Abstract
Poor cell engraftment in the myocardium is a limiting factor towards the use of bone marrow-derived cells (BMCs) to treat myocardial infarction (MI). In order to enhance the engraftment of circulating BMCs in the myocardium following MI, we have developed in situ forming hyaluronic acid (HA) hydrogels with degradable crosslinks to sustain the release of recombinant stromal cell-derived factor-1 alpha (rSDF-1α) and HA to the injured myocardium. Both rSDF-1α and the crosslinkable HA macromer stimulate BMC chemotaxis up to 4-fold in vitro through CXCR4 and CD44 receptor signaling, respectively. Moreover, the HA macromer binds rSDF-1α with a dissociation constant of 36 ± 5 µM through electrostatic interaction. When formed into hydrogels via photoinitiated crosslinking, release of encapsulated rSDF-1α and crosslinked HA was sustained for 7 days, and these molecules significantly increased BMC chemotaxis in vitro. When applied to the heart following experimental MI in mice, the HA gel containing rSDF-1α significantly increased the number of systemically infused BMCs in the heart by ~8.5 fold after 7 days, likely through both systemic and local effects of released molecules. We conclude that sustained release of rSDF-1α and HA from our engineered HA hydrogels enhances BMC homing to the remodeling myocardium better than delivery of rSDF-1α alone.
Keywords: hyaluronic acid, hydrogel, cardiac repair, bone marrow, stem cells, chemotaxis
1. Introduction
Nearly 1 million Americans suffer a myocardial infarction (MI) each year and many patients progress to heart failure due to limited treatment options to attenuate the remodeling response post-MI [1]. Following MI, a dynamic tissue remodeling process, characterized by cell death and the formation of avascular scar tissue, induces global changes to ventricle geometry (i.e., wall thinning and chamber dilation), which ultimately compromise heart function [2]. In order to attenuate the progression to heart failure, therapeutics using bone marrow-derived cells (BMCs) are being widely explored to promote repair in the remodeling myocardium. In particular, autologous BMC transplantation has received the greatest attention in a clinical setting; yet, only modest improvements in ventricular function have been demonstrated after intracoronary BMC delivery, potentially due to limited cell engraftment in the myocardium [3]. Inconsistencies in cell isolation and storage procedures [4], the timing of BMC administration following MI [5], and the location of BMC administration [6] complicate outcomes by altering the delicate signaling mechanisms that are critical for cell engraftment.
BMC engraftment in the bone marrow microenvironment (i.e., BMC homing) is regulated by the expression of chemokines and extracellular matrix (ECM) molecules under steady state conditions. In response to injury or under pathological conditions, elevated concentrations of these regulatory molecules in the peripheral circulation mobilize BMCs and direct their engraftment in remodeling tissues to mediate repair. In particular, stromal-derived factor-1 alpha (SDF-1α), along with its receptor (CXCR4), are critical regulators of BMC homing to the bone marrow, but also orchestrate BMC mobilization into the peripheral circulation and local engraftment in the heart following MI [7]. Myocardial SDF-1α expression post MI is accompanied by a concomitant increase in bone marrow derived CXCR4+ circulating cells, suggesting that these cells are mobilized and respond to the myocardial SDF-1α signal [8].
Despite evidence that the mobilized BMCs home to the myocardium via SDF-1α and contribute to tissue repair, the extent of this endogenous response is insufficient to prevent the eventual onset of heart failure following MI. Enhancing endogenous BMC homing (and therefore repair) through myocardial delivery of SDF-1α increases progenitor cell markers in the heart, stimulates angiogenesis, and attenuates global ventricular remodeling [9]. However, due to its very low molecular weight (8kDa) and proteolytic susceptibility, SDF-1α delivery to the myocardium following MI is challenging. Researchers have addressed this challenge by covalently linking recombinant SDF-1α (rSDF-1α) [10] or SDF-1α peptide analogs [11] to scaffolds that incorporate sites for integrin mediated adhesion. Beyond integrin mediated interactions, there are other components of the bone marrow niche, such as interactions with glycosaminoglycans (GAGs) that might aid in SDF-1α delivery and BMC engraftment with improved efficacy.
Naturally, local concentrations of small, cationic chemokines like SDF-1α are regulated by GAGs in the ECM [12]. SDF-1α binds to GAGs through ionic interactions between basic amino acid residues and acidic groups along the disaccharide backbone [13]. The affinity between SDF-1α and GAGs plays an important role in localizing SDF-1α on the endothelium to direct cell homing [14] and sustaining SDF-1α activity in the presence of proteases [15]. In addition to their roles in chemokine presentation, some GAGs play a more direct role in BMC homing through receptor interactions. For example, hyaluronic acid (HA) is abundant in the bone marrow, where it serves as an anchoring molecule for BMC homing through binding to the CD44 receptor [16]. Interestingly, HA is localized to regions of SDF-1α expression in the bone marrow and HA and CD44 mediate cell responsiveness to SDF-1α during cell migration in vitro and cell homing in vivo [17].
In this work, we exploit the cooperative roles of SDF-1α and HA in order to enhance endogenous BMC homing to the heart following MI. Specifically, recombinant SDF-1α was encapsulated in degradable HA hydrogels that form in situ on the heart, in order to localize SDF-1α and HA homing cues to the remodeling heart for enhanced engraftment of circulating BMCs in the myocardium.
2. Materials and Methods
2.1 Animals
Adult BALB/CAnNHsd mice (Charles River, Production) were housed in a dedicated vivarium under conventional conditions. Mice received a standard diet (LabDiet 5010) and water ad libitum. All experimental procedure were performed according to the NIH Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania.
2.2 Macromer synthesis
A degradable HA macromer with methacrylate functionality was synthesized through hydroxyethyl methacrylate (HEMA) modification of the HA backbone in a three step process. (1) Sodium hyaluronate (74 kDa, Lifecore) was converted to a tetrabutylammonium (TBA) salt by acidic ion exchange with Dowex resin (50W×8–200, Sigma), followed by neutralization with TBA-OH, and lyophilization. (2) 2-hydroxyethyl methacrylate was reacted with succinic anhydride in dichloroethane (DCE) via a ring opening reaction (65°C, 16hrs) in the presence of N-methylimidazole to obtain HEMA-COOH. HEMA-COOH was purified via hydrochloric acid wash, DI H2O wash, and DCE evaporation with a rotary evaporator. (3) HEMA-COOH was coupled to HA-TBA in anhydrous dimethyl sulfoxide through an esterification reaction in the presence of 4-dimethylaminopyridine (DMAP) and di-tert-butyl dicarbonate (BOC2O) activating agents (45°C, 20hrs). The following molar ratios of reactants were used: HA-TBA (1), HEMA-COOH (1.4), BOC2O (1.2), DMAP (0.1). The modified HEMA-HA macromer was purified via overnight dialysis in DI H2O at 4°C, acetone precipitation, another 5 days of dialysis in DI H2O at 4°C, followed by lyophilization. 1H NMR was used to determine the degree of modification and purity of the HEMA-HA.
