1. DISEASE CHARACTERISTICS
1.1 Name of the disease (synonyms)
Glanzmann thrombasthenia (Glanzmann–Naegeli syndrome, platelet αIIbβ3 deficiency, platelet fibrinogen receptor deficiency).
1.2 OMIM# of the disease
273800.
1.3 Name of the analyzed genes or DNA/chromosome segments
ITGA2B (αIIb) and ITGB3 (β3) – chromosome 17q21.32.
1.4 OMIM# of the gene(s)
607759 (ITGA2B) and 173470 (ITGB3).
1.5 Mutational spectrum
Glanzmann thrombasthenia (GT) is a rare autosomal recessive disorder caused by the absence or the dysfunction of the αIIbβ3 integrin (formerly termed the GPIIb–IIIa complex) on the platelet surface. The most abundant receptor on platelets, αIIbβ3, mediates platelet aggregation through its binding of adhesive proteins, such as fibrinogen, von Willebrand factor, vitronectin and fibronectin.1 First described in 1918 by Glanzmann as ‘hereditary hemorrhagic thrombasthenia', this disease is characterized by spontaneous and trauma-related mucocutaneous bleeding, with variable expression ranging from easy bruising to fatal hemorrhages.2 Heterozygotes are asymptomatic.
The ITGA2B gene encodes for the αIIb subunit, whereas the ITGB3 gene encodes for β3. ITGA2B spanning 17 kb has 30 exons, whereas ITGB3 has 15 exons, but spans 46 kb; they are closely located on chromosome 17q21.32 without evidence for coordinated expression.3 Mutations causing GT affect either ITGA2B or ITGB3. As GT is an autosomal recessive disease, patients are mostly compound heterozygotes for ITGA2B or ITGB3 mutations in the absence of consanguinity. An Internet database (http://med.mssm.edu/glanzmanndb) that is regularly updated contains clinical, biological and genetic data in referenced patients. To date, more than 150 different mutations have been described, with a tendency for more mutations in ITGA2B, perhaps reflecting its higher number of exons. GT is caused by a wide spectrum of defects, including nonsense, missense, splice site mutations, and less commonly, small insertions and deletions.3 Large deletions are rare.
Whereas expression of αIIb is specific to the megakaryocyte lineage, β3 has a widespread tissue distribution as part of the vitronectin receptor (αvβ3). Despite the wider expression of the β3 subunit, the bleeding phenotype continues to dominate for ITGB3 mutations. In rare patients, gain-of-function mutations in either ITGA2B or ITGB3 can also interfere with megakaryocytopoiesis and lead to platelet anisocytosis and moderate thrombocytopenia.3
1.6 Analytical methods
The anucleate platelet contains low amounts of RNA that is often unstable; therefore, mutation screening in GT is largely based on DNA analysis. A range of DNA screening methods have been described, including PCR single-strand conformation polymorphism analysis and denaturing gradient gel electrophoresis.4 More recently, high-resolution melting (HRM) screening has been used.5 Nonetheless, none of these methods are 100% guaranteed, and standard PCR of genomic DNA followed by direct sequencing of essential coding and flanking regions of both ITGA2B and ITGB3 is recommended.
1.7 Analytical validation
Analyses of mutated and non-mutated control samples are recommended. Quality control is also important, particularly for DNA isolated in different laboratories. Both strands of DNA should be sequenced and variations confirmed by an independent method (eg, restriction enzyme analysis, ASA-PCR; HRM and so on) and/or by DNA sequencing of a second sample.5 Identified variations can be directly compared with available databases and bioinformatic softwares, to establish whether they correspond to known mutations and to assess their potential pathological nature. Transmission of the mutation(s) should be established by analysis of family members whenever possible. Molecular modeling on the basis of the known crystal structure of the αIIbβ3 and αvβ3 extracellular domains can help to assess the effect of missense mutations on integrin structure.6, 7 Expression of recombinant mutated integrin in heterologous cell lines can be informative on expression and function, but is only available on a research basis in specialized laboratories. RT-PCR may be used to confirm potential splicing defects of RNA expression. Large deletions may be looked for by quantitative multiplex fluorescence-PCR, especially when standard DNA sequencing reveals a single heterozygous mutation in a patient with a clear GT phenotype.
