Introduction
The last several years have witnessed a dramatic increase in our understanding of the roles osteocytes play in skeletal biology. Although the potential importance of these cells has been recognized for decades [reviewed in (1)], there has until recently been limited evidence for the functional role of osteocytes in specific processes. Several factors have contributed to recent progress. First, loss-of-function studies for genes expressed predominantly in osteocytes have revealed important roles for these genes in skeletal biology (2;3). Second, mutations in the SOST gene and its regulatory elements were identified in the human diseases sclerosteosis and Van Buchem disease; and sclerostin, the product of the SOST gene, was found to be expressed predominantly by osteocytes and to suppress bone formation (4–8). Lastly, the identification of DNA fragments of the dentin matrix protein 1 (DMP1) gene that can drive osteocyte-specific expression of transgenes has led to the development of genetic tools that can be utilized for gain-and loss-of-function studies specifically in this cell type (9–11). Using such tools, two independent studies have recently implicated osteocytes as a major source of the osteoclastogenic cytokine receptor activator of NFκB ligand (RANKL) (12;13). The goal of this review is to highlight this evidence and to discuss how these findings may impact concepts related to bone remodeling.
Osteoblast-lineage cells and osteoclastogenesis
The idea that osteoblast-lineage cells control osteoclast differentiation and function originated more than 30 years ago with the observation that receptors for osteoclastogenic hormones, such as parathyroid hormone (PTH), are present on cells with osteoblastic characteristics but not on osteoclast progenitors (14). Subsequent work demonstrated that cell lines with osteoblastic characteristics or cell preparations rich in osteoblast progenitors support osteoclast formation in co-cultures with osteoclast progenitors (15;16). Eventually, M-CSF and RANKL were identified as the factors produced by support cells that are essential for osteoclast differentiation in vivo, thereby providing a mechanistic explanation for the requirement of osteoblastic cells for osteoclast formation (17–19).
However, there are several stages within the osteoblast lineage at which these cells might be involved in osteoclastogenesis. Osteoblasts are derived from mesenchymal progenitors via a process that requires the sequential action of the transcription factors Runx2 and osterix (20). Matrix-synthesizing osteoblasts are recognized by their cuboidal shape, their location on newly formed bone matrix, and the expression of relatively osteoblast-specific genes such as osteocalcin. During the process of bone formation, matrix-synthesizing osteoblasts have at least three potential fates (21). Some of them become embedded within the bone matrix and are thereafter referred to as osteocytes, which continue to live and function within the mineralized tissue. At the end of the remodeling cycle, some osteoblasts become flattened and then remain as quiescent lining cells at the bone surface. Lastly, some matrix-synthesizing osteoblasts die by apoptosis.
Several approaches have been used to identify the stages of osteoblast differentiation that may be involved in the support of osteoclast formation. One of the few in vivo approaches used conditional ablation of matrix-synthesizing osteoblasts to determine whether they play any role in bone resorption (22). In this study, Karsenty and co-workers induced the death of replicating osteoblast progenitors by administering gancyclovir to mice harboring a thymidine-kinase transgene under the control of the osteocalcin promoter. This maneuver killed replicating progenitors and thereby blocked the supply of new osteoblasts. Thus, over time osteoblasts were depleted from the bone surface as they became osteocytes, lining cells, or died by apoptosis. After several weeks of gancyclovir administration, these mice completely lacked osteoblasts and had no bone formation, yet osteoclast surface and bone resorption markers were unaffected. More recently, we have performed a similar study using a collagen I promoter – thymidine kinase transgenic model and shown that depletion of osteoblasts also had no effect on basal or PTH-stimulated RANKL mRNA levels in bone (23). These results demonstrate that matrix-synthesizing osteoblasts, and their immediate replicating progenitors, are not required for osteoclast differentiation, and thus cannot be essential osteoclast support cells.
