Summary
Many studies have demonstrated that GABAergic inhibition within the basolateral amygdala (BLA) plays an integral role in the regulation of anxiety, an important behavioral component in the etiology of alcoholism. Although ethanol has recently been shown to enhance BLA GABAergic inhibition via two distinct populations of inhibitory cells, local and lateral paracapsular (lpcs) interneurons, little is known about the mechanisms underlying ethanol potentiation of these two inhibitory pathways. Ethanol is known to enhance GABAergic inhibition in many brain regions via a complex array of pre- and postsynaptic mechanisms. In addition, ethanol’s presynaptic effects are often subject to GABAB autoreceptor (GABAB-R) modulation. Therefore, in this study, we characterized GABAB-R function and modulation of ethanol actions at local and lpcs GABAergic synapses. At local synapses, we found significant paired-pulse depression (PPD, 250 msec interpulse interval) which was abated by SCH-50911 (GABAB-R antagonist). No significant PPD was detected at lpcs synapses, but SCH-50911 significantly potentiated lpcs-evoked IPSCs. Baclofen (GABAB-R agonist) had similar depressant effects on local- and lpcs-evoked IPSCs, however baclofen pretreatment only reduced ethanol potentiation at local synapses. Ethanol also significantly enhanced the frequency of spontaneous and miniature IPSCs, and these effects were also sensitive to GABAB-R modulators. Collectively, these data suggest that stimulus-independent inhibitory responses recorded from BLA principal neurons primarily reflect the activity of local GABAergic interneurons and provide additional evidence that ethanol potentiates local BLA inhibitory synapses primarily via a presynaptic enhancement of GABA release that is tightly regulated by GABAB-R. In contrast, ethanol potentiation of lpcs GABAergic synapses is not sensitive to GABAB-R activation and does not appear to involve increased presynaptic GABA release.
Keywords: Ethanol, Anxiety, GABAergic interneurons, Basolateral Amygdala, IPSCs, GABAB receptors
Introduction
Converging lines of clinical, behavioral, and pharmacological evidence suggest that the relationship between ethanol (EtOH) drinking and anxiety disorders may be an important component in the development and maintenance of alcoholism (Kushner et al., 2000). Indeed, alcohol use disorders are often found to be more prevalent in patients with many types of stress and anxiety disorders, such as generalized anxiety disorder (Grant et al., 2005), social anxiety (Carrigan and Randall, 2003), panic disorder (Cosci et al., 2007), or posttraumatic stress disorder (Stewart, 1996). In addition, stress and anxiety are also known to be important mediators in relapse to alcoholism (Breese et al., 2005). In the preclinical setting, it has been shown that episodes of stress can substitute for initial cycles of EtOH withdrawal in a behavioral model that increases EtOH-withdrawal anxiety in rats (Breese et al., 2004). Similarly, exposure to stress has been shown to increase anxiety and alcohol craving in recently abstinent human alcoholics (Fox et al., 2007), a response that was greater in those dependent on alcohol than in social drinkers (Sinha et al., 2008). While the exact etiology of this connection between anxiety and alcoholism is not clearly defined, determining the neuropharmacological effects of EtOH in brain regions associated with anxiety-like behaviors may lead to a better understanding of the mechanisms underlying the development of alcoholism.
One brain region that is thought to play a critical role in the regulation of anxiety-like behaviors is the basolateral complex of the amygdala (BLA) (LeDoux, 1996). Located in the medial temporal lobe, the entire amygdaloid complex is comprised of many structurally diverse nuclei. Together, the lateral, basal, and accessory basal nuclei form the main portions of the BLA. The BLA receives sensory information from cortical and subcortical areas, integrates these inputs, and then projects to the central nuclei of the amygdala and other brain regions, ultimately eliciting the appropriate behavioral response to external stimuli. As such, the BLA is thought to play an important role in controlling the output of processed information from the entire amygdaloid complex (Sah et al., 2003). Glutamatergic pyramidal cells comprise ~85–95% of the neurons within the BLA, while the remaining cells are thought to be GABAergic interneurons (Washburn and Moises, 1992). Typically, increases in BLA excitation are associated with elevated levels of a broad spectrum of anxiety-like behaviors while decreases in BLA activity are usually associated with anxiolysis (Davis et al., 1994). Although GABAergic interneurons only comprise a small portion of the total neurons in this brain region, these cells are thought to play a critical role in regulating BLA excitability and the appropriate output of the region to other structures and thus are likely to play an integral role in regulating anxiety-like behaviors.
The importance of BLA GABAergic transmission in regulating anxiety has been established in both the clinical and preclinical settings. Preclinically, numerous studies have shown that direct infusion of GABAA receptor modulators into the BLA can alter some measures of anxiety-like behaviors (Bueno et al., 2005; Sanders and Shekhar, 1995). In the clinical setting, fMRI studies have shown BLA activity to be correlated with trait anxiety levels (Etkin et al., 2004). Furthermore, many drugs that enhance GABAergic inhibition in the central nervous system are often the first line of pharmacotherapy used to treat anxiety disorders (Allison and Pratt, 2003), thus implicating increased BLA inhibition in the treatment of anxiety. Interestingly, many of the acute pharmacological properties of EtOH may be attributable to an enhancement of GABAergic synaptic transmission (Criswell and Breese, 2005; Weiner and Valenzuela, 2006), leading to the hypothesis that EtOH enhancement of BLA GABAergic transmission may contribute to its well-known anxiolytic properties. Therefore, determining the mechanisms through which EtOH alters GABAergic synaptic transmission in the BLA may provide insight into the pathophysiology of alcoholism and provide new avenues for the development of pharmacotherapies to help alleviate this disease.
Previous research from our lab and others has shown that pyramidal cells in the BLA receive inhibitory input from at least two distinct populations of GABAergic interneurons, distinguishable by their pharmacological and morphological properties (Fuxe et al., 2003; Marowsky et al., 2005; Silberman et al., 2008). The first group of cells is comprised mainly of local circuit interneurons, which are sparsely dispersed throughout the BLA, stain positive for typical interneuronal markers such as parvalbumin, and are thought to mediate local feed-back inhibition (Marowsky et al., 2005; McDonald and Mascagni, 2001). Recently, a second group of interneurons have been characterized in the BLA: the lateral paracapsular cells (lpcs). These cells are located in dense clusters along the BLA-external capsule border, do not stain for parvalbumin or other classical interneuron markers such as CCK or calbindin, and are thought to mediate feed-forward inhibition from cortical inputs (Marowsky et al., 2005). Importantly, while EtOH potentiates inhibitory post-synaptic currents (IPSCs) evoked from both local and lpcs interneurons to a similar extent, the mechanisms underlying EtOH enhancement of these two groups of interneurons appear to be distinct.
We have recently shown that EtOH potentiation of locally-evoked IPSCs is associated with a decrease in paired pulse ratio and is enhanced by pretreatment with a GABAB receptor antagonist, SCH-50911 (Silberman et al., 2008). This confirms earlier findings that SCH-50911 can enhance EtOH’s ability to increase spontaneous IPSC frequency in isolated synaptic boutons from BLA pyramidal cells (Zhu and Lovinger, 2006). Collectively, these findings suggest that EtOH enhancement of local interneuronal inhibition in the BLA may be primarily due to a presynaptic mechanism that can be regulated by GABAB autoreceptors. In contrast, EtOH potentiation of lpcs IPSCs was not associated with a change in paired pulse ratio and was not altered by pretreatment with SCH-50911. Interestingly SCH-50911 application alone significantly potentiated lpcs IPSCs while having no effect on local IPSCs. These findings suggest that EtOH facilitation of lpcs IPSCS may not be mediated by an increase in GABA release probability and, importantly, that there may be significant differences in presynaptic GABAB receptor sensitivity or activity between local and lpcs synapses. Therefore, in this study, we sought to further characterize presynaptic GABAB autoreceptor regulation of GABA release in these two distinct inhibitory pathways in the BLA. Our second goal was to utilize the GABAB receptor system to further characterize the distinct mechanisms by which EtOH enhances GABAergic inhibition in the BLA. Our findings reveal significant differences in synaptic- but not exogenous agonist- activation of GABAB receptors at local and lpcs BLA GABAergic synapses. Our findings also provide further evidence that EtOH facilitation of local IPSCs is mediated by a presynaptic facilitation of GABA release that is under the tight regulation of GABAB autoreceptors. In contrast, our data suggest that EtOH enhancement of lpcs synaptic transmission does not result from an increase in GABA release and is not modulated by synaptic or exogenous agonist-mediated activation of GABAB receptors.
