Abstract
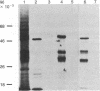
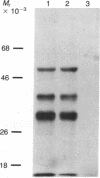
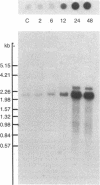
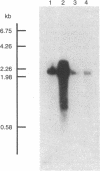
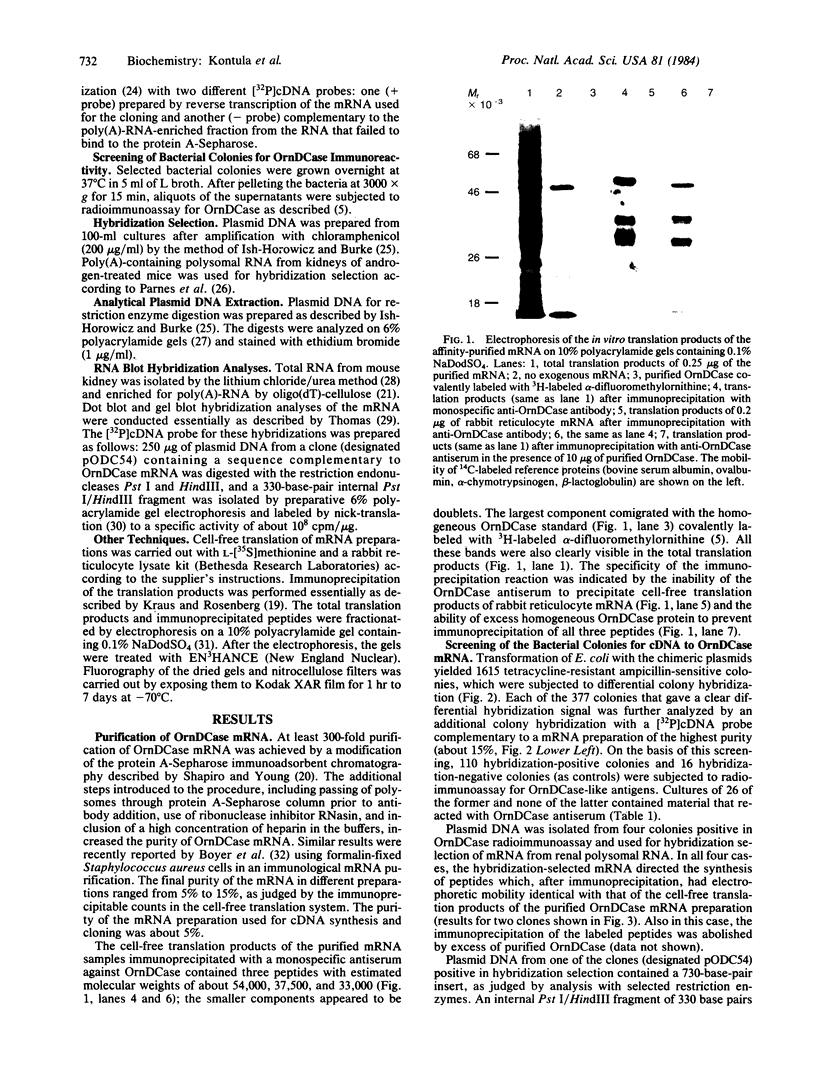
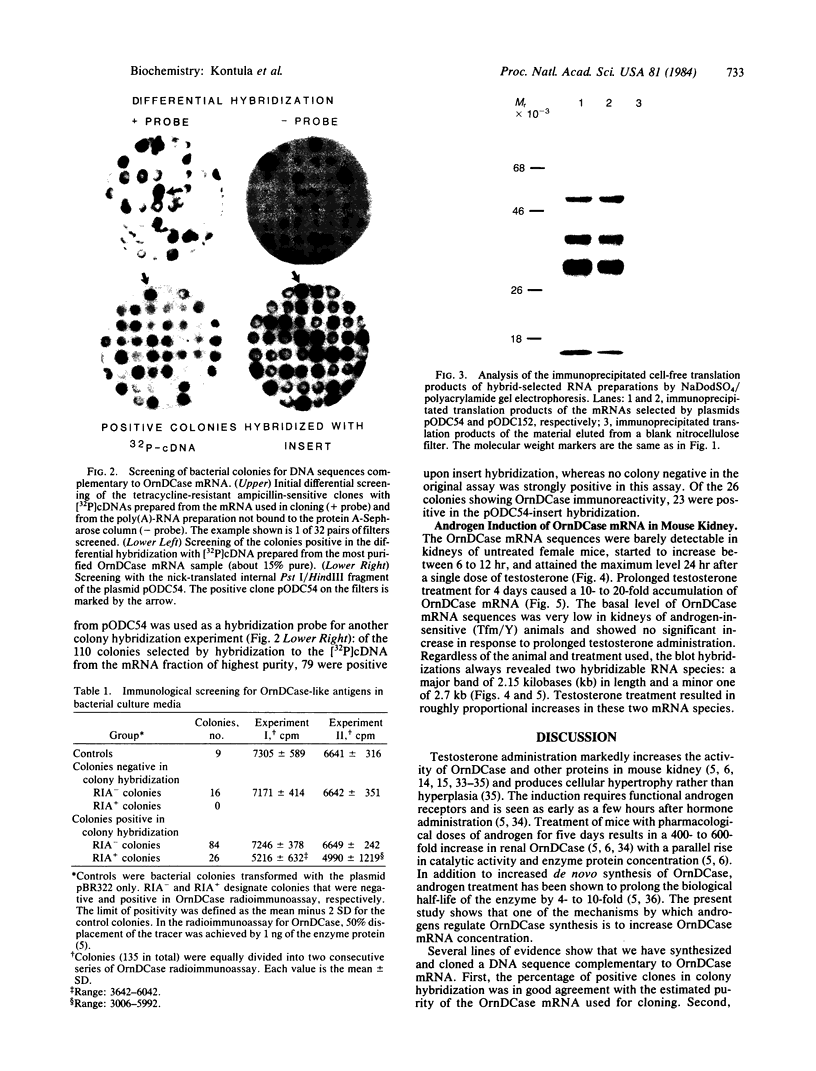
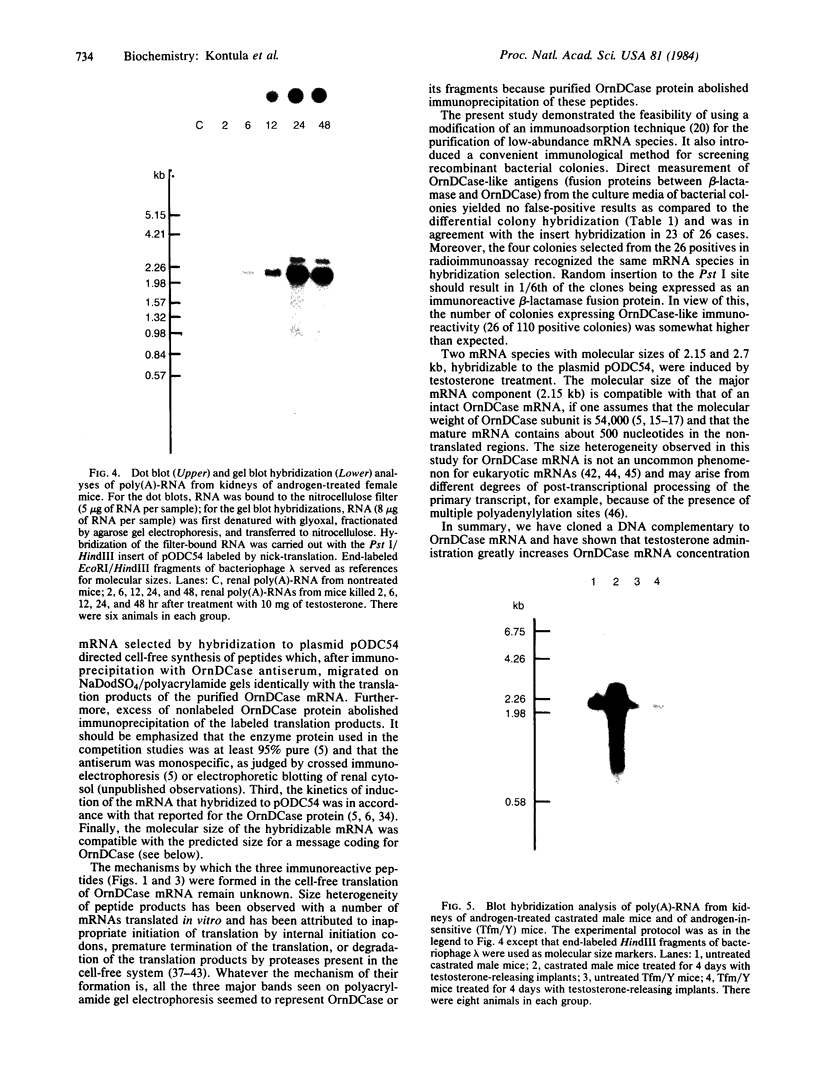
To investigate the mechanisms by which androgens regulate ornithine decarboxylase (OrnDCase; L-ornithine carboxy-lyase, EC 4.1.1.17) in mouse kidney, a cDNA clone encoding OrnDCase mRNA was prepared. Purification of OrnDCase mRNA from kidneys of androgen-treated mice was accomplished by immunoadsorption of renal polysomes to a protein A-Sepharose column and enrichment for poly(A)-containing RNA by oligo(dT)-cellulose. Double-stranded cDNA synthesized from this mRNA was inserted into the Pst I site of plasmid pBR322 by using oligo(dG . dC)-tailing and was propagated in Escherichia coli. Plasmids containing cDNA sequences coding for OrnDCase were identified by differential colony hybridization, by radioimmunological detection of OrnDCase-like antigens in bacterial cultures, and by cell-free translation of hybrid-selected mRNA followed by immunoprecipitation with monospecific OrnDCase antiserum. A restriction endonuclease fragment of the selected plasmid DNA (pODC54) was labeled by nick-translation and used to study changes in OrnDCase mRNA concentration. After a single dose of testosterone, renal OrnDCase mRNA concentration increased as soon as 6 hr and peaked 24 hr after steroid injection, as measured by RNA blot hybridization. Continuous androgen treatment for 4 days resulted in a 10- to 20-fold increase in OrnDCase mRNA concentration in normal animals, but no induction of this mRNA was detected in mice that have an inherent defect of the androgen receptor (testicular feminization). These results indicate that androgens regulate OrnD-Case synthesis in mouse kidney, at least in part, by increasing OrnDCase mRNA accumulation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F. G., Gross K. W., Watson G. Isolation and characterization of a DNA sequence complementary to an androgen-inducible messenger RNA from mouse kidney. J Biol Chem. 1981 Jul 10;256(13):7006–7013. [PubMed] [Google Scholar]