Another approach to identify osteoclast support cells has been correlation of RANKL expression with the relative stage of osteoblast differentiation in various in vitro cell models. BMP treatment of C2C12 cells confers on them the ability to support osteoclast differentiation and express RANKL and M-CSF, suggesting that commitment to the osteoblast lineage may be involved in osteoclast support (24;25). In addition, the MLO-Y4 cell line, which displays many characteristics of authentic osteocytes, expresses high levels of RANKL and supports osteoclast formation in the absence of any external stimulus, suggesting that cells at the other end of the osteoblast spectrum also support osteoclast formation (26). However, genetic deletion of Runx2, which is required for commitment to the osteoblast lineage, or suppression of its essential cofactor Cbfβ, has no effect on RANKL expression in multiple cell models (23). Moreover, promotion of osteoblast differentiation in human primary bone cells or in an osteoblastic cell line reduces RANKL expression and inhibits the ability of the cells to support osteoclast differentiation (27;28). In contrast, osteoblast differentiation of primary murine cells has been shown to either suppress osteoclastogenesis without changing RANKL expression (29), or to stimulate osteoclast formation as well as RANKL expression (30). Thus these in vitro approaches have led to conflicting conclusions regarding the stage of osteoblast commitment and differentiation at which RANKL may be expressed and thereby support osteoclast formation.
Identification of osteoclast support cells has also been attempted by histological localization of RANKL expressing cells in bone sections. In situ hybridization and immunohistochemistry have detected RANKL expression in many different bone cell types, including cells at the endosteal surface, spindle-shaped mesenchymal cells at the periosteum, mature osteoblasts, osteocytes, and hypertrophic chondrocytes (17;31–33). While in situ hybridization and immunolocalization can be useful for determining whether a particular cell in a tissue section expresses a gene of interest, both techniques yield only qualitative endpoints due to the low dynamic range of their signals. Thus, it is difficult to compare the relative abundance of a gene of interest in different cell types using such techniques. Moreover, with the exception of chondrocytes and osteocytes, the identity of the cell types expressing RANKL can be difficult to determine conclusively. Most importantly, these techniques do not indicate whether RANKL expression in a particular cell type is functionally relevant.
Osteocyte RANKL is essential for cancellous bone remodeling
To address the functional significance of RANKL expression in various cell types, we have developed mice in which RANKL, encoded by the tnfsf11 gene, can be deleted in genetically-defined cell populations using Cre-loxP technology (13). We reasoned that crossing these mice with mice that begin to express the Cre recombinase at different stages of osteoblast differentiation should reveal which stages, if any, are essential sources of the RANKL involved in osteoclast formation. The laboratory of Hiroshi Takayanagi has independently developed mice harboring a similar RANKL conditional allele (12).
To determine whether any cells of the mesenchymal lineage are important sources of RANKL, we began by deleting the RANKL conditional allele using Prx1-Cre transgenic mice, which express the Cre recombinase in mesenchymal progenitors in developing limbs (34). Mice lacking RANKL in Prx1-Cre-expressing cells developed severe osteopetrosis with retention of calcified cartilage in limb bones caused by a complete absence of osteoclasts (13). This phenotype is identical to that caused by germline deletion of RANKL (35), demonstrating that mesenchyme-derived cells are an essential source of RANKL for osteoclastogenesis.
In our initial attempt to examine the role of osteoblast lineage cells, the RANKL gene was inactivated using the osterix1-Cre and osteocalcin-Cre transgenes, which become active at the commitment stage of osteoblastogenesis and in more mature osteoblasts, respectively (36;37). RANKL deletion with both of these transgenes also caused severe osteopetrosis associated with failure of tooth eruption, retention of calcified cartilage in bones formed by endochondral bone formation, and dramatically reduced osteoclast staining (13). Moreover, the cartilage retention phenotype was also obtained by deleting RANKL with a collagen X-Cre transgene, which expresses the Cre recombinase in hypertrophic chondrocytes (38). In each of these models, the strong RANKL immunostaining that occurs in hypertrophic chondrocytes of control mice was eliminated in the conditional knockout animals (13). These results strongly suggest that hypertrophic chondrocytes are the major source of RANKL driving the formation of the osteoclasts that resorb calcified cartilage during endochondral bone formation.
Our next step was to utilize a DMP1-Cre transgene, which is active predominantly in osteocytes (10), to delete the RANKL conditional allele. Tooth eruption was normal in mice lacking RANKL in DMP1-Cre-expressing cells. When analyzed at 5 weeks of age, resorption of calcified cartilage was also normal and there was only a mild increase in bone mass (13). In a separate study, Takayanagi and colleagues used the same DMP1-Cre transgene to delete their RANKL conditional allele and also observed normal skeletal development and bone mass when the mice were analyzed at birth (12). However, both studies demonstrated that bone mass increases with age in mice lacking RANKL in DMP1-Cre-expressing cells. Importantly, deletion of RANKL from DMP1-Cre-expressing cells reduced the number of osteoclasts on cancellous bone as well as circulating markers of bone resorption. In addition, both bone formation (12) and circulating osteocalcin (13) were reduced in the conditional knockout mice due to the coupling of bone formation to bone resorption. These results strongly suggest that osteocytes are an essential source of the RANKL that controls osteoclast formation during cancellous bone remodeling.