Methods
Slice preparation
Transverse amygdala slices (400 μm) were prepared from 4- to 6-week-old male Sprague Dawley rats. Slices were maintained at ambient temperature for at least 2 hr in oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): 124 NaCl, 3.3 KCl, 2.4 MgCl2, 2.5 CaCl2, 1.2 KH2PO4, 10 D-glucose, and 25 NaHCO3, saturated with 95% O2 and 5% CO2.
Electrophysiological recordings
Slices were transferred to a recording chamber and superfused with aerated aCSF at 2 ml/min using a calibrated flowmeter (Gilmont Instruments, Racine, WI). Experiments were performed at ambient temperature as our previous studies have found that this promotes the stability of patch-clamp recordings in brain slices and does not influence EtOH enhancement of GABAA IPSCs (Ariwodola and Weiner, 2004). Recording electrodes were prepared from filamented borosilicate glass capillary tubes (inner diameter, 0.86 mm) using a horizontal micropipette puller (P-97; Sutter Instruments, Novato, CA). Whole-cell patch-clamp recordings of evoked IPSCs (eIPSCs) were made using a filling solution containing 130 mM K-gluconate, 10 mM KCl, 1 mM EGTA, 100 μM CaCl2, 2 mM Mg-ATP, 200 μM Tris-guanosine 5′-triphosphate, and 10 mM HEPES, pH adjusted with KOH, 275–280 mOsm. Recordings of spontaneous IPSCs (sIPSCs) and TTX-resistant miniature IPSCS (mIPSCs) were made using a similar filling solution exchanging equimolar CsCl for K-gluconate and KCl. In all experiments, 5 mM N-(2,6-dimethyl-phenylcarbamoylmethyl)-triethylammonium chloride (QX-314) was included in the recording solution to block voltage-gated sodium currents and GABAB IPSCs in the BLA neurons being recorded (Horn et al., 1980; Nathan et al., 1990). Whole-cell patch-clamp recordings were made from BLA pyramidal neurons voltage-clamped at −30 to −40 mV for eIPSCs and at −60 to −70 mV for s/mIPSCs (not corrected for junction potential). Only cells with a stable access resistance of 5–20 MΩ were used in these experiments. Whole-cell currents were acquired using an Axoclamp 2B or Axopatch 200B amplifier, digitized (Digidata 1200 or Digidata 1321A; Axon Instruments, Union City, CA), and analyzed on- and off-line using an IBM-compatible personal computer and pClamp 9.0 software (Axon Instruments).
Pharmacological isolation of synaptic currents
In many experiments, GABAA IPSCs were evoked every 20 sec by electrical stimulation (0.2 msec duration) using a concentric bipolar stimulating electrode (FHC, Bowdoinham, ME) placed near (50–100μm) the recording electrode to target local interneurons (“local” stimulation) or along the external capsule to target lpcs interneurons (“distal” stimulation). In some experiments designed to assess the effect of synaptic activation of GABAB autoreceptor activity on local or lpcs synapses, a paired-pulse protocol with an interstimulus interval (ISI) of 250 msec was utilized. In these experiments, GABAA IPSC pairs were evoked every 45 sec. In both of these experimental procedures, stimulation intensity was adjusted to evoke responses that were 10–20% of maximal currents (typically 80–120 pA). s/mIPSCs were recorded in the absence of external electrical stimulation and were digitized at 5–10kHz in continuous 3 min epochs. In all experiments, GABAA IPSCs were pharmacologically isolated using a mixture of 50 μM APV and 20 μM DNQX to block NMDA and AMPA/kainate receptors, respectively. mIPSCs were recorded in the presence of 0.5 μM TTX. Unless otherwise stated, all drugs used were purchased from Sigma (St. Louis, MO). Drugs were made up as 100- to 400-fold concentrates and applied to slices via calibrated syringe pumps (Razel Scientific Instruments, Stamford, CT). A 4M EtOH solution was prepared immediately before each experiment from a 95% stock solution (Aaper Alcohol and Chemical, Shelbyville, KY) kept in a glass storage bottle.
Statistics
EtOH and other drug effects on eIPSCs were quantified as the percentage change in the area under the curve of synaptic currents relative to the mean of control and washout values. The effect of EtOH on paired pulse depression (PPD) was assessed by calculating ratios from averages of five consecutive sweeps recorded in the presence and absence of the drug to eliminate problems with spurious IPSCs (Kim and Alger, 2001). PPD was calculated as the amplitude of the second response divided by the amplitude of the first, using an ISI of 250 msec. s/mIPSC events were first identified using Clampfit event detection software (pClamp 9.0) and then visually inspected to avoid inclusion of spurious IPSCs. s/mIPSCs in each epoch were then averaged and the amplitude and decay time of the averaged traces were calculated. Frequency and kinetic distributions were calculated using the statistic functions included in the Clampfit program. Effects of EtOH and other drugs on s/mIPSCs were quantified as the percentage change in area, decay, and frequency of spontaneous events relative to the mean of control and washout values. Statistical analysis of drug or stimulation effects were performed using the two-tailed Student’s paired, unpaired t tests, or two-way ANOVA followed by the Bonferroni post hoc test, where applicable, with a minimal level of significance of p < 0.05. Statistical analysis of s/mIPSC data was performed using the two-tailed Student’s t test for group results, with a minimum level of significance of p < 0.05, and confirmed by the Kolmogorov-Smirnov (KS) test on individual cells, with a minimal level of significance of p < 0.01.
Results
Synaptic and exogenous agonist activation of GABAB autoreceptors at local and lpcs GABAergic Synapses
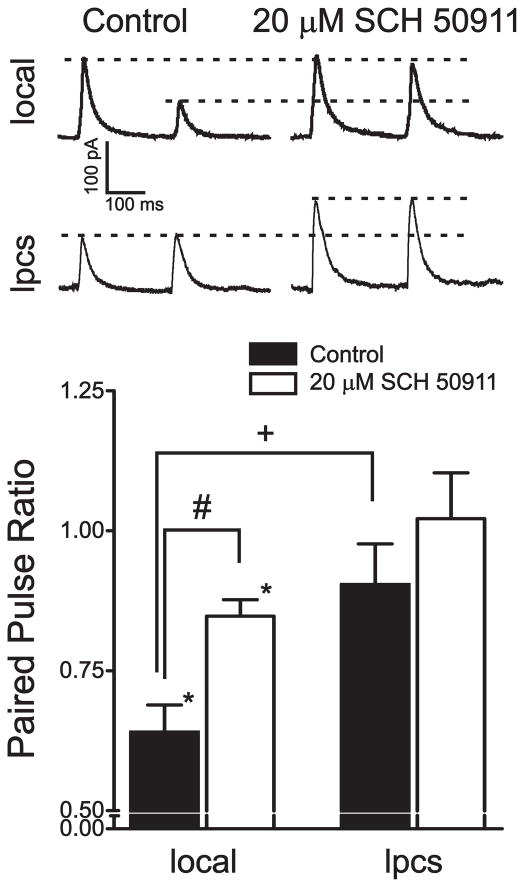
Previous data from our lab (Silberman et al., 2008) suggested that there may be differences in basal GABAB autoreceptor activity between local and lpcs synapses, as the GABAB receptor antagonist SCH-50911 had distinct effects on GABAA IPSCs evoked from these two populations of interneurons. Specifically, 20 μM SCH-50911 alone had no effect on local eIPSCs while this same concentration of SCH-50911 significantly potentiated lpcs eIPSCs, suggesting that presynaptic GABAB receptors may be tonically active at lpcs synapses. This may be due to a number of possible mechanisms such as differing pharmacological or functional coupling properties of GABAB autoreceptors or differences in ambient GABA levels between local and lpcs synapses. To begin to evaluate these possibilities, we first sought to examine whether there were any differences in synaptic activation of presynaptic GABAB receptors at these two populations of synapses. GABAB autoreceptors at most GABAergic synapses can be synaptically activated using paired-pulse stimulation protocols at inter-pulse intervals between 100–500 msec (Pearce et al., 1995). In these protocols, GABA spillover from the first pulse activates presynaptic GABAB receptors which, in turn, reduce GABA release during the second pulse. This phenomenon, termed paired-pulse depression (PPD), is typically measured as the ratio of the second IPSC peak amplitude compared to the first. Paired local stimulation, at an ISI of 250 ms, resulted in a significant depression of the amplitude of the second IPSC relative to the first (36.0 ± 4.9%, p < 0.05, n=13; Fig 1), similar to our previous reports (Silberman et al., 2008). We then tested the effects of 20 μM SCH-50911 to determine if this PPD is dependent on GABAB autoreceptor activity at local synapses. Bath application of SCH-50911 for 8–10 minutes significantly reduced the magnitude of PPD relative to control conditions (15.3 ± 2.9%, p < 0.05), suggesting that a significant component of PPD at local synapses is due to the activation of presynaptic GABAB autoreceptors.