1.8 Estimated frequency of the disease (incidence at birth (‘birth prevalence') or population prevalence)
Unknown. No epidemiological data are currently available for GT on a world-wide basis.8 It is more common in Europe and the Middle East than in the United States, and is clustered within certain ethnic groups (see below). In France, a recent census suggests approximately 300 cases, half of whom are of Manouche Gypsy origin and share the same mutation (M Fiore, data not published).
1.9 If applicable, prevalence in the ethnic group of investigated persons
GT occurs in high frequency in certain ethnic populations, with an increased incidence of consanguinity, such as Iraqi Jews, Palestinian Arabs or Jordanian Arabs.9, 10 The unusual high frequency of GT among these groups is largely the result of founder mutations. In France, GT is particularly frequent in the Gypsy population, mainly represented by the Manouche tribe, with a founder mutation estimated by us to have occurred 300–400 years ago.11
1.10 Diagnostic setting

Comment:
A.Although very rare, acquired thrombasthenia has been reported in leukemia with chromosome 15–17 translocations; it is more commonly associated with autoantibodies directed against αIIbβ3, some of which also induce thrombocytopenia, as well as blocking αIIbβ3 function.12, 13 It is also to be noted that some anti-thrombotic therapies involve the use of αIIbβ3-blocking drugs that transiently induce a GT-like state.In leukocyte adhesion deficiency-3, patients manifest a bleeding phenotype with immune deficiency and recurrent infections, but demonstrate a normal expression of β3 integrins. Mutations in the FERMT3 gene (encoding kindlin-3) account for the phenotype.14 Kindlin-3 belongs to a family of proteins that cooperate in integrin activation.3Platelet aggregation defects restricted to specific agonists may imply abnormalities of a signaling pathway and should be distinguished from GT, where aggregation is defective for all physiological agonists in the presence of a normal platelet number and size. GPIb-related function as assessed by ristocetin-induced platelet agglutination is normal, although it can be cyclic.
B.To date, studies have failed to find any correlation between frequency and severity of bleeding and the nature of the gene defect in GT. Bleeding tendencies can differ considerably between affected persons, even within the same family or ethnic group.3
C.Mutation screening in patients and family members is essential for genetic counseling, for most patients will have their own private mutation (unless in an ethnic group). DNA analysis allows identification of asymptomatic heterozygous carriers, as these cannot be identified with certainty by phenotypic studies alone.4 Expression of αIIbβ3 in platelets of normal healthy subjects is known to vary by more than two-fold.
2. TEST CHARACTERISTICS
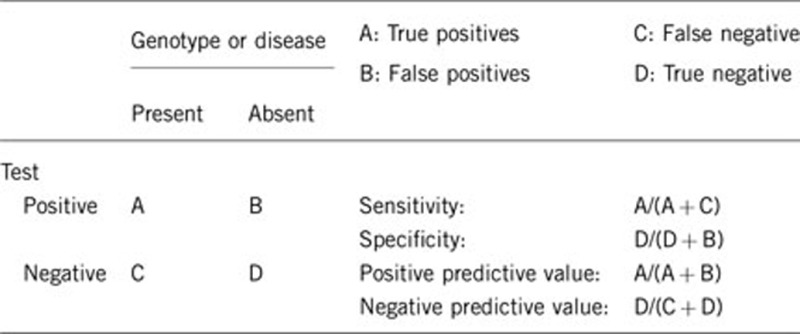
2.1 Analytical sensitivity
(proportion of positive tests if the genotype is present)
For cases with a classic GT phenotype, the success of mutation analysis by DNA sequencing of ITGA2B and ITGB3 ranges from 80 to 90%. Other possible causes of GT, such as miRNAs and intronic mutations, are not currently researched, although an involvement of other genes (eg, chaperone defects) cannot be ruled out. Once a mutation is found the success of genotyping should be 100%.