Osteocytes versus other cell types
Osteoclasts are formed at different skeletal sites for different purposes. The results of the conditional RANKL deletion studies demonstrate that the osteoclasts that form at these different sites require different support cells in each case. Specifically, the finding that osteocyte-derived RANKL is not required for tooth eruption or resorption of calcified cartilage during endochondral bone formation demonstrates that other cell types must supply the RANKL required for osteoclast formation in these processes (13). In addition, femurs in mice with germline deletion of RANKL display the Erlenmeyer-flask or club shape that is typical of severe osteopetrosis (35). However, femurs in mice lacking RANKL in osteocytes are normally shaped indicating that modeling of the metaphyseal cortex of long bones is controlled by cells other than osteocytes. Thus the role of osteocyte-derived RANKL may be limited to bone remodeling.
Bone lining cells have also been proposed as an important source of RANKL for bone remodeling (39;40). Although the cellular lineage and function of lining cells are not entirely clear (41), preliminary lineage tracing studies confirm the long-standing idea that they are former osteoblasts, at least at the periosteum of murine calvaria and long bones (42). This evidence, together with the finding that the DMP1-Cre transgene used in the RANKL deletion studies leads to recombination in matrix-synthesizing osteoblasts (13), raises the possibility that at least some of the reduction in osteoclast number in these conditional knockout mice is due to loss of RANKL in lining cells. Nonetheless, Takayanagi and colleagues were able to detect RANKL protein in osteocytes by immunostaining (12) and we demonstrated that PTH stimulates RANKL mRNA abundance in cortical bone that had been extensively digested with collagenase to remove surface cells, which presumably included lining cells (13). While these findings indicate that osteocytes produce RANKL, they cannot exclude the possibility that RANKL produced by osteoblast-derived lining cells also contributes to osteoclastogenesis in cancellous bone. To directly address this issue, it will be necessary to develop Cre-deleter strains that have activity in osteocytes but not in osteoblasts or lining cells. In preliminary studies, we have found that deletion of RANKL using a Sost-Cre transgene produced a skeletal phenotype similar to that produced using the DMP1-Cre transgene (Xiong and O’Brien, unpublished results). Importantly, the Sost-Cre transgene is active in osteocytes but not osteoblasts, supporting the contention that RANKL produced by osteocytes but not lining cells controls cancellous bone remodeling.
The conclusion that osteocytes are a major source of RANKL leads to the question of how osteocyte-derived RANKL reaches osteoclast progenitors. The discovery that osteocytes control bone formation via secretion of sclerostin has established that osteocyte products can effectively reach precursor cells in the bone marrow (8). RANKL is produced as an integral membrane protein that can be shed to produce a soluble form (17). Although some in vitro studies have suggested that cell-to-cell contact between osteoclast progenitors and support cells is required for osteoclastogenesis (15), other studies have demonstrated that conditioned medium from the MLO-Y4 osteocytic cell line, which produces large amounts of RANKL (26), is sufficient to stimulate osteoclast formation (43). Moreover, expression of soluble RANKL (sRANKL) via a liver-specific promoter was sufficient to increase bone resorption in transgenic mice (44). Thus osteocytes may control osteoclast formation via production of sRANKL. On the other hand, osteocyte projections extend to the bone surface (45) leaving open the possibility that RANKL on the membrane of such projections interacts with osteoclast progenitors.
Osteocyte RANKL in biomechanics
Changes in mechanical load alter both bone mass and shape (46). Osteocytes within the lacunocanalicular network have long been proposed as the cells best suited to detect changes in load and then send signals that cause bone to be resorbed or formed in response to such changes (1). Mechanical strain regulates several molecular signals produced by osteocytes, including nitric oxide, prostaglandins, and sclerostin, which can influence bone resorption and formation (47–49). The recently demonstrated role of osteocyte RANKL in controlling cancellous bone remodeling suggests that RANKL may be another factor used by osteocytes to achieve skeletal adaptation.