Figure 1.
Electrophysiological and pharmacological evidence of distinct GABAB autoreceptor activity at local and lpcs synapses. Bar graph summarizing the effect of a GABAB antagonist, SCH-50911, on paired pulse depression (250 msec interstimulus interval) at local and lpcs synapses, expressed as a ratio of the amplitude of the second pulse relative to the first pulse. Traces above the graph are averages of five consecutive sweeps from a single representative cell illustrating the difference in basal paired pulse depression as well as differences in the GABAB receptor dependence of paired pulse depression at local and lpcs synapses. 13 cells were used in this experiment.*, significant difference from null control (ratio of 1.0, p < 0.05). #, significant effect of SCH-50911 compared to baseline levels (p < 0.05). +, significant difference between groups (p < 0.05).
In contrast, paired distal stimulation (250 msec ISI), targeting lpcs synapses, in the same cells tested above, did not result in a significant reduction in the amplitude of the second IPSC relative to the first (9.6 ± 7.3 %, p > 0.05). This finding was significantly different from PPD observed at local synapses (p < 0.05). Furthermore, bath application of 20 μM SCH-50911 did not result in any significant differences to the original paired pulse ratio (−0.02 ± 0.08%, p > 0.05). Although SCH-50911 had no significant effect on the paired pulse ratio, it did significantly increase the peak amplitude of both the first and second IPSCs, relative to control levels (40.6 ± 18.3% and 44.4 ± 24.5%, respectively, p < 0.05). Together, these findings suggest that lpcs GABAB autoreceptors may be tonically active under basal recording conditions and therefore may not be further activated by synaptic stimulation protocols. Overall, these findings are also consistent with the hypothesis that ambient GABA levels may be higher at lpcs synapses. However, more intense stimulation protocols can lead to increased GABAB mediated responses at some GABAergic synapses (Thompson and Gahwiler, 1989). Since a single paired-pulse stimulation protocol was not able to induce synaptic activation of lpcs GABAB receptors, we therefore tested whether multiple stimulations could elicit PPD. Interestingly, stimulus trains ranging from 2 to 30 stimuli, separated by 10 msec per stimulus, did not cause a depression of a test pulse 250 msec after the stimulus train (data not shown). As such, more intense stimulation was still not able to activate presynaptic GABAB receptors at lpcs synapses.
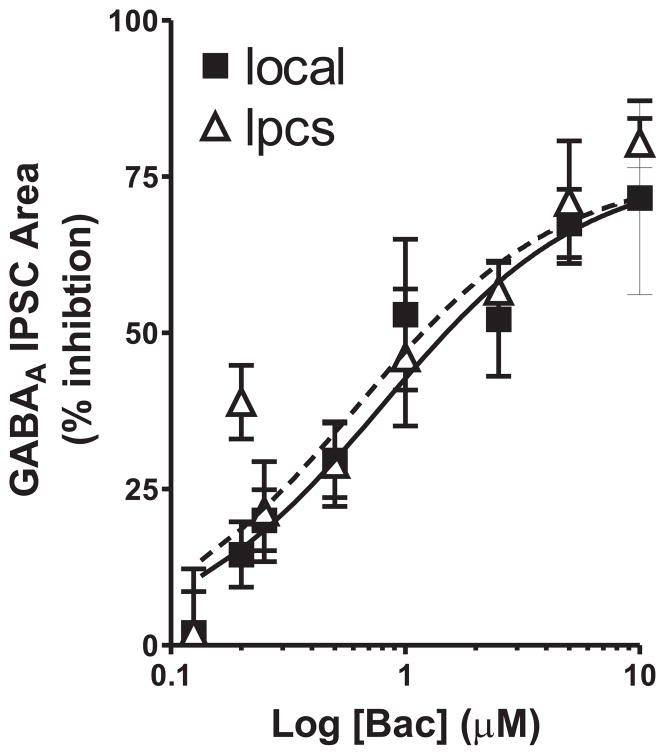
The preceding experiment revealed significant differences in the level of synaptic activation of presynaptic GABAB receptors at local and lpcs BLA GABAergic synapses. We next examined the concentration-dependence of baclofen (a GABAB receptor agonist) inhibition of local and lpcs eIPSCs to determine if these two synapses also differed in their response to exogenous agonist-mediated activation of GABAB autoreceptors. We chose to look at the area of eIPSCs in this, and subsequent eIPSC experiments as this measurement is more reflective of overall changes in total charge transfer during synaptic responses than peak response amplitudes. The concentrations of baclofen ranged from 125 nM to 10 μM and between 3 and 10 cells were tested at each concentration for each stimulation locale. Bath application of baclofen inhibited the area of both local and lpcs eIPSCs in a concentration dependent manner, with significant inhibition observed at concentrations of 200 nM and higher at both populations of synapses (Fig 2). Statistical analysis of the data revealed a significant overall effect of baclofen concentration (F =18.51, p < 0.0001) with no significant effect of stimulus location (F = 0.94, p > 0.05) and no stimulus-baclofen interaction effect (F = 0.55, p > 0.05).
Figure 2.
Concentration dependence of baclofen inhibition of local and lpcs evoked IPSCs. Scatter plot summarizing the effect of baclofen (0.125–10 μM) on the area of GABAA IPSCs evoked by either local or lpcs stimulation in the BLA. Solid and dashed lines represent curve fitting analysis for local and lpcs stimulation, respectively. Three to 12 cells were tested at each concentration. Inhibition by baclofen was significant at both cell types at concentrations of 200 nM and higher (p < 0.05).
Effects of baclofen on EtOH potentiation of local and lpcs synapses
Collectively, the result of the previous experiments suggest that while local and lpcs GABAergic synapses exhibit profound differences in synaptic activation of GABAB autoreceptors, these differences do not appear to reflect alterations in the functional properties of these receptors at these two populations of synapses. Previous research from our laboratory suggests that EtOH enhancement of GABAergic synaptic transmission at local BLA synapses may be due to an increase in GABA release and that the magnitude of this effect is limited by presynaptic GABAB autoreceptors, a finding also observed at GABAergic synapses onto hippocampal CA1 pyramidal neurons (Ariwodola and Weiner, 2004; Wan et al., 1996). At hippocampal synapses, EtOH enhancement of GABAergic transmission can be significantly reduced by pretreatment with low concentrations of baclofen, a GABAB receptor agonist. We therefore next examined the effect of baclofen pretreatment on EtOH potentiation of local and lpcs eIPSCs.
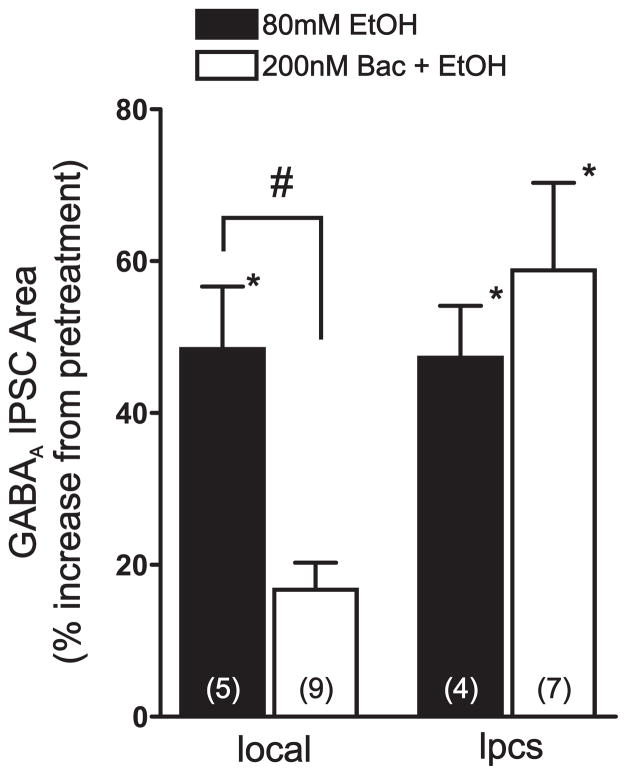
As observed in our previous studies, bath application of 80 mM EtOH significantly potentiated the area of both local (48.4 ± 8.2%, p < 0.05, n=5) and lpcs eIPSCs (47.3 ± 6.8%, p < 0.05, n = 4) to a similar level (p > 0.05, Fig 3). Pretreating slices with 200 nM baclofen had a modest but significant inhibitory effect on local and lpcs eIPSCs. Once a stable level of baclofen inhibition was obtained, 80mM EtOH was then applied. Baclofen pretreatment significantly reduced the potentiating effect of 80 mM EtOH on local eIPSC area by about 65% (16.7 ± 3.6% increase from post-baclofen levels, p < 0.05, n = 9). In contrast, baclofen pretreatment did not antagonize the facilitatory effect of 80 mM EtOH on lpcs eIPSCs (58.8 ± 11.5% increase from post-baclofen levels, p < 0.05, n = 7).