2.3 SDF-1α electrostatics
Electrostatic surface potentials of SDF-1α were visualized with Adaptive Poisson-Boltzmann Solver (APBS) software [18]. The PDB file for SDF-1α (2KEC, [19]) was first converted to a PQR file with PDB2PQR software [20] using a PARSE forcefield and PROPKA software [21] to assign protonation states at pH = 7.4. All calculations were performed on a web server hosted by the National Biomedical Computation Resource and surface potentials were visualized with Jmol [22].
2.4 Macromer binding affinity
Binding affinity between rSDF-1α and HEMA-HA was quantified using an intrinsic protein fluorescence quenching technique [23]. Fluorescence titration experiments were performed on a Tecan infinite m200 plate reader (Grödig, Austria) at 25°C. The fluorescence of a 2 µM rSDF-1α (460-SD, R&D Systems) solution in 100 mM HEPES buffer (pH 7.4) was recorded at 280/350nm excitation/emission. Fluorescence quenching was monitored by increasing the concentration of HEMA-HA in rSDF-1α solution in 13.5 µM increments. Dry macromer was dissolved in the rSDF-1α solution, mixed, and incubated for a 2 min equilibration period before measuring fluorescence. The normalized changes in fluorescence with each HEMA-HA titration were plotted against HEMA-HA concentration and resulting binding isotherms were fit by nonlinear regression (Mathematica, Wolfram Research, Inc.) to the equation describing bimolecular association:
where F is the change in fluorescence over initial fluorescence (−ΔF/Fo), Fi is the initial value for (−ΔF/Fo), Ff is the final value for (−ΔF/Fo), and Kd is the dissociation constant [23].
2.5 Hydrogel formation
Hydrogels were formed upon visible light exposure using a previously established initiator system consisting of eosin Y, triethanolamine (TEOA), 1-vinyl-2-pyrrolidinone (VP), and a halogen curing light (Elipar 2500, 3M) [24]. 50 µL hydrogel precursor solutions were prepared by mixing HEMA-HA and initiators in PBS at final concentrations of: 6 wt% HEMA-HA, 0.02 wt% eosin Y, 225 mM TEOA, and 37 mM VP. 200 ng rSDF-1α was mixed into the 50 µL hydrogel precursor solution for HA Gel/rSDF-1α groups. Hydrogels were formed in cylindrical molds upon light exposure for 90 seconds. For release studies, gels were incubated in 1 mL of chemically defined (CD) media comprised of α-MEM media (Invitrogen) supplemented with 20 mM L-glutamine, 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.1% bovine serum albumin (BSA). Gels were moved to fresh media at the indicated time points and media containing eluted molecules were stored at −20°C. rSDF-1 α was quantified with ELISA (DY350, R&D Systems) and HA was quantified with a previously established uronic acid assay [25]. The effect of HEMA-HA on rSDF-1α ELISA detection was investigated with a competition assay as described in the Supplementary Information.
2.6 BMC isolation
Adult BALB/CAnNHsd mice (10–12 weeks old, 22–26 g) were anesthetized with 100 mg/kg ketamine and 20 mg/kg xylazine. Unfractionated BMCs were obtained by removing the femurs, and flushing the marrow cavity with sterile PBS over a 40 µm filter. The cells were pelleted by centrifugation and resuspended in lysis buffer (BD Biosciences) for 1 min to lyse red blood cells. Cells were again pelleted and resuspended in 1 mL PBS to remove remaining lysis buffer. To visualize the cells in vitro and in vivo, PKH fluorescent linker kits were employed (Sigma).
2.7 In vitro BMC chemotaxis
The chemotactic activities of rSDF-1α and HEMA-HA were quantified using freshly isolated BMCs in microchemotaxis chambers (NeuroProbe) following a modified boyden chamber assay [26]. Briefly, BMCs (2×106 cells/mL) in CD media were separated from active molecules in CD media by a porous polycarbonate membrane (5 µm pores) coated with type 1 collagen (PureCol, Advanced BioMatrix). After 4 hrs at 37°C, non-migrated cells on the top side of the membrane were scraped away, while migrated cells on the bottom side were fixed in 4% formalin and imaged. PKH67GL fluorescence was used to visualize cells and Image J Particle Analysis software was used to quantify cells as described in the Supplementary Information. For each experiment, the average number of migrated cells per well for each condition were normalized to the average number of migrated cells per well for control wells containing only CD media (n=6 wells per condition). A monoclonal antibody to CD44 (ab25064, abcam) was used for HA specific blocking at 3 µg/mL, while a CXCR4 antagonist (AMD3100, Calbiochem) was used for SDF-1α specific blocking at 0.5 µg/mL. Human mesenchymal stem cell (hMSC) culture and in vitro chemotaxis conditions are described in the Supplementary Information.
2.8 Experimental MI
A cryoinjury model of MI was used to initiate MI remodeling [27]. Adult BALB/CAnNHsd mice (10–12 weeks old, 22–26 g) were anesthetized with 20 mg/kg ketamine and 4 mg/kg xylazine, shaved and disinfected with alcohol. The mice were kept under anesthesia (1.5% isoflurane), incubated and ventilated with 95% O2 using a mechanical ventilator (MiniVent, Harvard Apparatus). The heart was exposed through a left lateral thoracotomy and cryoinjury was introduced by applying a round 2 mm diameter stainless steel probe frozen with liquid nitrogen to the epicardial surface of the ventricle for 10 sec. After removing the probe, the cryoinjured area was confirmed by a white disk-shaped region. For mice receiving hydrogels, 25 µL of the liquid hydrogel precursor solutions (same as section 2.5) were applied to the surface of the injured ventricle through a 27G syringe in the presence of curing lamp exposure. For mice receiving SDF-1α only, 10 µL of a 10 µg/mL rSDF-1α solution was injected into the ventricle wall using a 30G syringe. The order of therapeutic intervention was rotated from animal to animal. Following manipulations to the heart, the intercostal space and skin were closed with sutures. Mice received 100% oxygen until responding to interdigital pinch, after which the endotracheal tube was withdrawn. Mice were kept warm with a heating blanket during recovery. Meloxicam (5 mg/kg) was administered via intraperitoneal injection 1 hr after surgery and once a day for 1 week following surgery to minimize pain.