The loss of mechanical load, such as occurs with bed rest or space flight, results in bone loss in part due to increased bone resorption (46;50). Conversely, excessive or fatigue loading induces formation of microcracks in areas of cortical bone which are subsequently resorbed (51). Importantly, both situations increase osteocyte apoptosis in specific regions of bone (50;51). Indeed, tail-suspension stimulates osteocyte apoptosis which is followed by bone resorption targeted to areas containing the apoptotic osteocytes in mice (50). Similarly, osteocyte apoptosis increases in fatigue-loaded rat ulnas in areas surrounding microcracks (51). In the latter case, inhibition of osteocyte apoptosis prevents the intra-cortical resorption that occurs in response to microcracks (52), suggesting that osteocyte apoptosis controls osteoclast recruitment to the damaged area. Consistent with this idea, Tatsumi and colleagues have demonstrated that stimulation of osteocyte apoptosis, in-and-of-itself, is sufficient to stimulate bone resorption that is associated with an increase in RANKL production in bone, but the cellular source of the RANKL was not determined (39).
Taken together, these studies suggest that osteocyte apoptosis generates one or more signals that increase bone resorption and direct it towards the area of bone containing the dead or dying cells. Apoptosis induced by serum starvation, or cell death induced by mechanical damage, increases production of sRANKL by MLO-Y4 cells as well as their ability to support osteoclast formation (43;53). In our recent study using mice with a conditional RANKL allele, we found that hindlimb unloading increases RANKL mRNA levels in cortical bone and that deletion of RANKL from DMP1-Cre expressing cells blunts the cortical bone loss associated with hindlimb unloading (13). These results, together with the finding that unloading induces osteocyte apoptosis (50), are consistent with the idea that osteocyte apoptosis stimulates bone resorption by increasing production of RANKL by osteocytes. However, it is unknown whether the elevated levels of RANKL are produced by dying osteocytes, healthy neighboring osteocytes, or both. Based on these lines of evidence, we have proposed the more general concept that cells embedded within mineralized matrix, such as osteocytes and hypertrophic chondrocytes, are ideally suited not only to detect the need for resorption, but also to target the resorption process to specific areas.
Osteocyte RANKL in Hormone Action
PTH controls the rate of bone remodeling and is a potent stimulator of RANKL expression (54–56). We have shown that PTH stimulates RANKL expression via a distant transcriptional enhancer, which we have termed the distal control region (57), and that mice lacking this enhancer have low bone turnover (58). These results demonstrate that one of the main mechanisms that PTH uses to control bone turnover is stimulation of RANKL production. However, the identity of the cells in which PTH controls RANKL expression in bone is not clear. Recently, we demonstrated that transgenic expression of a constitutively active PTH receptor in osteocytes was sufficient to increase RANKL production in bone as well as increase the rate of bone remodeling (59). In addition, two preliminary studies have demonstrated that deletion of the PTH receptor from osteocytes is sufficient to increase bone mass in adult mice (60;61). In one of these studies, the high bone mass was associated with reduced bone resorption and reduced RANKL mRNA abundance in bone (60). Thus, studies using gain- and loss-of-function of the PTH receptor are consistent with the contention that PTH controls bone remodeling by stimulating RANKL production in osteocytes.
Sex steroids also control the rate of bone remodeling, predominantly by suppressing osteoclastogenesis (21). Compelling genetic studies have shown that estrogen receptor (ER) action in osteoclasts mediates much of the effect of estrogen on osteoclastogenesis (62;63). Nonetheless, increased osteocyte apoptosis is another consistent finding in sex steroid deficiency (64–68). Moreover, Schaffler and colleagues have demonstrated that blockade of osteocyte apoptosis prevented the increase in cortical bone resorption that occurs in ovariectomized mice (65). Thus, similar to fatigue loading, loss of sex steroids may stimulate bone resorption in part by increasing osteocyte apoptosis and thereby RANKL expression.
There is very little evidence suggesting that estrogens or androgens directly control RANKL transcription (33). However, cell surface RANKL is elevated in lymphocytes, as well as in cells that may be osteoblast progenitors, in the bone marrow of early postmenopausal women (69). Consistent with this, preliminary studies using the RANKL conditional allele have shown that the ovariectomy-induced loss of cancellous bone is blunted in mice lacking RANKL in B lymphocytes (70). Moreover, orchiectomy increases the level of sRANKL in the bone marrow of rats, possibly due to increased shedding of membrane-bound RANKL (71;72). Thus, some evidence suggests that loss of sex steroids increases the abundance of RANKL protein and that this contributes to the increase in bone resorption associated with this condition. However, additional genetic deletion studies will be necessary to determine whether osteocytes are a significant source of this increase in RANKL.