Figure 3.
Baclofen pretreatment reduces EtOH potentiation of local, but not lpcs, synapses. Bar graph summarizing the effect of baclofen pretreatment on EtOH potentiation of local and lpcs GABAA IPSC area. Note that, while EtOH potentiates both local and lpcs IPSCs to a similar extent, baclofen pretreatment significantly reduces the effect of EtOH only at local synapses. Numbers in parentheses indicate the number of cells tested in each group.*, significant difference from control or baclofen pretreatment (p < 0.05). #, significant difference between groups (p < 0.05).
Effects of EtOH and GABAB receptor modulators on spontaneous IPSCs
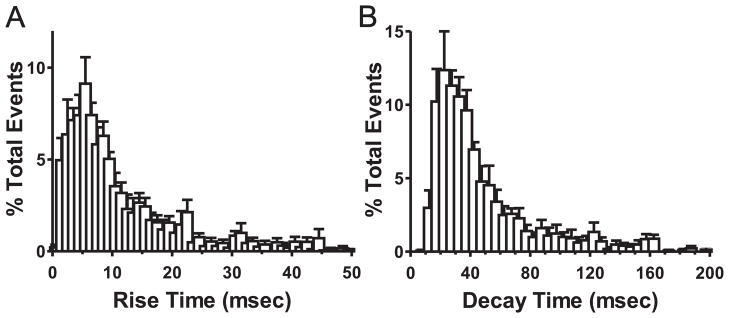
The experiments described above extend our previous work and further support the hypothesis that EtOH potentiation of local GABAergic synapses is due to a presynaptic mechanism, while the effect of EtOH at lpcs synapses may not involve increased GABA release and is not modulated by presynaptic GABAB receptor activity. However, these previous data did not directly assess the synaptic locus of EtOH action at local and lpcs synapses. To address this issue, we used an analysis of sIPSCs, which can more directly distinguish between pre- and post- synaptic mechanisms of a drug effect. Typically, changes in the frequency of sIPSCs are indicative of a presynaptic mechanism while changes in the amplitude or decay of these events are regarded as a postsynaptic alteration (Siggins et al., 2005). We therefore wanted to determine how EtOH impacts sIPSCs in the BLA and how pharmacological alterations of GABAB receptor activity may modify these effects. However, since these events reflect spontaneous synaptic activity and are not evoked by electrical stimulation, it is difficult to discern the origin of these events a priori, as they may potentially arise from either local or lpcs synapses, or both. Interestingly, previous evidence from Marowsky, et al (2005), utilizing paired recordings of lpcs- and local interneuron-pyramidal cell unitary IPSCs, showed that lpcs-mediated unitary IPSCs have slow kinetic properties relative to local IPSCs. These findings suggest that lpcs interneurons may synapse at distal dendritic sites on pyramidal neurons and could be subject to more extensive cable filtering. In contrast, a large proportion of local interneurons in the BLA are thought to form peri-somatic synapses onto BLA pyramidal neurons (Woodruff and Sah, 2007), which may contribute to their relatively fast kinetic properties. We therefore hypothesized that if sIPSCs recorded from BLA pyramidal neurons reflect the activation of both local and lpcs synapses, this might by revealed by an analysis of the distribution of the kinetic properties of these events. To test this hypothesis, we analyzed the distribution of basal sIPSC rise time and decay parameters from a representative group of cells. Although decay and rise time distributions did not fall entirely under a Gaussian distribution, a very large proportion of events for both parameters (>85%) did fall under a single Gaussian peak (Fig 4). This analysis suggests that a large majority of sIPSCs display a homogenous distribution of kinetic parameters indicating that they likely arise from a single interneuronal source (i.e. local or lpcs GABAergic cells).
Figure 4.
Evidence of a homogeneous distribution of spontaneous IPSC kinetic parameters recorded from BLA pyramidal neurons. Average distribution of rise (A) and decay (B) times (msec) of sIPSC events during control recording conditions represented as % of total events recorded for 6 representative cells. Note that for both parameters, greater than 85% of all events fall along a single Gaussian distribution. Bin sizes were (A) 1 msec and (B) 5 msec.
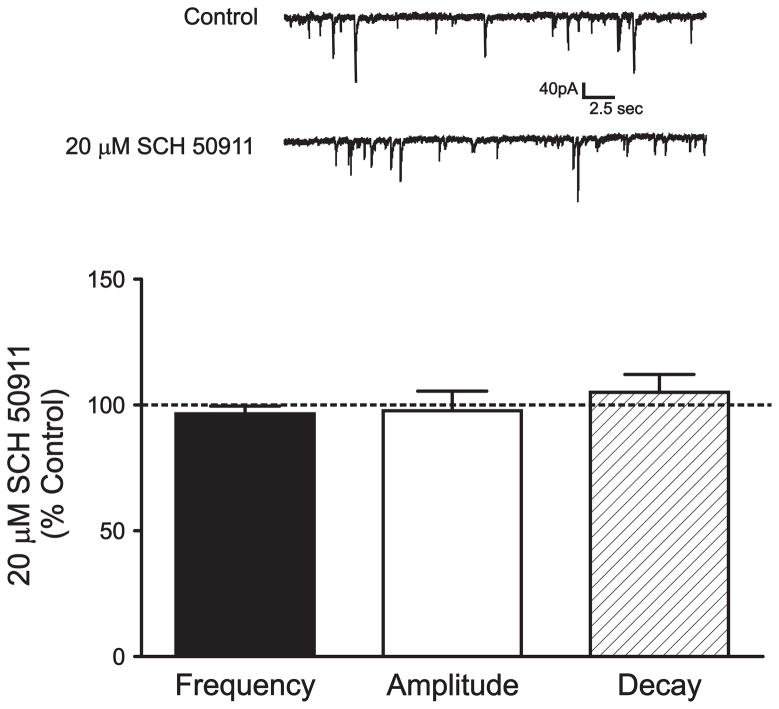
We next utilized a number of pharmacological interventions to further determine the source of sIPSC events as well as to characterize the mechanism and synaptic locus of EtOH’s modulatory effects on BLA GABAergic inhibition. Importantly, our previous experiments have shown that SCH-50911 alone can potentiate lpcs eIPSCs while having no effect on local eIPSCs. Bath application of 20 μM SCH-50911 for 8–10 minutes had no significant effect on the frequency (3.6 ± 3.1% decrease, p > 0.05, n = 6, Fig 5), amplitude (2.3 ± 7.8% decrease, p > 0.05), or decay (5.0 ± 7.2% increase, p > 0.05) of sIPSC events. We next tested the effect of EtOH on sIPSCs. Bath application of 40 mM EtOH for 8–10 minutes significantly increased the frequency of sIPSCs compared to control conditions (41.9 ± 9.2%, p < 0.05, n= 6, Fig. 6) while causing no significant change in either the amplitude or decay of these events (3.9 ± 7.1% and 22.7 ± 15.4 %, respectively, p > 0.05). KS test confirmed that EtOH significantly increased sIPSC frequency in 4 out of 6 cells.
Figure 5.
Application of a GABAB receptor antagonist has no effect on spontaneous IPSCs. Bar graph summarizing the effect of 20 μM SCH-50911, a GABAB receptor antagonist, on the frequency, amplitude, and decay of spontaneous IPSCs. Traces above the graph are sample recordings of spontaneous IPSCs from a representative cell under control conditions and during SCH-50911 application. Six cells were tested under these conditions. Note that SCH-50911 has no significant effect on its own on any measure tested.
Figure 6.