2.9 In vivo BMC homing
After a recovery period of 3 hrs following MI induction, mice were anesthetized with 20 mg/kg ketamine and 5 mg/kg xylazine. Freshly isolated BMCs from a donor mouse were fluorescently tagged with PKH linker kit, counted on a Vi-CELL (Beckman Coulter, Inc.), and 9×106 cells in 300 µL sterile PBS was injected into the femoral vein of the MI mouse. Blood samples were collected 1 day after BMC injection via retro orbital bleed, treated for RBC lysis, and analyzed for PKH+ cells using flow cytometry. Briefly, cells were gated for viability using 4’,6-diamidino-2-phenylindole (DAPI, Invitrogen), size and granularity using characteristic BMC distributions of forward scatter (FSC) and size scatter (SSC), respectively, and fluorescent intensity using appropriate controls (Supp. Fig. 4) [28]. PKH+ cells in the blood were reported as a percentage of total cells in the blood within the characteristic BMC size distribution. Mice were anesthetized 7 days after experimental MI with 100 mg/mL ketamine and xylazine, blood samples were collected from the abdomen, and hearts were excised and either perfusion digested with collagenase (150 U/mL type 2 collagenase in Krebs buffer, Worthington) for PKH+ cell quantification using flow cytometry [28] or flash frozen in embedding medium for PKH+ cell visualization using histology. Quantified PKH+ cells in the blood were again reported as a percentage of total BMCs in the blood, while quantified PKH+ cells in the heart were reported as a total number of cells/heart. Embedded hearts were sectioned at a 12 µm thickness and imaged immediately upon thawing at room temperature.
2.10 Statistical analysis
Values are reported as mean ± standard error of the mean. Statistical differences between groups were determined using ANOVA in conjunction with a student’s two-tailed t-test (Microsoft Excel), with P < *0.05, **0.01, and ***0.001 considered as significant.
3. Results
3.1 HEMA-HA synthesis
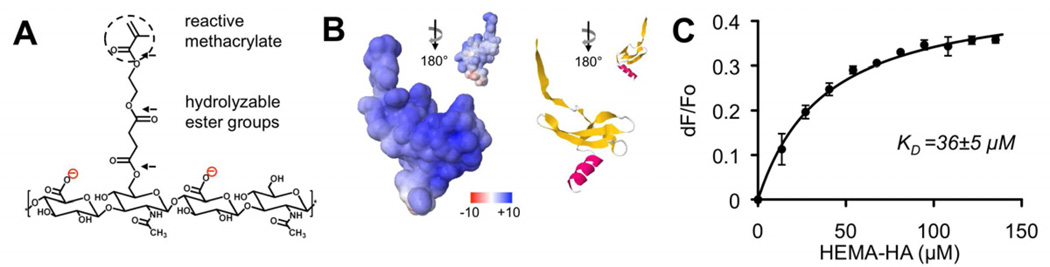
HA macromers were synthesized to facilitate photoinitiated crosslinking into hydrogels through a terminal methacrylate group and degradation through hydrolysis of ester groups between the methacrylate and the HA backbone (Fig. 1A). The extent of HEMA modification was determined to be ~25% of disaccharide repeat units along the HA backbone via 1H NMR (Supp. Fig. 1). This percent can be easily tuned with the molar ratio of HEMA-COOH to BOC2O in the coupling reaction [29].
Fig. 1.
Electrostatic interactions between HEMA-HA and rSDF-1α. A) Repeat unit of HEMA-HA macromer synthesized with reactive methacrylate group and hydrolyzable ester groups (black arrows) between the methacrylate functionality and HA backbone. The macromer carries a negative charge due to carboxylic acid groups along the HA backbone. B) SDF-1α surface potentials visualized using APBS software package (left) that correspond to the beta sheet rich in basic amino acids (right). Scale for surface potentials is in units of kT/e where k is Boltzman’s constant, T is temperature, and e is the charge of an electron. C) Fluorescence quenching of rSDF-1α with HEMA-HA titration. Data fit to bimolecular association equation to calculate KD [59] (n = 3 independent experiments).
3.2 HEMA-HA and SDF-1α binding
To investigate ionic interactions between the HEMA-HA macromer and rSDF-1α, the electrostatic surface potentials of SDF-1α were visualized and binding association constants were experimentally determined. Visualization of SDF-1α using APBS revealed an overall positive surface charge with the most positive charges of nearly +10 kT/e concentrated along the beta sheet of the protein structure (Fig. 1B). This charge distribution is attributed to an abundance of basic amino acids (i.e., arginine and lysine residues) in the beta sheet, which have important roles in SDF-1α binding to negatively charged GAGs [13]. Indeed, binding between rSDF-1α and the purified HEMA-HA macromer was observed with a dissociation constant (KD) of 36 ± 5 µM (Fig. 1C). This interaction was significantly stronger than the measured interaction between HEMA-HA and BSA (KD = 157 ± 4 µM, data not shown), illustrating the specificity of small, positively charged chemokines for negatively charged GAGs (i.e., HA) in the ECM.
3.3 BMC chemotaxis to rSDF-1α and HEMA-HA
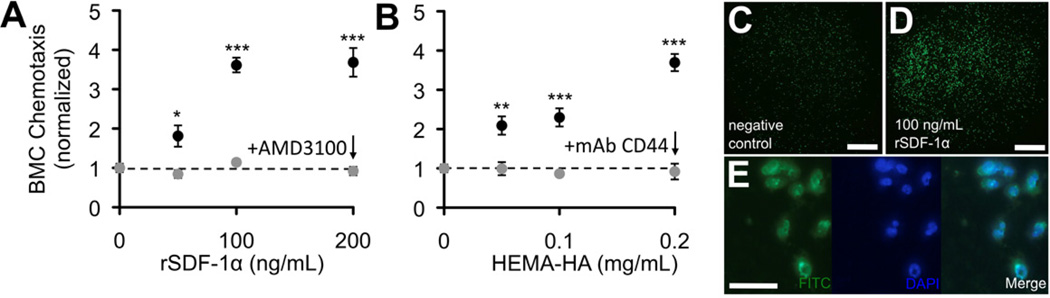
In order to model endogenous BMC homing, unfractionated BMCs were isolated from mouse femurs, fluorescently tagged with PKH linker dyes, and used to assess the activity of rSDF-1α and HEMA-HA in vitro and in vivo. In vitro, both rSDF-1α and HEMA-HA stimulated chemotaxis of BMCs in a dose dependent manner, nearly 4-fold at the highest concentrations investigated (Fig. 2, ***P = 0.0001 and ***P = 0.0008, respectively). The observed chemotaxis was specific to cell surface receptors for SDF-1α (CXCR4) and HA (CD44) as evidenced by antagonist and antibody blocking, respectively. Specifically, the introduction of the antagonist or antibody completely diminished any increased migration in response to the molecule and was indistinguishable from control groups. The chemotactic activity of rSDF-1α and HEMA-HA was also observed using a purified population of bone marrow derived stem cells, human mesenchymal stem cells (hMSCs) (Supp. Fig. 2). The hMSC response to rSDF-1α and HEMA-HA was also diminished in response to the introduction of a CXCR4 antagonist and CD44 antibody blocking, respectively.