Impact on remodeling theory
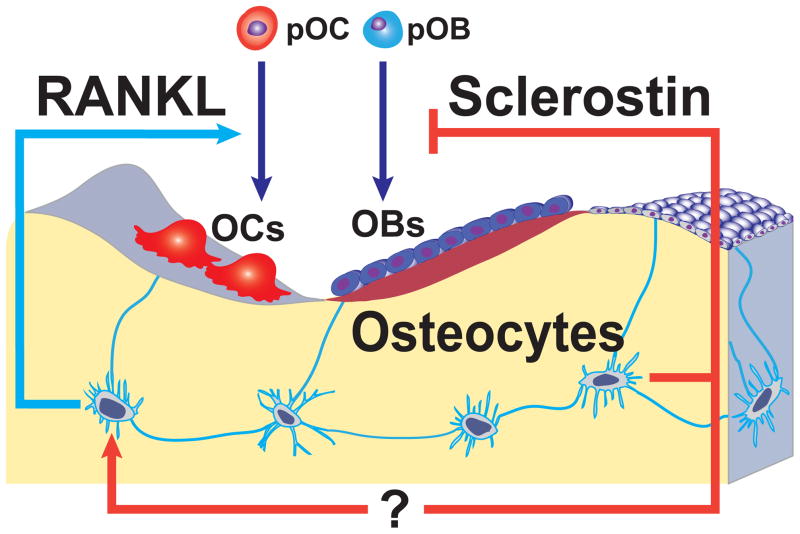
It is generally thought that the purposes of bone remodeling are to maintain skeletal mechanical properties and support mineral homeostasis (73). Bone remodeling is accomplished by teams of osteoclasts and osteoblasts acting within the anatomical structure known as the basic multicellular unit (BMU) (74). The large reduction in osteoclast number in mice lacking RANKL in osteocytes indicates that the number of BMUs is reduced in these mice. One possible conclusion based on this finding is that RANKL produced by osteocytes is a major stimulus for BMU origination and thus the overall rate of bone remodeling. Jilka and colleagues have recently reported that infusion of sRANKL or PTH stimulates bone resorption to similar levels, but that sRANKL does not increase bone formation to the same extent as PTH (75). These findings suggest that when remodeling is increased in response to RANKL, coupling mechanisms alone are not sufficient to maintain balanced remodeling, and that other mechanisms, such as suppression of the Wnt antagonist sclerostin, are necessary to increase bone formation to maintain focal balance. Work over the last several years has demonstrated that osteocytes are a major source of sclerostin (8;48;76;77). Thus the ability of osteocytes to independently control the rate of bone remodeling and the magnitude of bone formation may endow osteocytes with the unique ability to tailor the response of the remodeling apparatus to meet specific needs. In other words, osteocytes may control the number of BMUs via RANKL and the focal balance between resorption and formation within the BMUs via sclerostin (Figure 1). Additional levels of control may also be involved as suggested by a recent study showing that sclerostin stimulates RANKL expression in MLO-Y4 cells and primary human osteoblasts (78). A more complete understanding of the signals and mechanisms that control RANKL and sclerostin expression in osteocytes should help to clarify the extent to which these proteins work together to control physiological or pathological bone remodeling.
Figure 1.
Model depicting mechanisms by which osteocytes may independently control bone resorption and bone formation. Osteoclasts (OCs) and osteoblasts (OBs) within a cancellous BMU are shown as being derived from precursors (pOC and pOB). Osteocytes alter the rate of bone remodeling by controlling osteoclast formation via production of RANKL. On the other hand, osteocytes also control the balance between formation and resorption by regulating osteoblast formation via production of sclerostin. Sclerostin may have additional actions such as stimulation of RANKL expression by osteocytes.
Acknowledgments
The authors would like to thank Maria Almeida, Haibo Zhao, Robert Weinstein, Robert Jilka, and Stavros Manolagas for critical reading of this manuscript, and the National Institutes of Health (AR049794 and AG13918), the Department of Veterans Affairs (VA Merit Award), and the UAMS Translational Research Institute (1UL1RR029884) for their support.