2.2 Analytical specificity
(proportion of negative tests if the genotype is not present)
Genetic testing is expected to be negative in the absence of the disease with a specificity of 100%. However, both αIIb and β3 are polymorphic glycoproteins, so that each new amino acid substitution has to have its pathogenicity proven (or non-proven).
2.3 Clinical sensitivity
(proportion of positive tests if the disease is present)
The clinical sensitivity can be dependent on variable factors, such as age or family history. In such cases, a general statement should be given, even if a quantification can only be made case by case.
A sensitivity of 100% for patients with a classical GT phenotype of the disorder (absence of platelet aggregation and αIIbβ3 expression) can be achieved. However, some mutations may only partially block integrin expression/function, and consequently the bleeding syndrome can be negligible. In such cases, the characterization of αIIbβ3 may require specialized procedures. The clinical severity of GT tends to diminish with age, although the bleeding manifestations persist and are life-long.
2.4 Clinical specificity
(proportion of negative tests if the disease is not present)
The clinical specificity can be dependent on variable factors, such as age or family history. In such cases, a general statement should be given, even if a quantification can only be made case by case.
In the absence of GT the proportion of negative tests is expected to be high, although the prevalence of other disorders interfering with platelet function is relatively high, and mucocutaneous bleeding is symptomatic for all of them.
2.5 Positive clinical predictive value
(life-time risk of developing the disease if the test is positive)
Not applicable. GT shows complete penetrance, and most patients are diagnosed at birth or during early infancy.2
2.6 Negative clinical predictive value
(probability of not developing the disease if the test is negative)
If an index case has been genotyped negatively (with normal platelet function and full αIIbβ3 expression), the probability of not developing the disease by the propositus and other family members is 100%.
Even if an index case in a given family has not been tested genetically, phenotypic characterization can exclude the diagnosis in 100% of family members. However, this is not the case if members of the family have another bleeding disorder. In such a case, genetic analysis is indispensable.
3. CLINICAL UTILITY
3.1 (Differential) diagnosis: The tested person is clinically affected
(To be answered if in 1.10 ‘A' was marked)
3.1.1 Can a diagnosis be made other than through a genetic test?

3.1.2 Describe the burden of alternative diagnostic methods to the patient
GT is readily identifiable by platelet function testing, and a lack of platelet aggregation in response to all physiological agonists, such as ADP, collagen, epinephrine or thrombin, is unique for this disease. Clot retraction is also abnormal for most patients with GT. Flow cytometry allows rapid identification of both αIIbβ3 deficiency and (with specific tests) non-functional αIIbβ3.1 These provide minimal inconvenience to the patient and can be performed with 10–30 ml blood. However, only DNA analysis can confirm that the disease is caused by a genetic defect.
3.1.3 How is the cost effectiveness of alternative diagnostic methods to be judged?
There are no studies available to determine the cost effectiveness of alternative diagnostic approaches to GT. Nevertheless, biological analyses have to be performed in a specialized laboratory and their cost effectiveness is high. Moreover, platelet samples must be processed and analyzed within 4 h of blood collection, which is not always possible when they are sent from far away. So, often the patient has to visit a specialized center. In contrast, samples for DNA testing can be sent by mail.
3.1.4 Will disease management be influenced by the result of a genetic test?

3.2 Predictive Setting: The tested person is clinically unaffected but carries an increased risk based on family history
(To be answered if in 1.10 ‘B' was marked)
3.2.1 Will the result of a genetic test influence lifestyleand prevention?
No.
3.2.2 Which options in view of lifestyle and prevention does a person at-risk have if no genetic test has been done (please describe)?
Not applicable.
3.3 Genetic risk assessment in family members of a diseased person
(To be answered if in 1.10 ‘C' was marked)
3.3.1 Does the result of a genetic test resolve the genetic situation in that family?
Yes. If mutations were found and shown to be responsible for the disease, then no further tests are necessary.