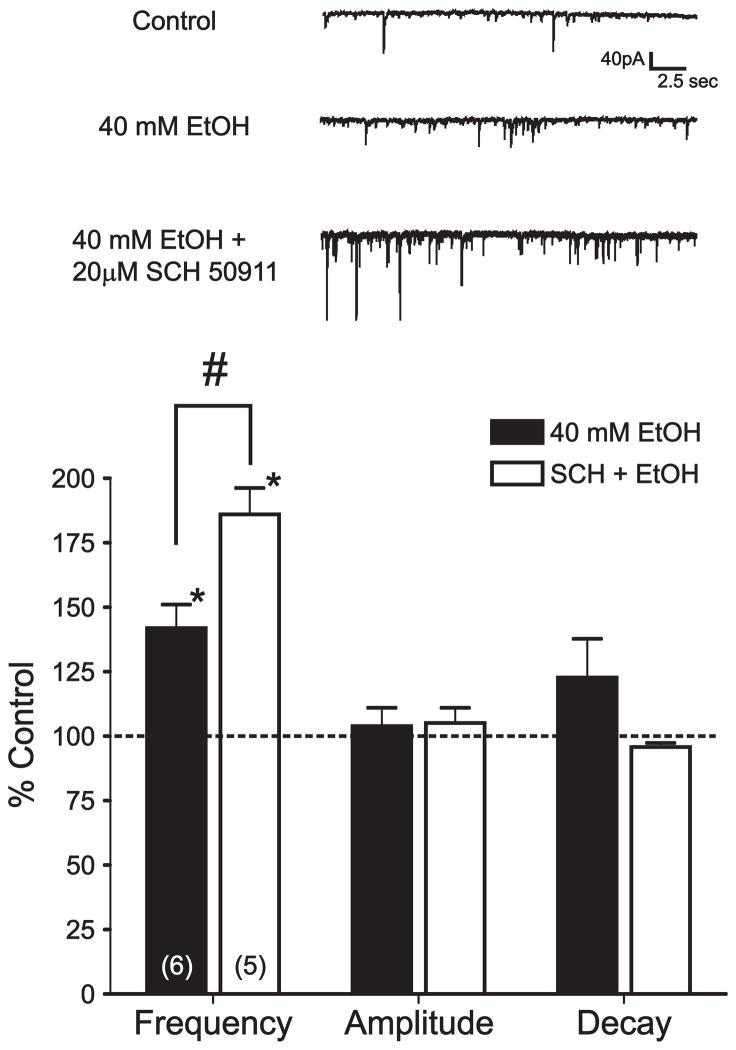
Pretreatment with a GABAB receptor antagonist enhances EtOH potentiation of sIPSC frequency. Top, sIPSCs recorded from a representative BLA pyramidal neuron during control, EtOH, and 20 μM SCH-50911 pretreatment + EtOH conditions. Bottom, bar graph summarizing the effect of EtOH alone and following pretreatment with SCH-50911 on sIPSC frequency, amplitude, and decay. Numbers in parentheses represent the number of cells tested under each experimental condition.*, significant difference from control conditions (p < 0.05). #, significant difference between groups (p < 0.05).
The preceding experiments are consistent with the idea that sIPSCs reflect primarily the activity of local GABAergic synapses and that EtOH enhances these synapses via a presynaptic mechanism. To further validate this hypothesis, we next examined the effect of GABAB receptor modulators on EtOH potentiation of BLA sIPSCs. If sIPSCs indeed largely reflect the activity of local GABAergic synapses, then GABAB receptor modulators should regulate EtOH potentiation of sIPSCs in a manner similar to that observed with local eIPSCs. Our previous work has shown that blockade of GABAB receptors significantly facilitates EtOH potentiation of local, but not lpcs, IPSCs. Therefore we next tested the effects of SCH-50911 pretreatment on EtOH enhancement of sIPSC frequency. Importantly, pretreatment with 20 μM SCH-50911 significantly enhanced the effect of 40 mM EtOH on sIPSC frequency (86.0 ± 10.3%, p < 0.05 compared to control and EtOH alone, n = 5, fig 6) and this effect was not accompanied by any significant changes in sIPSC amplitude or decay (5.1 ± 5.9 % and −4.2 ± 1.6 % respectively, p < 0.05, n = 5). KS test confirmed that the combination of 20 μM SCH-50911 and 40 mM EtOH significantly increased sIPSC frequency in 4 out of 5 cells. In addition, an analysis of sIPSC kinetics revealed that the combination of SCH-50911 and EtOH did not significantly alter the distribution of sIPSC rise time or decay parameters relative to control conditions (data not shown).
We also examined the interaction between SCH-50911 and 80 mM EtOH on sIPSCs. Bath application of 80 mM EtOH resulted in a larger increase in the frequency of sIPSC events compared to control conditions (95.1 ± 27.3%, p < 0.05, n = 8, Fig 7) while there was no significant effect on amplitude or decay (42.6 ± 19.7% and 0.5 ± 5.9% respectively, p > 0.05). Although pretreatment with SCH-50911 did result in a modest facilitation of the effect of 80 mM EtOH on sIPSC frequency, this effect was not significantly greater than the effect of 80 mM EtOH alone (114 ± 20.2%, p > 0.05, n = 8), suggesting a possible ceiling effect of EtOH on sIPSC frequency at higher concentrations.
Figure 7.
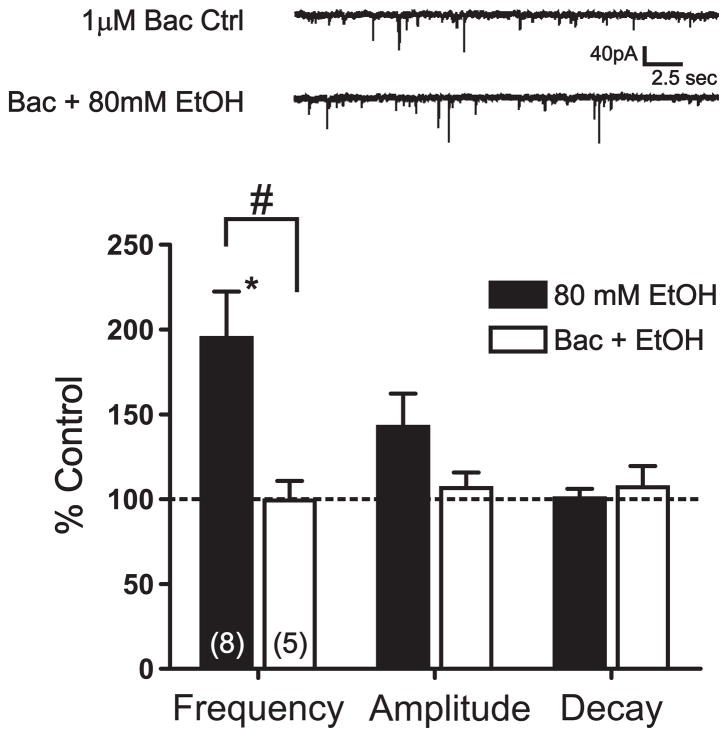
Pretreatment with a GABAB agonist reduces EtOH potentiation of sIPSC frequency. Top, sIPSCs recorded from a representative cell during 1 μM baclofen pretreatment and during baclofen + EtOH conditions. Bottom, bar graph summarizing the effect of EtOH alone and following pretreatment with 1 μM baclofen on sIPSC frequency, amplitude, and decay. Numbers in parentheses represent the number of cells tested under each experimental condition.*, significant difference from control conditions (p < 0.05). #, significant difference between groups (p < 0.05).
We next hypothesized that baclofen pretreatment would limit the facilitatory effects of EtOH on sIPSC frequency; similar to its inhibitory effect on EtOH potentiation of local eIPSCs (Fig 3). Bath application of 1 μM baclofen alone resulted in a modest, but nonsignificant inhibition of sIPSC frequency (23.8 ± 9.4%, p = 0.07, n = 5) with no significant change in sIPSC amplitude or decay measures (3.8 ± 2.7% decrease and 7.6 ± 7.0% increase, respectively, p > 0.05). In these same cells, 10 μM baclofen greatly reduced sIPSC frequency in a highly significant manner (57.0 ± 10.08%, p < 0.01) while still resulting in no significant changes in the amplitude or decay of sIPSC events (6.6 ± 4.4% and 11.6 ± 7.9% decreases respectively, p > 0.05). These results are in accordance with our earlier findings that baclofen significantly reduced local eIPSCs. Importantly, pretreatment with 1 μM baclofen significantly blocked the near maximal potentiating effect of 80 mM EtOH on sIPSC frequency (0.7 ± 11.4%, p < 0.05, n = 5; Fig 7) with no significant effects on amplitude or decay (6.6 ± 9.2% and 8.0 ± 12.5%, respectively, p > 0.05). Accordingly, lower concentrations of baclofen resulted in lower inhibition of the typical potentiating effects of 80 mM EtOH on sIPSC frequency (data not shown).