Fig. 2.
Chemotactic activity of rSDF-1α and HEMA-HA. A) rSDF-1α stimulated significant dose-dependent chemotaxis of unfractionated BMCs and was blocked with a CXCR4 antagonist, AMD3100. B) HEMA-HA stimulated similar dose-dependent chemotaxis of BMCs and was blocked with a monoclonal antibody to CD44. For each experiment, the average number of migrated cells per well for each condition was normalized to the average number of migrated cells per well for negative control wells containing only chemically defined media (mean ± SEM, n = 6 wells per condition). C) Representative negative control well with migrated PKH+ BMCs. D) Representative 100 ng/mL rSDF-1α well containing migrated PKH+ BMCs. E) Fluorescent visualization of migrated PKH+ BMCs. Scale bars: C–D = 500 µm; E = 50 µm.
3.4 Molecule release from HEMA-HA hydrogels
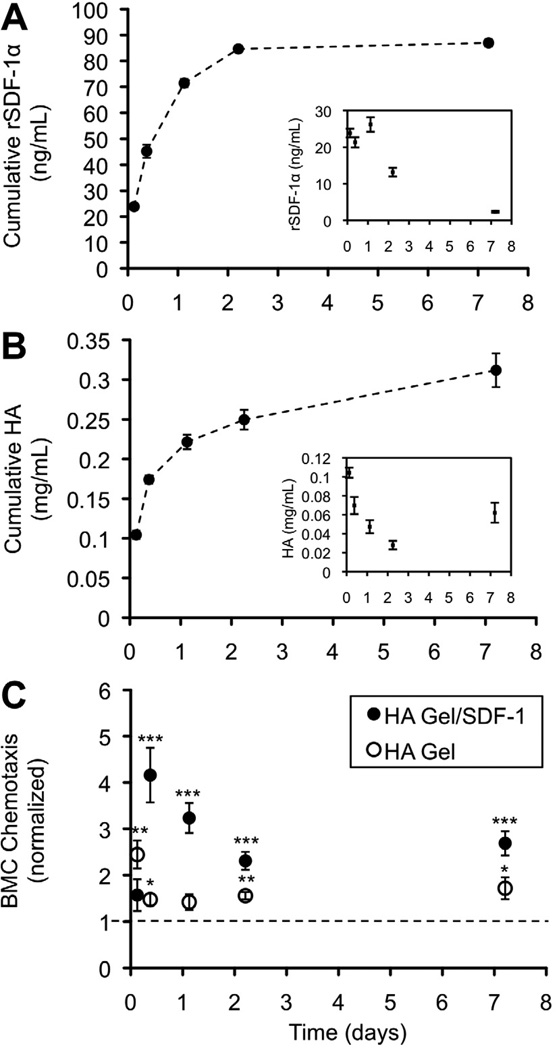
To sustain release of rSDF-1α and HEMA-HA in the setting of MI, a visible light initiator system was used to crosslink the HEMA-HA macromer into hydrogels from a liquid precursor solution (containing rSDF-1α, HEMA-HA, and photoinitiator) upon blue light exposure. Photocrosslinked HEMA-HA hydrogels sustained the release of rSDF-1α and HA, measured over 7 days in vitro (Fig. 3A–B). HEMA-HA hydrogels exhibit hydrolytic degradation of ester groups in the HEMA side groups, releasing HA, entrapped rSDF-1α, and poly(methacrylic acid) kinetic chains with time. Generally, the release of rSDF-1α and HA was rapid in the first few days, due to diffusion of peripheral rSDF-1α and uncrosslinked HEMA-HA, and then slowed as release of encapsulated molecules was mediated by hydrogel degradation. Gels were still intact after 7 days in buffer at 37°C, while little additional rSDF-1 α release was measured. While the ELISA kit provides a low limit of detection for rSDF-1α, this antibody “sandwich” technique was unable to detect rSDF-1α in the presence of soluble HEMA-HA, which acted as a binding competitor to the monoclonal antibodies with 44% inhibition at 0.08 mg/mL HEMA-HA (Supp. Fig. 3). Therefore, we suspect that reported concentrations of released rSDF-1α are for the free, unbound protein in solution, and that rSDF-1α/HEMA-HA complexes are also present in the release samples. Importantly, released molecules from HA hydrogels and HA hydrogels containing rSDF-1α both stimulated chemotaxis of BMCs over 7 days (Fig. 3C), indicating the influence of both HA and rSDF-1α components on BMC chemotaxis.
Fig. 3.
Molecule release from photocrosslinked HA Gels. A) Cumulative release of encapsulated rSDF-1α was sustained for 7 days in vitro as determined by ELISA. B) Cumulative release of uncrosslinked HEMA-HA and degradation products of the hydrogel was sustained for 7 days in vitro as determined by uronic acid analysis. Quantity of rSDF-1α and HA was measured from buffer collected and replaced at specified time points. Gels remained intact after the 7-day incubation. Insets indicate quantity of molecules in each sample (n = 3 gels, mean ± SEM). C) The HA Gel significantly enhanced BMC chemotaxis for up to 7 days, which was further enhanced with release of encapsulated rSDF-1α. For each experiment, the average number of migrated cells per well for each condition was normalized to the average number of migrated cells per well for negative control wells containing only chemically defined media (mean ± SEM, n = 6 wells per condition).
3.5 BMC homing to the remodeling heart
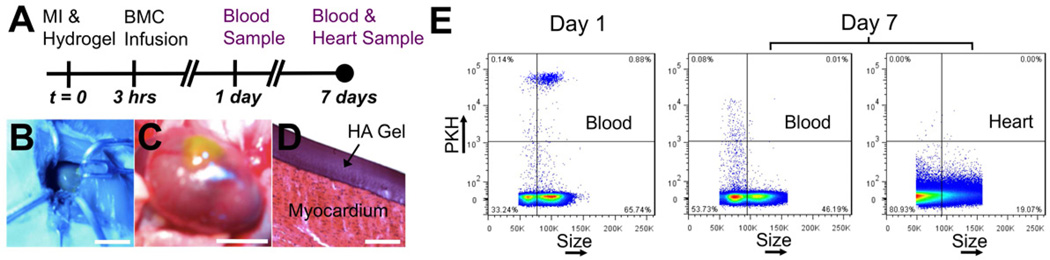
In order to quantify BMC homing in vivo, fluorescently tagged BMCs were infused into the circulation following experimental MI in mice and tracked in the blood and the heart using flow cytometry (Fig. 4A). Myocardial delivery of homing factors (HA and rSDF-1α) from hydrogels (formed on heart using blue light, Fig. 4B) was assessed for the ability to enhance BMC homing to the heart and compared to controls of MI only and MI with intramyocardial injection of rSDF-1α. Hydrogels were localized to the injury site and adhered to the myocardium for sustained delivery of homing molecules (Fig. 4C–D).