- Boyer S. H., Smith K. D., Noyes A. N., Young K. E. Adjuvants to immunological methods for mRNA purification. Application to the isolation of mRNA for carbonic anhydrase I from rabbit reticulocytes. J Biol Chem. 1983 Feb 25;258(4):2068–2071. [PubMed] [Google Scholar]
- Bullock L. P., Bardin W. C. Androgen receptors in testicular feminization. J Clin Endocrinol Metab. 1972 Dec;35(6):935–937. doi: 10.1210/jcem-35-6-935. [DOI] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Faust J. R., MacDonald R. J., Brown M. S., Goldstein J. L. Molecular cloning of 3-hydroxy-3-methylglutaryl coenzyme a reductase and evidence for regulation of its mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7704–7708. doi: 10.1073/pnas.79.24.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- El-Dorry H. A., Pickett C. B., MacGregor J. S., Soffer R. L. Tissue-specific expression of mRNAs for dipeptidyl carboxypeptidase isoenzymes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4295–4297. doi: 10.1073/pnas.79.14.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong W. F., Heller J. S., Canellakis E. S. The appearance of an ornithine decarboxylase inhibitory protein upon the addition of putrescine to cell cultures. Biochim Biophys Acta. 1976 Apr 23;428(2):456–465. doi: 10.1016/0304-4165(76)90054-4. [DOI] [PubMed] [Google Scholar]
- Fujita K., Murakami Y., Hayashi S. A macromolecular inhibitor of the antizyme to ornithine decarboxylase. Biochem J. 1982 Jun 15;204(3):647–652. doi: 10.1042/bj2040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. S., Canellakis E. S. Cellular control of ornithine decarboxylase activity by its antizyme. J Cell Physiol. 1981 May;107(2):209–217. doi: 10.1002/jcp.1041070206. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsson S., Persson L., Rosengren E. Polyamines and nucleic acids in the mouse kidney induced to growth by testosterone propionate. Acta Physiol Scand. 1978 Apr;102(4):385–393. doi: 10.1111/j.1748-1716.1978.tb06085.x. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isomaa V. V., Pajunen A. E., Bardin C. W., Jänne O. A. Ornithine decarboxylase in mouse kidney. Purification, characterization, and radioimmunological determination of the enzyme protein. J Biol Chem. 1983 Jun 10;258(11):6735–6740. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Kallio A., Löfman M., Pösö H., Jänne J. Diamine-induced inhibition of liver ornithine decarboxylase. Biochem J. 1979 Jan 1;177(1):63–69. doi: 10.1042/bj1770063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameji T., Murakami Y., Fujita K., Hayashi S. Purification and some properties of ornithine decarboxylase from rat liver. Biochim Biophys Acta. 1982 Jul 16;717(1):111–117. doi: 10.1016/0304-4165(82)90387-7. [DOI] [PubMed] [Google Scholar]
- Kitani T., Fujisawa H. Purification and properties of ornithine decarboxylase from rat liver. J Biol Chem. 1983 Jan 10;258(1):235–239. [PubMed] [Google Scholar]
- Kraus J. P., Rosenberg L. E. Purification of low-abundance messenger RNAs from rat liver by polysome immunoadsorption. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4015–4019. doi: 10.1073/pnas.79.13.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagacé L., Chandra T., Woo S. L., Means A. R. Identification of multiple species of calmodulin messenger RNA using a full length complementary DNA. J Biol Chem. 1983 Feb 10;258(3):1684–1688. [PubMed] [Google Scholar]
- Lizardi P. M., Mahdavi V., Shields D., Candelas G. Discontinuous translation of silk fibroin in a reticulocyte cell-free system and in intact silk gland cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6211–6215. doi: 10.1073/pnas.76.12.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley D. V. Regulation of polyamine biosynthesis. Biochem Pharmacol. 1979;28(2):153–161. doi: 10.1016/0006-2952(79)90496-9. [DOI] [PubMed] [Google Scholar]
- Medford R. M., Wydro R. M., Nguyen H. T., Nadal-Ginard B. Cytoplasmic processing of myosin heavy chain messenger RNA: evidence provided by using a recombinant DNA plasmid. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5749–5753. doi: 10.1073/pnas.77.10.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. M., Jr, Nilson J. H., Jenik R. A., Winberry L. K., McDevitt M. A., Goodridge A. G. Molecular cloning of gene sequences for avian fatty acid synthase and evidence for nutritional regulation of fatty acid synthase mRNA concentration. J Biol Chem. 1982 Mar 25;257(6):3225–3229. [PubMed] [Google Scholar]