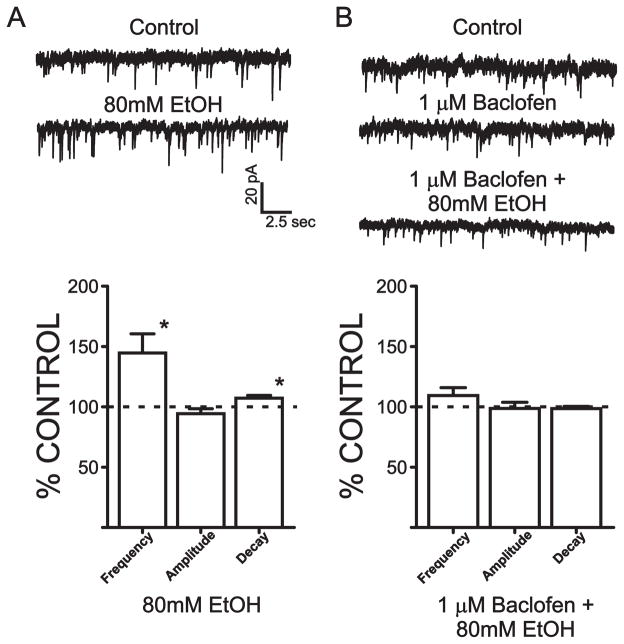
Effects of EtOH and GABAB receptor modulators on miniature IPSCs
Collectively, the sIPSC experiments suggested that ethanol enhances local synaptic inhibition in the BLA primarily via a facilitation of GABA release from local interneurons. To determine if this presynaptic effect results from an increase in the firing rate of local interneurons or reflects an increase in terminal GABA release probability, we next examined the effect of ethanol on TTX-resistant mIPSCs. In the presence of 500 nM TTX, 80 mM EtOH significantly increased the frequency of mIPSCs compared to control conditions (44.6±16.0%, n= 11, p < 0.05; Fig 8), while causing no significant change to the amplitude of these events (5.6±4.1% decrease from baseline, p > 0.05). KS test confirmed that EtOH significantly increased mIPSC frequency in 10 out of 11 cells. EtOH did cause a small, albeit significant, increase in mIPSC decay (7.2±2.3% increase from control, n=11, p <0.05). However, since the predominant effect of EtOH on mIPSCs was to increase their frequency, we hypothesized that this presynaptic effect would be sensitive to GABAB autoreceptor modulation, as observed with ethanol facilitation of sIPSCs. Bath application of 1 μM baclofen had no significant effect on the frequency, amplitude or decay of mIPSCs (1.0±5.4%, 4.3±3.0%, 0.4±2.7% decreases from control, respectively, n=8, p>0.05). However, baclofen pretreatment completely blocked the facilitatory effect of ethanol on mIPSC frequency (9.4±6.5%, n=9, p> 0.05) and decay (1.4±1.8% decrease, p >0.05).
Figure 8.
EtOH enhancement of mIPSC frequency is reduced by pretreatment with a GABAB agonist. A) Top, mIPSCs recorded from a representative cell during control conditions and during EtOH application. Bottom, bar graph summarizing the effect of EtOH on mIPSC frequency, amplitude, and decay. B) Top, mIPSCs recorded from a representative cell during control, 1 μM baclofen, and during baclofen + EtOH conditions. Bottom, bar graph summarizing the effect of baclofen + EtOH on mIPSC frequency, amplitude, and decay. Between 8–11 cells were used in each group.*, significant difference from control conditions (p < 0.05).
Discussion
The results of this study provide new insight into the mechanisms through which EtOH enhances two distinct populations of GABAergic synapses in the rat BLA. Our data also provide further insight into the regulation of these synapses by GABAB autoreceptors. Specifically, the present findings provide further support for the hypothesis that EtOH enhances local BLA GABAergic synapses via a presynaptic increase in terminal GABA release and that this enhancement is tightly regulated by presynaptic GABAB autoreceptors. Interestingly, while EtOH also enhances GABAergic inhibition via the lpcs-mediated pathway, our findings further suggest that the mechanism of EtOH action at lpcs synapses is not due to an increase in GABA release nor is it regulated by GABAB receptor activity. In addition, pharmacological studies on sIPSCs and mIPSCs recorded from BLA pyramidal cells suggest that local populations of interneurons are the primary source of sIPSCs and mIPSCs onto these cells and provide additional evidence that EtOH enhances local BLA GABAergic synapses via a presynaptic facilitation of GABA release.
As noted above, the data presented in this study provide further evidence of significant differences in GABAB autoreceptor activity between local and lpcs synapses in the BLA. We have previously shown that SCH-50911, a GABAB receptor antagonist, significantly enhances IPSCs evoked by lpcs stimulation while having no effect on local eIPSCs (Silberman et al., 2008). These findings suggest that lpcs GABAergic synapses may be tonically regulated by presynaptic GABAB receptor activity, at least under our standard recording conditions. This could be due to a number of possible mechanisms, such as higher ambient GABA levels and/or enhanced GABAB receptor function or agonist sensitivity at lpcs vs. local synapses. To begin to distinguish between these possible mechanisms, we studied synaptic and exogenous agonist activation of GABAB autoreceptors at local and lpcs synapses. Using an inter-pulse interval of 250 msec, we observed significant PPD at local synapses, with no such depression at lpcs synapses under our basal recording conditions. Importantly, the GABAB receptor antagonist SCH-50911 significantly reduced PPD at local synapses, suggesting that a significant portion of PPD at local synapses is dependent on GABAB autoreceptor activation, as observed at GABAergic synapses in many other brain regions (Jia et al., 2004; Kolaj et al., 2004; Pearce et al., 1995).
Although we found no evidence of synaptic activation of GABAB autoreceptors at lpcs synapses, bath application of SCH-50911 did significantly potentiate the peak amplitude of the paired lpcs responses compared to baseline levels, similar to our previous findings with individually-evoked lpcs IPSCs. These findings are consistent with the hypothesis that there may be higher ambient GABA levels at lpcs synapses which may result in tonic GABAB receptor activation at lpcs synapses under our basal recording conditions. This higher basal activity may, in turn, occlude the ability of these receptors to be further activated by GABA released via experimental stimulation protocols, such as the paired-pulse procedure employed in this study. Interestingly, increasing the amount and duration of conditioning stimuli prior to a test pulse also failed to induce significant PPD at lpcs synapses. These data suggest that, at least under our recording conditions, even supra-physiological stimulation protocols cannot cause additional activation of presynaptic GABAB autoreceptors at lpcs GABAergic synapses beyond that observed under basal conditions.
The differences in PPD and SCH-50911 activity at local and lpcs synapses could also be due to differences in the pharmacological properties or functional coupling of GABAB receptors present at these two populations of synapses. However, the lack of any significant differences in the concentration dependence of baclofen inhibition of local and lpcs GABAA eIPSCs argues against this possibility. Interestingly one might have expected that increased ambient GABA levels at lpcs synapses under basal conditions would have resulted in a shift in the baclofen concentration-response curve, particularly at low concentrations. One possible explanation for the absence of such a shift at lpcs synapses may be that ambient endogenous GABA and exogenous agonist application may be activating distinct subsets of presynaptic GABAB receptors or, alternatively, that ambient GABA only activates a small subset of presynaptic GABAB receptors on lpcs interneurons. GABAB receptors may be present at synaptic, perisynaptic and/or somatodendritic loci (Davies and Collingridge, 1993; Li and Guyenet, 1995; Stuart and Redman, 1992) and activation of any of these receptor populations could potentially modulate evoked GABA release. Additional studies will be needed to further resolve the localization of presynaptic GABAB receptors on both local and lpcs interneurons and to identify the population(s) of GABAB receptors responsible for the apparent ambient GABAB receptor-dependent modulation of lpcs eIPSCs. Nevertheless, our findings suggest that the basic pharmacological properties of presynaptic GABAB receptors are similar at local and lpcs GABAergic synapses and that the observed differences in PPD and tonic GABAB receptor activity between these local and lpcs synapses may be due, at least in part, to differences in ambient GABA levels between these two populations of inhibitory synapses.