References
- 1.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, Simmons HA, Crawford DT, Chidsey-Frink KL, Ke HZ, McNeish JD, Brown TA. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278:1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- 4.Brunkow ME, Gardner JC, Van NJ, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68:577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balemans W, Ebeling M, Patel N, Van HE, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den EJ, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van HW. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 6.Balemans W, Patel N, Ebeling M, Van HE, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van HW. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staehling-Hampton K, Proll S, Paeper BW, Zhao L, Charmley P, Brown A, Gardner JC, Galas D, Schatzman RC, Beighton P, Papapoulos S, Hamersma H, Brunkow ME. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110:144–152. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- 8.Van Bezooijen RL, Roelen BAJ, Visser A, Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CWGM. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W, Lu Y, Kalajzic I, Guo D, Harris MA, Gluhak-Heinrich J, Kotha S, Bonewald LF, Feng JQ, Rowe DW, Turner CH, Robling AG, Harris SE. Dentin matrix protein 1 gene cis-regulation: use in osteocytes to characterize local responses to mechanical loading in vitro and in vivo. J Biol Chem. 2005;280:20680–20690. doi: 10.1074/jbc.M500104200. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res. 2007;86:320–325. doi: 10.1177/154405910708600404. [DOI] [PubMed] [Google Scholar]
- 11.Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg MS, Mina M, Harris MA, Harris SE, Rowe DW. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35:74–82. doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 13.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodan GA, Martin TJ. Role of osteoblasts in hormonal control of bone resorption--a hypothesis. Calcif Tissue Int. 1981;33:349–351. doi: 10.1007/BF02409454. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 16.Udagawa N, Takahashi N, Akatsu T, Sasaki T, Yamaguchi A, Kodama H, Martin TJ, Suda T. The bone marrow-derived stromal cell lines MC3T3-G2/PA6 and ST2 support osteoclast-like cell differentiation in cocultures with mouse spleen cells. Endocrinology. 1989;125:1805–1813. doi: 10.1210/endo-125-4-1805. [DOI] [PubMed] [Google Scholar]
- 17.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi, Guo J, Delaney J, Boyle WJ. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo, Shultz LD. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- 20.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 21.Manolagas SC. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis [Review] Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- 22.Corral DA, Amling M, Priemel M, Loyer E, Fuchs S, Ducy P, Baron R, Karsenty G. Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc Natl Acad Sci U S A. 1998;95:13835–13840. doi: 10.1073/pnas.95.23.13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galli C, Fu Q, Wang W, Olsen BR, Manolagas SC, Jilka RL, O’Brien CA. Commitment to the osteoblast lineage is not required for RANKL gene expression. J Biol Chem. 2009;284:12654–12662. doi: 10.1074/jbc.M806628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otsuka E, Notoya M, Hagiwara H. Treatment of myoblastic C2C12 cells with BMP-2 stimulates vitamin D-induced formation of osteoclasts. Calcif Tissue Int. 2003;73:72–77. doi: 10.1007/s00223-002-1071-0. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh-Choudhury N, Singha PK, Woodruff K, St CP, Bsoul S, Werner SL, Choudhury GG. Concerted action of Smad and CREB-binding protein regulates bone morphogenetic protein-2-stimulated osteoblastic colony-stimulating factor-1 expression. J Biol Chem. 2006;281:20160–20170. doi: 10.1074/jbc.M511071200. [DOI] [PubMed] [Google Scholar]
- 26.Zhao S, Kato Y, Zhang Y, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res. 2002;17:2068–2079. doi: 10.1359/jbmr.2002.17.11.2068. [DOI] [PubMed] [Google Scholar]
- 27.Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, Riggs BL. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology. 2000;141:4768–4776. doi: 10.1210/endo.141.12.7840. [DOI] [PubMed] [Google Scholar]
- 28.Atkins GJ, Kostakis P, Pan BQ, Farrugia A, Gronthos S, Evdokiou A, Harrison K, Findlay DM, Zannettino ACW. RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res. 2003;18:1088–1098. doi: 10.1359/jbmr.2003.18.6.1088. [DOI] [PubMed] [Google Scholar]