- Pajunen A. E., Isomaa V. V., Jänne O. A., Bardin C. W. Androgenic regulation of ornithine decarboxylase activity in mouse kidney and its relationship to changes in cytosol and nuclear androgen receptor concentrations. J Biol Chem. 1982 Jul 25;257(14):8190–8198. [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Persson L. Decarboxylation of ornithine and lysine by ornithine decarboxylase from kidneys of testosterone treated mice. Acta Chem Scand B. 1981;35(6):451–459. doi: 10.3891/acta.chem.scand.35b-0451. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Seely J. E., Pegg A. E. Changes in mouse kidney ornithine decarboxylase activity are brought about by changes in the amount of enzyme protein as measured by radioimmunoassay. J Biol Chem. 1983 Feb 25;258(4):2496–2500. [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Effect of androgens on turnover of ornithine decarboxylase in mouse kidney. Studies using labeling of the enzyme by reaction with [14C] alpha-difluoromethylornithine. J Biol Chem. 1982 Jul 10;257(13):7549–7553. [PubMed] [Google Scholar]
- Seely J. E., Pösö H., Pegg A. E. Purification of ornithine decarboxylase from kidneys of androgen-treated mice. Biochemistry. 1982 Jul 6;21(14):3394–3399. doi: 10.1021/bi00257a023. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Young J. R. An immunochemical method for mRNA purification. Application to messenger RNA encoding trypanosome variable surface antigen. J Biol Chem. 1981 Feb 25;256(4):1495–1498. [PubMed] [Google Scholar]
- Stein J. P., Catterall J. F., Woo S. L., Means A. R., O'Malley B. W. Molecular cloning of ovomucoid gene sequences from partially purified ovomucoid messenger RNA. Biochemistry. 1978 Dec 26;17(26):5763–5772. doi: 10.1021/bi00619a025. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach M., Boeke J. D. In vitro translation and characterization of a unique histidine-rich protein mRNA in the avian malaria parasite Plasmodium lophurae. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1867–1871. doi: 10.1073/pnas.80.7.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., van der Zwaag-Gerritsen J., Mulder J., Ab G., Gruber M. Translation in vivo and in vitro of mRNAs coding for vitellogenin, serum albumin and very-low-density lipoprotein II from chicken liver. A difference in translational efficiency. Eur J Biochem. 1981 Mar;114(3):635–641. doi: 10.1111/j.1432-1033.1981.tb05191.x. [DOI] [PubMed] [Google Scholar]