Fig. 4.
In vivo model to quantify BMC homing. A) HA Gels were applied to the heart immediately following experimental MI. Freshly isolated BMCs were fluorescently tagged with PKH linker dyes and infused into the circulation via a femoral vein injection 3 hrs after MI. Blood samples were collected 1 and 7 days following MI and hearts were digested 7 days following MI to quantify PKH+ BMCs. B) Hydrogels formed in situ by applying a liquid precursor solution to the epicardial surface of the ventricle upon blue light exposure. C) This technique allowed the hydrogels to be localized on the injured myocardium for localized molecule delivery. D) The hydrogels adhered to the myocardium while processing the tissue for histology and H&E staining. E) Representative flow cytometry with constant threshold to quantify PKH+ BMCs in the blood and heart. Scale bars: B-10 mm; C-5 mm; D-100 µm.
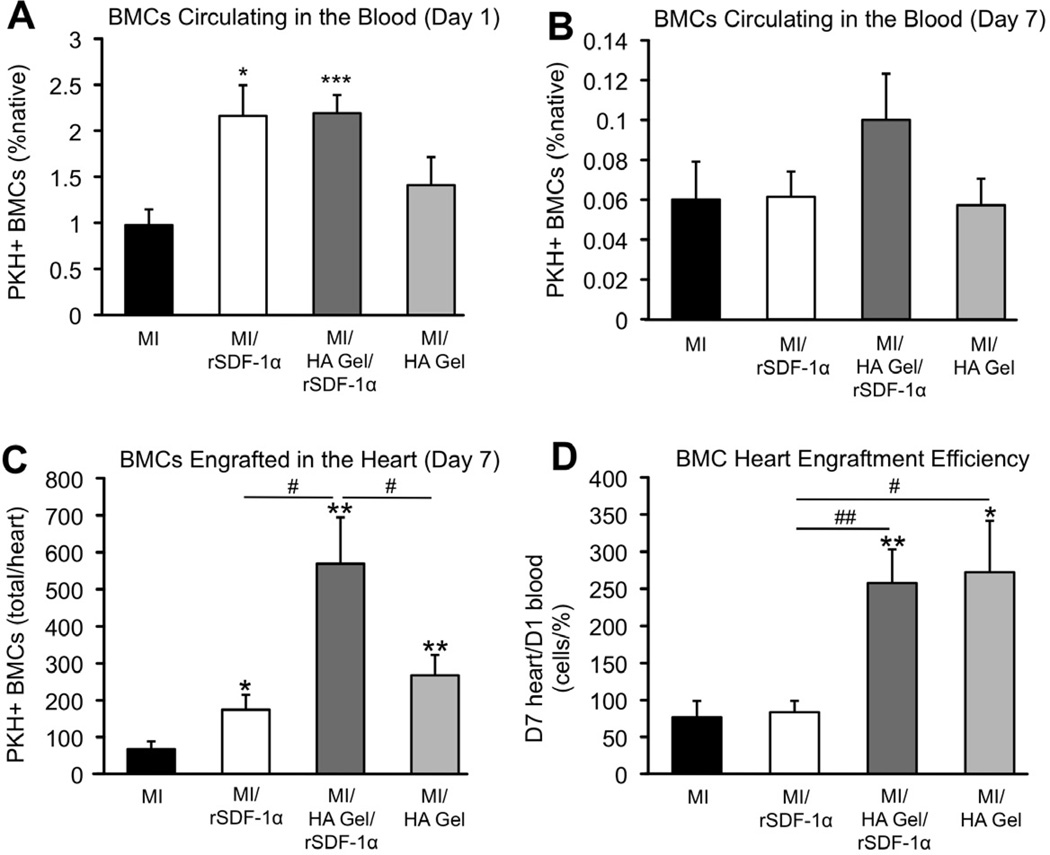
The circulating PKH+ BMCs were readily quantified in the blood and heart using flow cytometry (Fig. 4E). The fluorescence intensity of the cells varied over a wide range, so a PKH+/PKH- threshold was chosen to quantify PKH+ BMCs while minimizing PKH- false-positive events in the blood and the heart (Supp. Fig. 4). Using this threshold, we observed a decrease in fluorescence intensity of the PKH+ BMCs in the blood from day 1 to day 7 (Fig. 4E). Considering that the PKH linker dyes are stable in vivo for weeks, the decrease in fluorescent intensity could be due to cell proliferation, or simply due to preferential retaining of cell types in the blood that do not uptake PKH dyes as effectively as others [30]. Interestingly, BMCs quantified in the blood on day 1 were primarily larger cells around the size and granularity of granulocytes, while the BMCs quantified in the blood on day 7 were primarily smaller cells around the size and granularity of lymphocytes (Supp. Fig. 5); however, both size phenotypes show similar ranges of PKH staining intensity immediately after exposure to the PKH linker dye.
Delivery of the HA Gel with encapsulated rSDF-1α and delivery of rSDF-1α as a bolus injection significantly increased the number of infused BMCs circulating in the blood 1 day after infusion (Fig. 5A, ***P < 0.001 and *P = 0.01, respectively). Both rSDF-1α delivery groups more than doubled the number of circulating BMCs on day 1 from 1% to 2% of native BMCs; however, this effect was only apparent in the HA Gel/rSDF-1α group on day 7, although not statistically significant (Fig. 5B). All intervention groups significantly increased the number of BMCs engrafted in the heart compared to MI only, and the HA Gel with encapsulated rSDF-1α significantly increased BMC engraftment compared to rSDF-1α alone and the HA Gel alone (Fig. 5C, #P = 0.01 and 0.047, respectively), indicating synergy between the released rSDF-1α and HA. When normalized to the percentage of BMCs circulating in the blood, the HA Gel (with and without rSDF-1α) enhanced myocardial engraftment of circulating BMCs at a significantly improved rate, over 3-fold greater than the bolus rSDF-1α injection and MI control, which showed the same engraftment rate (Fig. 5D). While both the HA Gel/rSDF-1α and bolus rSDF-1α injection groups had the same effect on circulating BMCs 1 day after infusion (2.2 ± 0.2 and 2.2 ± 0.3% native BMCs, respectively), they had significantly different effects on enhancing BMC engraftment in hearts 7 days after infusion, 570 ± 130 and 170 ± 40 BMCs per heart, respectively.