- 29.Thomas GP, Baker SU, Eisman JA, Gardiner EM. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J Endocrinol. 2001;170:451–460. doi: 10.1677/joe.0.1700451. [DOI] [PubMed] [Google Scholar]
- 30.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, Nissenson RA. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–244. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 31.Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JMW, Niforas P, Ng KW, Martin TJ, Gillespie MT. Localization of RANKL (Receptor activator of NF kappa B ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone. 1999;25:525–534. doi: 10.1016/s8756-3282(99)00214-8. [DOI] [PubMed] [Google Scholar]
- 32.Silvestrini G, Ballanti P, Patacchioli F, Leopizzi M, Gualtieri N, Monnazzi P, Tremante E, Sardella D, Bonucci E. Detection of osteoprotegerin (OPG) and its ligand (RANKL) mRNA and protein in femur and tibia of the rat. J Mol Histol. 2005;36:59–67. doi: 10.1007/s10735-004-3839-1. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda T, Utsuyama M, Hirokawa K. Expression profiles of receptor activator of nuclear factor kappa B ligand, receptor activator of nuclear factor kappa B, and osteoprotegerin messenger RNA in aged and ovariectomized rat bones. J Bone Miner Res. 2001;16:1416–1425. doi: 10.1359/jbmr.2001.16.8.1416. [DOI] [PubMed] [Google Scholar]
- 34.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 35.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira dSA, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 36.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- 38.Gebhard S, Hattori T, Bauer E, Schlund B, Bosl MR, de CB, von der MK. Specific expression of Cre recombinase in hypertrophic cartilage under the control of a BAC-Col10a1 promoter. Matrix Biol. 2008;27:693–699. doi: 10.1016/j.matbio.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–475. doi: 10.1016/j.cmet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Eriksen EF, Eghbali-Fatourechi GZ, Khosla S. Remodeling and vascular spaces in bone. J Bone Miner Res. 2007;22:1–6. doi: 10.1359/jbmr.060910. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Liu Y, Kalajzic Z, Jiang X, Rowe DW. Heterogeneity of engrafted bone-lining cells after systemic and local transplantation. Blood. 2005;106:3650–3657. doi: 10.1182/blood-2005-02-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Pajevic PD, Seling M, Kronenberg H. Intermittent PTH of short term can activate quiescent lining cells to mature osteoblasts: unproven mechanism of the anabolic action of intermittent PTH. J Bone Miner Res. 2010;25:S46. [Google Scholar]
- 43.Kurata K, Heino TJ, Higaki H, Vaananen HK. Bone marrow cell differentiation induced by mechanically damaged osteocytes in 3D gel-embedded culture. J Bone Miner Res. 2006;21:616–625. doi: 10.1359/jbmr.060106. [DOI] [PubMed] [Google Scholar]
- 44.Mizuno A, Kanno T, Hoshi M, Shibata O, Yano K, Fujise N, Kinosaki M, Yamaguchi K, Tsuda E, Murakami A, Yasuda H, Higashio K. Transgenic mice overexpressing soluble osteoclast differentiation factor (sODF) exhibit severe osteoporosis. J Bone Miner Metab. 2002;20:337–344. doi: 10.1007/s007740200049. [DOI] [PubMed] [Google Scholar]
- 45.Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28:145–149. doi: 10.1016/s8756-3282(00)00421-x. [DOI] [PubMed] [Google Scholar]
- 46.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 47.Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res. 1996;11:1688–1693. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- 48.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 49.Aguirre J, Buttery L, O’Shaughnessy M, Afzal F, Fernandez dMI, Hukkanen M, Huang P, MacIntyre I, Polak J. Endothelial nitric oxide synthase gene-deficient mice demonstrate marked retardation in postnatal bone formation, reduced bone volume, and defects in osteoblast maturation and activity. Am J Pathol. 2001;158:247–257. doi: 10.1016/S0002-9440(10)63963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–615. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- 51.Verborgt O, Gibson GJ, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15:60–67. doi: 10.1359/jbmr.2000.15.1.60. [DOI] [PubMed] [Google Scholar]
- 52.Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24:597–605. doi: 10.1359/JBMR.081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Dujaili SA, Lau E, Al-Dujaili H, Tsang K, Guenther A, You L. Apoptotic osteocytes regulate osteoclast precursor recruitment and differentiation in vitro. J Cell Biochem. 2011;112:2412–2423. doi: 10.1002/jcb.23164. [DOI] [PubMed] [Google Scholar]
