in our institution, biopsy by nasal endoscopy provides a diagnosis of fungal sinusitis in >80% of the cases. Sinus involvement is a common manifestation of IFI in neutropenic patients. Mortality of acute fulminant invasive fungal rhinosinusitis can be up to 50–80%, secondary to invasion of the orbit and intracranial cavity and the initial symptoms are often subtle (reviewed in [31]). In up to 90% of patients, the most common symptom is persistent fever that fails to respond to appropriate broad-spectrum antibiotics. Localising symptoms such as facial and/or periorbital pain, nasal congestion, rhinorrhoea and headache are variably present in 20–60% of patients. In this study, among the five patients diagnosed with invasive fungal sinusitis, only two reported localising sinus symptoms. Thus, clinicians evaluating patients with febrile neutropenia must maintain a high index of suspicion for fungal sinusitis and have a low threshold to obtain sinus imaging studies. Although the patients who underwent sinus imaging in this study were a subset of those identified through abnormal chest CT, a review of our entire database of neutropenic febrile patients did not identify any other individuals who had isolated invasive fungal rhinosinusitis without concomitant chest CT abnormalities. In our experience, diagnostic biopsy by nasal endoscopy has a higher yield than bronchoscopy or transthoracic lung biopsy for IFI. Furthermore, for those patients with fungal sinusitis, early diagnosis with appropriate antibiotic treatment and aggressive surgical debridement may also improve their outcome.
Recently, the detection of Aspergillus galactomannan, β-glucan or fungal DNA (by real-time PCR) in serum or BAL has been added to the diagnostic tools for invasive pulmonary aspergillosis.32 However, these methodologies are currently not well standardised and have variable sensitivities and specificities. Furthermore, it has been reported that the detection of galactomannan does not precede detection of major radiological findings suggestive of IPA.33 Therefore, given the high morbidity and mortality associated with IFI, early evaluation of neutropenic febrile patients with chest CT imaging remains paramount in their care. In fact, for our patient population, the performance of chest CT imaging led to a change of their antifungal management in over half of our patients. Further studies are central to establish what the best algorithms are to combine the use of chest CT with newer diagnostic modalities including serum/BAL galactomannan and PCR.
Finally, only about a quarter of our patients were diagnosed with proven or probable fungal infections, while 47% were categorised as having possible fungal infection per diagnostic criteria put forth by EORTC/MSG.6 The patients with proven/probable fungal infection were noted to have a significantly longer duration of neutropenia, which might then lead to larger pulmonary nodules with increased fungal burden and higher diagnostic yield, thus fulfilling the criteria for proven/probable IFI. Alternatively, patients with protracted neutropenic fever might also be more likely to receive diagnostic procedures. Recent studies noted that the original EORTC/MSG diagnosis algorithm34 lack sensitivity. Studying series of autopsy proven IPA cases, only 25–47% would receive antemortem diagnoses of proven or probable IPA.35–37 In our cohort, one of the two patients that underwent autopsy was also found to have IFI diagnosed only postmortem. The definitions of IFI have been revised by EORTC/MSG recently with expanded definition of ‘proven’ IFI, but stricter inclusion criteria for the ‘possible’ category.6 How these changes will affect the sensitivity and specificity of the IFI definitions remains to be evaluated. Nonetheless, such guidelines provide a standardisation of definitions used in clinical studies, but should not be used for clinical decision-making.6,36
In conclusion, we have studied a cohort of haematological patients with neutropenic fever and abnormal chest CT imaging. This study is limited by its retrospective nature, the small size of the cohort and the fact that only a quarter of our patients had proven or probable IFI per revised EORTC/MSG criteria. Nevertheless, chest CT imaging is an important modality for the evaluation of IFI in such patients and it leads to a change of antifungal management in over half of our patients. For abnormal chest CT findings, we noted that close to 80% of the patients had pulmonary nodules, but only a quarter of them were associated with the halo sign, which, in our cohort, has a low sensitivity. In our experience, diagnostic biopsy by nasal endoscopy for invasive fungal disease has a higher yield than lung biopsy, while bronchoscopy has the lowest yield. Furthermore, since patients with fungal sinusitis or pulmonary IFI may not have any localising symptoms, clinicians evaluating neutropenic febrile patients should have a low threshold for sinus and/or chest imaging. If indicated and safe, a biopsy of the abnormal sites should also be considered.
Acknowledgments
This work was supported by postdoctoral NIH training grants ‘Applied Genomics and Infectious Diseases’ (T32 AI07502) to Dora Y. Ho and Joanna Schaenman, as well as ‘Epi of Emerging Infections/Bioterrorism’ (1 T32 AI052073) to Joanna Schaenman. We thank Emily Cooper, Brian Doan, Jeanne Talucod and Ann Vongspanich in their assistance in data collection.