Fig. 5.
Quantification of BMC homing with molecule delivery to the heart. A) rSDF-1α delivery to the heart significantly increased the number of circulating PKH+ BMCs 1 day after infusion. B) This effect was sustained, although not statistically significant, 7 days after infusion when rSDF-1α was delivered from the HA Gel. PKH+ BMCs in the blood are reported as a percentage of total cells in the blood within the BMC size and granularity gating. C) Intramyocardial injection of rSDF-1α and application of the HA Gel alone following MI significantly enhanced the number of PKH+ BMCs in the heart compared to MI only 7 days after MI and cell infusion. Application of the HA Gel with encapsulated rSDF-1α further enhanced PKH+ BMC engraftment in the heart compared to delivering rSDF-1α or the HA gel individually. D) When normalized to the percent of PKH+ BMCs in the blood 1 day after infusion, the HA Gel enhances engraftment of circulating PKH+ BMC with a constant efficiency. Values reported are mean ± SEM, n = 7 animals for all groups except for MI only, n = 6.
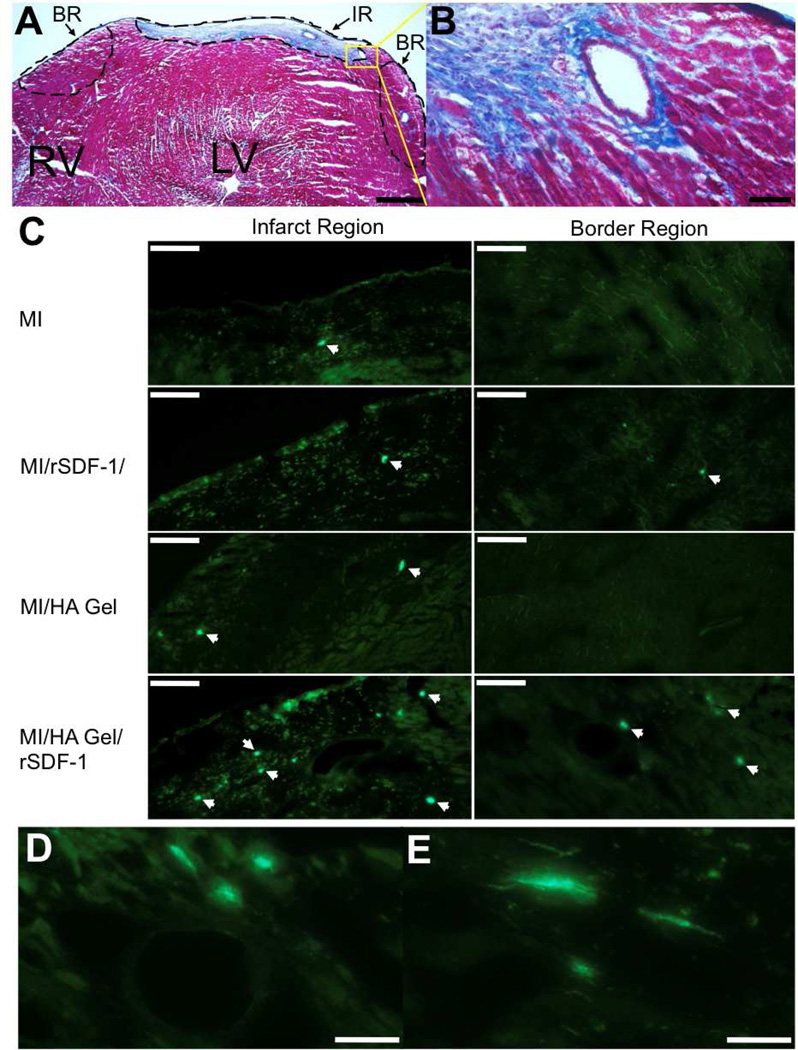
Engrafted BMCs localized to the infarct region (IR) and border region (BR) in the heart following MI (Fig. 6), while PKH+ BMCs in other regions of the myocardium were very rare. The infarct region was characterized by dense collagen staining with Masson’s Trichrome (Fig. 6A–B). This collagen scar tissue autofluoresced, allowing the infarct region to be easily identified for characterizing engrafted BMC locations within the heart, and PKH+ BMC fluorescence was bright enough to distinguish the BMCs against the tissue background as indicated by white arrows (Fig. 6C). PKH+ BMCs were identified in the infarct region in all groups, but were rare in the border region for all groups except for the HA Gel/rSDF-1α group. Border region BMCs in this group were commonly localized within and around vascular structures (Fig. 6D). Some cells also appeared to integrate into the tissue and align with the native tissue morphology (Fig. 6E).
Fig. 6.
Visualization of PKH+ BMCs in the myocardium 7 days after systemic infusion. A) The cryoinjury resulted in the formation of a nontransmural collagen rich scar tissue (blue region) 7 days following MI as visualized with Masson’s Trichrome. An infarct region (IR) and border region (BR) were defined to assess PKH+ BMC locations within the heart. B) Vasculature in the IR and BR remained intact following the cryoinjury. C) PKH+ BMCs were identified in the IR of all groups, while PKH+ BMCs in the BR were rare except in the HA Gel/rSDF-1α group. PKH+ BMCs were not found in remote regions of the myocardium for all groups. White arrowheads indicate PKH+ BMCs. D) PKH+ BMCs in the BR were often localized in and around vascular structures. E) In addition, cells in the BR appeared to align with the myocardial tissue structure. Scale bars: A-500 µm; B-50 µm; C-100 µm; D and E-50 µm.
4. Discussion
The objective of this study was to develop a biomaterial system to enhance BMC homing to the remodeling myocardium through exogenous delivery of the chemokine rSDF-1α. To accomplish this, we synthesized an in situ crosslinkable and degradable hydrogel system based on the molecule HA to encapsulate and sustain the local release of rSDF-1α to the myocardium. Our rationale for choosing HA as our biomaterial was two-fold: (1) HA is a negatively charged GAG that naturally regulates chemokines in tissues, and (2) HA is the major ligand for CD44, which mediates cell motility processes that govern BMC homing.