3.3.2 Can a genetic test in the index patient save genetic or other tests in family members?
No. Even in the case of known familial mutations, carriers need to be individually identified (or excluded). Heterozygous expression is asymptomatic.
3.3.3 Does a positive genetic test result in the index patient enable a predictive test in a family member?
Yes, DNA testing can be rapid, as it is restricted to the identified mutation.
3.4 Prenatal diagnosis
(To be answered if in 1.10 ‘D' was marked)
3.4.1 Does a positive genetic test result in the index patient enable a prenatal diagnosis?
Prenatal diagnosis is possible, but testing requires special procedures, such as chorionic villi sampling. Fetal blood sampling should be avoided, as it can lead to death due to bleeding in affected fetuses.21, 22, 23
4. IF APPLICABLE, FURTHER CONSEQUENCES OF TESTING
Please assume that the result of a genetic test has no immediate medical consequences. Is there any evidence that a genetic test is nevertheless useful for the patient or his/her relatives? (Please describe)
Diagnosis of GT is based first on the clinical and phenotypic characterization, including specific biological studies designed to assess quantitative and qualitative deficiencies of αIIbβ3. Biological testing originally resulted in the classification of GT into three subtypes: type I with little or no expression of αIIbβ3, no fibrinogen in α-granules and no clot retraction; type II with 5 to 20% αIIbβ3, and as a consequence, fibrinogen in α-granules and a subnormal to normal clot retraction; and variant GT with 50% or more of the normal expression of αIIbβ3 unable to support aggregation.2 Nonetheless, genetic testing should be proposed to families because: (i) a positive test helps to remove any doubt in the diagnosis; (ii) it is essential for carrier detection; the asymptomatic carriers display a normal phenotype; and (iii) in some cases, it may be useful for prediction of anti-platelet antibody formation. Genetic testing also provides more precise information on the cause of the αIIbβ3 deficiency or non-functioning.
Acknowledgments
This work was supported by EuroGentest2 (Unit 2: ‘Genetic testing as part of health care'), a Coordination Action under FP7 (Grant Agreement Number 261469) and the European Society of Human Genetics.
The authors declare no conflict of interest.
References
- Nurden AT. Glanzmann thrombasthenia. Orphanet J Rare Dis. 2006;1:10. doi: 10.1186/1750-1172-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George JN, Caen JP, Nurden AT. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990;75:1383–1395. [PubMed] [Google Scholar]
- Nurden AT, Fiore M, Nurden P, Pillois X. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood. 2011;118:5996–6005. doi: 10.1182/blood-2011-07-365635. [DOI] [PubMed] [Google Scholar]
- Nurden AT, Fiore M, Pillois X, Nurden P. Genetic testing in the diagnostic evaluation of inherited platelet disorders. Semin Thromb Hemost. 2009;35:204–212. doi: 10.1055/s-0029-1220328. [DOI] [PubMed] [Google Scholar]
- Fiore M, Nurden AT, Vinciguerra C, Nurden P, Pillois X. Rapid diagnosis of the French gypsy mutation in Glanzmann thrombasthenia using high-resolution melting analysis. Thromb Haemost. 2010;104:1076–1077. doi: 10.1160/TH10-05-0268. [DOI] [PubMed] [Google Scholar]
- Coller BS, Shattil SJ. The GPIIb/IIIa (integrin αIIbβ3) odyssey: a technology-driven saga of a receptor with twists and turns and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden AT. Glanzmann thrombasthenia: the need for epidemiological studies. J Thromb Haemost. 2009;7:1875–1877. doi: 10.1111/j.1538-7836.2009.03597.x. [DOI] [PubMed] [Google Scholar]