References
- 1.Caillot D, Mannone L, Cuisenier B, Couaillier JF. Role of early diagnosis and aggressive surgery in the management of invasive pulmonary aspergillosis in neutropenic patients. Clin Microbiol Infect. 2001;7:54–61. doi: 10.1111/j.1469-0691.2001.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 2.Kuhlman JE, Fishman EK, Burch PA, Karp JE, Zerhouni EA, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia. The contribution of CT to early diagnosis and aggressive management. Chest. 1987;92:95–99. doi: 10.1378/chest.92.1.95. [DOI] [PubMed] [Google Scholar]
- 3.Blum U, Windfuhr M, Buitrago-Tellez C, et al. Invasive pulmonary aspergillosis. MRI, CT, and plain radiographic findings and their contribution for early diagnosis. Chest. 1994;106:1156–61. doi: 10.1378/chest.106.4.1156. [DOI] [PubMed] [Google Scholar]
- 4.Potente G. Computed tomography in invasive pulmonary aspergillosis. Acta Radiol. 1989;30:587–90. [PubMed] [Google Scholar]
- 5.Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19:253–9. doi: 10.1200/JCO.2001.19.1.253. [DOI] [PubMed] [Google Scholar]
- 6.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 8.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 9.Koneman EW, Roberts GD. Practical Laboratory Mycology. 3. London: Williams and Wilkins; 1985. [Google Scholar]
- 10.Larone DH. Medically Important Fungi: A Guide to Identification. 4. New York: Elsevier; 2002. [Google Scholar]
- 11.Horger M, Hebart H, Einsele H, et al. Initial CT manifestations of invasive pulmonary aspergillosis in 45 non-HIV immunocompromised patients: association with patient outcome? Eur J Radiol. 2005;55:437–44. doi: 10.1016/j.ejrad.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Kami M, Kishi Y, Hamaki T, et al. The value of the chest computed tomography halo sign in the diagnosis of invasive pulmonary aspergillosis. An autopsy-based retrospective study of 48 patients. Mycoses. 2002;45:287–94. doi: 10.1046/j.1439-0507.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- 13.Logan PM, Primack SL, Miller RR, Muller NL. Invasive aspergillosis of the airways: radiographic, CT, and pathologic findings. Radiology. 1994;193:383–8. doi: 10.1148/radiology.193.2.7972747. [DOI] [PubMed] [Google Scholar]
- 14.Won HJ, Lee KS, Cheon JE, et al. Invasive pulmonary aspergillosis: prediction at thin-section CT in patients with neutropenia – a prospective study. Radiology. 1998;208:777–82. doi: 10.1148/radiology.208.3.9722859. [DOI] [PubMed] [Google Scholar]
- 15.Kuhlman JE, Fishman EK, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985;157:611–4. doi: 10.1148/radiology.157.3.3864189. [DOI] [PubMed] [Google Scholar]
- 16.Althoff Souza C, Muller NL, Marchiori E, Escuissato DL, Franquet T. Pulmonary invasive aspergillosis and candidiasis in immunocompromised patients: a comparative study of the high-resolution CT findings. J Thorac Imaging. 2006;21:184–9. doi: 10.1097/01.rti.0000213552.16011.ad. [DOI] [PubMed] [Google Scholar]
- 17.Chamilos G, Marom EM, Lewis RE, Lionakis MS, Kontoyiannis DP. Predictors of pulmonary zygomycosis versus invasive pulmonary aspergillosis in patients with cancer. Clin Infect Dis. 2005;41:60–66. doi: 10.1086/430710. [DOI] [PubMed] [Google Scholar]
- 18.Franquet T, Muller NL, Gimenez A, Martinez S, Madrid M, Domingo P. Infectious pulmonary nodules in immunocompromised patients: usefulness of computed tomography in predicting their etiology. J Comput Assist Tomogr. 2003;27:461–8. doi: 10.1097/00004728-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Franquet T, Muller NL, Lee KS, Oikonomou A, Flint JD. Pulmonary candidiasis after hematopoietic stem cell transplantation: thin-section CT findings. Radiology. 2005;236:332–7. doi: 10.1148/radiol.2361031772. [DOI] [PubMed] [Google Scholar]
- 20.Primack SL, Hartman TE, Lee KS, Muller NL. Pulmonary nodules and the CT halo sign. Radiology. 1994;190:513–515. doi: 10.1148/radiology.190.2.8284408. [DOI] [PubMed] [Google Scholar]