GAGs are linear polysaccharides with an abundance of acidic groups that are negatively charged under physiologic conditions. Chemokines, on the other hand, are characterized by an abundance of basic amino acid residues that are positively charged under the same conditions. These complimentary charges induce binding between GAGs and chemokines with an affinity that is dependent on the specific GAG chemistry, the size of the GAG molecule, and the net charge of the chemokine [12]. In particular, heparin sulfate (HS) has been shown to bind SDF-1α with high affinity (KD~30 nM) [13]. Binding between SDF-1α and HS in the glycocalyx of the cell surface is important to localize SDF-1α and facilitate binding to CXCR4 [13]; however, soluble HS has been shown to inhibit the chemotactic activity of SDF-1α in a dose dependent manner (0.1 to 100 µg/mL HS) [31]. Since the CXCR4 binding domain remains exposed after HS binding [13], the soluble HS is likely acting as a binding competitor to glycocalyx HS, preventing SDF-1α from achieving close contact with CXCR4 on the cell surface. Therefore, GAG chemistries with less affinity for SDF-1α may be optimal as a carrier matrix to allow glycocaylx sequestration of released SDF-1α in the presence of the degraded matrix. HA has 1 carboxylic acid per repeat unit compared to 3 sulfate groups per repeat unit of HS; therefore, we expected HA to bind SDF-1α with much less affinity (higher KD) than HS. Indeed, we measured a KD of 36 µM between our HA macromer and SDF-1α (Fig. 1C), which was selective to the positively charged SDF-1α (KD,BSA=157 µM). This micromolar affinity was sufficient to slow SDF-1α diffusion within the HA Gels where the concentration of HA is very high (~100 mg/mL), without inhibiting cell chemotaxis to released SDF-1α in the presence of HA released from the gels (10 to 100 µg/mL HA) (Fig. 3B–C).
HA also interacts cooperatively with SDF-1α in directing cell motility through CD44 and CXCR4 receptor signaling crosstalk [17]. SDF-1α-CXCR4 binding on hematopoetic progenitor cells stimulated cell spreading on HA coated substrates through CD44 binding and CD44 polarized to the leading edge of cells migrating towards gradients of SDF-1α in vitro [17]. HA-CD44 binding initiates G-protein-dependent signal transduction that stimulates cell motility through Rho-ROCK signaling [32], as well as cell adhesion through integrin expression [33]. These signaling pathways are important regulators of BMC homing processes in the bone marrow [17], as well as in peripheral tissues during inflammation [34]. For example, intravenously injected MSCs home to the injured kidney in a CD44 dependent process [35]. The role of HA in this process was corroborated in vitro as the MSCs exhibited CD44 dependent chemotaxis to HA.
In line with these studies, we showed that our modified HA macromer stimulates chemotaxis of BMCs through CD44 (Fig. 2, Supp. Fig. 2). When delivered to the myocardium as an epicardial hydrogel immediately following experimental MI, the HA Gel enhanced engraftment of circulating BMCs in the myocardium (Fig. 5C). BMC engraftment was further enhanced by encapsulating rSDF-1α in the HA Gel (Fig. 5C). To form the hydrogels in situ, a photo-initiated crosslinking mechanism was utilized in order to localize molecule release to the myocardium while avoiding biomaterial injections that could cause further damage due to the small thickness of a mouse ventricle wall. In experimental models with larger ventricle wall thicknesses, a redox-initiated crosslinking mechanism can be used to form these hydrogels upon injection through a syringe [29].
Interestingly, delivering rSDF-1α to the heart had systemic effects in significantly increasing the number of PKH+ BMCs circulating in the blood, and sustained release of rSDF-1α from the HA Gel prolonged this effect (Fig. 5A/B). Separate studies have shown that an increase in circulating CXCR4+ cells coincides with the temporal expression of rSDF-1α in the heart following MI [8, 9]. In addition, elevating SDF-1α concentrations in the blood has been shown to mobilize BMCs into the circulation [36]. Since we report the number of PKH+ BMCs in the blood as a percentage of total BMCs in the blood, an increase in this percentage may indicate that systemic concentrations of SDF-1α are high enough to prevent circulating PKH+ BMCs from homing to other organs, such as the bone marrow or spleen [37, 38], but not high enough to mobilize native BMCs from SDF-1α gradients in the bone marrow. Indeed, mobilizing agents have been administered systemically following MI in order to mobilize CXCR4+ BMCs into the circulation and therefore enhance the response to SDF-1α signals in the heart [39].
To model BMCs mobilized from the bone marrow, unfractionated BMCs were injected directly into the systemic circulation. These cells were used with minimal manipulations to include all CXCR4+ and CD44+ BMC populations that are present in vivo, and avoid culture conditions that are known to affect cell responsiveness to chemokines [40]. Using this model system, we were able to definitively quantify BMC homing with molecule delivery to the heart; however, investigation of how homed BMCs contribute to myocardial repair is beyond the scope of the model. Nevertheless, a growing body of literature suggests that delivery of SDF-1α and HA would promote a beneficial healing response following MI. For example, CXCR4 is expressed on selective populations of BMCs that have been implicated in myocardial tissue repair, including hematopoetic progenitor cells [41], mesenchymal stem cells [42], dendritic cells [43], T lymphocytes [44], and even committed tissue-specific stem cells [45]. Furthermore, SDF-1α delivery has been shown to largely promote neovascularization in ischemic tissues through BMC homing [9–11]. While enhancing CXCR4 positive cells in the heart has been shown to significantly attenuate post-MI remodeling, this approach seems to require complimentary growth factor and cell adhesion molecule expression that occurs in the early stages of post MI remodeling [46]. Therefore, early therapeutic intervention may be a limiting factor in translating SDF-1α-based therapies to the clinic.
Like SDF-1α/CXCR4, HA/CD44 interactions have important roles in regenerative processes during tissue remodeling including cell migration, proliferation, and differentiation [47]. In the setting of MI, CD44 expression is abundant on infiltrating leukocytes, myofibroblasts and endothelial cells that contribute to tissue repair [48]. CD44 knockout animals showed a prolonged maldaptive inflammatory response and diminished collagen synthesis, causing further ventricle dilation following MI [48]. Here, we report that dual delivery of SDF-1α and HA from in situ forming hydrogels enhances BMC homing to the remodeling myocardium. This finding highlights the importance of using materials that mimic components of the bone marrow niche to provide BMC specific cell adhesion ligands that act synergistically with SDF-1α/CXCR. However, applying these systems beyond the early stages of post MI remodeling remains to be investigated.
5. Conclusions
We conclude that our HA Gel/rSDF-1α system enhances BMC homing to the remodeling myocardium through sustained delivery of rSDF-1α and HA. HA macromers with hydrolytically unstable ester bonds between the HA backbone and the reactive methacrylate group to allow hydrogel degradation and subsequent release of HA after in situ crosslinking were synthesized. The HA macromer binds rSDF-1α through electrostatic interactions to slow diffusion of the chemokine from the hydrogel. Moreover, the HA macromer stimulated chemotaxis of BMCs in vitro through CD44 receptor signaling. Finally, when applied to the heart following experimental MI, the HA Gel/rSDF-1α system significantly increased the number of systemically infused BMCs in the circulation and in the heart through sustained delivery of rSDF-1α and HA.