- Seligsohn U, Rososhansky S. A Glanzmann's thrombasthenia cluster among Iraqi Jews in Israel. Thromb Haemost. 1984;52:230–231. [PubMed] [Google Scholar]
- Awidi AS. Increased incidence of Glanzmann's thrombasthenia in Jordan as compared with Scandinavia. Scand J Haematol. 1983;30:218–222. doi: 10.1111/j.1600-0609.1983.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Fiore M, Pillois X, Nurden P, Nurden AT, Austerlitz F. Founder effect and estimation of the age of the French Gypsy mutation associated with Glanzmann thrombasthenia in Manouche families. Eur J Hum Genet. 2011;19:981–987. doi: 10.1038/ejhg.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloor AJ, Smith GA, Jaswon M, Parker NE, Ouwehand WH, Liesner R. Acquired thrombasthenia due to GPIIbIIIa platelet autoantibodies in a 4-yr-old child. Eur J Haematol. 2006;76:89–90. doi: 10.1111/j.1600-0609.2005.00574.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Wu QY, Wang Z, et al. Abnormalities of platelet membrane glycoproteins in acute nonlymphoblastic leukemia [abstract] Thromb Haemost. 1989;62:176. [Google Scholar]
- Svensson L, Howarth K, McDowall A, et al. Leukocyte adhesion deficiency-III is caused by mutations in KINDLIN3 affecting integrin activation. Nat Med. 2009;15:306–312. doi: 10.1038/nm.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton-Maggs PH, Chalmers EA, Collins PW, et al. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. Br J Haematol. 2006;135:603–633. doi: 10.1111/j.1365-2141.2006.06343.x. [DOI] [PubMed] [Google Scholar]
- Di Minno G, Coppola A, Di Minno MN, Poon MC. Glanzmann's thrombasthenia (defective platelet integrin alphaIIb-beta3): proposals for management between evidence and open issues. Thromb Haemost. 2009;102:1157–1164. doi: 10.1160/TH09-04-0225. [DOI] [PubMed] [Google Scholar]
- Santoro C, Rago A, Biondo F, et al. Prevalence of allo-immunization anti-HLA and anti-integrin alphaIIbbeta3 in Glanzmann Thromboasthenia patients. Haemophilia. 2010;16:805–812. doi: 10.1111/j.1365-2516.2010.02230.x. [DOI] [PubMed] [Google Scholar]
- Taaning E, Knudsen FU, Thorsen S, Jonsson V. Immunization against platelet glycoprotein IIb-IIIa in Glanzmann's thrombasthenia. Eur J Haematol. 1997;58:360–361. doi: 10.1111/j.1600-0609.1997.tb01684.x. [DOI] [PubMed] [Google Scholar]
- Poon MC, D'Oiron R, Von Depka M, et al. Prophylactic and therapeutic recombinant factor VIIa administration to patients with Glanzmann's thrombasthenia: results of an international survey. J Thromb Haemost. 2004;2:1096–1103. doi: 10.1111/j.1538-7836.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- Fiore M, Firah N, Pillois X, Nurden P, Heilig R, Nurden AT. Natural history of platelet antibody formation against alphaIIbbeta3 in a French cohort of Glanzmann thrombasthenia patients. Haemophilia. 2012;18:e201–e219. doi: 10.1111/j.1365-2516.2011.02744.x. [DOI] [PubMed] [Google Scholar]
- Seligsohn U, Mibashan RS, Rodeck CH, Nicolaides KH, Millar DS, Coller BS. Prenatal diagnosis of Glanzmann thrombasthenia. Lancet. 1985;28:1419. doi: 10.1016/s0140-6736(85)92579-6. [DOI] [PubMed] [Google Scholar]
- French DL, Coller BS, Usher S, et al. Prenatal diagnosis of Glanzmann thrombasthenia using the polymorphic markers BRCA1 and THRA1 on chromosome 17. Br J Haematol. 1998;102:582–587. doi: 10.1046/j.1365-2141.1998.00798.x. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Usher S, Nelson EJ, et al. Prenatal diagnosis of Glanzmann thrombasthenia. Natl Med J India. 2003;16:207–208. [PubMed] [Google Scholar]