- 54.Ueno Y, Shinki T, Nagai Y, Murayama H, Fujii K, Suda T. In vivo administration of 1,25-dihydroxyvitamin D-3 suppresses the expression of RANKL mRNA in bone of thyroparathyroidectomized rats constantly infused with PTH. J Cell Biochem. 2003;90:267–277. doi: 10.1002/jcb.10623. [DOI] [PubMed] [Google Scholar]
- 55.Miao DS, He B, Lanske B, Bai XY, Tong XK, Hendy GN, Goltzman D, Karaplis AC. Skeletal abnormalities in Pth-null mice are influenced by dietary calcium. Endocrinology. 2004;145:2046–2053. doi: 10.1210/en.2003-1097. [DOI] [PubMed] [Google Scholar]
- 56.Fu Q, Jilka RL, Manolagas SC, O’Brien CA. Parathyroid hormone stimulates receptor activator of NFkappa B ligand and inhibits osteoprotegerin expression via protein kinase A activation of cAMP-response element-binding protein. J Biol Chem. 2002;277:48868–48875. doi: 10.1074/jbc.M208494200. [DOI] [PubMed] [Google Scholar]
- 57.Fu Q, Manolagas SC, O’Brien CA. Parathyroid Hormone Controls Receptor Activator of NF-{kappa}B Ligand Gene Expression via a Distant Transcriptional Enhancer. Mol Cell Biol. 2006;26:6453–6468. doi: 10.1128/MCB.00356-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galli C, Zella LA, Fretz JA, Fu Q, Pike JW, Weinstein RS, Manolagas SC, O’Brien CA. Targeted deletion of a distant transcriptional enhancer of the receptor activator of nuclear factor-kappaB ligand gene reduces bone remodeling and increases bone mass. Endocrinology. 2008;149:146–153. doi: 10.1210/en.2007-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tu X, Edwards R, Olivos N, Benson J, Galli C, Pellegrini G, Bivi N, Plotkin L, Bellido T. Conditional deletion of the parathyroid hormone (PTH) receptor 1 from osteocytes results in decreased bone resorption and a progressive increase in cancellous bone mass. J Bone Miner Res. 2011;26:S16. [Google Scholar]
- 61.Saini V, Barry K, Fulzele K, Feng J, Divieti PP. PTH/PTHrP (PPR) Receptor signaling in osteocytes regulate bone development in temporal manner. J Bone Miner Res. 2011;26:S16. [Google Scholar]
- 62.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 63.Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O’Brien CA, Manolagas SC. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24:323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82:3128–3135. doi: 10.1210/jcem.82.9.4200. [DOI] [PubMed] [Google Scholar]
- 65.Emerton KB, Hu B, Woo AA, Sinofsky A, Hernandez C, Majeska RJ, Jepsen KJ, Schaffler MB. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2010;46:577–583. doi: 10.1016/j.bone.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res. 1998;13:1243–1250. doi: 10.1359/jbmr.1998.13.8.1243. [DOI] [PubMed] [Google Scholar]
- 67.Kousteni S, Bellido T, Plotkin LI, O’Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 68.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O’Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 69.Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest. 2003;111:1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Onal M, Xiong J, Cazer P, Manolagas S, O’Brien C. RANKL production by B lymphocytes contributes to the bone loss induced by inflammation and ovariectomy. J Bone Miner Res. 2011;26:S142. [Google Scholar]
- 71.Proell V, Xu H, Schuler C, Weber K, Hofbauer LC, Erben RG. Orchiectomy upregulates free soluble RANKL in bone marrow of aged rats. Bone. 2009;45:677–681. doi: 10.1016/j.bone.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 72.Li X, Ominsky MS, Stolina M, Warmington KS, Geng Z, Niu QT, Asuncion FJ, Tan HL, Grisanti M, Dwyer D, Adamu S, Ke HZ, Simonet WS, Kostenuik PJ. Increased RANK ligand in bone marrow of orchiectomized rats and prevention of their bone loss by the RANK ligand inhibitor osteoprotegerin. Bone. 2009;45:669–676. doi: 10.1016/j.bone.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 73.Parfitt AM. Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone. 2002;30:5–7. doi: 10.1016/s8756-3282(01)00642-1. [DOI] [PubMed] [Google Scholar]
- 74.Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. [Review] [52 refs] J Cell Biochem. 1994;55:273–286. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- 75.Jilka RL, O’Brien CA, Bartell SM, Weinstein RS, Manolagas SC. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and -independent mechanisms. J Bone Miner Res. 2010;25:2427–2437. doi: 10.1002/jbmr.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 77.Poole KE, Van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 78.Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin Stimulates Osteocyte Support of Osteoclast Activity by a RANKL-Dependent Pathway. PLoS One. 2011;6:e25900. doi: 10.1371/journal.pone.0025900. [DOI] [PMC free article] [PubMed] [Google Scholar]