Regardless of the reasons for the differences in tonic GABAB receptor activity between local and lpcs synapses, it is clear from our previous work (Ariwodola and Weiner, 2004; Silberman et al., 2008) that GABAB receptor modulators might represent a useful tool to further characterize the apparent differential mechanisms of EtOH action at these two synapses. To that end, we employed GABAB receptor agonists and antagonists to further characterize the synaptic locus of EtOH action at local and lpcs GABAergic synapses. We found that pretreatment with a low concentration of baclofen significantly reduced EtOH potentiation of GABAergic transmission at local synapses. This effect is similar to that observed at GABAergic synapses in the hippocampus (Ariwodola and Weiner, 2004) and suggests that EtOH may enhance presynaptic GABA release at local synapses under the regulation of GABAB autoreceptors. These data are also in accordance with our previous research that showed that blockade of GABAB receptors can enhance the potentiating effects of EtOH at local BLA synapses (Silberman et al., 2008), a finding that was also observed in the hippocampus (Ariwodola and Weiner, 2004; Wan et al., 1996). Interestingly, even though 200 nM baclofen did cause a modest but significant inhibition of lpcs eIPSCs, baclofen pretreatment had no effect on EtOH potentiation of lpcs synapses. Thus, although exogenous activation of presynaptic GABAB receptors did inhibit lpcs GABAergic transmission, this treatment had no effect on EtOH enhancement of these synapses. Collectively, these findings further support the hypothesis that EtOH acts to increase GABA release at local interneuron-mediated synapses in the rat BLA, while EtOH enhancement of lpcs mediated inhibition may be due to a different mechanism entirely. Baclofen has recently been reported to be an important pharmacological tool to help reduce alcohol intake and craving in recovering alcoholics in clinical settings (Addolorato et al., 2000; Addolorato et al., 2002; Flannery et al., 2004). In the preclinical setting, baclofen has also been shown to reduce EtOH intake, relative to water consumption, in rats selectively bred for increased alcohol drinking (Colombo et al., 2006; Quintanilla et al., 2008), although this effect may be nonselective in outbred rat lines (Anstrom et al., 2003; Czachowski et al., 2006). The selectivity of baclofen’s effects on EtOH intake and craving to those with proclivities to alcohol dependence is an important consideration as recent findings suggest that the negatively reinforcing properties of EtOH on anxiety-like behaviors may play a critical role in the development and maintenance of alcohol use disorders (Koob, 2003). Therefore, the mechanism by which baclofen may reduce EtOH intake in the alcoholic population may be due, in part, to its ability to block the facilitatory effects of EtOH on GABA release at local synapses, thereby reducing the overall negatively rewarding anxiolytic aspects of EtOH drinking during withdrawal.
In regards to the synaptic locus of EtOH action, many studies have suggested that EtOH enhancement of GABAergic inhibition may be due to a number of possible pre- or postsynaptic mechanisms in a brain region specific manner (Siggins et al., 2005; Weiner and Valenzuela, 2006). Our initial evidence suggested that EtOH may be working through a presynaptic mechanism at local, but not lpcs, synapses to enhance GABAergic inhibition in the rat BLA. To gain more insight into these possible mechanisms of EtOH action, we examined the effects of EtOH on sIPSCs. However, since spontaneous events are, by definition, not evoked, it was not possible to identify the source of these events a priori. We therefore first conducted an analysis of the distribution of sIPSC kinetic parameters. Based on previous studies, local and lpcs IPSCs are thought to have different kinetic properties (Marowsky et al., 2005; Woodruff and Sah, 2007), which could be due to differences in the location of these synapses (proximal vs. distal) and/or the subunit composition of GABAA receptors at these two groups of synapses. If both populations of BLA interneurons contributed as significant sources of sIPSCs, we might predict two peaks in the distribution of parameters such as rise time and decay. However, under our standard recording conditions, analysis of sIPSC rise time and decay distributions revealed that greater than 85% of all sIPSC events fall within one Gaussian peak, suggesting that a large proportion of sIPSC events likely arise from a single synaptic population.
To begin to identify which population of synapses was the likely source of sIPSCs, we characterized the pharmacological profile of these events using GABAB receptor modulators. Pretreatment with the GABAB receptor antagonist SCH-50911, alone, had no effect on sIPSC frequency, decay or amplitude. This is an important observation as SCH-50911 did significantly enhance lpcs eIPSCs(Silberman et al., 2008). Therefore, this finding provides initial pharmacological evidence that lpcs synapses may not be a key source of sIPSC events under our recording conditions. We then demonstrated that 40 mM EtOH significantly increased the frequency of sIPSCs with no change in the amplitude or decay of these events. In addition, pretreatment with SCH-50911 significantly facilitated EtOH enhancement of sIPSC frequency. Together, these findings provide further evidence that sIPSCs likely reflect mainly the activity of local BLA GABAergic synapses and that EtOH enhances these synapses primarily via a presynaptic mechanism. Moreover, pretreatment with baclofen completely blocked the facilitatory effects of a near maximal concentration of EtOH on sIPSC frequency. Since baclofen had no effect on EtOH potentiation of lpcs eIPSCs, the effect of baclofen on sIPSCs appears to be more akin to its effects on local eIPSCs. Taken together, our kinetic analysis and pharmacological evidence suggest that, as a whole, local GABAergic interneurons are the primary source of sIPSCs under our standard recording conditions. This finding is further supported by a recent report (Jiang et al., 2008) which suggested that a 5HT2A receptor-mediated increase in sIPSCs frequency in the BLA of juvenile rats may be mostly due to increased firing rates of parvalbumin-containing local interneurons. However, it should be noted that a number of studies have demonstrated that local BLA interneurons are actually comprised of heterogeneous groups of cells that can be distinguished based on their differential expression of peptides and calcium binding proteins (McDonald and Mascagni, 2002). For example, recent studies have identified discrete populations of local interneurons that express somatostatin (Muller et al., 2007), substance P (Levita et al., 2003), or cholecystokinin (Mascagni and McDonald, 2003). In addition, at least four distinct subpopulations of parvalbumin positive interneurons have also been identified that differ in their physiological properties (Woodruff and Sah, 2007). While we cannot at present distinguish between these subpopulations of local interneurons under our recording conditions, the observation that EtOH consistently potentiates local eIPSC area and sIPSC frequency in almost all cells tested strongly suggests that the majority of local interneuronal subpopulations are acutely enhanced by EtOH.
Interestingly, EtOH also enhanced the frequency of mIPSCs, suggesting that EtOH can facilitate action potential-independent GABA release from presynaptic terminals, a finding similar to that previously reported in the BLA (Zhu and Lovinger, 2006) and other brain regions (Roberto et al., 2003; Theile et al., 2008). These data further support the results of our previous PPR experiments (Silberman et al., 2008) suggesting that EtOH primarily acts to enhance the probability of transmitter at local interneuron synapses. In addition, baclofen was able to reduce the potentiating effects of EtOH on mIPSC frequency, similar to our results with sIPSCs, consistent with the idea that mIPSCs likely arise from the diverse populations of local interneurons.
Taken together, the data presented here provide further evidence that EtOH enhances local BLA GABAergic synapses, at least in part, by increasing GABA release from presynaptic terminals and that this enhancement is regulated by GABAB autoreceptors. Conversely, although GABAB receptor activation can modulate lpcs synapses, synaptic and exogenous agonist activation of GABAB receptors have no effect on EtOH potentiation of these inhibitory responses. Future studies will be needed to fully elucidate the mechanism(s) underlying EtOH potentiation of the lpcs inhibitory pathway. Interestingly, our previous studies suggest a possible role for the noradrenergic receptor system as bath application of norepinephrine (NE) significantly enhanced lpcs eIPSCs. Furthermore, blockade of NE receptors, using a cocktail of α1, α2, and β adrenoceptor antagonists, while having no effect on its own, blocked the EtOH potentiation of lpcs, but not local, synapses. Studies are in progress to further understand the role of the NE receptor system in EtOH modulation of lpcs GABAergic synapses.
Collectively, these data provide additional evidence that acute exposure to pharmacologically-relevant concentrations of ethanol can significantly enhance both feed-forward (lpcs) and local feed-back inhibition onto BLA pyramidal neurons. These synaptic alterations likely contribute to the well-known anxiolytic effects of acute ethanol, effects that may well underlie some of the negative reinforcing properties of this drug that are thought to be an important factor in the development of alcoholism. Our findings also provide new insight into some of the neurophysiological mechanisms that mediate and modulate EtOH enhancement of local- and lpcs interneuron-mediated GABAergic inhibitory pathways in the rat BLA. Future research will be needed to further examine the complex mechanisms of EtOH action at these two pathways and how these effects may be altered by chronic EtOH exposure and withdrawal. Additionally, these and other ongoing studies provide potential avenues for the development of new treatment targets for the alleviation of alcoholism, such as the GABAB and NE receptor systems. Additional studies will also be needed to evaluate the role of these distinct BLA inhibitory pathways in the regulation of anxiety-like behaviors and to assess the effects of GABAB and NE receptor regulation of these pathways on EtOH-mediated anxiolysis and drinking behavior during the development of alcoholism.