Supplementary Material
Acknowlegements
We are grateful for support from a Fellowship in Science and Engineering from the David and Lucile Packard Foundation (JAB), the Ruth L. Kirschstein National Research Service Award 2T32HL007954 from the NIH-NHLBI (BPP and JAE), and NIH grants R01HL107938 and R01HL089847.
Appendix. Supplementary Information
Supplementary information associated with this article can be found, in the online version.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial-infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 4.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Sievers RE, Prasad M, Mirsky R, Shih H, Wong ML, et al. Timing of bone marrow cell therapy is more important than repeated injections after myocardial infarction. Cardiovasc Pathol. 20:204–212. doi: 10.1016/j.carpath.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Penn MS, Ellis S, Gandhi S, Greenbaum A, Hodes Z, Mendelsohn FO, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: phase I clinical study. Circ Res. 110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 7.Dai SJ, Yuan FP, Mu JY, Li CX, Chen N, Guo SZ, et al. Chronic AMD3100 antagonism of SDF-1 alpha-CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. J Mol Cell Cardiol. 2010;49:587–597. doi: 10.1016/j.yjmcc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wojakowski W, Michalowska A, Majka M, Kucia M, Maslankievvicz K, Wyderka R, et al. The mobilization of tissue-committed (CD34(+), CD117(+), CXCR4(+), c-met(+)) stem cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in acute myocardial infarction: REGENT study. Circulation. 2004;110:238–239. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 9.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang G, Nakamura Y, Wang XH, Hu QS, Suggs LJ, Zhang JY. Controlled release of stromal cell-derived factor-1alpha in situ increases C-kit(+) cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 11.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 12.Kuschert GSV, Coulin F, Power CA, Proudfoot AEI, Hubbard RE, Hoogewerf AJ, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 13.Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, et al. Stromal cell-derived factor-1 alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 14.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 15.Sadir R, Imberty A, Baleux F, Lortat-Jacob H. Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem. 2004;279:43854–43860. doi: 10.1074/jbc.M405392200. [DOI] [PubMed] [Google Scholar]
- 16.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 17.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34(+) stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 18.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldkamp CT, Ziarek JJ, Su JD, Basnet H, Lennertz R, Weiner JJ, et al. Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Sci. 2009;18:1359–1369. doi: 10.1002/pro.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolinsky TJ, Nielsen JE, McCammon JA, Baker NA. PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004;32:W665–W667. doi: 10.1093/nar/gkh381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Robertson AD, Jensen JH. Very fast empirical prediction and rationalization of protein pK(a) values. Proteins. 2005;61:704–721. doi: 10.1002/prot.20660. [DOI] [PubMed] [Google Scholar]
- 22.Jmol: an open-source Java viewer for chemical structures in 3D. http://www.jmol.org/
- 23.Nomanbhoy TK, Cerione RA. Characterization of the interaction between RhoGDI and Cdc42Hs using fluorescence spectroscopy. J Biol Chem. 1996;271:10004–10009. doi: 10.1074/jbc.271.17.10004. [DOI] [PubMed] [Google Scholar]
- 24.Pathak CP, Sawhney AS, Hubbell JA. Rapid photopolymerization of immunoprotective gels in contact with cells and tissue. J Am Chem Soc. 1992;114:8311–8312. [Google Scholar]
- 25.Bitter TM, H M. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 26.Goncharova EA, Goncharov DA, Krymskaya VP. Assays for in vitro monitoring of human airway smooth muscle (ASM) and human pulmonary arterial vascular smooth muscle (VSM) cell migration. Nat Protoc. 2006;1:2933–2939. doi: 10.1038/nprot.2006.434. [DOI] [PubMed] [Google Scholar]
- 27.van Amerongen MJ, Hamsen MC, Petersen AH, Popa ER, van Luyn MJA. Cryoinjury: a model of myocardial regeneration. Cardiovasc Pathol. 2008;17:23–31. doi: 10.1016/j.carpath.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 28.Elser JA, Purcell BP, Allana IA, Burdick JA, Margulies KB. Ischemia induces P-selectin-mediated selective progenitor cell engraftment in the isolated-perfused heart. J Mol Cell Cardiol. 52:105–112. doi: 10.1016/j.yjmcc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tous E, Ifkovits JL, Koomalsingh KJ, Shuto T, Soeda T, Kondo N, et al. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules. 2011;12:4127–4135. doi: 10.1021/bm201198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusumbe AP, Bapat SA. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Res. 2009;69:9245–9253. doi: 10.1158/0008-5472.CAN-09-2802. [DOI] [PubMed] [Google Scholar]
- 31.Murphy JW, Cho Y, Sachpatzidis A, Fan CP, Hodsdon ME, Lolis E. Structural and functional basis of CXCL12 (strornal cell-derived factor-1 alpha) binding to heparin. J Biol Chem. 2007;282:10018–10027. doi: 10.1074/jbc.M608796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuda M, Makino Y, Iwahara T, Nishihara H, Sawa H, Nagashima K, et al. Crk associates with ERM proteins and promotes cell motility toward hyaluronic acid. J Biol Chem. 2004;279:46843–46850. doi: 10.1074/jbc.M401476200. [DOI] [PubMed] [Google Scholar]
- 33.Nandi A, Estess P, Siegelman M. Bimolecular complex between rolling and firm adhesion receptors required for cell arrest; CD44 association with VLA-4 in T cell extravasation. Immunity. 2004;20:455–465. doi: 10.1016/s1074-7613(04)00077-9. [DOI] [PubMed] [Google Scholar]
- 34.Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HAdependent primary adhesion. J Clin Invest. 1998;101:97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, et al. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 36.Kuraitis D, Zhang PC, McEwan K, Zhang J, Mckee D, Sofrenovic T, et al. Controlled release of stromal cell-derived factor-1 for enhanced progenitor response in ischemia. J Control Release. 2011;152:E216–E218. doi: 10.1016/j.jconrel.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 38.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misao Y, Takemura G, Arai M, Ohno T, Onogi H, Takahashi T, et al. Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 2006;71:455–465. doi: 10.1016/j.cardiores.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 41.Mohle R, Bautz F, Rafii S, Moore MAS, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34(+) hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- 42.Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 43.Anzai A, Anzai T, Nagai S, Maekawa Y, Naito K, Kaneko H, et al. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation. 125:1234–1245. doi: 10.1161/CIRCULATIONAHA.111.052126. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 45.Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–1199. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 47.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Bi. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 48.Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, et al. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol. 2008;180:2625–2633. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.