- 21.Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–9. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 22.Brodoefel H, Vogel M, Hebart H, et al. Long-term CT followup in 40 non-HIV immunocompromised patients with invasive pulmonary aspergillosis: kinetics of CT morphology and correlation with clinical findings and outcome. AJR Am J Roentgenol. 2006;187:404–13. doi: 10.2214/AJR.05.0513. [DOI] [PubMed] [Google Scholar]
- 23.Reichenberger F, Habicht J, Matt P, et al. Diagnostic yield of bronchoscopy in histologically proven invasive pulmonary aspergillosis. Bone Marrow Transplant. 1999;24:1195–9. doi: 10.1038/sj.bmt.1702045. [DOI] [PubMed] [Google Scholar]
- 24.Shelhamer JH, Toews GB, Masur H, et al. NIH conference. Respiratory disease in the immunosuppressed patient. Ann Intern Med. 1992;117:415–31. doi: 10.7326/0003-4819-117-5-415. [DOI] [PubMed] [Google Scholar]
- 25.Carrafiello G, Lagana D, Nosari AM, et al. Utility of computed tomography (CT) and of fine needle aspiration biopsy (FNAB) in early diagnosis of fungal pulmonary infections. Study of infections from filamentous fungi in haematologically immunodeficient patients. Radiol Med (Torino) 2006;111:33–41. doi: 10.1007/s11547-006-0004-9. [DOI] [PubMed] [Google Scholar]
- 26.Wong PW, Stefanec T, Brown K, White DA. Role of fine-needle aspirates of focal lung lesions in patients with hematologic malignancies. Chest. 2002;121:527–32. doi: 10.1378/chest.121.2.527. [DOI] [PubMed] [Google Scholar]
- 27.Pearce JG, Patt NL. Fatal pulmonary hemorrhage after percutaneous aspiration lung biopsy. Am Rev Respir Dis. 1974;110:346–9. doi: 10.1164/arrd.1974.110.3.346. [DOI] [PubMed] [Google Scholar]
- 28.Tobin EH, Westenfeld F, Dietrich PA. Cutaneous infection due to Aspergillus species after transthoracic lung biopsy. Clin Infect Dis. 1993;17:955–6. doi: 10.1093/clinids/17.5.955. [DOI] [PubMed] [Google Scholar]
- 29.White DA, Wong PW, Downey R. The utility of open lung biopsy in patients with hematologic malignancies. Am J Respir Crit Care Med. 2000;161:723–9. doi: 10.1164/ajrccm.161.3.9904016. [DOI] [PubMed] [Google Scholar]
- 30.Zihlif M, Khanchandani G, Ahmed HP, Soubani AO. Surgical lung biopsy in patients with hematological malignancy or hematopoietic stem cell transplantation and unexplained pulmonary infiltrates: improved outcome with specific diagnosis. Am J Hematol. 2005;78:94–99. doi: 10.1002/ajh.20258. [DOI] [PubMed] [Google Scholar]
- 31.Gillespie MB, O’Malley BW. An algorithmic approach to the diagnosis and management of invasive fungal rhinosinusitis in the immunocompromised patient. Otolaryngol Clin North Am. 2000;33:323–34. doi: 10.1016/s0030-6665(00)80008-0. [DOI] [PubMed] [Google Scholar]
- 32.Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–22. doi: 10.1016/S1473-3099(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 33.Weisser M, Rausch C, Droll A, et al. Galactomannan does not precede major signs on a pulmonary computerized tomographic scan suggestive of invasive aspergillosis in patients with hematological malignancies. Clin Infect Dis. 2005;41:1143–9. doi: 10.1086/444462. [DOI] [PubMed] [Google Scholar]
- 34.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 35.Chamilos G, Luna M, Lewis RE, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003) Haematologica. 2006;91:986–9. [PubMed] [Google Scholar]
- 36.Hachem R, Sumoza D, Hanna H, Girgawy E, Munsell M, Raad I. Clinical and radiologic predictors of invasive pulmonary aspergillosis in cancer patients: should the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) criteria be revised? Cancer. 2006;106:1581–6. doi: 10.1002/cncr.21755. [DOI] [PubMed] [Google Scholar]
- 37.Subira M, Martino R, Rovira M, Vazquez L, Serrano D, De La Camara R. Clinical applicability of the new EORTC/MSG classification for invasive pulmonary aspergillosis in patients with hematological malignancies and autopsy-confirmed invasive aspergillosis. Ann Hematol. 2003;82:80–82. doi: 10.1007/s00277-002-0599-4. [DOI] [PubMed] [Google Scholar]