Acknowledgments
This work was funded by AA 13960, AA 17056, and AA 17039.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addolorato G, Caputo F, Capristo E, Colombo G, Gessa GL, Gasbarrini G. Ability of baclofen in reducing alcohol craving and intake: II--Preliminary clinical evidence. Alcohol Clin Exp Res. 2000;24:67–71. [PubMed] [Google Scholar]
- Addolorato G, Caputo F, Capristo E, Domenicali M, Bernardi M, Janiri L, Agabio R, Colombo G, Gessa GL, Gasbarrini G. Baclofen efficacy in reducing alcohol craving and intake: a preliminary double-blind randomized controlled study. Alcohol Alcohol. 2002;37:504–508. doi: 10.1093/alcalc/37.5.504. [DOI] [PubMed] [Google Scholar]
- Allison C, Pratt JA. Neuroadaptive processes in GABAergic and glutamatergic systems in benzodiazepine dependence. Pharmacol Ther. 2003;98:171–195. doi: 10.1016/s0163-7258(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Anstrom KK, Cromwell HC, Markowski T, Woodward DJ. Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res. 2003;27:900–908. doi: 10.1097/01.ALC.0000071744.78580.78. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology (Berl) 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno CH, Zangrossi H, Jr, Viana MB. The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Braz J Med Biol Res. 2005;38:1697–1701. doi: 10.1590/s0100-879x2005001100019. [DOI] [PubMed] [Google Scholar]
- Carrigan MH, Randall CL. Self-medication in social phobia: a review of the alcohol literature. Addict Behav. 2003;28:269–284. doi: 10.1016/s0306-4603(01)00235-0. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MA, Gessa GL. Baclofen-induced suppression of alcohol deprivation effect in Sardinian alcohol-preferring (sP) rats exposed to different alcohol concentrations. Eur J Pharmacol. 2006;550:123–126. doi: 10.1016/j.ejphar.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Cosci F, Schruers KR, Abrams K, Griez EJ. Alcohol use disorders and panic disorder: a review of the evidence of a direct relationship. J Clin Psychiatry. 2007;68:874–880. doi: 10.4088/jcp.v68n0608. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Stansfield KH. Ethanol and sucrose seeking and consumption following repeated administration of the GABA(B) agonist baclofen in rats. Alcohol Clin Exp Res. 2006;30:812–818. doi: 10.1111/j.1530-0277.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Davies CH, Collingridge GL. The physiological regulation of synaptic inhibition by GABAB autoreceptors in rat hippocampus. J Physiol. 1993;472:245–265. doi: 10.1113/jphysiol.1993.sp019945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17:208–214. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, Hirsch J. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–1055. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Garbutt JC, Cody MW, Renn W, Grace K, Osborne M, Crosby K, Morreale M, Trivette A. Baclofen for alcohol dependence: a preliminary open-label study. Alcohol Clin Exp Res. 2004;28:1517–1523. doi: 10.1097/01.alc.0000141640.48924.14. [DOI] [PubMed] [Google Scholar]
- Fox HC, Bergquist KL, Hong KI, Sinha R. Stress-induced and alcohol cue-induced craving in recently abstinent alcohol-dependent individuals. Alcohol Clin Exp Res. 2007;31:395–403. doi: 10.1111/j.1530-0277.2006.00320.x. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Jacobsen KX, Hoistad M, Tinner B, Jansson A, Staines WA, Agnati LF. The dopamine D1 receptor-rich main and paracapsular intercalated nerve cell groups of the rat amygdala: relationship to the dopamine innervation. Neuroscience. 2003;119:733–746. doi: 10.1016/s0306-4522(03)00148-9. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, June Ruan W, Goldstein RB, Smith SM, Saha TD, Huang B. Prevalence, correlates, co-morbidity, and comparative disability of DSM-IV generalized anxiety disorder in the USA: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2005;35:1747–1759. doi: 10.1017/S0033291705006069. [DOI] [PubMed] [Google Scholar]
- Horn R, Brodwick MS, Dickey WD. Asymmetry of the acetylcholine channel revealed by quaternary anesthetics. Science. 1980;210:205–207. doi: 10.1126/science.6251552. [DOI] [PubMed] [Google Scholar]
- Jia F, Xie X, Zhou Y. Short-term depression of synaptic transmission from rat lateral geniculate nucleus to primary visual cortex in vivo. Brain Res. 2004;1002:158–161. doi: 10.1016/j.brainres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xing G, Yang C, Verma A, Zhang L, Li H. Stress Impairs 5-HT(2A) Receptor-Mediated Serotonergic Facilitation of GABA Release in Juvenile Rat Basolateral Amygdala. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.71. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci. 2001;21:9608–9618. doi: 10.1523/JNEUROSCI.21-24-09608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaj M, Bai D, Renaud LP. GABAB receptor modulation of rapid inhibitory and excitatory neurotransmission from subfornical organ and other afferents to median preoptic nucleus neurons. J Neurophysiol. 2004;92:111–122. doi: 10.1152/jn.00014.2004. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotional networks and motor control: a fearful view. Prog Brain Res. 1996;107:437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- Levita L, Mania I, Rainnie DG. Subtypes of substance P receptor immunoreactive interneurons in the rat basolateral amygdala. Brain Res. 2003;981:41–51. doi: 10.1016/s0006-8993(03)02870-1. [DOI] [PubMed] [Google Scholar]
- Li YW, Guyenet PG. Neuronal inhibition by a GABAB receptor agonist in the rostral ventrolateral medulla of the rat. Am J Physiol. 1995;268:R428–437. doi: 10.1152/ajpregu.1995.268.2.R428. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Yanagawa Y, Obata K, Vogt KE. A specialized subclass of interneurons mediates dopaminergic facilitation of amygdala function. Neuron. 2005;48:1025–1037. doi: 10.1016/j.neuron.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Mascagni F, McDonald AJ. Immunohistochemical characterization of cholecystokinin containing neurons in the rat basolateral amygdala. Brain Res. 2003;976:171–184. doi: 10.1016/s0006-8993(03)02625-8. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Colocalization of calcium-binding proteins and GABA in neurons of the rat basolateral amygdala. Neuroscience. 2001;105:681–693. doi: 10.1016/s0306-4522(01)00214-7. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Immunohistochemical characterization of somatostatin containing interneurons in the rat basolateral amygdala. Brain Res. 2002;943:237–244. doi: 10.1016/s0006-8993(02)02650-1. [DOI] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Postsynaptic targets of somatostatin-containing interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;500:513–529. doi: 10.1002/cne.21185. [DOI] [PubMed] [Google Scholar]
- Nathan T, Jensen MS, Lambert JD. The slow inhibitory postsynaptic potential in rat hippocampal CA1 neurones is blocked by intracellular injection of QX-314. Neurosci Lett. 1990;110:309–313. doi: 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- Pearce RA, Grunder SD, Faucher LD. Different mechanisms for use-dependent depression of two GABAA-mediated IPSCs in rat hippocampus. J Physiol. 1995;484 ( Pt 2):425–435. doi: 10.1113/jphysiol.1995.sp020675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla ME, Perez E, Tampier L. Baclofen reduces ethanol intake in high-alcohol-drinking University of Chile bibulous rats. Addict Biol. 2008 doi: 10.1111/j.1369-1600.2008.00102.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced Negative Emotion and Alcohol Craving, and Altered Physiological Responses Following Stress and Cue Exposure in Alcohol Dependent Individuals. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH. Alcohol abuse in individuals exposed to trauma: a critical review. Psychol Bull. 1996;120:83–112. doi: 10.1037/0033-2909.120.1.83. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Redman SJ. The role of GABAA and GABAB receptors in presynaptic inhibition of Ia EPSPs in cat spinal motoneurones. J Physiol. 1992;447:675–692. doi: 10.1113/jphysiol.1992.sp019023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res. 2008;32:1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Gahwiler BH. Activity-dependent disinhibition. III. Desensitization and GABAB receptor-mediated presynaptic inhibition in the hippocampus in vitro. J Neurophysiol. 1989;61:524–533. doi: 10.1152/jn.1989.61.3.524. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci U S A. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn MS, Moises HC. Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci. 1992;12:4066–4079. doi: 10.1523/JNEUROSCI.12-10-04066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: The view from the slice. Pharmacol Ther. 2006 doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Ethanol Potentiates GABAergic Synaptic Transmission in a Postsynaptic Neuron/Synaptic Bouton Preparation from Basolateral Amygdala. J Neurophysiol. 2006 doi: 10.1152/jn.01380.2005. [DOI] [PubMed] [Google Scholar]